Thermostable α-Glucan Phosphorylase-Catalyzed Enzymatic Copolymerization to Produce Partially 2-Deoxygenated Amyloses
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Thermostable α-Glucan Phosphorylase-Catalyzed Enzymatic Copolymerization of d-Glucal with Glc-1-P
2.3. Film Formation from Partially 2-Deoxygenated Amylose (Entry 4)
2.4. Measurements
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Berg, J.M.; Tymoczko, J.L.; Stryer, L. Biochemistry, 7th ed.; W. H. Freeman: New York, NY, USA, 2012. [Google Scholar]
- Schuerch, C. Polysaccharides. In Encyclopedia of Polymer Science and Engineering, 2nd ed.; Mark, H.F., Bilkales, N., Overberger, C.G., Eds.; John Wiley & Sons: New York, NY, USA, 1986; Volume 13, pp. 87–162. [Google Scholar]
- Stephen, A.M.; Phillips, G.O.; Williams, P.A. Food Polysaccharides and Their Applications, 2nd ed.; CRC/Taylor & Francis: Boca Raton, FL, USA, 2006; p. 733. [Google Scholar]
- DeAngelis, P.L. Glycosaminoglycan polysaccharide biosynthesis and production: Today and tomorrow. Appl. Microbiol. Biotechnol. 2012, 94, 295–305. [Google Scholar] [CrossRef]
- Gómez, B.; Míguez, B.; Yáñez, R.; Alonso, J.L. Manufacture and Properties of Glucomannans and Glucomannooligosaccharides Derived from Konjac and Other Sources. J. Agric. Food Chem. 2017, 65, 2019–2031. [Google Scholar] [CrossRef]
- Kobayashi, S.; Uyama, H.; Kimura, S. Enzymatic polymerization. Chem. Rev. 2001, 101, 3793–3818. [Google Scholar] [CrossRef]
- Shoda, S.; Izumi, R.; Fujita, M. Green process in glycotechnology. Bull. Chem. Soc. JPN 2003, 76, 1–13. [Google Scholar] [CrossRef]
