Analysis of Chemical and Biochemical Parameters of Petrol-Contaminated Soil after Biostimulation with an Enzyme Reagent
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Determination of Soil Chemical Parameters
2.3. Determination of Soil Enzyme Activity
2.4. Data Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nwankwegu, A.S.; Onwosi, C.O. Bioremediation of gasoline contaminated agricultural soil by bioaugmentation. Environ. Technol. Innov. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Almansoory, A.F.; Hasan, H.A.; Idris, M.; Abdullah, S.R.S.; Anuar, N. Potential application of a biosurfactant in phytoremediation technology for treatment of gasoline-contaminated soil. Ecol. Engin. 2015, 84, 113–120. [Google Scholar] [CrossRef]
- Stręk, M.; Telesiński, A. Change in oxidoreductase activity of selected microbial enzymes in gasoline-contaminated light soil in presence of selenium. Ochr. Środ. 2015, 37, 43–47. (In Polish) [Google Scholar]
- Nishiwaki, J.; Kawabe, Y.; Komai, T.; Zhang, M. Decomposition of gasoline hydrocarbons by natural microorganisms in Japanese soils. Geosciences 2018, 8, 35. [Google Scholar] [CrossRef]
- Broóijmans, R.J.E.; Pastink, M.I.; Siezen, R.J. Hydrocarbon-degrading bacteria: The oil spill clean-up crew. Microb. Biotechnol. 2009, 2, 587–594. [Google Scholar] [CrossRef]
- Chaillan, F.; Flèche, A.L.; Bury, E.; Phantavong, Y.-H.; Grimont, P.; Sliot, A.; Oudot, J. Identification and biodegradation potential of tropic aerobic hydrocarbon-degrading microorganisms. Res. Microbiol. 2004, 155, 587–595. [Google Scholar] [CrossRef]
- Das, N.; Chandran, P. Microbial biodegradation of petroleum hydrocarbon contaminants: An overview. Biotechnol. Res. Int. 2010, 2011, 941810. [Google Scholar]
- Marschal, R.; Penet, S.; Solano-Selena, F.; Vandecasteele, J.P. Gasoline and diesel oil biodegradation. Oil Gas Sci. Technol. 2003, 58, 441–448. [Google Scholar] [CrossRef]
- Telesiński, A.; Krzyśko-Łupicka, T.; Cybulska, K.; Wróbel, J. Response of soil phosphatase activities to contamination with two types of tar oil. Environ. Sci. Pollut. Res. 2018, 25, 28642–28653. [Google Scholar] [CrossRef]
- Liu, Q.; Li, Q.; Wang, N.; Liu, D.; Zan, L.; Chang, L.; Gou, X.; Wang, P. Bioremediation of petroleum-contaminated soil using aged refuse from landfills. Waste Manag. 2018, 77, 576–585. [Google Scholar] [CrossRef]
