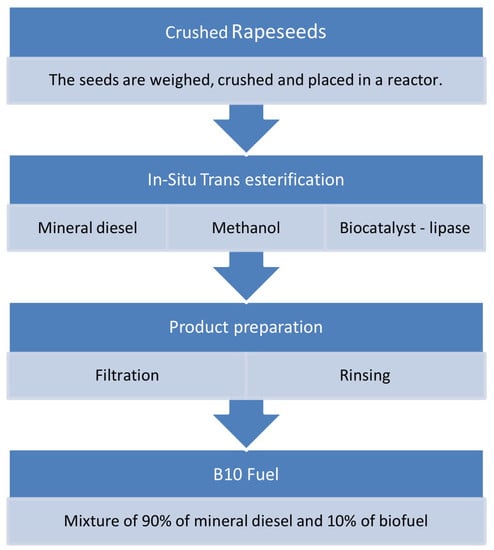
Lipase-Catalysed In Situ Transesterification of Waste Rapeseed Oil to Produce Diesel-Biodiesel Blends
Abstract
1. Introduction
2. Materials and Methods
2.1. Seed Quality Analysis
2.2. Selection of Lipase for In Situ Transesterification
2.3. Optimization of the Lipase-Catalysed In Situ Transesterification
2.4. Determination of the Rapeseed Oil Extraction Effectiveness and Methyl Ester Yield in the Reaction Product
2.5. Determination of Transesterification Degree
3. Results and Discussion
3.1. Seed Quality Analysis
3.2. Selection of Lipase
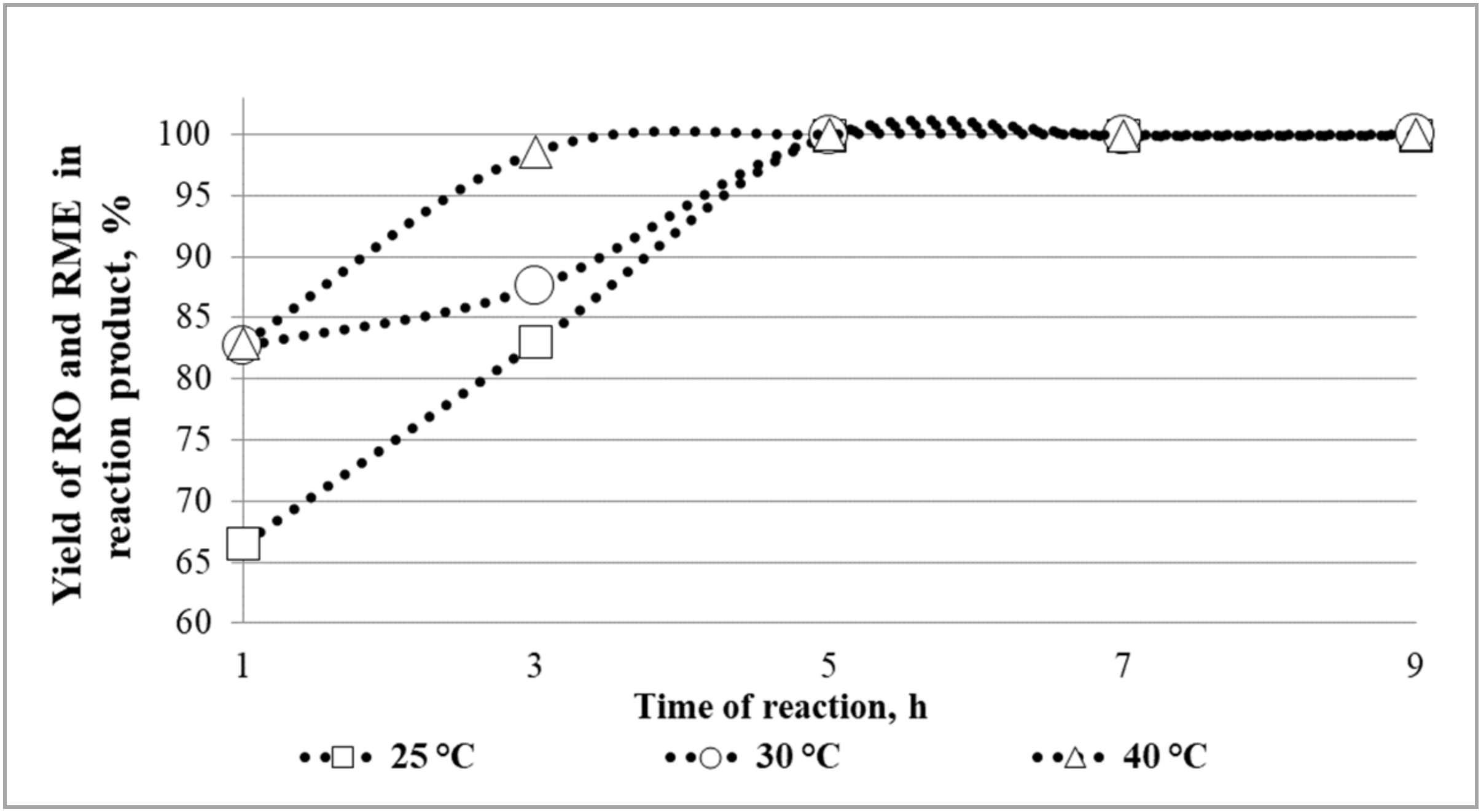
3.3. Optimization of the Lipase-Catalysed In Situ Transesterification Process Parameters
4. Conclusions
- A diesel–biodiesel blend can be produced directly from prospective non-edible renewable raw material-rapeseed containing the oil of high acidity, by the application of simultaneous oil extraction and transesterification in situ with methanol and diesel mixture and catalysed by the lipase Lipozyme TL IM.
- The optimal conditions for in situ transesterification process when the methanol to oil molar ratio is 5:1 are as follows: temperature of the reaction of 25 °C, time of the reaction of 5 h, and lipase concentration of 5%.
- Under optimal conditions 99.90% (w/w) of oil is extracted from the seed and transesterified. The degree of transesterification of 98.76% (w/w) is obtained.
- These results indicate that waste rapeseeds are a suitable raw material to produce biodiesel with an in situ transesterification process using the lipase Lipozyme TL IM as the catalyst and methanol as acyl receptor. The resulting biodiesel fulfils the requirements for the degree of transesterification and the requirements for glyceride content in biodiesel fuel.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Go, A.W.; Sutanto, S.; Ong, L.K.; Tran-Nguyen, P.L.; Ismadji, S.; Ju, Y.H. Developments in in-situ (trans) esterification for biodiesel production: A critical review. Renew. Sustain. Energy Rev. 2016, 60, 284–305. [Google Scholar] [CrossRef]
