MCM-41 Supported Co-Based Bimetallic Catalysts for Aqueous Phase Transformation of Glucose to Biochemicals
Abstract
1. Introduction
2. Experimental Part
2.1. Catalysts Synthesis
2.2. Catalysts Characterisation
2.2.1. Nitrogen Physisorption
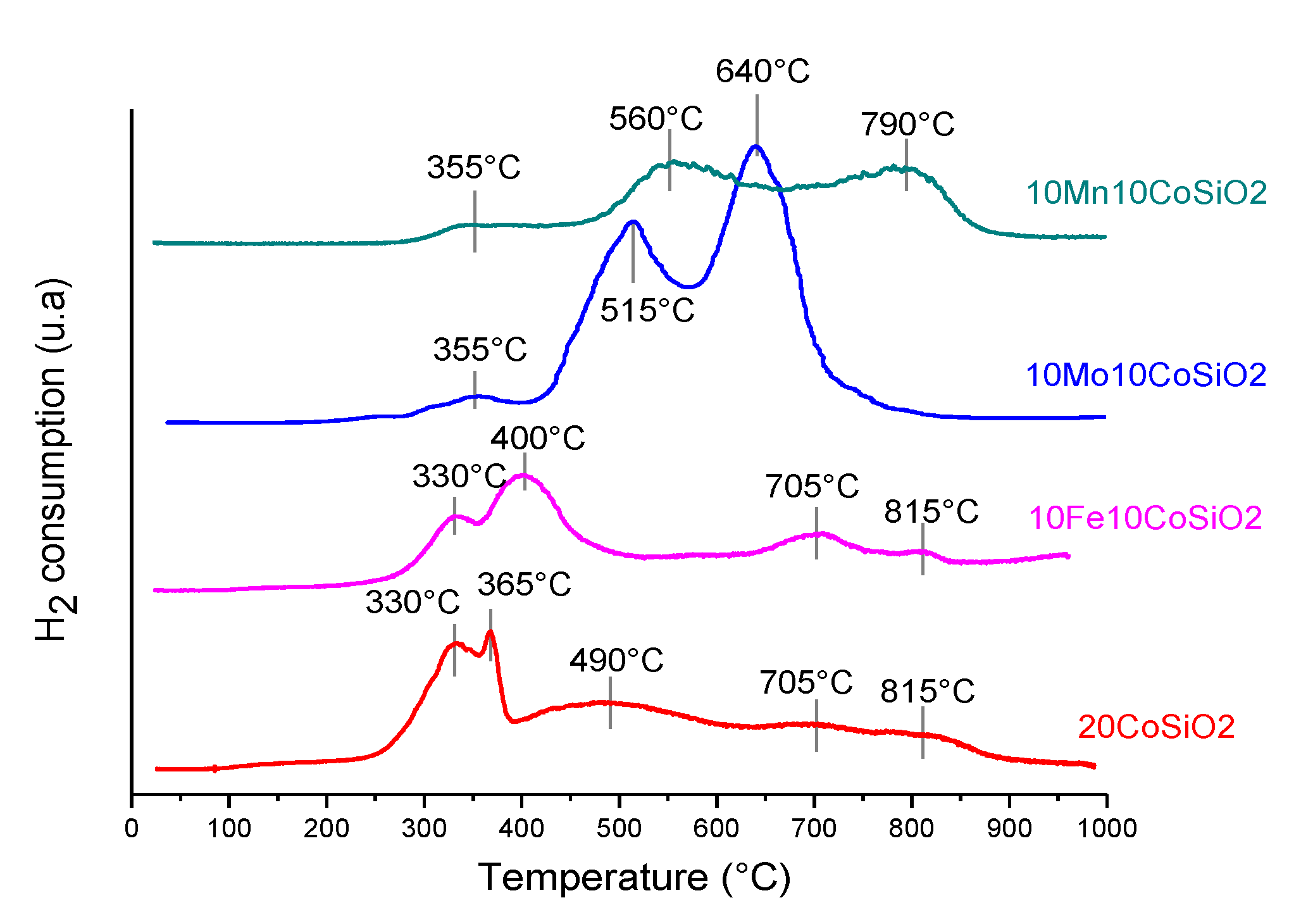
2.2.2. Temperature Programmed Reduction (TPR)
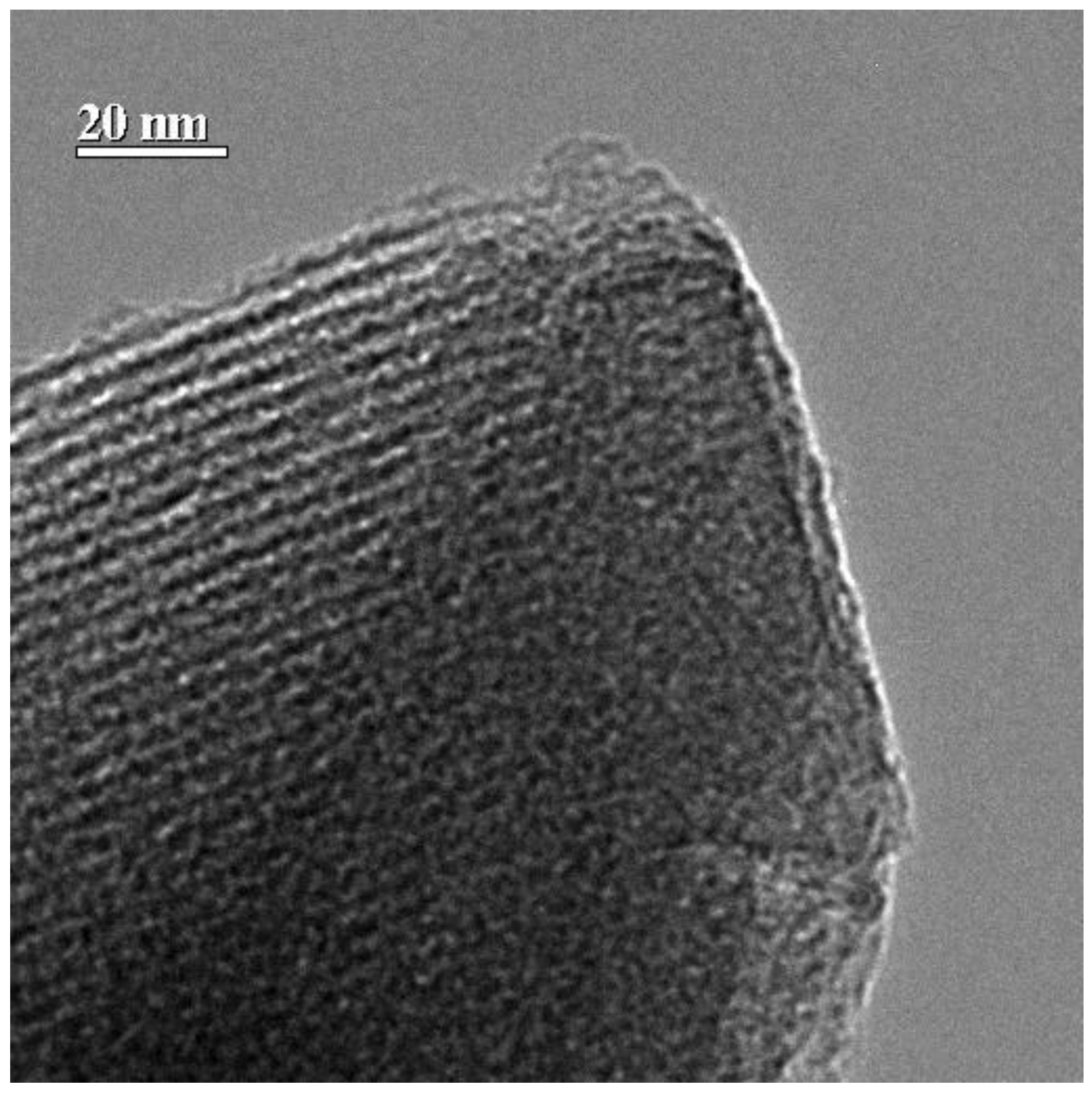
2.2.3. X-ray Powder Diffraction (XRD)
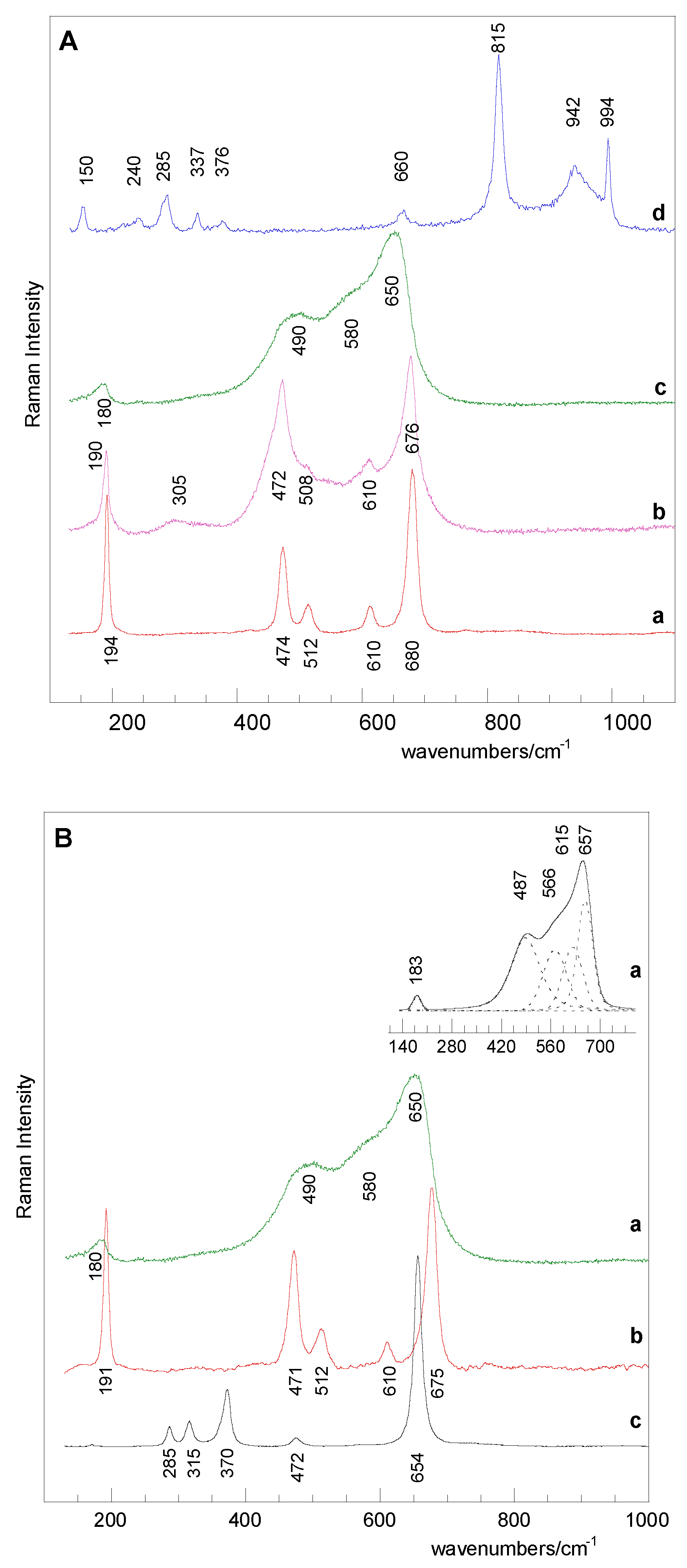
2.2.4. Raman Spectroscopy
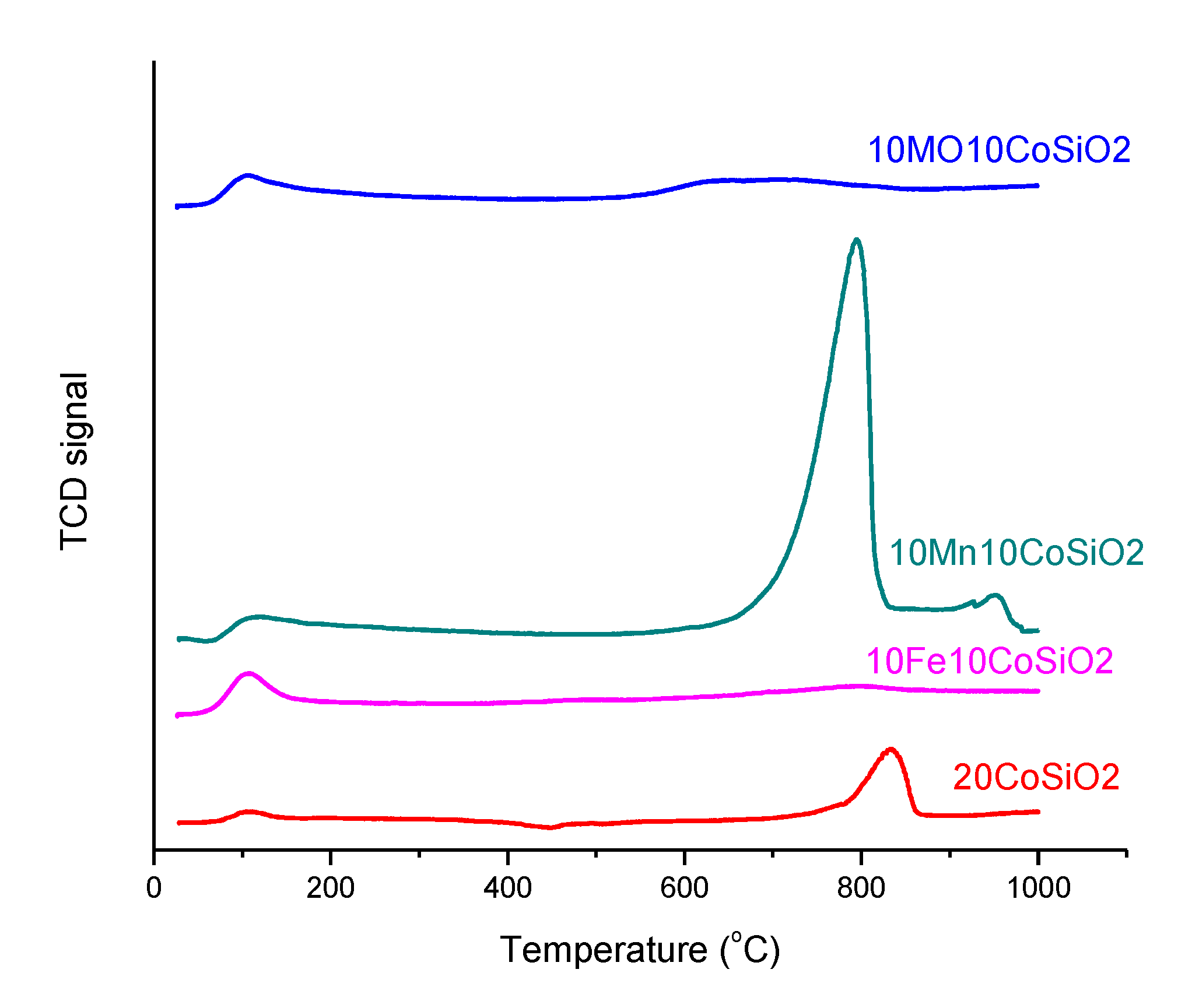
2.2.5. Temperature-programmed Desorption (NH3-TPD)
2.3. Aqueous Phase Transformation Catalytic Tests
3. Result and Discussion
3.1. Catalysts Characterization
3.2. Catalytic Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wang, J.; Xi, J.; Wang, Y. Recent advances in the catalytic production of glucose from lignocellulosic biomass. Green Chem. 2015, 17, 737–751. [Google Scholar] [CrossRef]