- Kobayashi, S.; Makino, A. Enzymatic polymer synthesis: An opportunity for green polymer chemistry. Chem. Rev. 2009, 109, 5288–5353. [Google Scholar] [CrossRef] [PubMed]
- Kadokawa, J. Precision polysaccharide synthesis catalyzed by enzymes. Chem. Rev. 2011, 111, 4308–4345. [Google Scholar] [CrossRef] [PubMed]
- Shoda, S.; Uyama, H.; Kadokawa, J.; Kimura, S.; Kobayashi, S. Enzymes as green catalysts for precision macromolecular synthesis. Chem. Rev. 2016, 116, 2307–2413. [Google Scholar] [CrossRef]
- Kadokawa, J. Synthesis of non-natural oligosaccharides by α-glucan phosphorylase-catalyzed enzymatic glycosylations using analogue substrates of α-D-glucose 1-phosphate. Trends Glycosci. Glycotechnol. 2013, 25, 57–69. [Google Scholar] [CrossRef]
- Kadoakwa, J. Enzymatic synthesis of non-natural oligo- and polysaccharides by phosphorylase-catalyzed glycosylations using analogue substrates. In Green Polymer Chemistry: Biobased Materials and Biocatalysis; ACS Symposium Series 1192; Cheng, H.N., Gross, R.A., Smith, P.B., Eds.; American Chemical Society: Washington, DC, USA, 2015; pp. 87–99. [Google Scholar]
- Kadokawa, J. Precision synthesis of functional polysaccharide materials by phosphorylase-catalyzed enzymatic reactions. Polymers 2016, 8, 138. [Google Scholar] [CrossRef]
- Kadokawa, J. α-Glucan phosphorylase: A useful catalyst for precision enzymatic synthesis of oligo- and polysaccharides. Curr. Org. Chem. 2017, 21, 1192–1204. [Google Scholar] [CrossRef]
- Kadokawa, J.I. α-Glucan phosphorylase-catalyzed enzymatic reactions using analog substrates to synthesize non-natural oligo-and polysaccharides. Catalysts 2018, 8, 473. [Google Scholar] [CrossRef]
