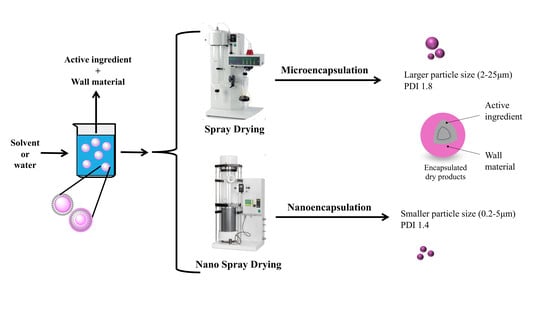
Encapsulation of Active Ingredients in Food Industry by Spray-Drying and Nano Spray-Drying Technologies
Abstract
:1. Introduction
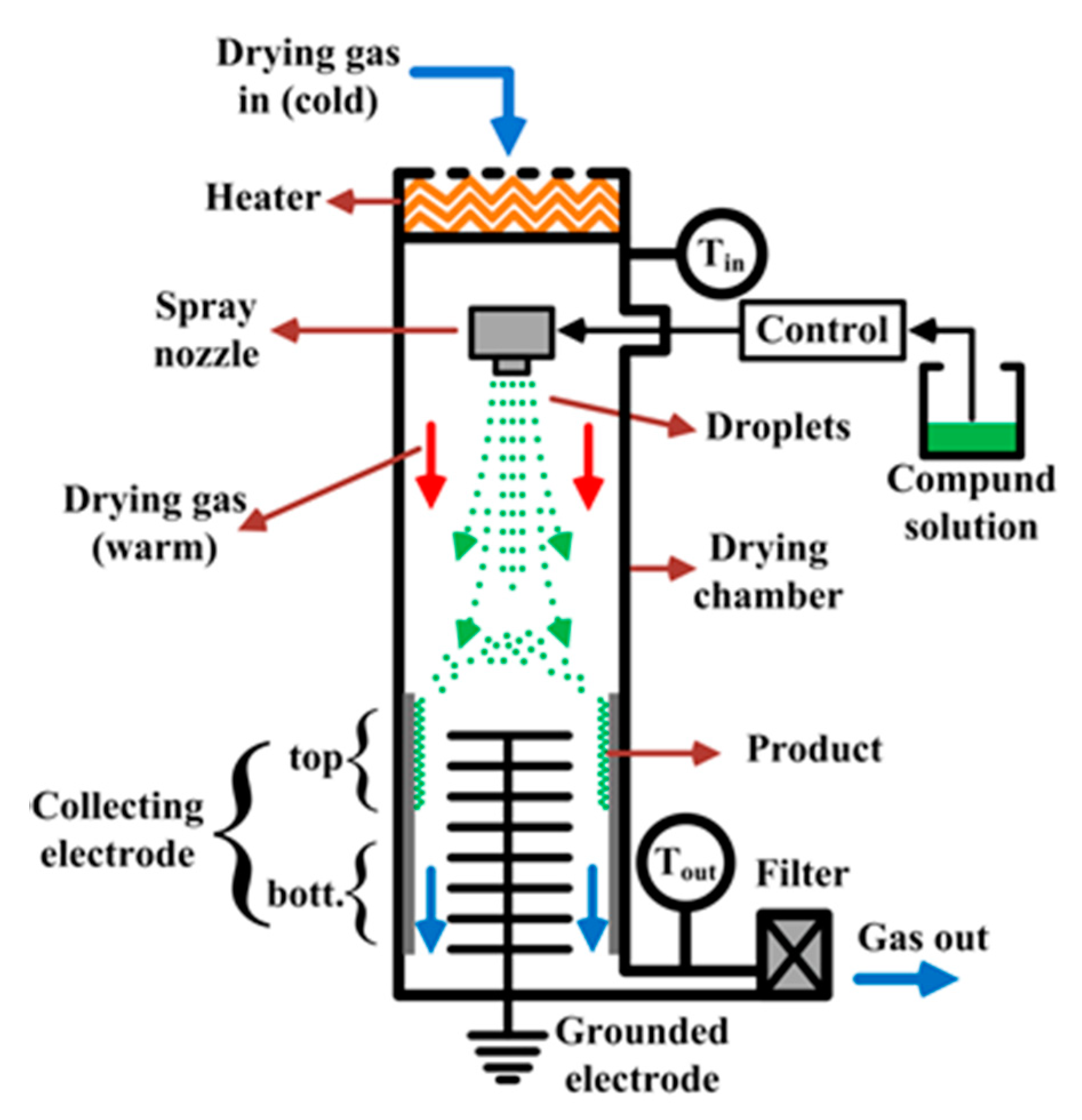
2. The Spray-Drying Process
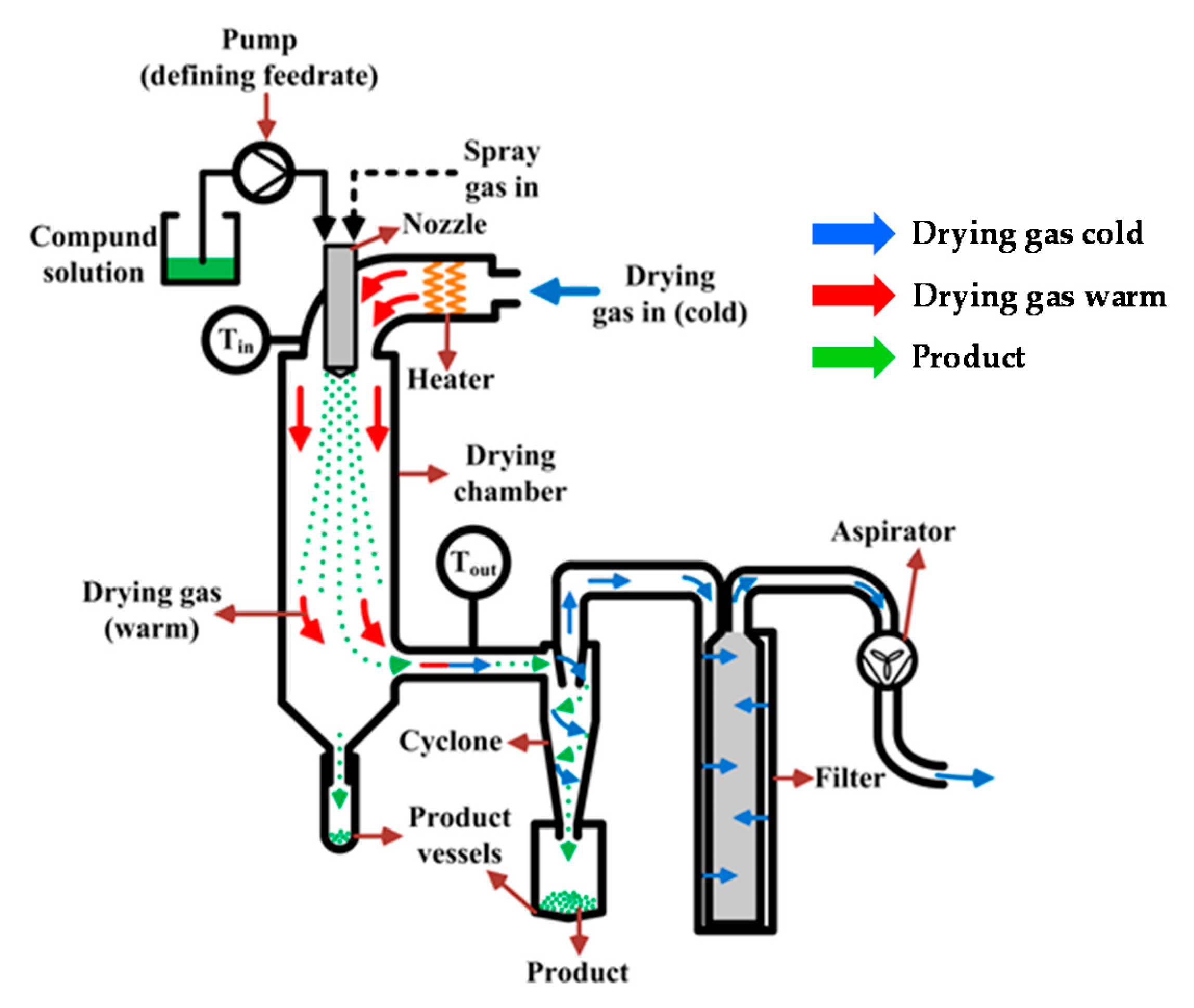
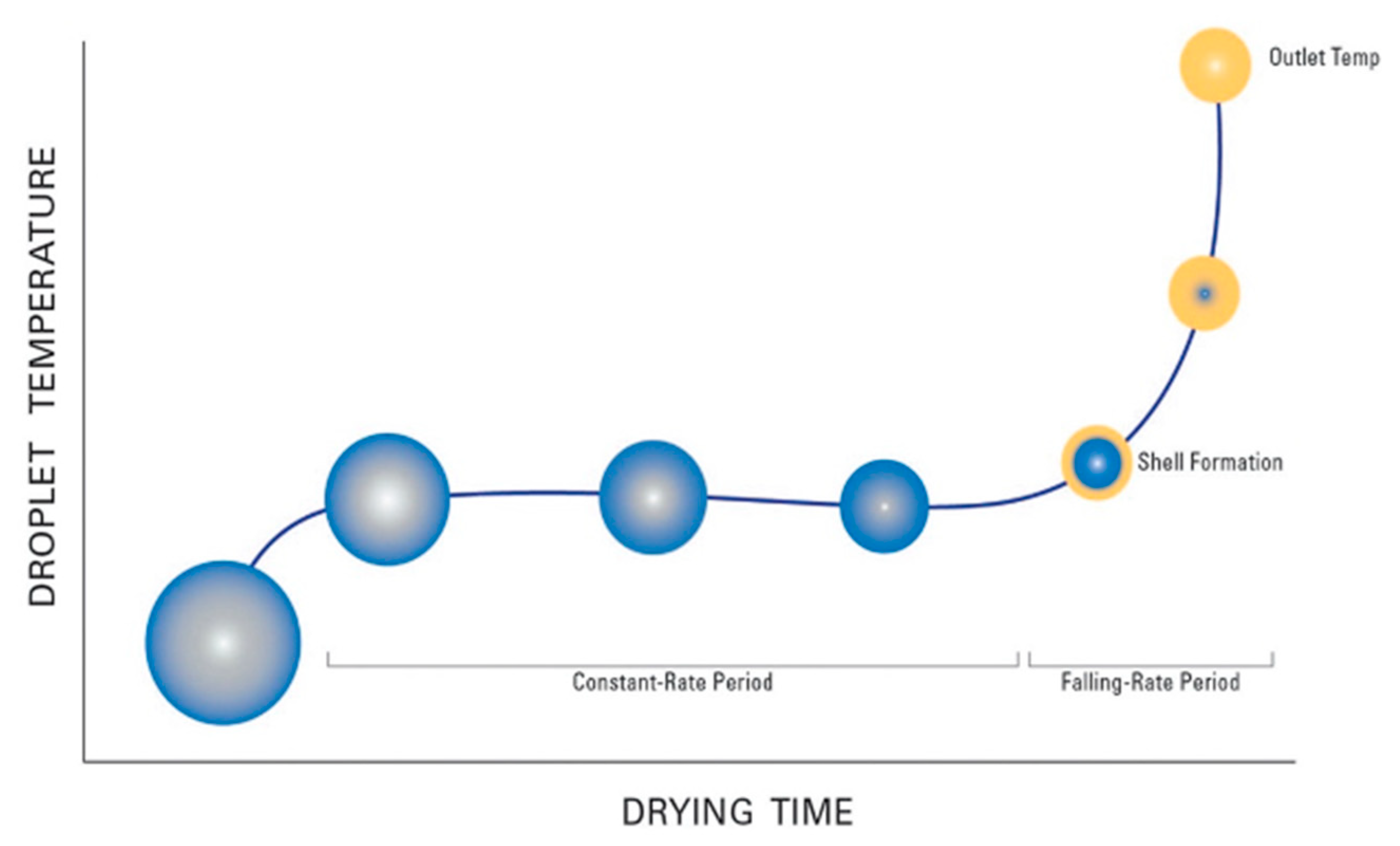
2.1. Factors Influencing the Spray-Drying Process
2.2. Microencapsulation of Food Active Ingredients
| Category | Core Material | Wall Material | Spray Drying Conditions | Major Outcomes | Reference |
|---|---|---|---|---|---|
| Oils and lipids | Fish oil | Fish protein hydrolysates from sardine (S.pilchardus) and horse mackerel (T. mediterraneus) | Inlet: 180 °C Outlet: 70 °C Air pressure: 4 bars Rotary speed: 22,000 rpm | Fish protein with a hydrolysate degree of 5% shows better performance for the stabilization of emulsions of fish oils. 98 ± 0.1% of encapsulation efficiency was reached with oxidative stability of the encapsulated oil over a period of 12 weeks. | [48] |
| Arabic gum, sodium caseinate, sage extract | Inlet: 160 °C Outlet: 80 °C Feed rate: 15–22 g/min Air pressure: 450 Kpa | The high encapsulation efficiency, oxidative stabilization, and low surface oil content for the encapsulation of fish oil was obtained with emulsions stabilized using Arabic gum and sage extract. Furthermore, the simulation of gastrointestinal condition revealed that more than 80% of fish oil encapsulated could be released. | [49] | ||
| Yeast cells (Saccharomyces cerevisiae) | Inlet: 150 °C Outlet: 60 °C Feed rate: 25 g/min | For optimization of the drying conditions, a statistical experiment design was applied, and the maximum efficiency of encapsulation was 82.7 ± 1.0%. The stability of encapsulated oil was of 30 days at relative humidity below 70%. Additionally, stability during storage was increased by coating with hydroxypropyl methylcellulose. | [50] | ||
| Brucea javanica oil (BJO) | Arabic gum: gelatin (1:2-8) | Inlet: 140, 150 and 160 °C Feed rate: 1.0, 1.5, and 2.0 mL/min. Air flow rate: 50, 65, and 80 L/min. | The best spray-drying conditions were an inlet temperature of 151.3 °C, a feed flow rate of 1.32 mL/min, and a drying air flow speed of 80 L/m in a relation of 1:6 Arabic gum: gelatin. It shows maximum encapsulation efficiency (82.9% w/w) and a loading capacity of 10.56% with high oxidative stability. | [51] | |
| Nigella sativa oil | Maltodextrin, sodium caseinate | Inlet: 150–190 °C Feed rate: 1 L/h Air pressure: 4.5 ± 0.1 bar | The microencapsulation efficiency was a function of the concentration of the wall material, the oil content, and the inlet temperature, showing the best results for 30%, 10%, and 160 °C, respectively. The dry product exhibited low moisture content, high solubility, and an encapsulation efficiency of 92.71%. | [52] | |
| Sardine oil | Vanillic acid grafted chitosan (Va-g-Ch) | Inlet: 140 °C Outlet: 77 °C | Obtaining sardine oil-loaded microparticles (75%) and a polydispersity index of 2.4 μ, the encapsulation efficiency was 84 ± 0.84%, and the loading efficiency was 67 ± 0.51%. The encapsulated product shows good oxidative stability. | [53] | |
| Flaxseed oil | Maltodextrin, arabic gum, whey protein, modified starch | Inlet: 180 °C Outlet: 110 °C Feed rate: 12 ± 2 g/min. Air flow rate: 73 m3/h | The best encapsulation efficiency was obtained for maltodextrin/modified starch (Hi-Cap 100TM), while the maltodextrin/whey protein combination exhibited better performance against lipid oxidation. Obtained hollow particles without cracks and fissures and with the active material embedded in the matrix. | [54] | |
| Flavors | D-limonene, ethyl hexanoate, citral and ethyl propionate | Yeast cells (Saccharomyces cerevisiae) | Inlet: 140–200 °C Outlet: 64–95 °C Feed rate: 10 mL/min Air flow rate: 35m3/h | Successful flavor encapsulation in partially β-glucans extracted from yeast cells. The incubation time and the temperature showed noticeable effects on the encapsulation of flavors. Flavor contents of d-limonene 37% and ethyl hexanoate 49%. | [55] |
| Lemon | Arabic gum and maltodextrin | Inlet:160 °C Outlet: 65 ± 2 °C Air flow rate: 1.42·10−6 m3/s Rotary speed: 39,000 rpm | Good stability of the oil–water emulsion for a period of 5 days. The increase in aroma addition caused an increase in emulsion viscosity. Increasing the aroma content, an increase in porosity, distribution of particle size, and total color differences was observed at the time that loose bulk density, solubility, and lightness decreased. The lowest water content was obtained for powder based on the emulsion with 6% of the aroma. | [56] | |
| Lime | Arabic gum and maltodextrin | Inlet: 180, 200, and 220 °C Outlet: 80, 90, and 100 °C Feed rate: 75–170 mL/min | The methodology of the response surface was used for optimization of the drying parameters, estimating an inlet air temperature of 220 °C and an outlet temperature of 85 °C to provide the maximum evaporative rate, volatile oil retention, and microencapsulation efficiency of 7.7 kg/h, 95.7%, and 99.9% respectively. | [57] | |
| Orange | Maltodextrin, modified starch and trehalose | Inlet: 175 ± 3 °C Outlet: 83 ± 3 °C Flow rate: 8 mL/min- Air pressure: 3.2 bar | The systems using trehalose were more effective in the encapsulation of orange oil with high aroma retention, since they presented higher Tg values in comparison with those containing sucrose. These systems could be stored in a variety of temperatures and relative humidity conditions without modifying the physical characteristics of the powders. | [58] | |