- Wang, S.; Wang, X. Bioremediation of petroleum contaminated soils collected all around China: The extensive application and the microbial mechanism. Petrol. Sci. Technol. 2018, 36, 974–980. [Google Scholar] [CrossRef]
- Konieczny, M.; Krzyśko-Łupicka, T. The influence of stimulation techniques on the microbiological changes and n-alkane transitions in the soil contaminated of petroleum-derived substances. Water Air Soil Pollut. 2019, 230, 81. [Google Scholar] [CrossRef]
- Juwarkar, A.A.; Singh, S.K.; Mudhoo, A. A comprehensive overview of elements in bioremediation. Rev. Environ. Sci. Biotechnol. 2010, 9, 215–288. [Google Scholar] [CrossRef]
- Pajares, S.; Gallardo, J.F.; Masciandro, G.; Ceccanti, B.; Etchevers, J.D. Enzyme activity as an indicator of soil quality changes in degraded cultivated Acrisols in the Mexican Trans-Volcanic Belt. Land Degrad. Dev. 2011, 22, 373–381. [Google Scholar] [CrossRef]
- Schloter, M.; Nannipieri, P.; Sørensen, S.J.; van Elsas, J.D. Microbial indicators for soil quality. Biol. Fertil. Soils 2018, 54, 1–10. [Google Scholar] [CrossRef]
- Telesiński, A.; Biczak, R.; Stręk, M.; Płatkowski, M.; Pawłowska, B.; Emin, N. A study of the fluoride content and enzymatic activity in soil exposed to inorganic ammonium salt and quaternary ammonium salts with hexafluorophosphate anions. Fluoride 2018, 51, 206–219. [Google Scholar]
- Borowik, A.; Wyszkowska, J.; Wyszkowski, M. Resistance of aerobic microorganisms and soil enzyme response to soil contamination with Ekodiesel Ultra fuel. Environ. Sci. Pollut. Res. 2017, 24, 24346–24363. [Google Scholar] [CrossRef]
- Jankauskas, B.; Jankauskiene, G.; Fullen, M.A.; Booth, C.A. International comparison of analytical methods of determining the soil organic matter content of Lithuanian Eutric Albeluvisols. Commun. Soil Sci. Plant Anal. 2006, 37, 707–720. [Google Scholar] [CrossRef]
- Jarnuszewski, G. Characterization of some physical and chemical properties of post-bog soils developed from limnic deposit in vicinity of lake Dąbie (West Pomerania, NW Poland). Soil Sci. Ann. 2016, 67, 24–31. [Google Scholar] [CrossRef]