- Atabani, A.E.; Silitonga, A.S.; Badruddin, I.A.; Mahlia, T.M.I.; Masjuki, H.H.; Mekhilef, S. A comprehensive review on biodiesel as an alternative energy resource and its characteristics. Renew. Sustain. Energy Rev. 2012, 16, 2070–2093. [Google Scholar] [CrossRef]
- Go, A.W.; Sutanto, S.; Liu, Y.T.; Tran-Nguyen, P.L.; Ismadji, S.; Ju, Y.H. In situ transesterification of Jatropha curcas L. seeds in subcritical solvent system. J. Taiwan Inst. Chem. 2014, 45, 1516–1522. [Google Scholar] [CrossRef]
- Patel, R.L.; Sankhavara, C.D. Biodiesel production from Karanja oil and its use in diesel engine a review. Renew. Sustain. Energy Rev. 2017, 71, 464–474. [Google Scholar] [CrossRef]
- Dasari, S.R.; Borugadda, V.B.; Goud, V.V. Reactive extraction of castor seeds and storage stability characteristics of produced biodiesel. Process. Saf. Environ. Prot. 2016, 100, 252–263. [Google Scholar] [CrossRef]
- Koutsouki, A.A.; Tegou, E.; Kontakos, S.; Kontominas, M.G.; Pomonis, P.J.; Manos, G. In situ transesterification of Cynara cardunculus L. seed oil via direct ultrasonication for the production of biodiesel. Fuel Process. Technol. 2015, 134, 122–129. [Google Scholar] [CrossRef]
- Wu, H.; Liu, Y.; Zhang, J.; Li, G. In situ reactive extraction of cottonseeds with methyl acetate for biodiesel production using magnetic solid acid catalysts. Bioresour. Technol. 2014, 174, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tu, Q.; Knothe, G.; Lu, M. Direct transesterification of spent coffee grounds for biodiesel production. Fuel 2017, 199, 157–161. [Google Scholar] [CrossRef]
- Tuntiwiwattanapuna, N.; Monono, E.; Wiesenborn, D.; Tongcumpou, C. In-situ transesterification process for biodiesel production using spent coffee grounds from the instant coffee industry. Ind. Crop. Prod. 2017, 102, 23–31. [Google Scholar] [CrossRef]
- Choi, O.K.; Song, J.S.; Cha, D.K.; Lee, J.W. Biodiesel production from wet municipal sludge: Evaluation of in situ transesterification using xylene as a cosolvent. Bioresour. Technol. 2014, 166, 51–56. [Google Scholar] [CrossRef]
- Salam, K.A.; Velasquez-Orta, S.B.; Harvey, A.P. A sustainable integrated in situ transesterification of microalgae for biodiesel production and associated co-product-a review. Renew. Sustain. Energy Rev. 2016, 65, 1179–1198. [Google Scholar] [CrossRef]