| Citral | Maltodextrin, sucrose, and trehalose | Inlet: 175 ± 3 °C Outlet: 83 ± 3 °C Flow rate: 8 mL/min- Air pressure: 3.2 bar | Among the citral retention with matrices of sucrose and trehalose, no differences were observed in the quantity and quality of powder obtained. However, the physical stability of the trehalose system was better than that of the sucrose system. | [59] | |
| Food additives | Goldenberry (Physalis peruviana L). | Maltodextrin, modified starch, inulin, alginate, and arabic gum. | Inlet: 140 °C Outlet: 70 °C Flow rate: 473 L/h Feed rate: 10 mL/min | Maximum yield of goldenberry powder of 67.2%. The obtained products exhibited low moisture content (<5.25%) and good solubility in cold water (>82%). The highest total carotenoid contents after spray drying were found using maltodextrin and cellobiose powder. Additionally, cellobiose powder showed the highest retention of carotenoids and encapsulation efficiency of 77.2% | [60] |
| β-carotene | Maltodextrin | Inlet: 170 °C Outlet: 95 °C Feed rate: 7.5 mL/min | Two different microencapsulation methods by β-carotene were compared; spray drying with maltodextrin and the structuration of beads with alginate and chitosan, as well as its bioavailability in a food matrix. The spray drying showed less encapsulation efficiency than beads of alginate and chitosan, being of 37.7% and 54.7% respectively. Nevertheless, into of a food matrix, the β-carotene microencapsulate obtained by spray drying was more bioavailable compare to beads of alginate and chitosan. | [61] | |
| Carotenoid Astaxanthin | Arabic gum, whey protein, maltodextrin, and inulin | Inlet: 120 °C Outlet: 70 °C Aspirator rate: 32.9 m3 h−1 Air pressure: 40 kg/cm2 | Obtaining yellow and orange pigments was evidence of the pigment contents. Whey protein alone or in combination with Arabic gum exhibited the best encapsulation yield (61.2–70.1%). The microencapsulates with 100% whey protein showed the highest temperature stability. However, the system with 100% whey protein showed the maximum stability as a function of the temperature. | [62] | |
| Vitamin A acetate | HI-CAP 100 (starch octenylsucciniate, OSA-starch) | Inlet: 182 °C Outlet: 82 °C Feed rate: 1000 mL/ min | The microcapsules exhibited spherical morphology with characteristic dents, and the maximum encapsulation efficiency (96.38 ± 0.71%) was obtained with a solution of total solids concentration at the core/wall material ratios of 40%. | [63] | |
| Quercetin 3-D-Galactoside | Maltodextrin | Inlet: 170–210 °C Flow rate: 35 m3/h Air pressure: 1.5 bar | Optimization of the spray-drying conditions for maximizing the yield, content, and retention of the antioxidant quercetin 3D galactoside, as well as evaluation of the effects of type and concentration of maltodextrin, such as the inlet temperature for drying. The yield and content of the antioxidant were mainly affected by the maltodextrin concentration, while temperature had a relatively low effect on the quantitative parameters. | [64] | |
| Orange juice | Maltodextrin | Inlet: 200 °C Outlet: 70 °C Flow rate: 7 mL/min Air flow: 28 m3/h Air pressure: 1.5 bar | Obtaining orange juice–maltodextrin powders and evaluating maltodextrins with different grades of polymerization to avoid structure collapse due to any change in appearance and the formation of particle agglomerates. | [46] | |
| Bioactive ingredients | β-galactosidase | Arabic gum, chitosan, modified chitosan, calcium alginate, and sodium alginate | Inlet: 115 °C Outlet: 56–61 °C Flow rate: 4 mL/min Air pressure: 6.5 bar | All the microencapsulates showed a spherical morphology with a mean diameter of 3 μm, but the particles obtained with chitosan and Arabic gum as wall material presented a rough surface. Regarding the enzymatic activity, this decreased with all the wall materials evaluated compared to the free enzyme activity. | [22] |
| Lactobacillus acidophilus NRRL B-4495 and Lactobacillus rhamnosus NRRL B-442 | Maltodextrin | Inlet: 100–130 °C Outlet: 67–97 °C Feed rate: 40–60 mL/min | Response surface methodology was used to evaluate the effect of the concentration of maltodextrin, inlet temperature, and feed rate during the spray drying of raspberry juice with a probiotic. The response variables were the culturability of probiotics and the color of the powder. The high temperatures during spray drying were detrimental to probiotics and may be circumvented by sub-lethal thermal shock (50 °C for L. acidophilus and 52.5 °C for L. rhamnosus). An increase in the concentration of maltodextrin favored the survival of the probiotics. | [65] | |
| Bifidobacterium BB-12 | Inulin, oligofructose, and oligofructose-enriched inulin | Inlet: 150 °C Outlet: 55 °C Feed rate: 6 mL/min Flow rate: 35 m3/h Air pressure: 0.7 MPa | Three prebiotics and their mixtures with reconstituted skim milk (RSM) were used to microencapsulate bifidobacterias BB-12. The system with prebiotics increased the survival rate of the microorganism during storage at the temperatures evaluated. Specifically, the microcapsules produced with a blend of oligofructose-enriched inulin with RSM and blending of oligofructose with RSM resulted in better protection of bifidobacteria during storage. | [66] |
2.3. Carrier Agents or Wall Materials
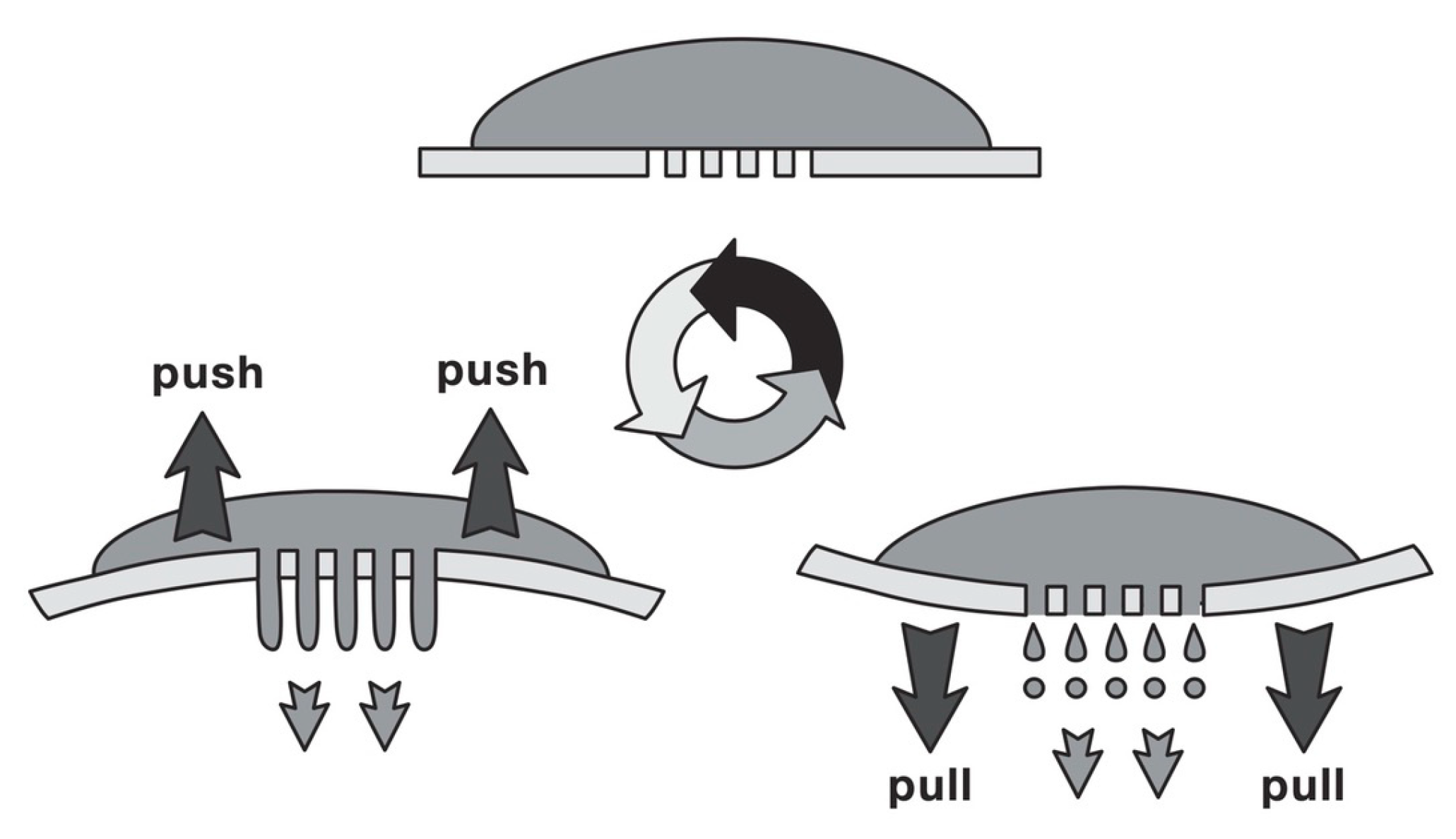
3. The Nano Spray-Drying Process
Nanoencapsulation of Food Active Ingredients
4. Advantages and Disadvantages of Conventional Spray Drying and Nano Spray Drying