- Casida, J.E., Jr.; Klein, D.A.; Santoro, T. Soil dehydrogenase activity. Soil Sci. 1964, 98, 371–376. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Kaczyńska, G.; Borowik, A.; Wyszkowska, J. Soil dehydrogenases as an indicator of contamination of the environment with petroleum products. Water Air Soil Pollut. 2015, 226, 372. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.T.E. Eta squared and partial eta squared as measures of effect size in educational research. Educ. Res. Rev. 2011, 6, 135–147. [Google Scholar] [CrossRef]
- Robin, X.; Turck, N.; Hainard, A.; Tiberti, N.; Lisacek, F.; Sanchez, J.-C.; Müller, M. pROC: An open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinform. 2011, 17, 77. [Google Scholar] [CrossRef]
- Alatorre, L.C.; Begueria, S. Identification of eroded areas using remote sensing in a badlands landscape on marls in the central Spanish Pyrenees. Catena 2009, 76, 182–190. [Google Scholar] [CrossRef]
- Lin, Y.-P.; Cheng, B.-Y.; Shyu, G.-S.; Chang, T.-K. Combining a finite mixture distribution model with indicator kriging to delineate and map the spatial patterns of soil heavy metal pollution in Chunghua County, central Taiwan. Environ. Pollut. 2010, 158, 235–244. [Google Scholar] [CrossRef]
- Wyszkowski, M.; Ziółkowska, A. Effect of petrol and diesel oil on content of organic carbon and mineral components in soil. Am.-Eurasian J. Sustain. Agric. 2008, 2, 54–60. [Google Scholar]
- Wyszkowski, M.; Sivitskaya, V. Changes in content of organic carbon and available forms of macronutrients in soil under the influence the soil contamination with fuel oil and application of different substances. J. Elem. 2012, 17, 139–148. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, J.; Lin, Q.; Lyu, X.; Wang, X.; Wang, G. Effects of crude oil contamination on soil physical and chemical properties in Momoge wetland of China. Chin. Geogr. Sci. 2013, 23, 708–715. [Google Scholar] [CrossRef]
- Devatha, C.P.; Vishal, A.V.; Rao, J.P.C. Investigation of physical and chemical characteristics on soil due to crude oil contamination and its remediation. Appl. Water Sci. 2019, 9, 89. [Google Scholar] [CrossRef]
- Stręk, M.; Telesiński, A. Comparison of selenite (IV) and selenate (VI) effect on some oxidoreductive enzymes in soil contaminated with spent engine oil. Plant Soil Environ. 2016, 62, 157–163. [Google Scholar] [CrossRef]
- Adipah, S. Introduction of petroleum hydrocarbons contaminants and its human effects. J. Environ. Sci. Public Health 2019, 3, 1–9. [Google Scholar] [CrossRef]
