1. Introduction
Refractory sulfurs in fuel oil are known to be a pollutant as they produce sulfur dioxide, SO
x when combusted. SO
x gasses can react with water vapor in the atmosphere forming sulfuric acid, otherwise known as acid rain. Acid rain can disrupt marine ecology, destroy forests, and deteriorate heritage buildings. Moreover, long-term exposure to SO
x can cause respiratory related illness, heart disease, and asthma [
1]. With the rapid growth of automobile industries, production of ultra-low sulfur fuel oil is indispensable to minimize SO
x emission.
Current technology uses hydrodesulfurization (HDS) to eliminate sulfur from fuel oil. This process however demands high temperature (300–400 °C) and high hydrogen gas pressure (30–130 atm) to operate, making it an energy-extensive and financially tasking separation process [
2]. Moreover, HDS is unable to intensely remove sterically hindered sulfurs such as dibenzothiophene (DBT), 1-benzothiophene (BT), 4-methyldibenzothiophene (MDBT), benzonaphthothiophene (BPT), and 4,6-dimethyldibenzothiophene (4,6-DMDBT) due to the existence of bulky substituent groups. Therefore, alternative separation technologies are highly desired to solve the drawbacks of conventional HDS.
Some of the non-conventional ways to remove sulfur are currently being tested including biodesulfurization (BDS) [
3,
4,
5], adsorptive desulfurization (ADS) [
6,
7,
8], extractive desulfurization (EDS) [
9,
10,
11,
12], and oxidative desulfurization (ODS) [
13,
14]. Compared to BDS and ADS, EDS could be operated at mild conditions with the use of suitable extractant to desulfurize fuel oil. On the other hand, integrating ODS in EDS system (EODS) will promote the oxidation of sulfur into sulfone components, which can be easily extracted into the solvent phase [
15,
16,
17,
18]. Selection of extractant is nonetheless challenging since some of the conventional solvents are highly flammable and volatile [
19,
20,
21]. Therefore, ionic liquids (ILs), which are composed of cationic and anionic salts, have been introduced as green solvent since it has extremely low vapor pressure, customizable polarity, minimum flammability, and substantial stability for various applications [
22,
23,
24,
25]. By using ILs in EODS, the performance of desulfurization could be greatly improved [
20,
26,
27]. Despite its unique solvent properties, the synthesis of IL requires tedious process and the raw materials are expensive, therefore it is not economically preferred by industrial practice.
Pioneered by Abbot et al., [
28,
29], a new class of green solvent called deep eutectic solvent (DES), which have similar properties with IL, have received considerable attention in the past few years. It is formed from the interaction of hydrogen bond acceptor (HBA), which is usually in the form of quaternary ammonium salt and hydrogen bond donors (HBD). The raw materials are widely available and affordable, while the synthesis of DES is straightforward compared to IL. Moreover, reports from the literature show that this type of solvent is environmentally benign due to the biodegradability properties of the raw materials [
30,
31].
Research of polyethylene glycol (PEG)-based DES in EDS is currently attracting researchers’ attentions due to its enormous performance in sulfur removal. In early 2013, Li et al. had synthesized PEG eutectic mixture with different HBAs namely choline chloride (ChCl), tetramethylammonium chloride (TMAC), and tetrabutylammonium chloride (TBAC) for application in EDS system. It was found that in a single extraction, 82.83% of sulfur could be removed from simulated fuel by using TBAC-PEG as an extractant [
32]. The higher extraction efficiency arose from the longer alkyl chain components in DES. Furthermore, Xu et al. had synthesized a metal-based DES using cobalt chloride (CoCl
2), ChCl, and PEG (number average molecular weight of 200 g mol
−1) in extractive and catalytic-oxidative desulfurization system (ECODS) [
33]. At optimal conditions, 90% of sulfur could be removed using this DES. Another finding on the application of PEG-based DES in EDS was also reported by Lima et al., where 85% of sulfur could be eliminated when using PEG with the molecular weight of 400 g mol
−1 as HBD [
34]. In their findings, low-molecular-weight liquid polymer was preferable for EDS system since higher molecular weight may hinder the mass transfer during the extraction process. According to these reports, several EDS parameters were analyzed to optimize the performance of EDS, which includes extraction time, extraction temperature, volume ratio of extractant to simulated fuel, and stirring speed. However, all important parameters were studied independently, and the relationship between the variables have not yet been studied statistically in the context of EDS performance. Therefore, a systematic way to study the effect of each variable is crucial to analyze the sensitivity of input parameters towards EDS.
Response surface methodology (RSM) is a statistical tool that can help users to empirically analyze the effect of input variables for optimizing a system [
35]. The three essential processes employed in RSM are the methodical design of experiments, regression modelling approach, and optimization technique. By implementing RSM, the total experiments can be minimized and a synergistic influence of each independent variable could be monitored along the process. A Box–Behnken design RSM was employed by Mokhtar et al., to optimize EDS using dimethylformamide as extractant [
19]. Based on the response contour of interacting variables, they found that the increase of extractant volume and extraction time both gave a positive effect towards EDS performance with a maximum removal of 67.5%. Rahma et al. utilized RSM in evaluating the performance of PEG-based DES using tetrabutylammonium bromide as HBA. According to their outcome, extraction efficiency could reach as high as 82.40% within one extraction [
36]. They concluded that the stirring speed was the most sensitive factor in EE. Meanwhile, Almashjary et al. reported an EE of 64.9% at optimized conditions using ChCl:propionic acid as EDS extractant [
37]. Likewise, many parameters have been studied to optimize the EDS conditions. Nonetheless, reports on the influence of DES composition in EDS are relatively scarce. Especially, as far as we know, no statistical models have been constructed for the performance of TBAC:PEG in EODS.
Herein, we report a systematic investigation of the influence of independent variables toward EDS performance via RSM. We first synthesize the TBAC:PEG mixture in different mole ratios. Next, important parameters are included in the design stage of experiment employing central composite design (CCD) to understand the mutual effect of different variables on EE. From the statistical analysis, suitable mathematical modelling is established and optimum points that yield the highest extraction efficiency are determined.
2. Materials and Methods
2.1. Chemicals and Software
Tetrabutylammonium chloride, TBAC (95%) and n-dodecane (99%) were obtained from Acros Organics (Geel West Zone 2, Janssen Pharmaceuticalaan 3a, B-2440 Geel, Belgium). Hydrogen peroxide (30% in water) and dibenzothiophene, DBT were purchased from Merck (Sunway Annexe Tower, Jalan Lagoon Timur, Sunway City, Selangor, Malaysia). Poly(ethylene glycol), PEG400 was supplied by Sigma Aldrich (Jalan PJS 7/21, Sunway City, Subang Jaya, Petaling Jaya, Selangor, Malaysia). DesignExpert (version: 12.0.0.6, 64-bit) software was provided by State-Ease Inc. (1300 Godward St NE, Suite 6400, Minneapolis, MN 55413, USA).