- Ziegast, G.; Pfannemüller, B. Linear and star-shaped hybrid polymers. Phosphorolytic syntheses with di-functional, oligo-functional and multifunctional primers. Carbohydr. Res. 1987, 160, 185–204. [Google Scholar]
- Kitaoka, M.; Hayashi, K. Carbohydrate-processing phosphorolytic enzymes. Trends Glycosci. Glycotechnol. 2002, 14, 35–50. [Google Scholar] [CrossRef]
- Fujii, K.; Takata, H.; Yanase, M.; Terada, Y.; Ohdan, K.; Takaha, T.; Okada, S.; Kuriki, T. Bioengineering and application of novel glucose polymers. Biocatal. Biotransform 2003, 21, 167–172. [Google Scholar] [CrossRef]
- Seibel, J.; Jordening, H.J.; Buchholz, K. Glycosylation with activated sugars using glycosyltransferases and transglycosidases. Biocatal. Biotransform 2006, 24, 311–342. [Google Scholar] [CrossRef]
- Yanase, M.; Takaha, T.; Kuriki, T. α-Glucan phosphorylase and its use in carbohydrate engineering. J. Sci. Food Agric. 2006, 86, 1631–1635. [Google Scholar] [CrossRef]
- Nakai, H.; Kitaoka, M.; Svensson, B.; Ohtsubo, K. Recent development of phosphorylases possessing large potential for oligosaccharide synthesis. Curr. Opin. Chem. Biol. 2013, 17, 301–309. [Google Scholar] [CrossRef]
- O’Neill, E.C.; Field, R.A. Enzymatic synthesis using glycoside phosphorylases. Carbohydr. Res. 2015, 403, 23–37. [Google Scholar] [CrossRef]
- Puchart, V. Glycoside phosphorylases: Structure, catalytic properties and biotechnological potential. Biotechnol. Adv. 2015, 33, 261–276. [Google Scholar] [CrossRef]