5. Unwanted Reactions and Physicochemical Changes Presented by Spray-Dried Products
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siriwardhana, N.; Kalupahana, N.S.; Cekanova, M.; LeMieux, M.; Greer, B.; Moustaid-Moussa, N. Modulation of adipose tissue inflammation by bioactive food compounds. J. Nutr. Biochem. 2013, 24, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.I.; Saraiva, J.M.A.; Vicente, A.A.; Moldão-Martins, M. Methods for determining bioavailability and bioaccessibility of bioactive compounds and nutrients. In Innovative Thermal and Non-Thermal Processing, Bioaccessibility and Bioavailability of Nutrients and Bioactive Compounds; Elsevier: Amsterdam, The Netherlands, 2019; pp. 23–54. [Google Scholar]
- Hassimotto, N.M.A.; Genovese, M.I.; Lajolo, F.M. Antioxidant capacity of Brazilian fruit, vegetables and commercially-frozen fruit pulps. J. Food Compos. Anal. 2009, 22, 394–396. [Google Scholar] [CrossRef]
- Juranić, Z.; Žižak, Ž. Biological activities of berries: From antioxidant capacity to anti-cancer effects. Biofactors 2005, 23, 207–211. [Google Scholar] [CrossRef]
- Ray, S.; Raychaudhuri, U.; Chakraborty, R. An overview of encapsulation of active compounds used in food products by drying technology. Food Biosci. 2016, 13, 76–83. [Google Scholar] [CrossRef]
- Burgain, J.; Gaiani, C.; Linder, M.; Scher, J. Encapsulation of probiotic living cells: From laboratory scale to industrial applications. J. Food Eng. 2011, 104, 467–483. [Google Scholar] [CrossRef]
- Arpagaus, C.; Schwartzbach, H. Scale-up from the Büchi Mini Spray Dryer B-290 to the Niro MOBILE MINOR, best@ buchi Information Bulletin. Number 2008, 52, 2008. [Google Scholar]
- Wang, B.; Zhang, W.; Zhang, W.; Mujumdar, A.S.; Huang, L. Progress in drying technology for nanomaterials. Dry. Technol. 2005, 23, 7–32. [Google Scholar] [CrossRef]
- Gharsallaoui, A.; Roudaut, G.; Chambin, O.; Voilley, A.; Saurel, R. Applications of spray-drying in microencapsulation of food ingredients: An overview. Food Res. Int. 2007, 40, 1107–1121. [Google Scholar] [CrossRef]
- Masters, K.; Part, V. Applications of spray drying in industry. In Spray Drying Handbook, 5th ed.; Longman Scientific & Technical UK: Harlow Essex, UK, 1991; pp. 491–680. [Google Scholar]
- Anandharamakrishnan, C. Spray Drying Techniques for Food Ingredient Encapsulation; John Wiley & Sons: Chicago, IL, USA, 2015. [Google Scholar]
- Assadpour, E.; Jafari, S.M. Advances in spray-drying encapsulation of food bioactive ingredients: From microcapsules to nanocapsules. Annu. Rev. Food Sci. Technol. 2019, 10, 103–131. [Google Scholar] [CrossRef]
- Chan, H.; Kwok, P.C.L. Production methods for nanodrug particles using the bottom-up approach. Adv. Drug Deliv. Rev. 2011, 63, 406–416. [Google Scholar] [CrossRef]
- Heng, D.; Lee, S.H.; Ng, W.K.; Tan, R.B. The nano spray dryer B-90. Expert Opin. Drug Deliv. 2011, 8, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Anton, N.; Arpagaus, C.; Belleteix, F.; Vandamme, T.F. Nanoparticles by spray drying using innovative new technology: The Büchi Nano Spray Dryer B-90. J. Control. Release 2010, 147, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Donsì, F.; Annunziata, M.; Vincensi, M.; Ferrari, G. Design of nanoemulsion-based delivery systems of natural antimicrobials: Effect of the emulsifier. J. Biotechnol. 2012, 159, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Tastan, Ö.; Ferrari, G.; Baysal, T.; Donsì, F. Understanding the effect of formulation on functionality of modified chitosan films containing carvacrol nanoemulsions. Food Hydrocoll. 2016, 61, 756–771. [Google Scholar] [CrossRef]
- Zhang, Z.; Vriesekoop, F.; Yuan, Q.; Liang, H. Effects of nisin on the antimicrobial activity of D-limonene and its nanoemulsion. Food Chem. 2014, 150, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Jafari, S.M.; Assadpoor, E.; He, Y.; Bhandari, B. Encapsulation efficiency of food flavours and oils during spray drying. Dry. Technol. 2008, 26, 816–835. [Google Scholar] [CrossRef]
- Reineccius, G.A. The spray drying of food flavors. Dry. Technol. 2004, 22, 1289–1324. [Google Scholar] [CrossRef]
- I Ré, M. Microencapsulation by spray drying. Dry. Technol. 1998, 16, 1195–1236. [Google Scholar] [CrossRef]
- Estevinho, B.N.; Rocha, F.; Santos, L.; Alves, A. Microencapsulation with chitosan by spray drying for industry applications–A review. Trends Food Sci. Technol. 2013, 31, 138–155. [Google Scholar] [CrossRef]
- Grenha, A.; Seijo, B.; Remunán-López, C. Microencapsulated chitosan nanoparticles for lung protein delivery. Eur. J. Pharm. Sci. 2005, 25, 427–437. [Google Scholar] [CrossRef]
- Masters, K. Spray drying handbook. In Spray Drying Handbook; George Godwin Ltd.: London, UK, 1985. [Google Scholar]
- Gauvin, W.; Katta, S. Basic concepts of spray dryer design. AIChE J. 1976, 22, 713–724. [Google Scholar] [CrossRef]
- Hasheminya, S.M.; Dehghannya, J. Spray dryers: Applications, performance, essential parts and classifications. Int. J. Farming Allied Sci. 2013, 2, 756–759. [Google Scholar]
- Vicente, J.; Pinto, J.; Menezes, J.; Gaspar, F. Fundamental analysis of particle formation in spray drying. Powder Technol. 2013, 247, 1–7. [Google Scholar] [CrossRef]
- BUCHI Labortechnik AG. Spray Drying & Encapsulation Solutions-Particle Formation for Lab Scale; BUCHI Labortechnik AG: Flawil, Switzerland, 2015; pp. 1–24. [Google Scholar]