- Schreier, C.G.; Walker, W.J.; Burns, J.; Wilkenfeld, R. Total organic carbon as a screening method for petroleum hydrocarbons. Chemosphere 1999, 39, 503–510. [Google Scholar] [CrossRef]
- Walworth, J.; Pond, A.; Snape, I.; Rayner, J.; Ferguson, S.; Harvey, P. Nitrogen requirements for maximizing petroleum bioremediation in a Sub-Arctic soil. Cold Reg. Sci. Technol. 2007, 48, 84–91. [Google Scholar] [CrossRef]
- Oje Obinna, A.; Ubani Chibuike, S.; Onwurah, I.N.E. Variation in the carbon (C), phosphorus (P) and nitrogen (N) utiliztion during the biodegradation of crude oil in soil. J. Petrol. Environ. Biotechnol. 2015, 6, 2. [Google Scholar]
- Krzyśko-Łupicka, T.; Ciesielczuk, T.; Chwałowska, M. The biodegradation of petroleum substances in contaminated soil in presence of Fyre-Zyme preparation. Acta Sci. Pol. Biotechnol. 2013, 12, 5–18. (In Polish) [Google Scholar]
- Wyszkowska, J.; Kucharski, J. Biochemical properties of soil contaminated by petrol. Pol. J. Environ. Stud. 2000, 9, 479–485. [Google Scholar]
- Telesiński, A.; Krzyśko-Łupicka, T.; Cybulska, K.; Pawłowska, B.; Biczak, R.; Śnieg, M.; Wróbel, J. Comparison of oxidoreductive enzyme activities in three coal tar creosote-contaminated soils. Soil Res. 2019, 57, 814–824. [Google Scholar] [CrossRef]
- Telesiński, A.; Zambrana, A.B.; Jarnuszewski, G.; Curyło, K.; Krzyśko-Łupicka, T.; Pawłowska, B.; Cybulska, K.; Wróbel, J.; Rynkiewicz, M. Effect of rhamnolipids on microbial biomass content and biochemical parameters in soil contaminated with coal tar creosote. Open Life Sci. 2019, 14, 537–548. [Google Scholar] [CrossRef]
- Zhang, N.; He, X.-D.; Gao, Y.-B.; Li, Y.-H.; Wang, H.-T.; Ma, D.; Zhang, R.; Yang, S. Pedogenic carbonate and soil dehydrogenase activity in response to soil organic matter in Artemisia ordosica community. Pedosphere 2010, 20, 229–235. [Google Scholar] [CrossRef]
- Moeskops, B.; Sukristiyonubowo; Buchan, D.; Sleutel, S.; Herawaty, L.; Husen, E.; Saraswati, R.; Setyorini, D.; Neve, S.D. Soil microbial communities and activities under intensive organic and conventional vegetable farming in West Java, Indonesia. Appl. Soil Ecol. 2010, 45, 112–120. [Google Scholar] [CrossRef]
- Thiele-Bruhn, S.; Schloter, M.; Wilke, B.-M.; Beaudette, L.A.; Martin-Laurent, F.; Cheviron, N.; Mougin, C.; Römbke, J. Identification of new microbial functional standards for soil quality assessment. Soil 2020, 6, 17–34. [Google Scholar] [CrossRef]