2.2. DES Synthesis
TBAC-PEG was prepared by weighing desired amount of TBAC salt, followed by PEG in a closed vial. The mixtures were heated to 50 °C and stirred at 500 rpm within 60 min. The obtained clear solution was left to cool down prior to the analysis.
2.3. Fourier Transformation Infrared (FTIR) Spectroscopy
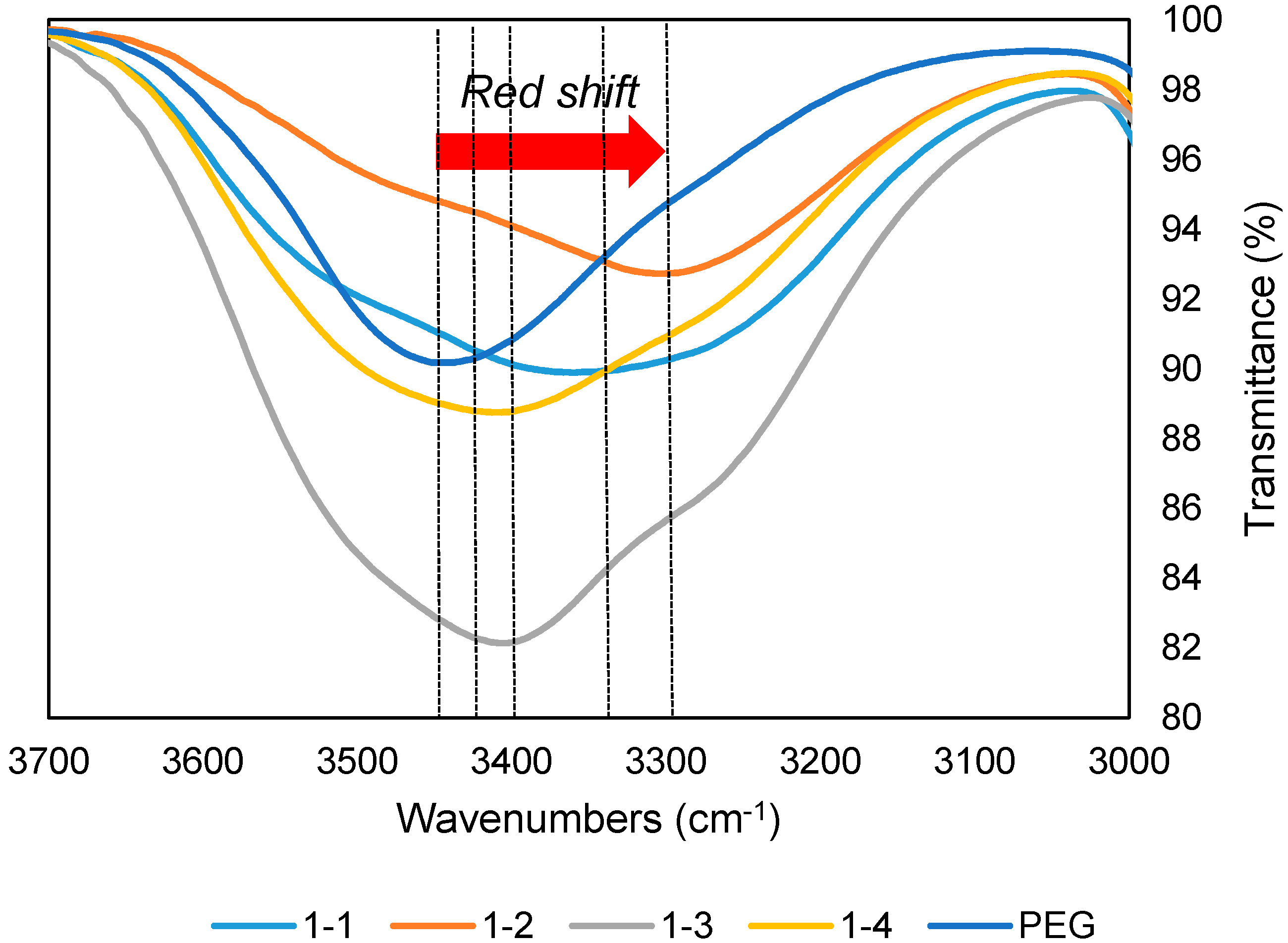
All samples of DES were characterized via Nicolet Series FTIR Spectrometer (Fisher Scientific (M) Sdn Bhd, Taman Perindustrian Axis, Shah Alam, Selangor, Malaysia) to confirm the formation of intermolecular forces between TBAC and PEG. A background spectrum was initially collected before sample characterization to get rid of undesirable residue peaks from the spectrum. The sample was dropped in a small amount on the diamond cell. For spectrum smoothing purpose, a total of 16 scans were used while the wavenumbers were collected from 550–4000 cm−1. All FTIR spectrums were reported in terms of transmittance.
2.4. Viscosity Measurement
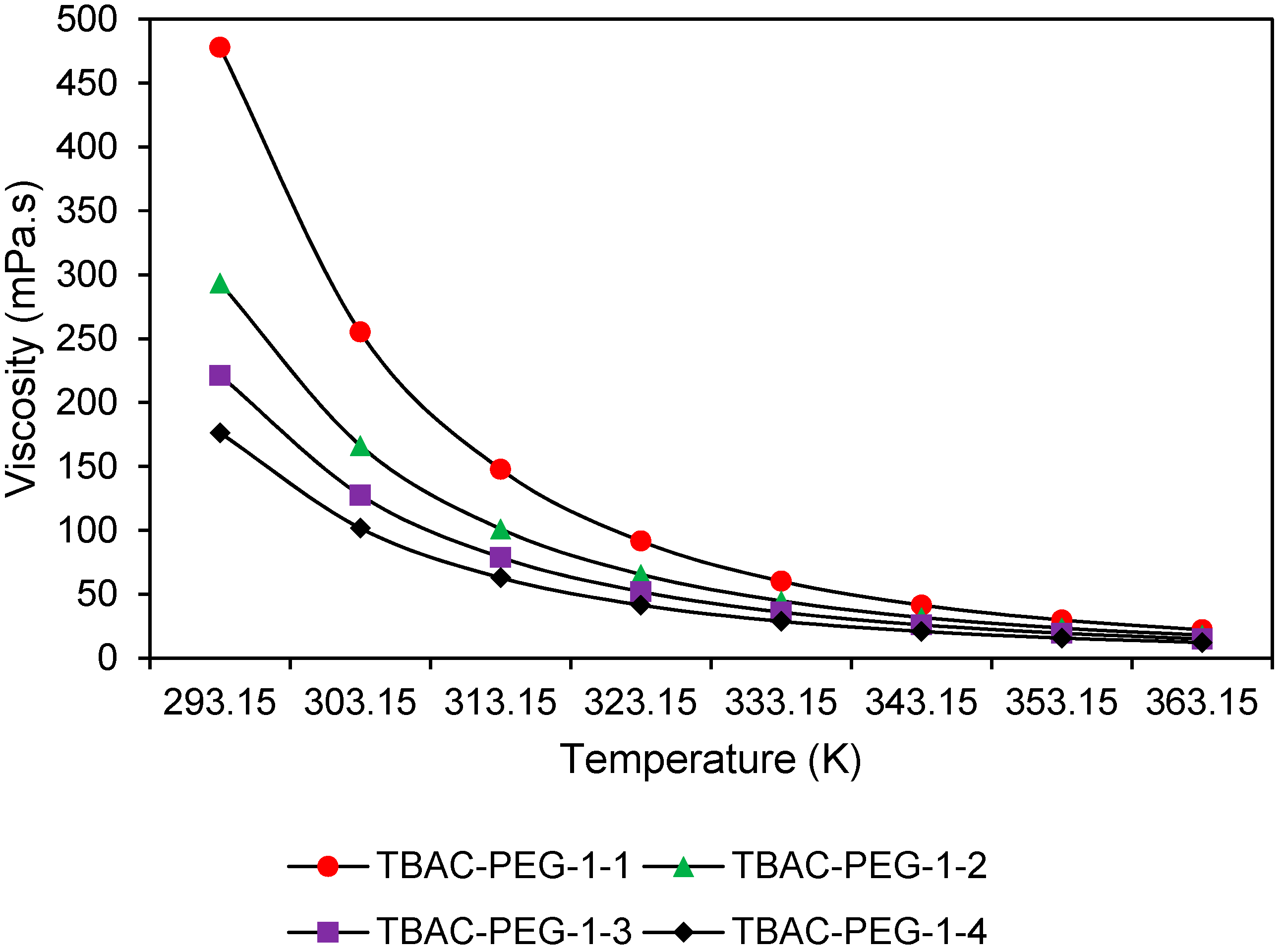
Evaluation of viscosity values was made via SVM 3000 Anton Paar viscometer (Anton Paar Malaysia Sdn Bhd, The Pinnacle, Persiaran Lagoon, Sunway City, Subang Jaya, Selangor, Malaysia). In general, 5 mL of DES sample was injected into an oscillating u-tube viscometer and the measurement was taken from 293.15 K to 363.15 K with temperature uncertainty of ± 0.01K. After each measurement was done, the tube was cleaned with acetone for three times before introducing the next sample.