- Adhikari, B.; Howes, T.; Bhandari, B.; Truong, V. Experimental studies and kinetics of single drop drying and their relevance in drying of sugar-rich foods: A review. Int. J. Food Prop. 2000, 3, 323–351. [Google Scholar] [CrossRef]
- Sano, Y.; Keey, R. The drying of a spherical particle containing colloidal material into a hollow sphere. Chem. Eng. Sci. 1982, 37, 881–889. [Google Scholar] [CrossRef]
- Crowe, C. Modelling spray-air contact in spray-drying systems. Adv. Dry. 1980, 1, 63–99. [Google Scholar]
- Mezhericher, M.; Levy, A.; Borde, I. Theoretical models of single droplet drying kinetics: A review. Dry. Technol. 2010, 28, 278–293. [Google Scholar] [CrossRef]
- Cal, K.; Sollohub, K. Spray drying technique. I: Hardware and process parameters. J. Pharm. Sci. 2010, 99, 575–586. [Google Scholar] [CrossRef]
- Lechanteur, A.; Evrard, B. Influence of Composition and Spray-Drying Process Parameters on Carrier-Free DPI Properties and Behaviors in the Lung: A review. Pharmaceutics 2020, 12, 55. [Google Scholar] [CrossRef] [Green Version]
- Lewandowski, A.; Jaskulski, M.; Zbicinski, I. Effect of foam spray drying process parameters on powder morphology. Dry. Technol. 2019, 37, 535–545. [Google Scholar] [CrossRef]
- Bednarska, M.A.; Janiszewska-Turak, E. The influence of spray drying parameters and carrier material on the physico-chemical properties and quality of chokeberry juice powder. J. Food Sci. Technol. 2020, 57, 564–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, I.; Niazi, M.B.K.; Jahan, Z.; Naqvi, S.R. Effect of drying parameters on the physical, morphological and thermal properties of spray-dried inulin. J. Polym. Eng. 2018, 38, 775–783. [Google Scholar] [CrossRef]
- Leyva-Porras, C.; Saavedra–Leos, M.; López-Pablos, A.; Soto-Guerrero, J.; Toxqui-Terán, A.; Fozado-Quiroz, R. Chemical, thermal and physical characterization of inulin for its technological application based on the degree of polymerization. J. Food Process. Eng. 2017, 40, e12333. [Google Scholar] [CrossRef]
- Fang, Z.; Bhandari, B. Encapsulation techniques for food ingredient systems. In Food Materials Science and Engineering; Wiley-Blackwell: Iowa, IA, USA, 2012; pp. 320–348. [Google Scholar]
- Rezvankhah, A.; Emam-Djomeh, Z.; Askari, G. Encapsulation and delivery of bioactive compounds using spray and freeze-drying techniques: A review. Dry. Technol. 2020, 38, 235–258. [Google Scholar] [CrossRef]
- Saavedra-Leos, M.; Leyva-Porras, C.; MartÃnez-Guerra, E.; Pérez-GarcÃa, S.; Aguilar-MartÃnez, J.; Ãlvarez-Salas, C. Physical properties of inulin and inulin–orange juice: Physical characterization and technological application. Carbohydr. Polym. 2014, 105, 10–19. [Google Scholar] [CrossRef]
- Chirife, J.; Iglesias, H.A. Equations for fitting water sorption isotherms of foods: Part 1—A review. Int. J. Food Sci. Technol. 1978, 13, 159–174. [Google Scholar] [CrossRef]
- Leyva-Porras, C.; López-Pablos, A.L.; Alvarez-Salas, C.; Pérez-Urizar, J.; Saavedra-Leos, Z. Physical Properties of Inulin and Technological Applications. In Polysaccharides; Ramawat, K.G., Merillon, J.M., Eds.; Springer: New York, NY, USA, 2015; pp. 959–984. [Google Scholar]
- Saavedra-Leos, Z.; Leyva-Porras, C.; Araujo-Díaz, S.B.; Toxqui-Terán, A.; Borrás-Enríquez, A.J. Technological application of maltodextrins according to the degree of polymerization. Molecules 2015, 20, 21067–21081. [Google Scholar] [CrossRef] [Green Version]
- Saavedra-Leos, M.Z.; Leyva-Porras, C.; Alvarez-Salas, C.; Longoria-Rodriguez, F.; Lopez-Pablos, A.L.; Gonzalez-Garcia, R.; Pwrez-Urizar, J.T. Obtaining orange juice–maltodextrin powders without structure collapse based on the glass transition temperature and degree of polymerization. CyTA J. Food 2018, 16, 61–69. [Google Scholar] [CrossRef] [Green Version]
- Araujo-Díaz, S.; Leyva-Porras, C.; Aguirre-Bañuelos, P.; Álvarez-Salas, C.; Saavedra-Leos, Z. Evaluation of the physical properties and conservation of the antioxidants content, employing inulin and maltodextrin in the spray drying of blueberry juice. Carbohydr. Polym. 2017, 167, 317–325. [Google Scholar] [CrossRef]
- Morales-Medina, R.; Tamm, F.; Guadix, A.; Guadix, E.; Drusch, S. Functional and antioxidant properties of hydrolysates of sardine (S. pilchardus) and horse mackerel (T. mediterraneus) for the microencapsulation of fish oil by spray-drying. Food Chem. 2016, 194, 1208–1216. [Google Scholar] [CrossRef]
- Binsi, P.; Nayak, N.; Sarkar, P.; Jeyakumari, A.; Ashraf, P.M.; Ninan, G.; Ravishankar, C. Structural and oxidative stabilization of spray dried fish oil microencapsulates with gum arabic and sage polyphenols: Characterization and release kinetics. Food Chem. 2017, 219, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Czerniak, A.; Kubiak, P.; Bialas, W.; Jankowski, T. Improvement of oxidative stability of menhaden fish oil by microencapsulation within biocapsules formed of yeast cells. J. Food Eng. 2015, 167, 2–11. [Google Scholar] [CrossRef]
- Hu, L.; Zhang, J.; Hu, Q.; Gao, N.; Wang, S.; Sun, Y.; Yang, X. Microencapsulation of brucea javanica oil: Characterization, stability and optimization of spray drying conditions. J. Drug Deliv. Sci. Technol. 2016, 36, 46–54. [Google Scholar] [CrossRef]
- Khorasani, M.T.; Joorabloo, A.; Moghaddam, A.; Shamsi, H.; MansooriMoghadam, Z. Incorporation of ZnO nanoparticles into heparinised polyvinyl alcohol/chitosan hydrogels for wound dressing application. Int. J. Biol. Macromol. 2018, 114, 1203–1215. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, N.K.; Tan, C.P.; Manap, Y.A.; Alhelli, A.M.; Hussin, A.S.M. Process conditions of spray drying microencapsulation of Nigella sativa oil. Powder Technol. 2017, 315, 1–14. [Google Scholar] [CrossRef]