- Filipović, L.; Romić, M.; Sikora, S.; Babić, K.K.; Filipović, V.; Gerke, H.H.; Romić, D. Response of soil dehydrogenase activity to salinity and cadmium species. J. Soil Sci. Plant Nutr. 2020, 20, 530–536. [Google Scholar]
- Lemanowicz, J.; Brzezińska, M.; Siwik-Ziomek, A.; Koper, J. Activity of selected enzymes and phosphorus content in soils of former sulphur mines. Sci. Total Environ. 2020, 708, 134545. [Google Scholar] [CrossRef]
- Holz, M.; Zarebanadkouki, M.; Carminati, A.; Becker, J.N.; Spohn, M. The effect of root hairs on rhizosphere phosphatase activity. J. Plant Nutr. Soil Sci. 2020, 183, 382–388. [Google Scholar] [CrossRef]
- Araujo, C.L.; Vihko, P.T. Structure of acid phosphatases. Methods Mol. Biol. 2013, 1053, 155–166. [Google Scholar]
- Antonious, G.F.; Turley, E.T.; Dawood, M.H. Monitoring soil enzymes activity before and after animal manure application. Agriculture 2020, 10, 166. [Google Scholar] [CrossRef]
- Furtak, K.; Gałązka, A.; Niedźwiecki, J. Changes in soil enzymatic activity caused by hydric stress. Pol. J. Environ. Stud. 2020, 29, 1–8. [Google Scholar] [CrossRef]
- Płatkowski, M.; Telesiński, A. Response of soil phosphatases to glyphosate and its formulations – Roundup (laboratory conditions). Plant Soil Environ. 2016, 62, 286–292. [Google Scholar] [CrossRef]
- Kuyukina, M.S.; Ivshina, I.B.; Makarov, S.O.; Litvinenko, L.V.; Cunningham, C.J.; Philp, J.C. Effect of biosurfactants on crude oil desorption and mobilization in soil system. Environ. Int. 2005, 31, 155–161. [Google Scholar] [CrossRef]
- Wang, L.M.; Liu, P.W.G.; Ma, C.C.; Cheng, S.S. Application of biosurfactants, rhamnolipids, and surfactin, for enhanced biodegradation of diesel-contaminated water and soil. J. Hazard. Mat. 2008, 151, 155–163. [Google Scholar] [CrossRef]
- Bustamante, M.; Durán, N.; Diez, M.C. Biosurfactants are usefull tools for the biodegradation of contaminated soil: A review. J. Soil Sci. Plant Nutr. 2012, 12, 667–687. [Google Scholar]
- Kołwzan, B. Possible biosurfactant applications in water and soil remediation processes. Ochr. Środ. 2014, 36, 3–18. (In Polish) [Google Scholar]
- Eivazi, F.; Mullings, N.; Banks, M.L. Effect of select surfactants on activities of soil enzymes involved in nutrient cycling. Comm. Soil Sci. Plant Anal. 2018, 49, 371–379. [Google Scholar] [CrossRef]