2.5. Differential Scanning Calorimetry (DSC)
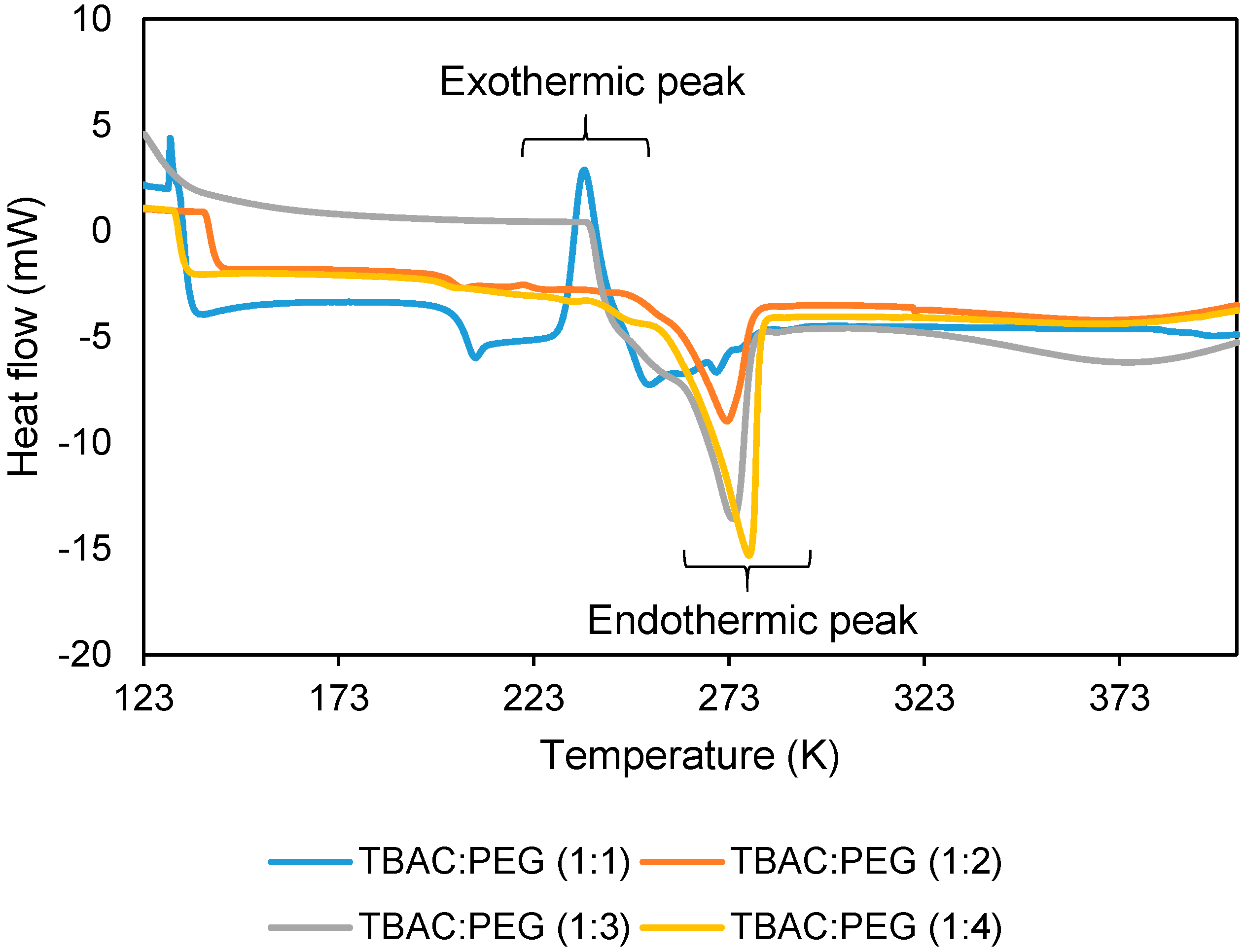
The melting point of DES was characterized via DSC Mettler-Toledo model SC822 (Mettler-Toledo Sdn Bhd, Bukit Jelutong, Shah Alam, Selangor, Malaysia). The temperature and heat flow of DSC was calibrated with zinc and indium reference sample. Approximately 10 mg of samples were weighed in a pan before exposed to flowing liquid nitrogen. The heating range was set from −150 °C to 130 °C at a heating/cooling rate of 10 °C min−1. The sample was subjected to four heating cycles, which started with cooling, followed by heating for elimination of thermal history.
2.6. Extractive Desulfurization System (EDS) Experiments
A 100 ppm simulated fuel was prepared by mixing fixed amount of DBT with n-dodecane in a volumetric flask. For the EDS set up, a desired amount of simulated fuel was mixed with DES and oxidant (oxidant to oil ratio of 2) in a reaction vial with a stirring speed of 200 rpm. Typically, phase separation into two layers occurred due to the immiscibility of DES and simulated fuel. A disposable syringe was used to transfer an aliquot of simulated fuel to a 2 mL screw-top vial followed by sulfur analysis.