- Gouveia, L.; Oliveira, A.C. Microalgae as a raw material for biofuels production. J. Ind. Microbiol. Biotechnol. 2009, 36, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Mendow, G.; Querini, C.A. High performance purification process of methyl and ethyl esters produced by transesterification. Chem. Eng. J. 2013, 228, 93–101. [Google Scholar] [CrossRef]
- Sendzikiene, E.; Makareviciene, V.; Gumbyte, M. Reactive extraction and fermental transesterification of rapeseed oil with butanol in diesel fuel media. Fuel Process. Technol. 2015, 138, 758–764. [Google Scholar] [CrossRef]
- Musa, I.A. The effects of alcohol to oil molar ratios and the type of alcohol on biodiesel production using transesterification process. Egypt J. Pet. 2016, 25, 21–31. [Google Scholar] [CrossRef]
- Shahid, E.M.; Jamal, Y. Production of biodiesel: A technical review. Renew. Sustain. Energy Rev. 2011, 15, 4732–4745. [Google Scholar] [CrossRef]
- Shuit, S.H.; Lee, K.T.; Kamaruddin, A.H.; Yusup, S. Reactive extraction and in situ esterification of Jatropha curcas L. seeds for the production of biodiesel. Fuel 2010, 89, 527–530. [Google Scholar] [CrossRef]
- Cao, H.; Zhang, Z.; Wu, X.; Miao, X. Direct Biodiesel Production from Wet Microalgae Biomass of Chlorella pyrenoidosa through In Situ Transesterification. BioMed Res. Int. 2013, 2013, 930686. [Google Scholar] [CrossRef]
- Atadashi, I.M.; Aroua, M.K.; Aziz, A.R.A.; Sulaiman, N.M.N. The effects of catalysts in biodiesel production: A review. J. Ind. Eng. Chem. 2013, 19, 14–26. [Google Scholar] [CrossRef]
- Abo El-Enin, S.A.; Attia, N.K.; El-Ibiari, N.N.; El-Diwani, G.I.; El-Khatib, K.M. In-situ transesterification of rapeseed and cost indicators for biodiesel production. Renew. Sustain. Energy Rev. 2013, 18, 471–477. [Google Scholar] [CrossRef]
- Ginting, M.S.A.; Azizan, M.T.; Yusup, S. Alkaline in situ ethanolysis of Jatropha curcas. Fuel 2012, 93, 82–85. [Google Scholar] [CrossRef]
- Thliveros, P.; Kiran, E.U.; Web, C. Microbial biodiesel production by direct methanolysis of oleaginous biomass. Bioresour. Technol. 2014, 157, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, H.; Kondo, A.; Noda, H. Biodiesel fuel production by transesterification of oils. J. Biosci. Bioeng. 2001, 92, 405–416. [Google Scholar] [CrossRef]