- Vishnu, K.; Chatterjee, N.S.; Ajeeshkumar, K.; Lekshmi, R.; Tejpal, C.; Mathew, S.; Ravishankar, C. Microencapsulation of sardine oil: Application of vanillic acid grafted chitosan as a bio-functional wall material. Carbohydr. Polym. 2017, 174, 540–548. [Google Scholar] [CrossRef]
- Carneiro, H.C.; Tonon, R.V.; Grosso, C.R.; Hubinger, M.D. Encapsulation efficiency and oxidative stability of flaxseed oil microencapsulated by spray drying using different combinations of wall materials. J. Food Eng. 2013, 115, 443–451. [Google Scholar] [CrossRef] [Green Version]
- Sultana, A.; Tanaka, Y.; Fushimi, Y.; Yoshii, H. Stability and release behavior of encapsulated flavor from spray-dried Saccharomyces cerevisiae and maltodextrin powder. Food Res. Int. 2018, 106, 809–816. [Google Scholar] [CrossRef]
- Janiszewska, E.; Jedlinska, A.; Witrowa-Rajchert, D. Effect of homogenization parameters on selected physical properties of lemon aroma powder. Food Bioprod. Process. 2015, 94, 405–413. [Google Scholar] [CrossRef]
- Bringasâ-Lantigua, M.; Valdes, D.; Pino, J.A. Influence of spray-dryer air temperatures on encapsulated lime essential oil. Int. J. Food Sci. Technol. 2012, 47, 1511–1517. [Google Scholar] [CrossRef]
- Sosa, N.; Zamora, M.C.; van Baren, C.; Schebor, C. New insights in the use of trehalose and modified starches for the encapsulation of orange essential oil. Food Bioprocess Technol. 2014, 7, 1745–1755. [Google Scholar] [CrossRef]
- Sosa, N.; Zamora, M.C.; Chirife, J.; Schebor, C. Spray-drying encapsulation of citral in sucrose or trehalose matrices: Physicochemical and sensory characteristics. Int. J. Food Sci. Technol. 2011, 46, 2096–2102. [Google Scholar] [CrossRef]
- Etzbach, L.; Meinert, M.; Faber, T.; Klein, C.; Schieber, A.; Weber, F. Effects of carrier agents on powder properties, stability of carotenoids, and encapsulation efficiency of goldenberry (Physalis peruviana L.) powder produced by co-current spray drying. Curr. Res. Food Sci. 2020, 3, 73–81. [Google Scholar] [CrossRef]
- Donhowe, E.G.; Flores, F.P.; Kerr, W.L.; Wicker, L.; Kong, F. Characterization and in vitro bioavailability of β-carotene: Effects of microencapsulation method and food matrix. LWT-Food Sci. Technol. 2014, 57, 42–48. [Google Scholar] [CrossRef]
- Bustos-Garza, C.; Yáñez-Fernández, J.; Barragán-Huerta, B.E. Thermal and pH stability of spray-dried encapsulated astaxanthin oleoresin from Haematococcus pluvialis using several encapsulation wall materials. Food Res. Int. 2013, 54, 641–649. [Google Scholar] [CrossRef]
- Moreno, M.A.; Orqueda, M.E.; Gomez-Mascaraque, L.G.; Isla, M.I.; Lopez-Rubio, A. Crosslinked electrospun zein-based food packaging coatings containing bioactive chilto fruit extracts. Food Hydrocoll. 2019, 95, 496–505. [Google Scholar] [CrossRef]
- Xie, Y.; Zhou, H.; Liang, X.; He, B.; Han, X. Study on the morphology, particle size and thermal properties of vitamin A microencapsulated by starch octenylsucciniate. Agric. Sci. China 2010, 9, 1058–1064. [Google Scholar] [CrossRef]
- Saavedra-Leos, M.Z.; Leyva-Porras, C.; López-Martínez, L.A.; González-García, R.; Martínez, J.O.; Compeán Martínez, I.; Toxqui-Terán, A. Evaluation of the Spray Drying Conditions of Blueberry Juice-Maltodextrin on the Yield, Content, and Retention of Quercetin 3-d-Galactoside. Polymers 2019, 11, 312. [Google Scholar] [CrossRef] [Green Version]
- Anekella, K.; Orsat, V. Optimization of microencapsulation of probiotics in raspberry juice by spray drying. LWT-Food Sci. Technol. 2013, 50, 17–24. [Google Scholar] [CrossRef]
- Fritzen-Freire, C.B.; Prudêncio, E.S.; Amboni, R.D.; Pinto, S.S.; Negrão-Murakami, A.N.; Murakami, F.S. Microencapsulation of bifidobacteria by spray drying in the presence of prebiotics. Food Res. Int. 2012, 45, 306–312. [Google Scholar] [CrossRef]
- BUCHI Labortechnik AG. Nano Spray Dryer B-90 Commercial Brochure; BUCHI Labortechnik AG: Flawil, Switzerland, 2009. [Google Scholar]
- Sosnik, A.; Seremeta, K.P. Advantages and challenges of the spray-drying technology for the production of pure drug particles and drug-loaded polymeric carriers. Adv. Colloid Interface Sci. 2015, 223, 40–54. [Google Scholar] [CrossRef] [PubMed]
- Arpagaus, C.; John, P.; Collenberg, A.; Rütti, D. Nanocapsules formation by nano spray drying. In Nanoencapsulation Technologies for the Food and Nutraceutical Industries; Elsevier: Gorgan, Iran, 2017; pp. 346–401. [Google Scholar]
- Schmid, K.; Arpagaus, C.; Friess, W. Evaluation of the Nano Spray Dryer B-90 for pharmaceutical applications. Pharm. Dev. Technol. 2011, 16, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Perdana, J.; Fox, M.B.; Schutyser, M.A.; Boom, R.M. Single-droplet experimentation on spray drying: Evaporation of a sessile droplet. Chem. Eng. Technol. 2011, 34, 1151–1158. [Google Scholar] [CrossRef]
- Arpagaus, C. Production of food bioactive-loaded nanoparticles by nano spray drying. In Nanoencapsulation of Food Ingredients by Specialized Equipment: Volume 3 in the Nanoencapsulation in the Food Industry Series; Academic Press: Cambridge, MA, USA, 2019; Volume 3, pp. 151–211. [Google Scholar]
- Augustin, M.A.; Sanguansri, L.; Lockett, T. Nano-and micro-encapsulated systems for enhancing the delivery of resveratrol. Ann. N. Y. Acad. Sci. 2013, 1290, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Garti, N.; McClements, D.J. Encapsulation Technologies and Delivery Systems for Food Ingredients and Nutraceuticals; Elsevier: Gorgan, Iran, 2012. [Google Scholar]