- Kadokawa, J.; Shimohigoshi, R.; Yamashita, K.; Yamamoto, K. Synthesis of chitin and chitosan stereoisomers by thermostable α-glucan phosphorylase-catalyzed enzymatic polymerization of α-D-glucosamine 1-phosphate. Org. Bimol. Chem. 2015, 13, 4336–4343. [Google Scholar] [CrossRef]
- Makino, A.; Kobayashi, S. Chemistry of 2-oxazolines: A crossing of cationic ring-opening polymerization and enzymatic ring-opening polyaddition. J. Polym. Sci. Polym. Chem. 2010, 48, 1251–1270. [Google Scholar] [CrossRef]
- Ochiai, H.; Fujikawa, S.; Ohmae, M.; Kobayashi, S. Enzymatic copolymerization to hybrid glycosaminoglycans: A novel strategy for intramolecular hybridization of polysaccharides. Biomacromolecules 2007, 8, 1802–1806. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, K.; Yamamoto, K.; Kadoakwa, J. Synthesis of non-natural heteroaminopolysaccharides by α-glucan phosphorylase-catalyzed enzymatic copolymerization: α(1->4)-linked glucosaminoglucans. Biomacromolecules 2015, 16, 3989–3994. [Google Scholar] [CrossRef] [PubMed]
- Baba, R.; Yamamoto, K.; Kadokawa, J. Synthesis of α(1-->4)-linked non-natural mannoglucans by alpha-glucan phosphorylase-catalyzed enzymatic copolymerization. Carbohydr. Polym. 2016, 151, 1034–1039. [Google Scholar] [CrossRef] [PubMed]
- Klein, H.W.; Palm, D.; Helmreich, E.J.M. General acid-base catalysis of α-glucan phosphorylases: Stereospecific glucosyl transfer from D-glucal is a pyridoxal 5′-phosphate and orthophosphate (arsenate) dependent reaction. Biochemistry 1982, 21, 6675–6684. [Google Scholar] [CrossRef] [PubMed]