- Jiang, Y.; Gu, H.; Zhou, L.; Cui, C.; Gao, J. Novel in situ batch reactor with a facile catalyst separation device for biodiesel production. Ind. Eng. Chem. Res. 2012, 51, 14935–14940. [Google Scholar] [CrossRef]
- Su, E.; You, P.; Wei, D. In situ lipase-catalyzed reactive extraction of oilseeds with short-chained dialkyl carbonates for biodiesel production. Bioresour. Technol. 2009, 100, 5813–5817. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, D.; Li, Y.; Gao, J.; Zhou, L.; He, Y. In situ self-catalyzed reactive extraction of germinated oilseed with short-chained dialkyl carbonates for biodiesel production. Bioresour. Technol. 2013, 150, 50–54. [Google Scholar] [CrossRef]
- Makareviciene, V.; Sendzikiene, E.; Gumbyte, M. Application of Simultaneous Oil Extraction and Transesterification in Biodiesel Fuel Synthesis: A Review. Energies 2020, 13, 2204. [Google Scholar] [CrossRef]
- Makareviciene, V.; Gumbyte, M.; Sendzikiene, E. Simultaneous extraction of microalgae Ankistrodesmus sp. oil and enzymatic transesterification with ethanol in the mineral diesel medium. Food Bioprod. Process. 2019, 116, 89–97. [Google Scholar] [CrossRef]
- Gumbytė, M.; Makareviciene, V.; Skorupskaite, V.; Sendzikiene, E.; Kondratavicius, M. Enzymatic microalgae oil transesterification with ethanol in mineral diesel fuel media. J. Renew. Sustain. Energy 2018, 10, 013105. [Google Scholar] [CrossRef]
- International Organization for Standardization. ISO 659:2009. Oilseeds—Determination of Oil Content (Reference Method); International Organization for Standardization: Geneva, Switzerland, 2009. [Google Scholar]
- International Organization for Standardization. EN ISO 5509:2000. Animal and Vegetable Fats and Oils—Preparation of Methyl Esters of Fatty Acids; International Organization for Standardization: Geneva, Switzerland, 2000. [Google Scholar]
- International Organization for Standardization. EN ISO 5508:1996. Animal and Vegetable Fats and Oils—Analysis by Gas Chromatography of Methyl Esters of Fatty Acids; International Organization for Standardization: Geneva, Switzerland, 1996. [Google Scholar]