2.7. Sulfur Content Analysis
To analyze the removal efficiency of sulfur from simulated fuel, high-performance liquid chromatography (1200 Series, Agilent Technologies, Damansara Uptown, Petaling Jaya, Selangor, Malaysia) was utilized. Determination of sulfur concentration was carried using external standard method and the UV-vis wavelength was fixed at 310 nm. Reversed-phase Zorbax SB-C18 was used as stationary phase with a column dimension of 4.6 × 150 mm. A mixture of methanol/deionized water/propan-2-ol (90%/8%/2%) was used as the mobile phase and the flow rate was set to 1 mL min
−1. Extraction efficiency (EE) was calculated using Equation (1):
where
and
are the initial and final concentration of sulfur, respectively.
2.8. Design of Experiments (DOE)
A systematic set of experiments was designed using central-composite design (CCD) approach. DES mole ratio (A), DES volume ratio (B), and extraction temperature (C) were incorporated as independent variables while EE% was selected as the output response. A face-centered design space with an axial spacing, α, of 1 was selected and a total of 20 experiments were generated in randomized order. All EDS parameters were coded as −1, 0, and ±1, which represents low level, central point, and high level (
Table 1).
Equation (2) shows the second order polynomial equation, which was used to fit all variables:
where
y is the predicted response,
is the constant coefficient,
is the linear coefficient,
is the squared coefficient,
denotes mutual interaction coefficient,
and
are both independent variables, and
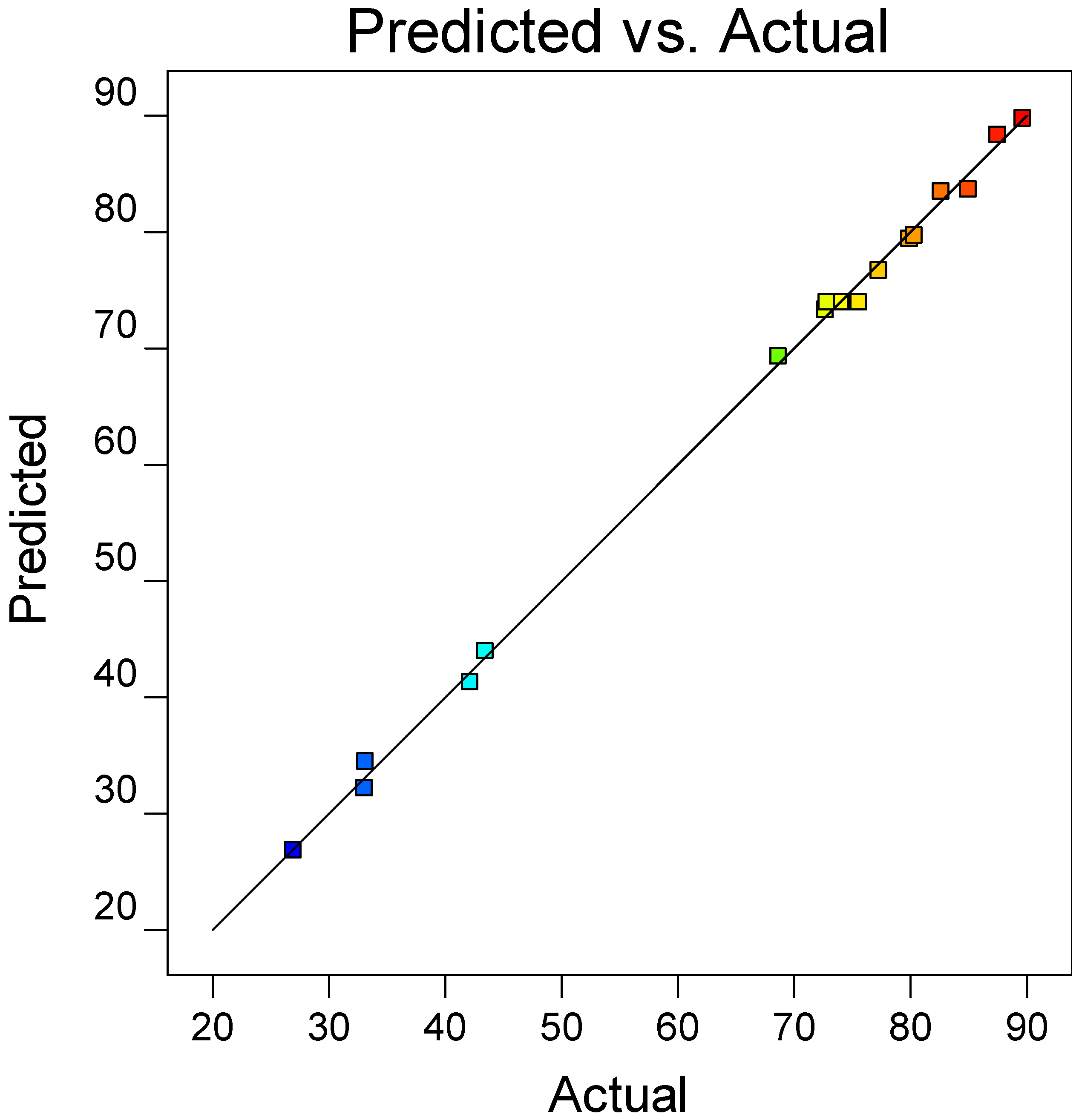