- Xu, X.; Liu, W.; Tian, S.; Wang, W.; Qi, Q.; Jiang, P.; Gao, X.; Li, F.; Li, H.; Yu, H. Petroleum hydrocarbon-degrading bacteria for the remediation of oil pollution under aerobic conditions: A perspective analysis. Front. Microbiol. 2018, 9, 2885. [Google Scholar] [CrossRef] [PubMed]


| Treatment | C | C1 × 40 | C2 × 20 | C4 × 10 | P | P1 × 40 | P2 × 20 | P4 × 10 |
|---|---|---|---|---|---|---|---|---|
| Petrol (g kg−1 DM) | 0 | 0 | 0 | 0 | 50 | 50 | 50 | 50 |
| 6% solution of FZ (cm3) | 0 | 1 × 40 | 2 × 20 | 4 × 10 | 0 | 1 × 40 | 2 × 20 | 4 × 10 |
| Corg (g kg−1) | Ntot (g kg−1) | C:N | Stot (g kg−1) |
|---|---|---|---|
| 5.99 ± 0.12 | 0.48 ± 0.01 | 12.48 ± 0.14 | 0.19 ± 0.01 |
| Day | Uncontaminated Soil | Soil Contaminated with Petrol | ||||
| 1 × 40 | 2 × 20 | 4 × 10 | 1 × 40 | 2 × 20 | 4 × 10 | |
| Corg | ||||||
| 1 | 1.111 ± 0.003 a | 1.001 ± 0.008 d | 1.059 ± 0.008 b | 1.094 ± 0.005 a | 1.095 ± 0.014 a | 1.031 ± 0.001 c |
| 56 | 1.144 ± 0.015 a | 1.022 ± 0.025 b | 1.023 ± 0.006 b | 1.128 ± 0.045 a | 1.148 ± 0.025 a | 1.164 ± 0.017 a |
| Ntot | ||||||
| 1 | 1.082 ± 0.036 a | 1.058 ± 0.056 a | 1.093 ± 0.041 a | 1.093 ± 0.086 a | 1.065 ± 0.104 a | 1.095 ± 0.096 a |
| 56 | 1.217 ± 0.072 a,b | 1.023 ± 0.049 c | 1.027 ± 0.053 c | 1.063 ± 0.101 b,c | 1.109 ± 0.083 b,c | 1.336 ± 0.033 a |
| C:N | ||||||
| 1 | 1.028 ± 0.032 a | 0.947 ± 0.046 a | 0.969 ± 0.032 a | 1.004 ± 0.076 a | 1.035 ± 0.102 a | 0.947 ± 0.085 a |
| 56 | 0.942 ± 0.064 a,b | 1.001 ± 0.075 a,b | 0.998 ± 0.051 a,b | 1.065 ± 0.064 a | 1.039 ± 0.055 a | 0.871 ± 0.015 b |
| Stot | ||||||
| 1 | 1.042 ± 0.056 b | 1.019 ± 0.035 b | 1.065 ± 0.029 b | 1.222 ± 0.081 a | 1.088 ± 0.046 a,b | 1.023 ± 0.053 b |
| 56 | 1.090 ± 0.038 a | 1.088 ± 0.008 a | 1.027 ± 0.065 a | 0.876 ± 0.029 b | 0.885 ± 0.041 b | 0.889 ± 0.050 b |
| Variable Factor | Corg | Ntot | C:N | Stot |
|---|---|---|---|---|
| Petrol (P) | 37.02 | 54.64 | 37.07 | 3.46 |
| Fyre-Zyme Dose (FZ) | 25.78 | 16.09 | 14.87 | 8.41 |
| Day of Experiment (D) | 12.46 | 0.43 | 13.57 | 7.72 |
| P × FZ | 9.02 | 9.96 | 16.42 | 6.17 |
| P × D | 9.55 | 5.58 | 0.34 | 34.09 |
| FZ × D | 1.24 | 1.97 | 2.45 | 12.87 |
| P × FZ × D | 4.32 | 10.58 | 13.04 | 26.12 |
| Error | 0.60 | 0.75 | 2.23 | 1.17 |
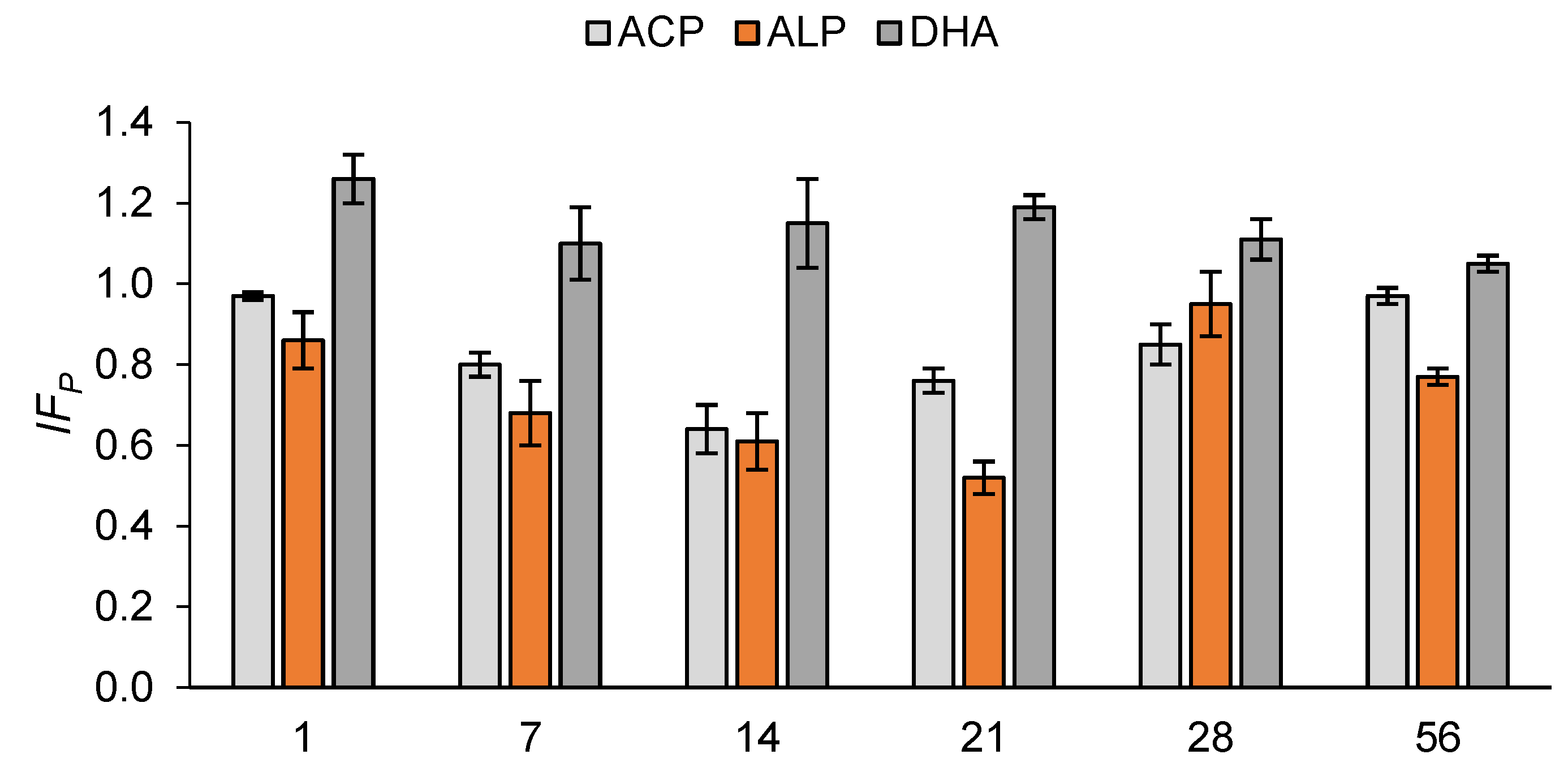
| Day | DHA (mg TPF kg−1 DM h−1) | ALP (mg p-NP kg−1 DM h−1) | ACP (mg p-NP kg−1 DM h−1) |
|---|---|---|---|
| 1 | 1.69 ± 0.04 a | 67.20 ± 6.65 c | 188.24 ± 10.21 a |