- International Organization for Standardization. ISO 660:2020. Animal and Vegetable Fats and Oils—Determination of Acid Value and Acidity; International Organization for Standardization: Geneva, Switzerland, 2020. [Google Scholar]
- International Organization for Standardization. ISO 665:2020. Oilseeds—Determination of Moisture and Volatile Matter Content; International Organization for Standardization: Geneva, Switzerland, 2020. [Google Scholar]
- European Committee for Standardization. EN 14078:2014. Liquid Petroleum Products. Determination of Fatty Acid Methyl Ester (FAME) Content in Middle Distillates. Infrared Spectrometry Method; European Committee for Standardization: Brussels, Belgium, 2014. [Google Scholar]
- Santaraite, M.; Sendzikiene, E.; Makareviciene, V.; Kazancev, K. Biodiesel Production by Lipase-Catalyzed in Situ Transesterification of Rapeseed Oil Containing a High Free Fatty Acid Content with Ethanol in Diesel Fuel Media. Energies 2020, 13, 2588. [Google Scholar] [CrossRef]
- European Committee for Standardization. EN 14105:2011. Fat and Oil Derivatives—Fatty Acid Methyl Esters (FAME)—Determination of Free and Total Glycerol and Mono-, Di-, Triglyceride Contents; European Committee for Standardization: Brussels, Belgium, 2011. [Google Scholar]
- Sendzikiene, E.; Sinkuniene, D.; Kazanceva, I.; Kazancev, K. Optimization of low quality rapeseed oil transesterification with butanol by applying the response surface methodology. Renew. Energy 2016, 87, 266–272. [Google Scholar] [CrossRef]
- Abbadi, A.; Leckband, G. Rapeseed breeding for oil content, quality, and sustainability. Eur. J. Lipid Sci. Technol. 2011, 113, 1198–1206. [Google Scholar] [CrossRef]
- Giakoumis, E.G. Analysis of 22 vegetable oils’ physico-chemical properties and fatty acid composition on a statistical basis, and correlation with the degree of unsaturation. Renew. Energy 2018, 126, 403–419. [Google Scholar] [CrossRef]
- Beyzi, E.; Gunes, A.; Buyukkilic Beyzi, S.; Konca, Y. Changes in fatty acid and mineral composition of rapeseed (Brassica napus ssp. oleifera L.) oil with seed sizes. Ind. Crop. Prod. 2019, 129, 10–14. [Google Scholar]