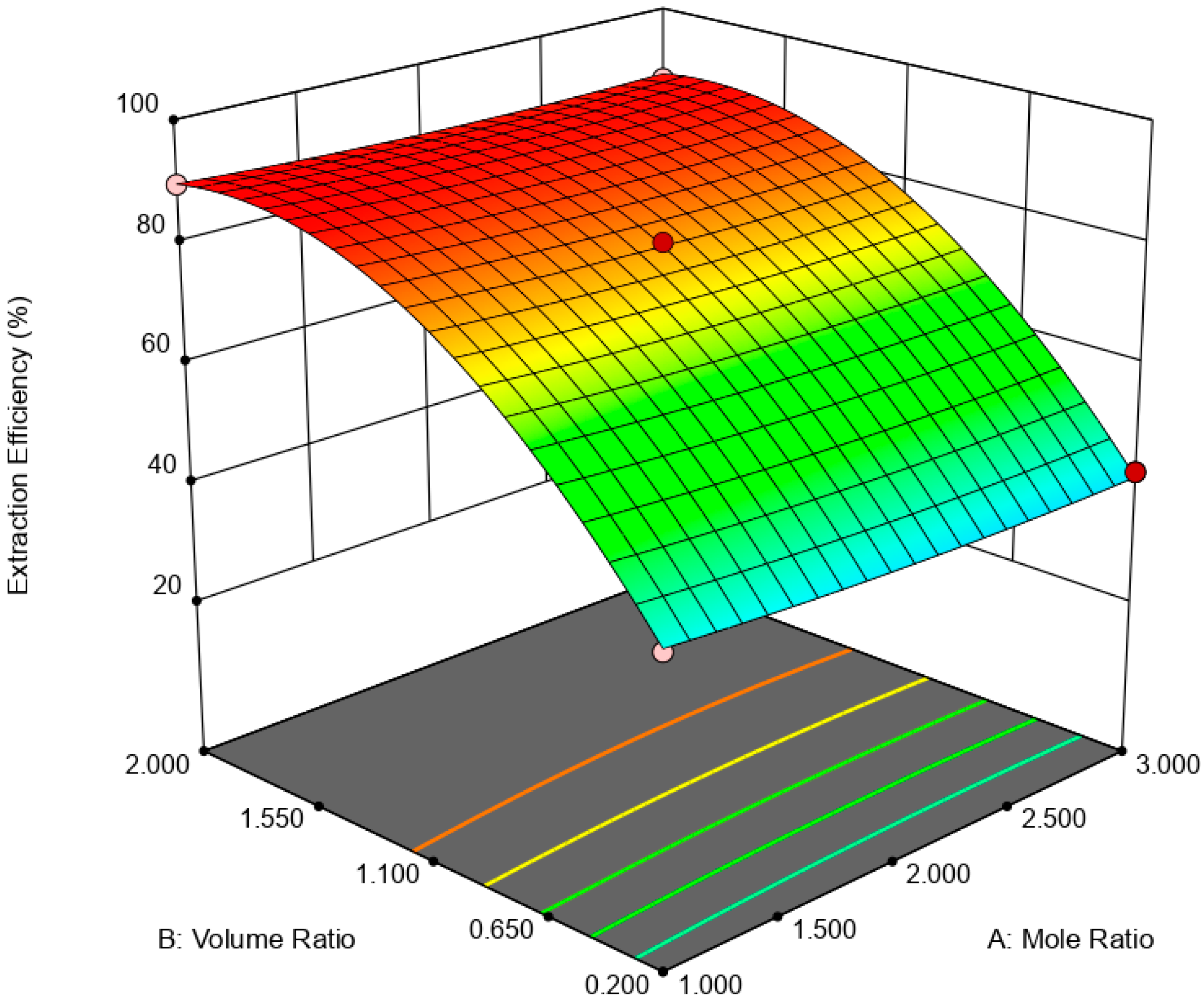
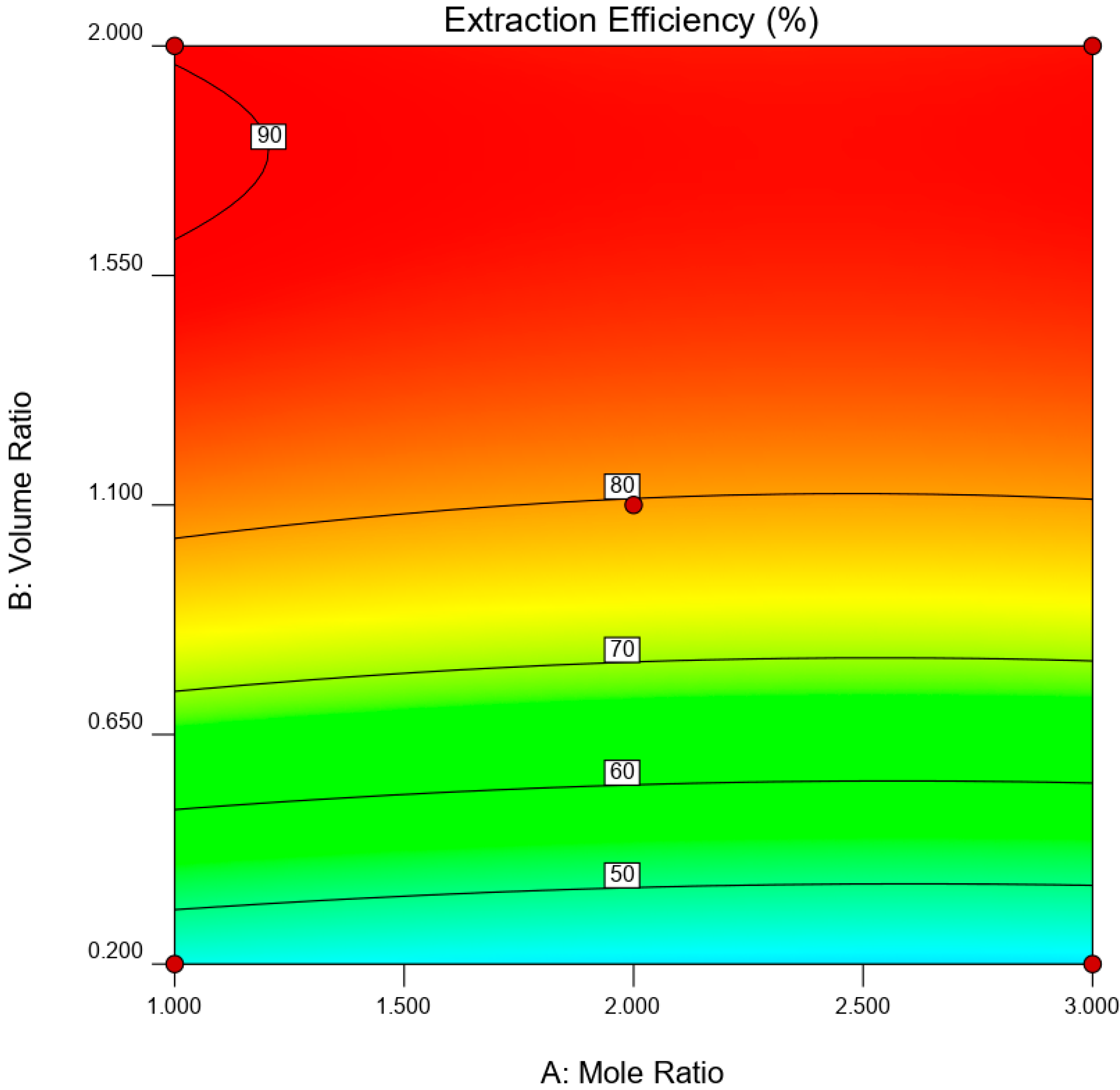
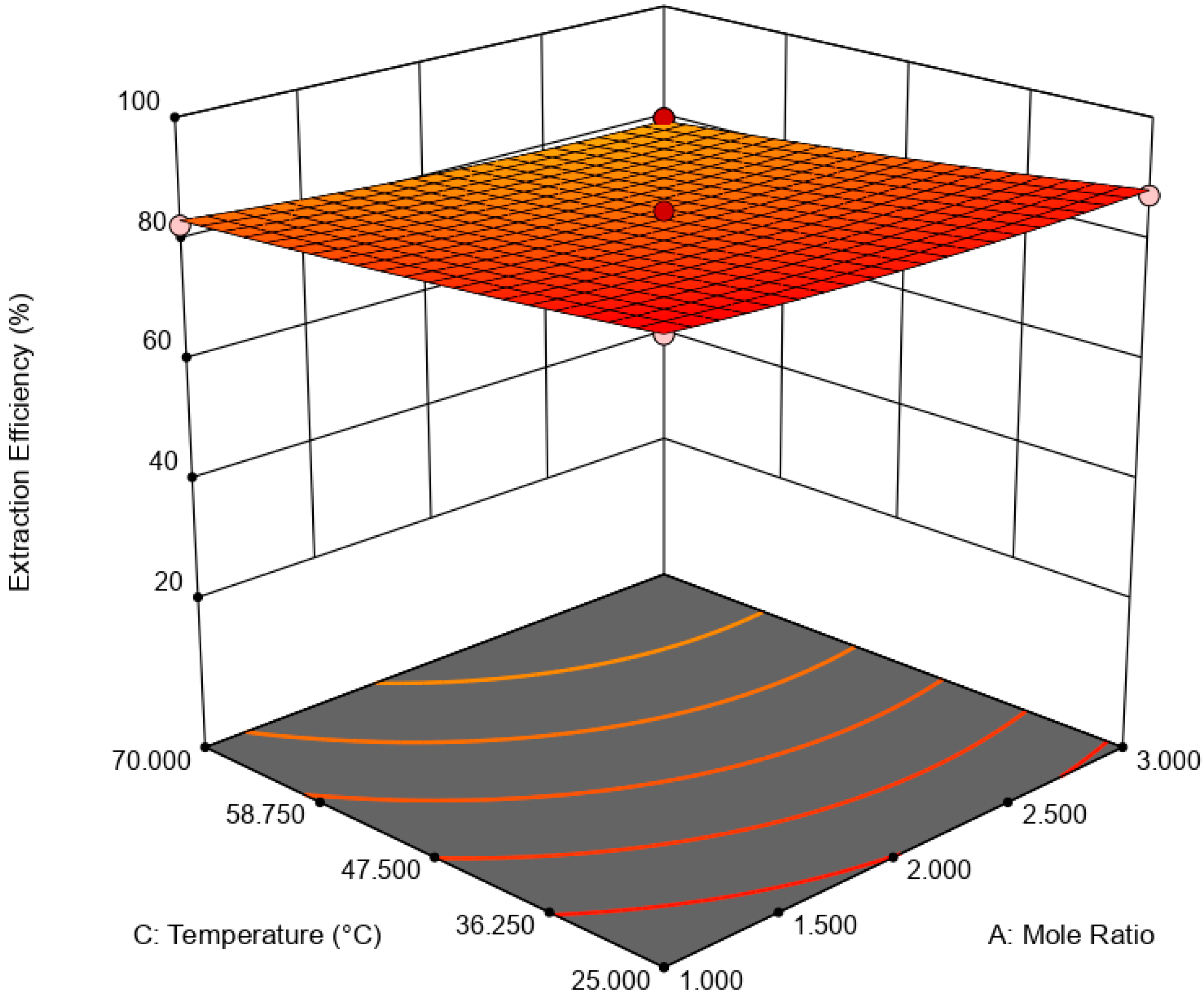
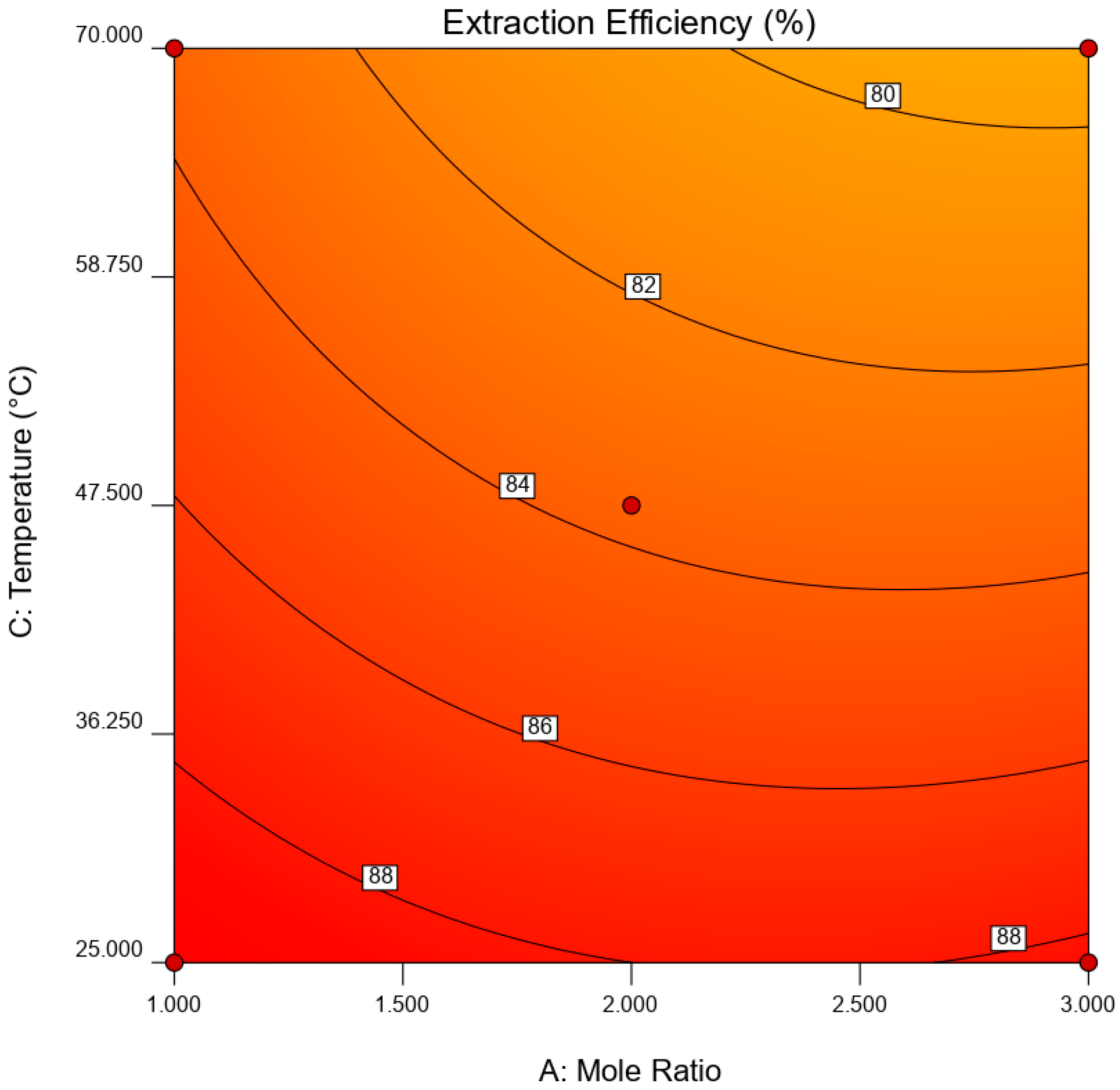
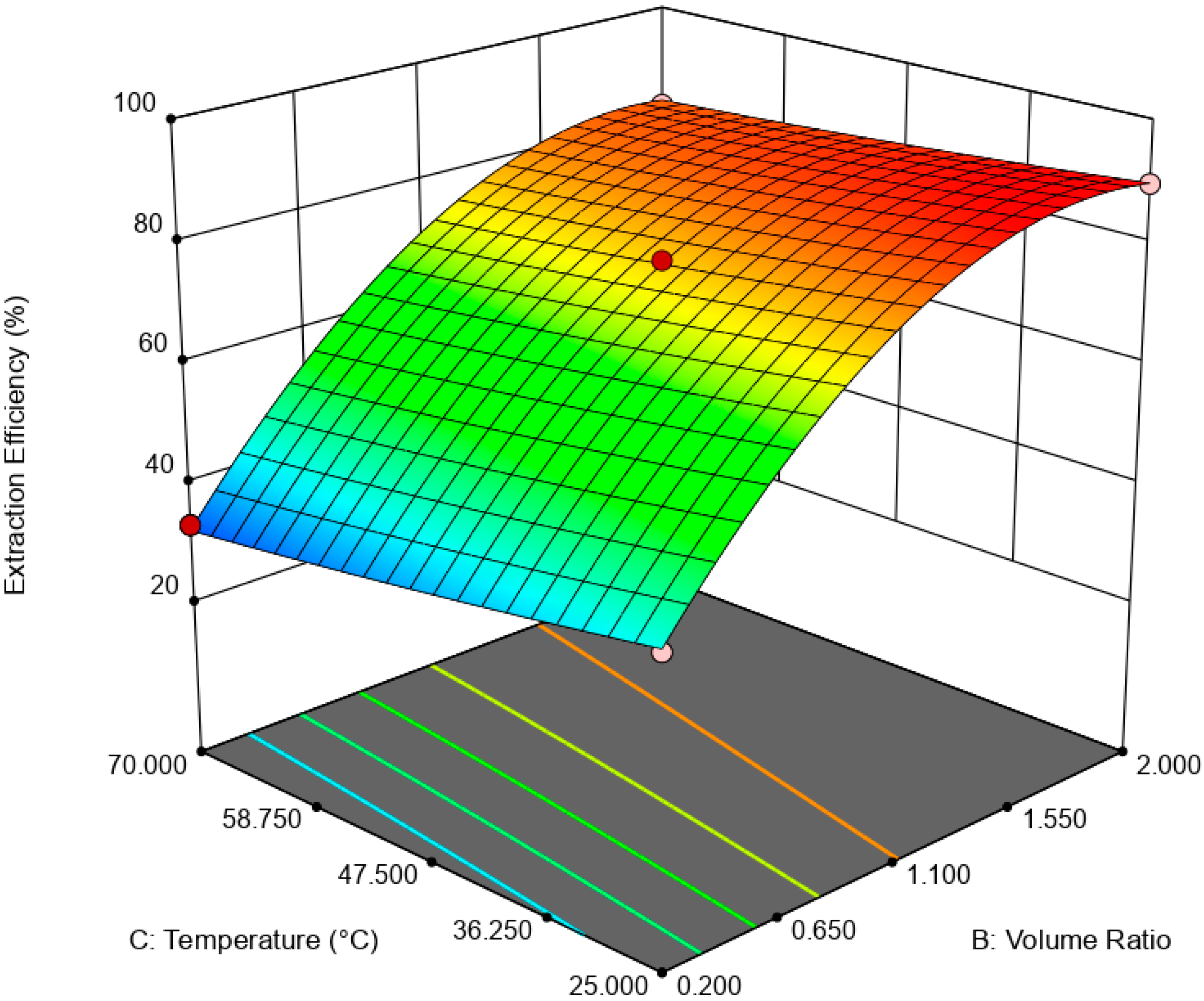
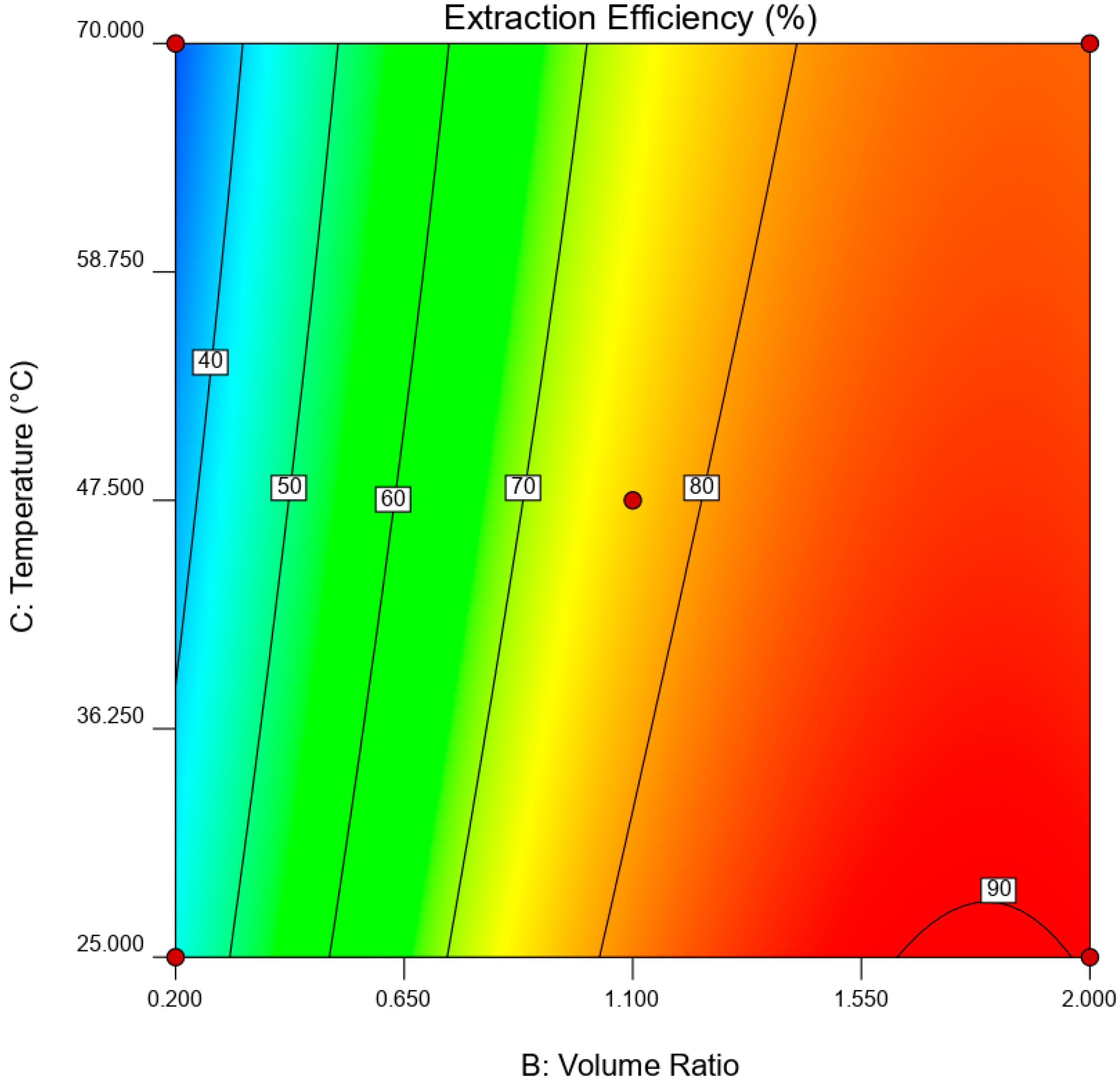
is the error term. EE was statistically analyzed via analysis of variance (ANOVA) while the significance of RSM model was quantified by regression analysis. A three-dimensional (3D) response surface and contour plot were used to study the interaction of independent variable towards EDS performances.