- Evers, B.; Mischnick, P.; Thiem, J. Synthesis of 2-deoxy-α-D-arabino-hexopyranosyl phosphate and 2-deoxy-maltooligosaccharides with phosphorylase. Carbohydr. Res. 1994, 262, 335–341. [Google Scholar] [CrossRef]
- Evers, B.; Thiem, J. Synthesis of 2-Deoxy-maltooligosaccharides with Phosphorylase and Their Degradation with Amylases. Starch Stärke 1995, 47, 434–439. [Google Scholar] [CrossRef]
- Evers, B.; Thiem, J. Further syntheses employing phosphorylase. Bioorg. Med. Chem. 1997, 5, 857–863. [Google Scholar] [CrossRef]
- Uto, T.; Nakamura, S.; Yamamoto, K.; Kadokawa, J. Evaluation of artificial crystalline structure from amylose analog polysaccharide without hydroxy groups at C-2 position. Carbohydr. Polym. 2020, 240, 116347. [Google Scholar] [CrossRef]
- Bhuiyan, S.H.; Rus’d, A.A.; Kitaoka, M.; Hayashi, K. Characterization of a Hyperthermostable Glycogen Phosphorylase from Aquifex aeolicus Expressed in Escherichia coli. J. Mol. Catal. B Enzym. 2003, 22, 173–180. [Google Scholar] [CrossRef]
- Yanase, M.; Takata, H.; Fujii, K.; Takaha, T.; Kuriki, T. Cumulative effect of amino acid replacements results in enhanced thermostability of potato type L α-glucan phosphorylase. Appl. Environ. Microbiol. 2005, 71, 5433–5439. [Google Scholar] [CrossRef] [PubMed]
- Kadokawa, J. Enzymatic synthesis of functional amylosic materials and amylose analog polysaccharides. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2019; Volume 627, pp. 189–213. [Google Scholar]
- Suzuki, S.; Shimahashi, K.; Takahara, J.; Sunako, M.; Takaha, T.; Ogawa, K.; Kitamura, S. Effect of addition of water-soluble chitin on amylose film. Biomacromolecules 2005, 6, 3238–3242. [Google Scholar] [CrossRef] [PubMed]