- Kwiecien, J.; Hájek, M.; Skopal, F. The effect of the acidity of rapeseed oil on its transesterification. Bioresour. Technol. 2009, 100, 5555–5559. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aamer, F.H.; Hameed, A.S.A. Industrial applications of microbial lipazes. Enzym. Microb. Technol. 2006, 39, 235–251. [Google Scholar]
- Nguyen, H.C.; Huong, D.T.M.; Juan, H.Y.; Su, C.H.; Chien, C.C. Liquid Lipase-Catalyzed Esterification of Oleic Acid with Methanol for Biodiesel Production in the Presence of Superabsorbent Polymer: Optimization by Using Response Surface Methodology. Energies 2018, 11, 1085. [Google Scholar] [CrossRef]
- Hernįndez-Martķn, E.; Otero, C. Different enzyme requirements for the synthesis of biodiesel: Novozym-35 and Lipozyme-TL IM. Bioresour. Technol. 2008, 99, 277–286. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Volpato, G.; Wada, K.; Ayub, M.A.Z. Enzymatic synthesis of biodiesel from transesterification reactions of vegetable oils and short chain alcohols. J. Am. Oil Chem. Soc. 2008, 85, 925–930. [Google Scholar] [CrossRef]
- Fjerbaek, L.; Christensen, K.V.; Norddahl, B. A review of the current state of biodiesel production using enzymatic transesterification. Biotechnol. Bioeng. 2009, 102, 1298–1315. [Google Scholar] [CrossRef]
- Stergiou, P.-Y.; Foukis, A.; Filippou, M.; Koukouritaki, M.; Parapouli, M.; Theodorou, L.G.; Papamichael, E.M. Advances in lipase-catalyzed esterification reactions. Biotechnol. Adv. 2013, 31, 1846–1859. [Google Scholar] [CrossRef] [PubMed]
- Nie, K.; Xie, F.; Wang, F.; Tan, T. Lipase catalyzed methanolysis to produce biodiesel: Optimization of the biodiesel production. J. Mol. Catal. B Enzym. 2006, 43, 142–147. [Google Scholar] [CrossRef]
- Antczak, M.S.; Kubiak, A.; Antczak, T.; Bielecki, S. Enzymatic biodiesel synthesis—Key factors affecting efficiency of the process. Renew. Energy 2009, 34, 1185–1194. [Google Scholar] [CrossRef]

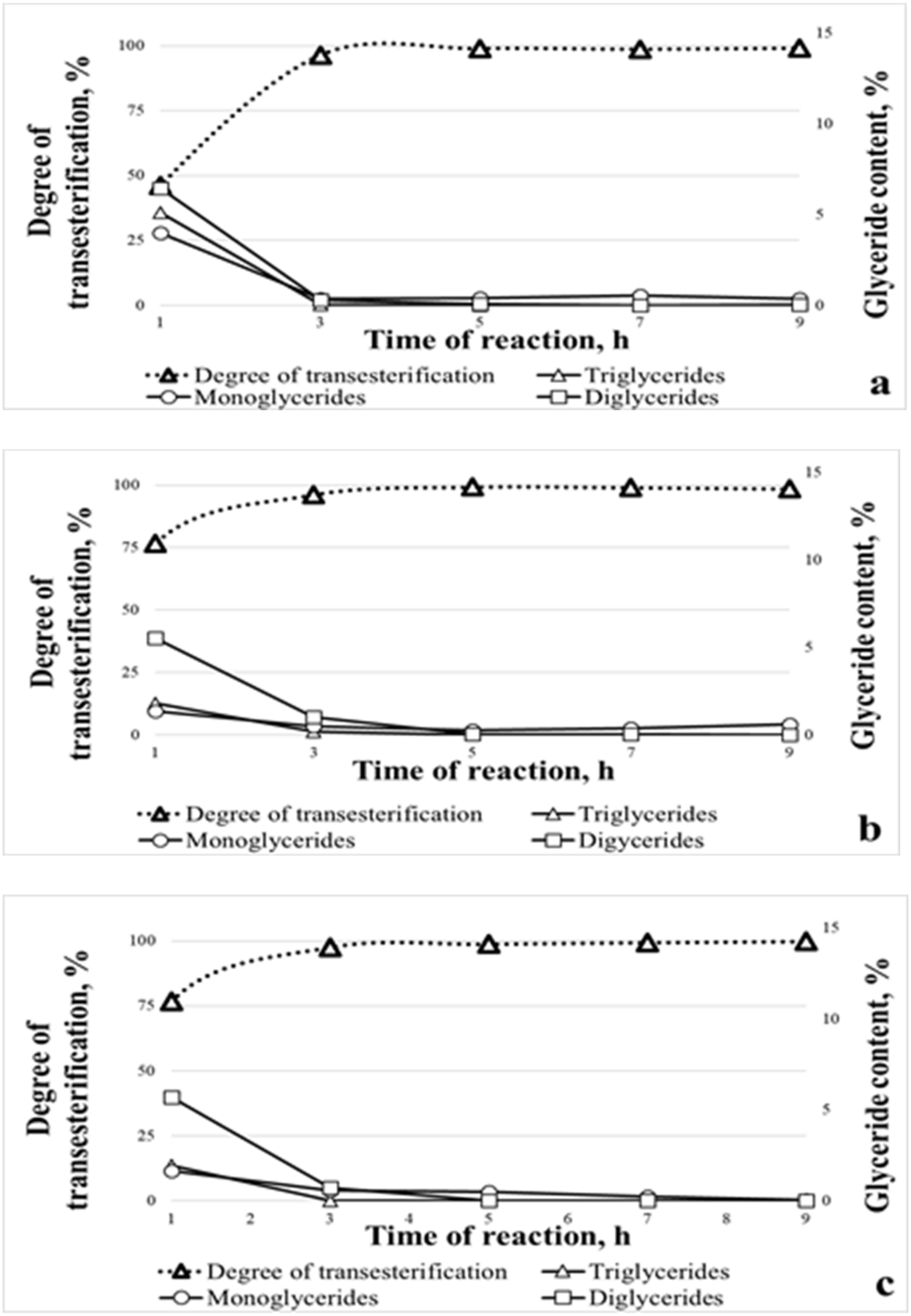
| Reaction Duration [h] | Reaction Temperature [°C] | Glyceride Contents in Biodiesel Fuel [%(w/w)] | ||
|---|---|---|---|---|
| Monoglycerides | Diglycerides | Triglycerides | ||
| 1 | 25 | 3.97 ± 0.06 | 6.43 ± 0.07 | 35.78 ± 0.10 |
| 1 | 30 | 1.35 ± 0.03 | 5.55 ± 0.05 | 12.54 ± 0.09 |
| 1 | 40 | 1.64 ± 0.03 | 5.70 ± 0.04 | 13.46 ± 0.09 |