4. Conclusions
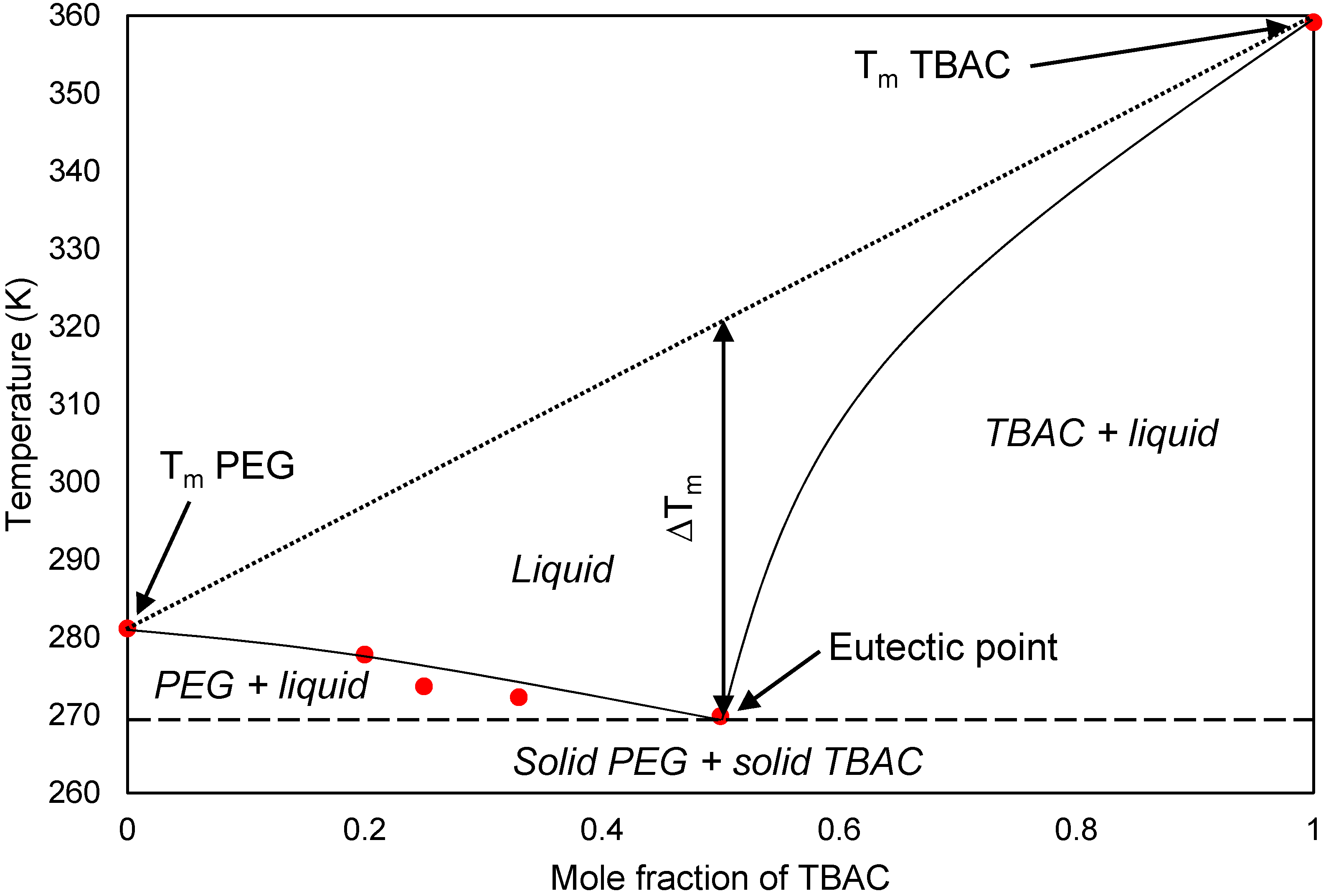
This paper demonstrated the application of TBAC:PEG as extractant in EDS system. The formation of TBAC:PEG led to the melting point depression, which resulted from the decrease in interaction energy between HBA and HBD component. Eutectic point in the eutectic system was observed in TBAC:PEG (1:1), the minimum PEG ratio tested. The performance of TBAC:PEG was analyzed via response surface methodology and the experiments were constructed by central-composite design. The statistical data revealed that the prediction model followed a second-order polynomial equation. Regression analysis also confirmed the good agreement of predicted value with actual data. The effect of three independent variables namely DES molar ratio, DES volume ratio, and extraction temperature towards EE were analyzed using response surface plot. In general, the most influential factor was DES volume ratio. This fact was further confirmed by the F-value of 4490.17, which implies the significance of term. Increasing the volume ratio could improve the EE, however at a volume ratio of 1.55, the effect was started to diminish. Meanwhile, high EE can be obtained at low DES molar ratio while excess PEG did not show significant improvement of the EE. The EDS system could be implemented at low temperature since high temperature decreases the EE tremendously. The desirability tool suggested that the optimum points for EDS system were located at DES volume ratio of 1.0, DES molar ratio of 1.0, and extraction temperature of 25 °C with predicted EE of 79.01%. Verification of model revealed that the experimental data has similar value with empirical data with relative error of 0.74%. As a conclusion, substituting conventional HDS with EDS could eliminate the use of energy-intensive process, which involves extremely high pressure and temperature in HDS and solve the problems to remove sterically hindered sulfur compounds. Although there are other non-conventional methods to remove sulfur, the advantages of EDS lied on its mild operating system, reusability of extractant, minimum loss of fuel quality, and practicability in industrial application. Extractant based on DES is likely to be a promising alternative compared to conventional organic solvent and ionic liquid due to its tunability, simple synthesis, low flammability, and effortless desulfurizing capability. Our findings show that implementing an optimization strategy in conducting experiments could yield best solution by studying the combined effect of two or more variables simultaneously. Analysis of their interrelationships permits the evaluation of interaction effects without the need for large and tedious set of experiments. Moreover, optimization based on factorial design permit the effect of a factor to be predicted at various levels of other factors, and hence providing valid conclusion over a range of experimental constrains. In a nutshell, these findings could provide valuable perspectives on the advancement of energy-friendly and economic EDS process for desulfurization technology as well as the importance of optimization process to maximize desulfurization efficiency.