- Qian, C.; Decker, E.A.; Xiao, H.; McClements, D.J. Nanoemulsion delivery systems: Influence of carrier oil on β-carotene bioaccessibility. Food Chem. 2012, 135, 1440–1447. [Google Scholar] [CrossRef] [PubMed]
- Bürki, K.; Jeon, I.; Arpagaus, C.; Betz, G. New insights into respirable protein powder preparation using a nano spray dryer. Int. J. Pharm. 2011, 408, 248–256. [Google Scholar] [CrossRef]
- Hu, Q.; Wang, T.; Zhou, M.; Xue, J.; Luo, Y. Formation of redispersible polyelectrolyte complex nanoparticles from gallic acid-chitosan conjugate and gum arabic. Int. J. Biol. Macromol. 2016, 92, 812–819. [Google Scholar] [CrossRef]
- O’Toole, M.G.; Henderson, R.M.; Soucy, P.A.; Fasciotto, B.H.; Hoblitzell, P.J.; Keynton, R.S.; Ehringer, W.D.; Gobin, A.S. Curcumin encapsulation in submicrometer spray-dried chitosan/tween 20 particles. Biomacromolecules 2012, 13, 2309–2314. [Google Scholar] [CrossRef]
- Lee, S.H.; Heng, D.; Ng, W.K.; Chan, H.; Tan, R.B. Nano spray drying: A novel method for preparing protein nanoparticles for protein therapy. Int. J. Pharm. 2011, 403, 192–200. [Google Scholar] [CrossRef]
- Pérez-Masiá, R.; López-Nicolás, R.; Periago, M.J.; Ros, G.; Lagaron, J.M.; López-Rubio, A. Encapsulation of folic acid in food hydrocolloids through nanospray drying and electrospraying for nutraceutical applications. Food Chem. 2015, 168, 124–133. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Soyama, S.; Luo, Y. Development of a novel functional drink from all natural ingredients using nanotechnology. LWT 2016, 73, 458–466. [Google Scholar] [CrossRef]
- Wang, T.; Hu, Q.; Zhou, M.; Xia, Y.; Nieh, M.; Luo, Y. Development of “All natural” layer-by-layer redispersible solid lipid nanoparticles by nano spray drying technology. Eur. J. Pharm. Biopharm. 2016, 107, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Wang, T.; Hu, Q.; Zhou, M.; Luo, Y. Insight into natural biopolymer-emulsified solid lipid nanoparticles for encapsulation of curcumin: Effect of loading methods. Food Hydrocoll. 2018, 79, 110–116. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, T.; Hu, Q.; Luo, Y. Low density lipoprotein/pectin complex nanogels as potential oral delivery vehicles for curcumin. Food Hydrocoll. 2016, 57, 20–29. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, A.; Guimarães, K.; Cerize, N.; Tunussi, A.; Poço, J. Nano spray drying as an innovative technology for encapsulating hydrophilic active pharmaceutical ingredients (API). J. Nanomed. Nanotechnol. 2013, 4. [Google Scholar] [CrossRef] [Green Version]
- Feng, A.; Boraey, M.; Gwin, M.; Finlay, P.; Kuehl, P.; Vehring, R. Mechanistic models facilitate efficient development of leucine containing microparticles for pulmonary drug delivery. Int. J. Pharm. 2011, 409, 156–163. [Google Scholar] [CrossRef]
- Veneranda, M.; Hu, Q.; Wang, T.; Luo, Y.; Castro, K.; Madariaga, J.M. Formation and characterization of zein-caseinate-pectin complex nanoparticles for encapsulation of eugenol. LWT 2018, 89, 596–603. [Google Scholar] [CrossRef]
- Kyriakoudi, A.; Tsimidou, M.Z. Properties of encapsulated saffron extracts in maltodextrin using the Büchi B-90 nano spray-dryer. Food Chem. 2018, 266, 458–465. [Google Scholar] [CrossRef]
- Suna, S.; Sinir, G.Ö.; Çopur, Ö. Nano-spray drying applications in food industry. In Proceedings of the 11th International Conference Of Food Physicists Food Physics And Innovative Technologies, Plovdiv, Bulgaria, 10–12 June 2014; pp. 10–12. [Google Scholar]
- Beck-Broichsitter, M.; Schweiger, C.; Schmehl, T.; Gessler, T.; Seeger, W.; Kissel, T. Characterization of novel spray-dried polymeric particles for controlled pulmonary drug delivery. J. Control. Release 2012, 158, 329–335. [Google Scholar] [CrossRef]
- Baker, C.; McKenzie, K. Energy consumption of industrial spray dryers. Dry. Technol. 2005, 23, 365–386. [Google Scholar] [CrossRef]
- Al-Mansour, H.; Al-Busairi, B.; Baker, C. Energy consumption of a pilot-scale spray dryer. Dry. Technol. 2011, 29, 1901–1910. [Google Scholar] [CrossRef]
- Motevali, A.; Minaei, S.; Banakar, A.; Ghobadian, B.; Khoshtaghaza, M.H. Comparison of energy parameters in various dryers. Energy Convers. Manag. 2014, 87, 711–725. [Google Scholar] [CrossRef]
- Huang, L.X.; Kumar, K.; Mujumdar, A. A comparative study of a spray dryer with rotary disc atomizer and pressure nozzle using computational fluid dynamic simulations. Chem. Eng. Process. Process. Intensif. 2006, 45, 461–470. [Google Scholar] [CrossRef]
- Drusch, S.; Serfert, Y.; Scampicchio, M.; Schmidt-Hansberg, B.; Schwarz, K. Impact of physicochemical characteristics on the oxidative stability of fish oil microencapsulated by spray-drying. J. Agric. Food Chem. 2007, 55, 11044–11051. [Google Scholar] [CrossRef]
- Linke, A.; Hinrichs, J.; Kohlus, R. Impact of the oil droplet size on the oxidative stability of microencapsulated oil. J. Microencapsul. 2020, 37, 170–181. [Google Scholar] [CrossRef]
- Majchrzak, T.; Wojnowski, W.; Dymerski, T.; Gębicki, J.; Namieśnik, J. Electronic noses in classification and quality control of edible oils: A review. Food Chem. 2018, 246, 192–201. [Google Scholar] [CrossRef]
- Linke, A.; Hinrichs, J.; Kohlus, R. Impact of the powder particle size on the oxidative stability of microencapsulated oil. Powder Technol. 2020, 364, 115–122. [Google Scholar] [CrossRef]