| 3 | 25 | 0.35 ± 0.01 | 0.27 ± 0.02 | 0.33 ± 0.04 |
| 3 | 30 | 0.47 ± 0.02 | 1.01 ± 0.03 | 1.23 ± 0.03 |
| 3 | 40 | 0.57 ± 0.02 | 0.69 ± 0.02 | 0 ± 0 |
| 5 | 25 | 0.38 ± 0.03 | 0.06 ± 0.02 | 0 ± 0 |
| 5 | 30 | 0.27 ± 0.02 | 0.04 ± 0.02 | 0 ± 0 |
| 5 | 40 | 0.47 ± 0.02 | 0.01 ± 0.01 | 0 ± 0 |
| 7 | 25 | 0.52 ± 0.03 | 0.01 ± 0.01 | 0 ± 0 |
| 7 | 30 | 0.38 ± 0.02 | 0.02 ± 0.01 | 0 ± 0 |
| 7 | 40 | 0.23 ± 0.03 | 0.01 ± 0.00 | 0 ± 0 |
| 9 | 25 | 0.34 ± 0.02 | 0.02 ± 0.01 | 0 ± 0 |
| 9 | 30 | 0.59 ± 0.02 | 0.01 ± 0.00 | 0 ± 0 |
| 9 | 40 | 0.04 ± 0.01 | 0.01 ± 0.00 | 0 ± 0 |
| Lipase Concentration [%] | Rapeseed Oil and Rapeseed Methyl Ester Yield in Biological Fraction of Reaction Product [%(w/w)] | Degree of Transesterification [%(w/w)] |
|---|---|---|
| 3 | 98.99 ± 0.08 | 93.06 ± 0.10 |
| 4 | 99.55 ± 0.10 | 94.84 ± 0.14 |
| 5 | 99.90 ± 0.09 | 98.75 ± 0.08 |
| 6 | 99.95 ± 0.05 | 98.76 ± 0.06 |
| Lipase Concentration [%] | Glycerides Contents in Biodiesel Fuel [%] | ||
|---|---|---|---|
| Monoglycerides | Diglycerides | Triglycerides | |
| 3 | 0.77 ± 0.06 | 1.58 ± 0.05 | 2.89 ± 0.06 |
| 4 | 0.79 ± 0.04 | 1.02 ± 0.03 | 1.83 ± 0.05 |
| 5 | 0.46 ± 0.02 | 0.07 ± 0.01 | 0 ± 0.0 |
| 6 | 0.47 ± 0.04 | 0.06 ± 0.02 | 0 ± 0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sendzikiene, E.; Santaraite, M.; Makareviciene, V. Lipase-Catalysed In Situ Transesterification of Waste Rapeseed Oil to Produce Diesel-Biodiesel Blends. Processes 2020, 8, 1118. https://doi.org/10.3390/pr8091118
Sendzikiene E, Santaraite M, Makareviciene V. Lipase-Catalysed In Situ Transesterification of Waste Rapeseed Oil to Produce Diesel-Biodiesel Blends. Processes. 2020; 8(9):1118. https://doi.org/10.3390/pr8091118
Chicago/Turabian StyleSendzikiene, Egle, Migle Santaraite, and Violeta Makareviciene. 2020. "Lipase-Catalysed In Situ Transesterification of Waste Rapeseed Oil to Produce Diesel-Biodiesel Blends" Processes 8, no. 9: 1118. https://doi.org/10.3390/pr8091118
APA StyleSendzikiene, E., Santaraite, M., & Makareviciene, V. (2020). Lipase-Catalysed In Situ Transesterification of Waste Rapeseed Oil to Produce Diesel-Biodiesel Blends. Processes, 8(9), 1118. https://doi.org/10.3390/pr8091118