| 7 | 1.71 ± 0.06 a | 139.94 ± 10.64 a | 177.31 ± 8.30 b |
| 14 | 1.66 ± 0.15 a | 81.08 ± 5.89 b | 180.45 ± 6.83 a,b |
| 21 | 1.77 ± 0.07 a | 73.48 ± 6.76 b,c | 177.76 ± 2.91 b |
| 28 | 1.71 ± 0.02 a | 71.49 ± 3.55 b,c | 183.53 ± 11.79 a,b |
| 56 | 1.79 ± 0.08 a | 67.06 ± 3.98 c | 180.56 ± 2.18 b |
| Day | Uncontaminated Soil | Soil Contaminated with Petrol | ||||
| 1 × 40 | 2 × 20 | 4 × 10 | 1 × 40 | 2 × 20 | 4 × 10 | |
| Dehydrogenases (DHA) | ||||||
| 1 | 0.918 ± 0.043 b,c | 0.967 ± 0.041 a,b | 1.051 ± 0.039 a | 0.839 ± 0.045 c | 0.978 ± 0.036 a,b | 0.876 ± 0.013 b,c |
| 7 | 0.806 ± 0.052 b | 0.936 ± 0.034 a,b | 0.990 ± 0.068 a | 0.929 ± 0.056 a,b | 1.052 ± 0.045 a | 1.002 ± 0.055 a |
| 14 | 0.951 ± 0.089 a | 1.005 ± 0.059 a | 1.041 ± 0.098 a | 0.916 ± 0.029 a | 1.020 ± 0.032 a | 1.002 ± 0.051 a |
| 21 | 0.926 ± 0.044 b | 0.984 ± 0.020 a,b | 1.010 ± 0.012 a | 0.805 ± 0.018 c | 0.954 ± 0.022 a,b | 0.905 ± 0.041 b |
| 28 | 0.951 ± 0.013 a | 1.004 ± 0.009 a | 1.039 ± 0.037 a | 0.909 ± 0.069 a | 1.039 ± 0.053 a | 1.021 ± 0.079 a |
| 56 | 0.997 ± 0.042 a | 0.987 ± 0.026 a | 0.926 ± 0.086 a | 0.935 ± 0.046 a | 1.013 ± 0.006 a | 1.040 ± 0.033 a |
| Alkaline Phosphatase (ALP) | ||||||
| 1 | 0.960 ± 0.091 a | 1.072 ± 0.102 a | 0.829 ± 0.081 a | 1.069 ± 0.103 a | 1.108 ± 0.066 a | 1.315 ± 0.114 a |
| 7 | 0.811 ± 0.076 a | 1.112 ± 0.109 a | 0.984 ± 0.038 a | 0.963 ± 0.093 a | 1.093 ± 0.099 a | 1.024 ± 0.087 a |
| 14 | 1.106 ± 0.101 b | 1.262 ± 0.114 b | 1.073 ± 0.098 b | 0.951 ± 0.056 b | 1.665 ± 0.124 a | 1.728 ± 0.132 a |
| 21 | 0.682 ± 0.056 c | 0.821 ± 0.078 b,c | 0.829 ± 0.081 b,c | 1.440 ± 0.129 a,b,c | 2.013 ± 0.187 a | 1.507 ± 0.091 a,b |
| 28 | 1.109 ± 0.059 a | 1.085 ± 0.104 a | 1.109 ± 0.102 a | 1.174 ± 0.027 a | 1.193 ± 0.041 a | 1.279 ± 0.061 a |
| 56 | 1.239 ± 0.106 a,b | 1.487 ± 0.123 a | 1.302 ± 0.107 a,b | 1.021 ± 0.023 b | 1.303 ± 0.099 a,b | 1.215 ± 0.111 a,b |
| Acid Phosphatase (ACP) | ||||||
| 1 | 0.674 ± 0.032 c | 0.992 ± 0.068 a,b | 1.137 ± 0.067 a | 0.683 ± 0.053 c | 0.973 ± 0.010 b | 1.057 ± 0.067 a,b |
| 7 | 0.607 ± 0.045 d | 1.014 ± 0.087 b | 1.178 ± 0.026 b | 0.812 ± 0.044 c | 1.118 ± 0.059 b | 1.352 ± 0.047 a |
| 14 | 0.742 ± 0.072 d | 0.958 ± 0.038 c | 1.142 ± 0.026 b | 0.836 ± 0.076 c,d | 1.321 ± 0.086 a | 1.371 ± 0.066 a |
| 21 | 0.809 ± 0.032 e | 1.052 ± 0.021 c,d | 1.193 ± 0.032 a,b | 0.945 ± 0.041 d | 1.103 ± 0.075 b,c | 1.241 ± 0.036 a |