- Karthik, P.; Anandharamakrishnan, C. Microencapsulation of docosahexaenoic acid by spray-freeze-drying method and comparison of its stability with spray-drying and freeze-drying methods. Food Bioprocess Technol. 2013, 6, 2780–2790. [Google Scholar] [CrossRef]
- Tonon, R.V.; Grosso, C.R.; Hubinger, M.D. Influence of emulsion composition and inlet air temperature on the microencapsulation of flaxseed oil by spray drying. Food Res. Int. 2011, 44, 282–289. [Google Scholar] [CrossRef]
- Haque, M.A.; Chen, J.; Aldred, P.; Adhikari, B. Denaturation and physical characteristics of spray-dried whey protein isolate powders produced in the presence and absence of lactose, trehalose, and polysorbate-80. Dry. Technol. 2015, 33, 1243–1254. [Google Scholar] [CrossRef]
- Ainis, W.N.; Ersch, C.; Ipsen, R. Partial replacement of whey proteins by rapeseed proteins in heat-induced gelled systems: Effect of pH. Food Hydrocoll. 2018, 77, 397–406. [Google Scholar] [CrossRef]
- Silva, J.V.; Balakrishnan, G.; Schmitt, C.; Chassenieux, C.; Nicolai, T. Heat-induced gelation of aqueous micellar casein suspensions as affected by globular protein addition. Food Hydrocoll. 2018, 82, 258–267. [Google Scholar] [CrossRef]
- Lechevalier, V.; Jeantet, R.; Arhaliass, A.; Legrand, J.; Nau, F. Egg white drying: Influence of industrial processing steps on protein structure and functionalities. J. Food Eng. 2007, 83, 404–413. [Google Scholar] [CrossRef]
- Tan, S.; Zhong, C.; Langrish, T. Encapsulation of caffeine in spray-dried micro-eggs for controlled release: The effect of spray-drying (cooking) temperature. Food Hydrocoll. 2020, 108, 105979. [Google Scholar] [CrossRef]
- Burgos-Díaz, C.; Opazo-Navarrete, M.; Soto-Añual, M.; Leal-Calderón, F.; Bustamante, M. Food-grade Pickering emulsion as a novel astaxanthin encapsulation system for making powder-based products: Evaluation of astaxanthin stability during processing, storage, and its bioaccessibility. Food Res. Int. 2020, 134, 109244. [Google Scholar] [CrossRef] [PubMed]
- Tupuna, D.S.; Paese, K.; Guterres, S.S.; Jablonski, A.; Flôres, S.H.; de Oliveira Rios, A. Encapsulation efficiency and thermal stability of norbixin microencapsulated by spray-drying using different combinations of wall materials. Ind. Crop. Prod. 2018, 111, 846–855. [Google Scholar] [CrossRef]
- Weber, F.; Boch, K.; Schieber, A. Influence of copigmentation on the stability of spray dried anthocyanins from blackberry. LWT 2017, 75, 72–77. [Google Scholar] [CrossRef]
- Marsilio, V.; Campestre, C.; Lanza, B. Phenolic compounds change during California-style ripe olive processing. Food Chem. 2001, 74, 55–60. [Google Scholar] [CrossRef]
- Rice-Evans, C.; Miller, N.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Ramírez, M.J.; Giraldo, G.I.; Orrego, C.E. Modeling and stability of polyphenol in spray-dried and freeze-dried fruit encapsulates. Powder Technol. 2015, 277, 89–96. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Ramirez, M.J.; Orrego, C.E.; Teixeira, J.A.; Mussatto, S.I. Encapsulation of antioxidant phenolic compounds extracted from spent coffee grounds by freeze-drying and spray-drying using different coating materials. Food Chem. 2017, 237, 623–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, A.; Xie, H.; Qi, Y.; Liu, C.; Guo, X.; Sun, J.; Liu, L. Effects of storage time and temperature on polyphenolic content and qualitative characteristics of freeze-dried and spray-dried bayberry powder. LWT 2017, 78, 235–240. [Google Scholar] [CrossRef]
- Leyva-Porras, C.; Saavedra-Leos, M.Z.; Cervantes-González, E.; Aguirre-Bañuelos, P.; Silva-Cázarez, M.B.; Álvarez-Salas, C. Spray drying of blueberry juice-maltodextrin mixtures: Evaluation of processing conditions on content of resveratrol. Antioxidants 2019, 8, 437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Category | Core Material | Wall Material | Spray Drying Conditions | Major Outcomes | Reference |
|---|---|---|---|---|---|
| Lyphophilic substances | Vitamin E acetate | Arabic gum, whey protein, polyvinyl alcohol, modified starch, and maltodextrin | Inlet: 100 °C Outlet: 41–58 °C | Obtaining of submicron particles with size as low as approximately 350 nm for the formulations prepared using Arabic gum as wall material at 0.1 wt % of solid concentration. The yield of the obtained dried products was between 70% and 90%. | [15] |
| Curcumin | Chitosan | Inlet: 100 °C Spray mesh: 4.0 μm Air flow rate: 150 L/min | The encapsulation of curcumin in submicrometric chitosan with spherical morphology and smooth surface with an encapsulation efficiency near 100%. The authors achieved a complete release of the active ingredient in a short period of time (2 h). | [79] | |
| Bovine serum albumin (BSA) | Polyoxyethylene and sorbitan monooleate | Inlet: 80–120 °C Outlet: 36–55 °C Spray meshes: 4.0, 5.5, and 7.0 μm Nitrogen flow rate: 120 L/min | Taguchi methodology was incorporated into an empirical study for the optimization of process parameters in the production of smooth, spherical nanoparticles with diameters around 460 nm and high-yield products (72%). | [80] | |
| Folic acid (Synthetic vitamin B9) | Guar gum, whey protein and resistant starch | Inlet: 90 °C Outlet: 45 °C Spray mesh: 0.7 μm Air flow rate: 140 L/h | Efficient encapsulation of folic acid (vitamin B9). Whey protein showed higher encapsulation efficiency and low degradation during storage compared to starch. The encapsulation efficiency from the whey protein/folic acid formulation was around 84%, although bigger average diameters and broader size distribution were observed in comparison with other drying technology (electrospraying). | [81] | |