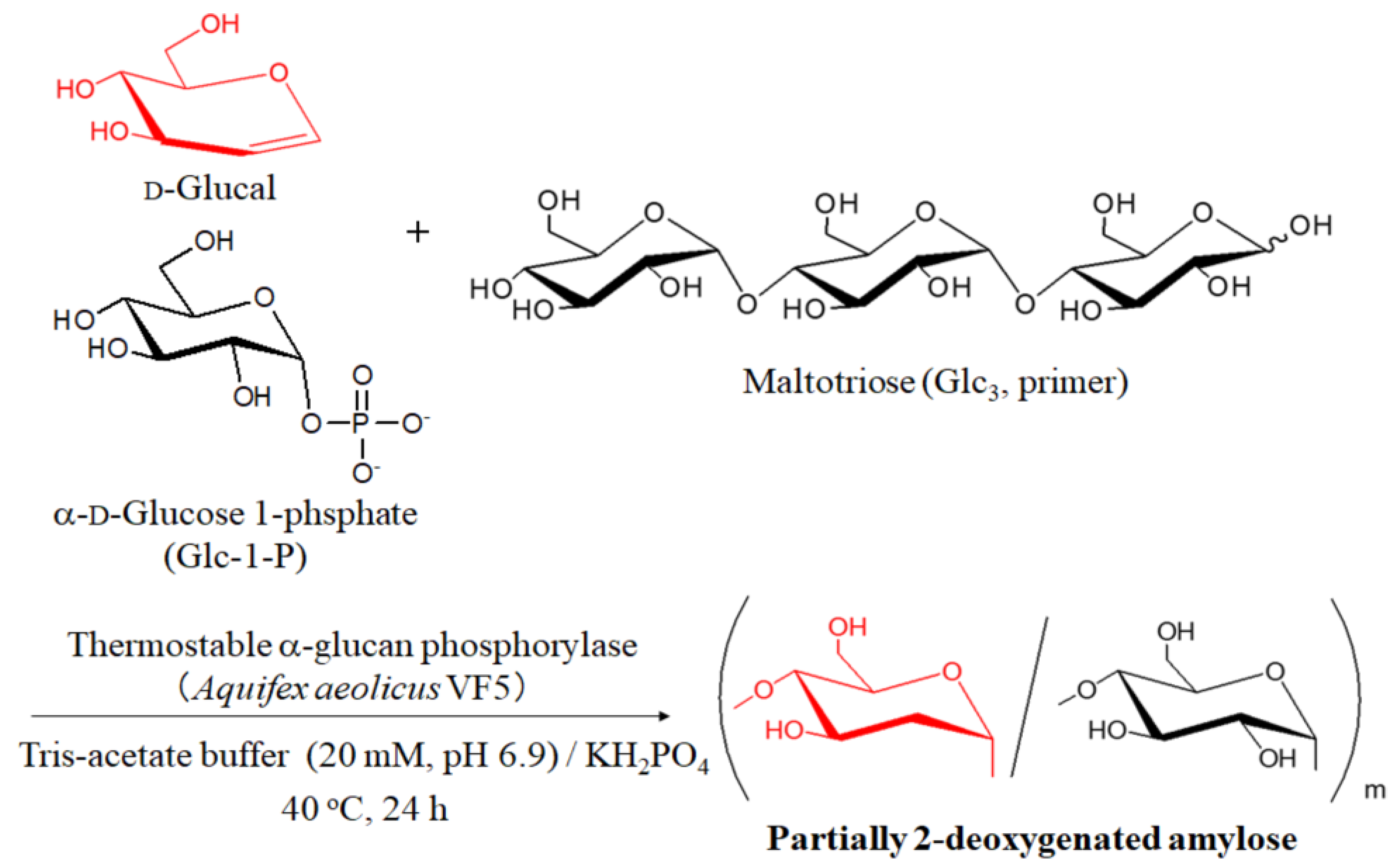
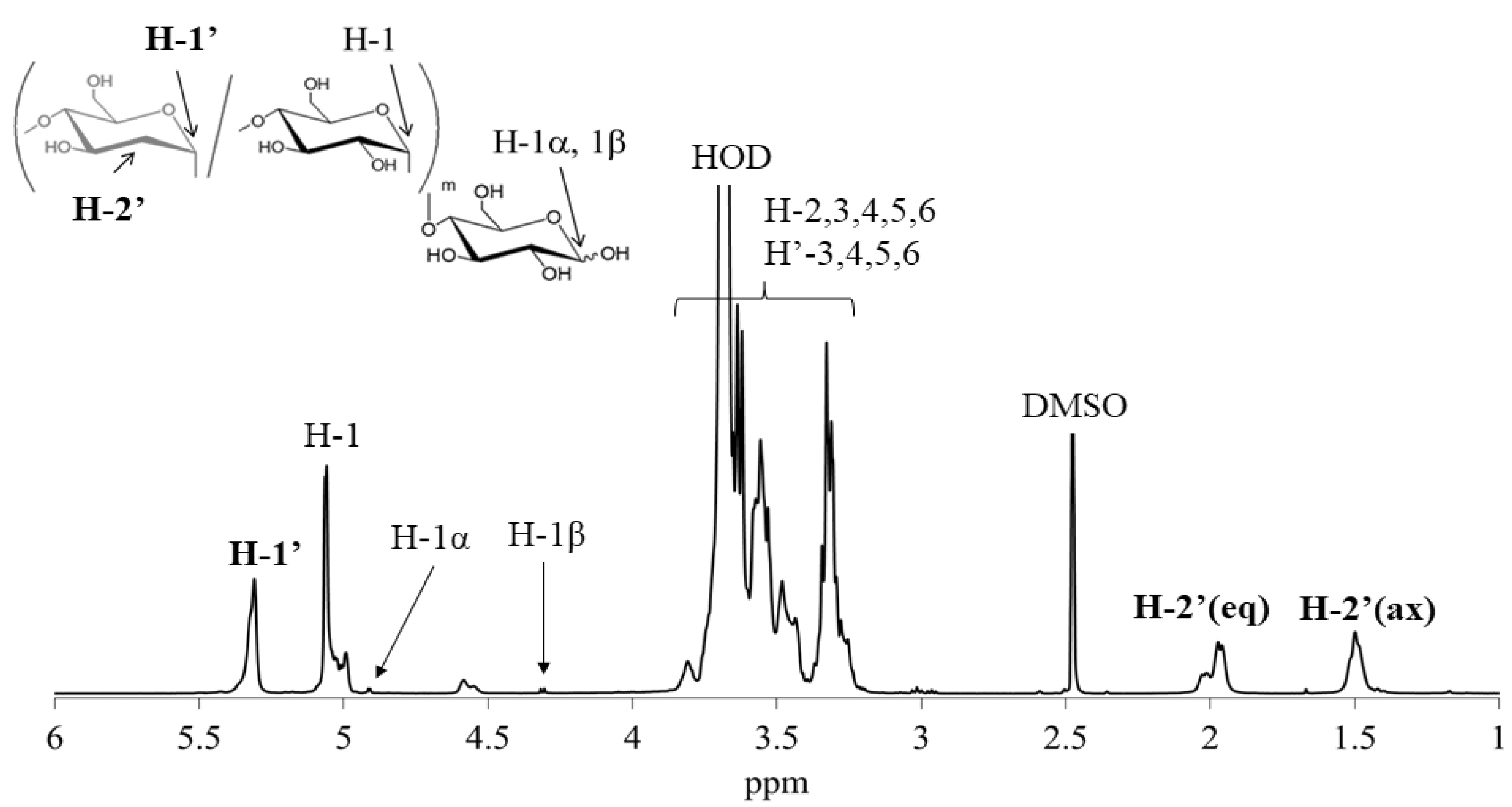
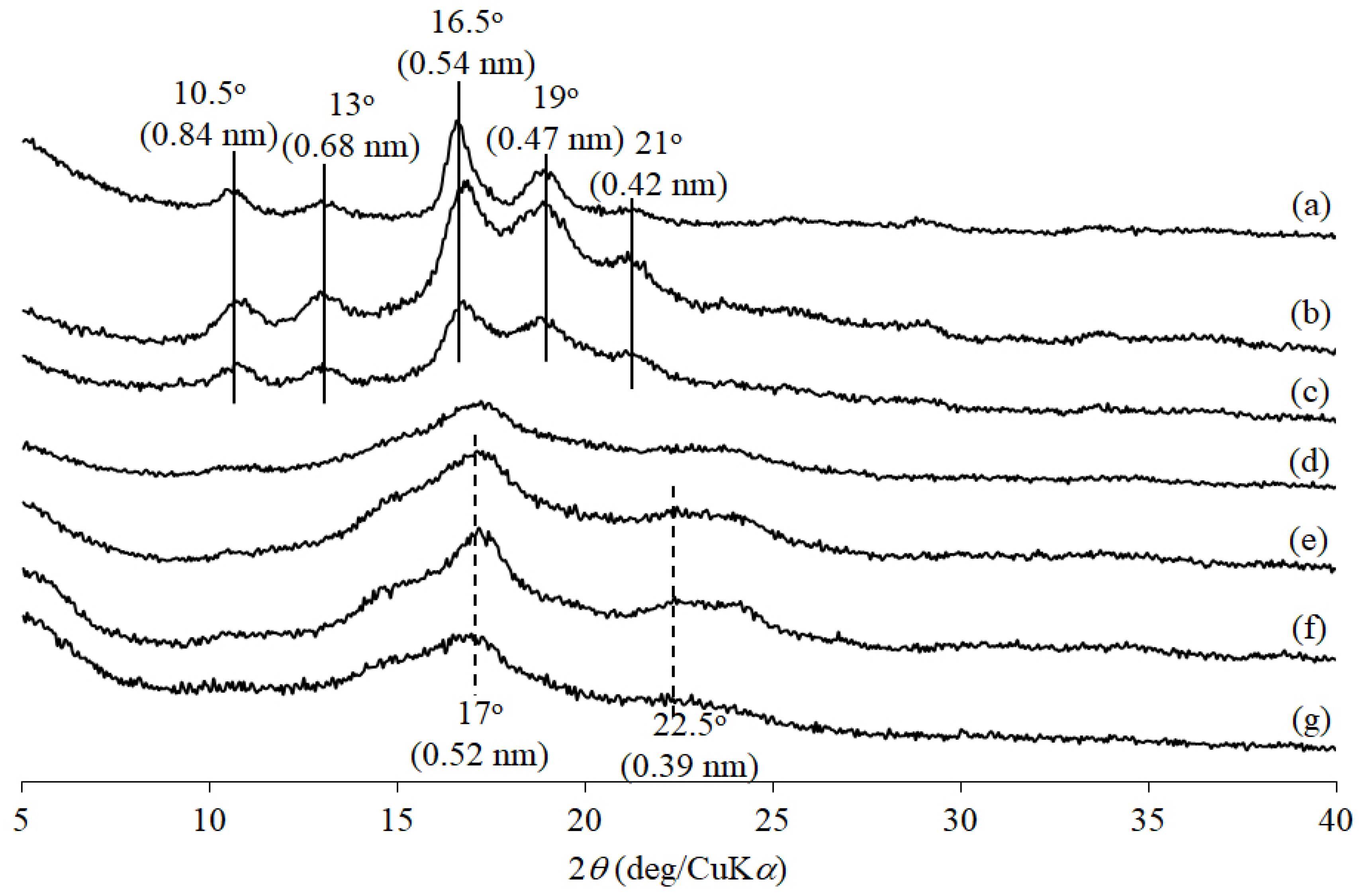
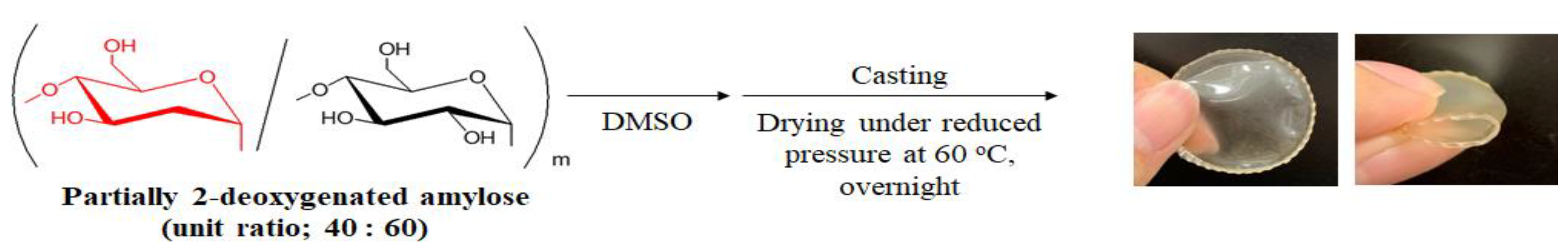
| Entry | Feed Ratio (b) Glc3:d-Glucal:Glc-1-P | Feed Ratio d-Glucal:Glc-1-P | Unit Ratio (c) dGlc:Glc | Mn(d) | Yield (%) (e) |
|---|---|---|---|---|---|
| 1 | 1:120:0 | 100:0 | 100:0 | 3810 | 19.6 |
| 2 | 1:100:20 | 83:17 | 62:38 | 7620 | 41.6 |
| 3 | 1:80:40 | 67:33 | 53:47 | 12820 | 55.7 |
| 4 | 1:60:60 | 50:50 | 40:60 | 16790 | 53.3 |
| 5 | 1:40:80 | 33:67 | 23:76 | 13560 | 49.2 |
| 6 | 1:20:100 | 17:83 | 6:94 | 11080 | 63.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kadokawa, J.-i.; Nakamura, S.; Yamamoto, K. Thermostable α-Glucan Phosphorylase-Catalyzed Enzymatic Copolymerization to Produce Partially 2-Deoxygenated Amyloses. Processes 2020, 8, 1070. https://doi.org/10.3390/pr8091070
Kadokawa J-i, Nakamura S, Yamamoto K. Thermostable α-Glucan Phosphorylase-Catalyzed Enzymatic Copolymerization to Produce Partially 2-Deoxygenated Amyloses. Processes. 2020; 8(9):1070. https://doi.org/10.3390/pr8091070
Chicago/Turabian StyleKadokawa, Jun-ichi, Shota Nakamura, and Kazuya Yamamoto. 2020. "Thermostable α-Glucan Phosphorylase-Catalyzed Enzymatic Copolymerization to Produce Partially 2-Deoxygenated Amyloses" Processes 8, no. 9: 1070. https://doi.org/10.3390/pr8091070
APA StyleKadokawa, J.-i., Nakamura, S., & Yamamoto, K. (2020). Thermostable α-Glucan Phosphorylase-Catalyzed Enzymatic Copolymerization to Produce Partially 2-Deoxygenated Amyloses. Processes, 8(9), 1070. https://doi.org/10.3390/pr8091070