| 28 | 0.928 ± 0.089 a | 0.982 ± 0.039 a | 1.071 ± 0.102 a | 0.991 ± 0.046 a | 1.108 ± 0.076 a | 1.086 ± 0.026 a |
| 56 | 1.004 ± 0.028 a | 1.014 ± 0.018 a | 1.032 ± 0.044 a | 1.018 ± 0.046 a | 1.002 ± 0.039 a | 1.035 ± 0.075 a |
| Variable Factor | ACP | ALP | DHA |
|---|---|---|---|
| Petrol (P) | 41.76 | 38.73 | 79.97 |
| Fyre-Zyme Dose (FZ) | 42.22 | 6.10 | 13.13 |
| Day of Experiment (D) | 7.07 | 42.16 | 2.05 |
| P × FZ | 1.79 | 2.51 | 1.94 |
| P × D | 3.43 | 7.95 | 1.27 |
| FZ × D | 3.23 | 1.04 | 0.50 |
| P × FZ × D | 0.40 | 1.17 | 0.89 |
| Error | 0.11 | 0.34 | 0.25 |
| Application of Fyre-Zyme | Cut-Off Value | AUC | SE | p |
|---|---|---|---|---|
| Acid phosphatase (ACP) | ||||
| 1 × 40 | 0.863 | 0.673 | 0.091 | 0.058 |
| 2 × 20 | 1.054 | 0.762 | 0.081 | 0.001 |
| 4 × 10 | 1.275 | 0.617 | 0.098 | 0.233 |
| Alkaline Phosphatase (ALP) | ||||
| 1 × 40 | 0.941 | 0.644 | 0.093 | 0.121 |
| 2 × 20 | 1.130 | 0.704 | 0.057 | 0.019 |
| 4 × 10 | 1.214 | 0.872 | 0.069 | 0.001 |
| Dehydrogenases (DHA) | ||||
| 1 × 40 | 0.807 | 0.387 | 0.096 | 0.241 |
| 2 × 20 | 1.015 | 0.685 | 0.089 | 0.038 |
| 4 × 10 | 0.877 | 0.389 | 0.096 | 0.247 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Curyło, K.; Telesiński, A.; Jarnuszewski, G.; Krzyśko-Łupicka, T.; Cybulska, K. Analysis of Chemical and Biochemical Parameters of Petrol-Contaminated Soil after Biostimulation with an Enzyme Reagent. Processes 2020, 8, 949. https://doi.org/10.3390/pr8080949
Curyło K, Telesiński A, Jarnuszewski G, Krzyśko-Łupicka T, Cybulska K. Analysis of Chemical and Biochemical Parameters of Petrol-Contaminated Soil after Biostimulation with an Enzyme Reagent. Processes. 2020; 8(8):949. https://doi.org/10.3390/pr8080949
Chicago/Turabian StyleCuryło, Kornel, Arkadiusz Telesiński, Grzegorz Jarnuszewski, Teresa Krzyśko-Łupicka, and Krystyna Cybulska. 2020. "Analysis of Chemical and Biochemical Parameters of Petrol-Contaminated Soil after Biostimulation with an Enzyme Reagent" Processes 8, no. 8: 949. https://doi.org/10.3390/pr8080949
APA StyleCuryło, K., Telesiński, A., Jarnuszewski, G., Krzyśko-Łupicka, T., & Cybulska, K. (2020). Analysis of Chemical and Biochemical Parameters of Petrol-Contaminated Soil after Biostimulation with an Enzyme Reagent. Processes, 8(8), 949. https://doi.org/10.3390/pr8080949