| Peppermint oil | Sodium caseinate and pectin | Inlet: 100 °C Spray mesh: 5.5 μm Air Flow rate: 120 L/min | Encapsulation of hydrophilic and hydrophobic nutrients by the development of a sodium caseinate/pectin/peppermint oil nanocomplex delivery system. Encapsulates were stable and had exceptional capability to preserve the antioxidant activity of nutrients under storage conditions. | [82] | |
| Not active compound encapsulated | Sodium caseinate, L-α-soya lecithin, and pectin | Inlet: 100 °C Spray mesh: 5.5 μm Air Flow rate: 120 L/min | The Box–Benhken design was applied in the spray drying of solid lipid nanoparticles with a bilayer of the biopolymers and stabilized with soya lecithin to achieve well-separated and spherical ultra-fine powders with excellent redispersibility in water. | [83] | |
| Curcumin and stearic acid | Sodium caseinate, pectin, and stearic acid | Inlet: 100 °C Spray mesh: 7 μm Air Flow rate: 120 L/min | Production of solid lipid nanoparticles loaded with curcumin with enhanced antioxidant activity, gastrointestinal stability, small particle, and low polydispersity index. | [84] | |
| Curcumin | Egg yolk low-density lipoprotein, pectin | Inlet: 70, 100, and 120 °C. Outlet: 50–60 °C Air Flow rate: 130 L/min | Optimization of the fabrication conditions for egg yolk low-density lipoprotein/pectin nanogels with a smooth surface and spherical shape with a diameter of 58 nm. The obtained ultra-fine powders were able to re-disperse into water and keep the nanoscale size. | [85] | |
| Hydrophilic substances | Vitamin B12 | Arabic gum, cashew nut gum, sodium alginate, sodium carboxymethyl cellulose, and Eudragit RS100 | Inlet: 120 °C Outlet: 50–60 °C Spray meshes: 4.0 and 7.0 μm Air flow rate: 130 L/min | Production of submicron particles by the highly diluted solutions. Eudragit RS100 showed more controlled release kinetics since it presented solubility dependent on the change in pH. | [86] |
| β-Galactoside | Trehalose | Inlet: 80–120 °C Outlet: 38–60 °C Spray meshes: 4.0, 5.5, and 7.0 μm Air flow rate: 100–110 L/min | Obtaining of submicrometric encapsulates between 2 and 4 μm of diameter at the optimized spray drying conditions of inlet temperature 80 °C and the lower spray mesh size and the flow rate of 4 μ and 100 L/min, respectively. The obtained morphology was spherical with a smooth surface and the yield product reached up to 90%. | [77] | |
| Not active compound encapsulated | Chitosan/gallic acid conjugate, Arabic gum, and polyethylene glycol | Inlet: 100 °C Spray mesh: 5.5 μm Air flow rate: 100–120 L/min | Obtaining spherical, homogeneous, and smooth powders of nanoparticles with improved water solubility and dispersibility properties. In comparison with the native chitosan (CS)/Arabic gum nanoparticles, the polyethylene glycol (PEG) complexes exhibited smaller size, narrower polydispersity index, and greater redispersibility behavior. | [78] | |
| Լ-leucine | α,α-trehalose | Inlet: 75 °C Outlet: 45 °C Spray mesh: 4 μm Air flow rate: 100 L/min | Presented a mechanistic model for the efficient experimental design for obtaining microparticles. Authors produced particles with spherical morphologies that begin to exhibit a corrugated surface as the percentage of crystalline leucine increases, lowering the density and improving the dispersibility properties of the dried products. | [87] | |
| Eugenol | Zein, sodium caseinate, and pectin | Inlet: 100 °C Spray mesh: 5 μm Air flow rate: 120 L/min | Under optimal preparation condition and formulation, eugenol-loaded complex nanoparticles with a size of 140 nm, spherical shape, and uniform size distribution and excellent storage stability were obtained. | [88] | |
| Saffron apocarotenoids | Maltodextrin | Inlet: 100 °C Spray meshes: 4 and 7 μm. Air flow rate: 100 L/min | Obtaining spherical particles found that the morphology is highly dependent on the mesh size employed. Product yield and encapsulation efficiency of saffron apocarotenoids were found to be satisfactory being approximately 70% and 80%, respectively. Thermal stability and bioaccessibility of the apocarotenoids was enhanced by the nanoencapsulation process. | [89] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piñón-Balderrama, C.I.; Leyva-Porras, C.; Terán-Figueroa, Y.; Espinosa-Solís, V.; Álvarez-Salas, C.; Saavedra-Leos, M.Z. Encapsulation of Active Ingredients in Food Industry by Spray-Drying and Nano Spray-Drying Technologies. Processes 2020, 8, 889. https://doi.org/10.3390/pr8080889
Piñón-Balderrama CI, Leyva-Porras C, Terán-Figueroa Y, Espinosa-Solís V, Álvarez-Salas C, Saavedra-Leos MZ. Encapsulation of Active Ingredients in Food Industry by Spray-Drying and Nano Spray-Drying Technologies. Processes. 2020; 8(8):889. https://doi.org/10.3390/pr8080889
Chicago/Turabian StylePiñón-Balderrama, Claudia I., César Leyva-Porras, Yolanda Terán-Figueroa, Vicente Espinosa-Solís, Claudia Álvarez-Salas, and María Z. Saavedra-Leos. 2020. "Encapsulation of Active Ingredients in Food Industry by Spray-Drying and Nano Spray-Drying Technologies" Processes 8, no. 8: 889. https://doi.org/10.3390/pr8080889
APA StylePiñón-Balderrama, C. I., Leyva-Porras, C., Terán-Figueroa, Y., Espinosa-Solís, V., Álvarez-Salas, C., & Saavedra-Leos, M. Z. (2020). Encapsulation of Active Ingredients in Food Industry by Spray-Drying and Nano Spray-Drying Technologies. Processes, 8(8), 889. https://doi.org/10.3390/pr8080889