- Chen, S.; Maneerung, T.; Tsang, D.C.; Ok, Y.S.; Wang, C.-H. Valorization of biomass to hydroxymethylfurfural, levulinic acid, and fatty acid methyl ester by heterogeneous catalysts. Chem. Eng. J. 2017, 328, 246–273. [Google Scholar] [CrossRef]
- Kang, S.; Fu, J.; Zhang, G. From lignocellulosic biomass to levulinic acid: A review on acid-catalyzed hydrolysis. Renew. Sustain. Energy Rev. 2018, 94, 340–362. [Google Scholar] [CrossRef]
- Somerville, C.; Youngs, H.; Taylor, C.; Davis, S.C.; Long, S. Feedstocks for Lignocellulosic Biofuels. Science 2010, 329, 790–792. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Lu, X.; Zhang, S.; Zhang, R.; Wang, X. Kinetic study for Fe (NO3)3 catalyzed hemicellulose hydrolysis of different corn stover silages. Bioresour. Technol. 2011, 102, 2936–2942. [Google Scholar] [CrossRef]
- Chen, H. Biotechnology of Lignocellulose; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Karnjanakom, S.; Guan, G.; Asep, B.; Hao, X.; Kongparakul, S.; Samart, C.; Abudula, A. Catalytic Upgrading of Bio-Oil over Cu/MCM-41 and Cu/KIT-6 Prepared by β-Cyclodextrin-Assisted Coimpregnation Method. J. Phys. Chem. C 2016, 120, 3396–3407. [Google Scholar] [CrossRef]
- Qib, X.; Watanabe, M.; Aida, T.M.; Smith, R.L. Fast Transformation of Glucose and Di-/Polysaccharides into 5-Hydroxymethylfurfural by Microwave Heating in an Ionic Liquid/Catalyst System. ChemSusChem 2010, 3, 1071–1077. [Google Scholar] [CrossRef]
- Choudhary, V.; Pinar, A.B.; Sandler, S.I.; Vlachos, D.G.; Lobo, R.F. Xylose Isomerization to Xylulose and its Dehydration to Furfural in Aqueous Media. ACS Catal. 2011, 1, 1724–1728. [Google Scholar] [CrossRef]
- Tanksale, A.; Beltramini, J.N.; Lu, G.Q. Reaction Mechanisms for Renewable Hydrogen from Liquid Phase Reforming of Sugar Compounds. Dev. Chem. Eng. Miner. Process. 2008, 14, 9–18. [Google Scholar] [CrossRef]
- Fasolini, A.; Cespi, D.; Tabanelli, T.; Cucciniello, R.; Cavani, F. Hydrogen from Renewables: A Case Study of Glycerol Reforming. Catalysts 2019, 9, 722. [Google Scholar] [CrossRef]
- Sasaki, M.; Goto, K.; Tajima, K.; Adschiri, T.; Arai, K. Rapid and selective retro-aldol condensation of glucose to glycolaldehyde in supercritical water. Green Chem. 2002, 4, 285–287. [Google Scholar] [CrossRef]
- Fasolini, A.; Cucciniello, R.; Paone, E.; Mauriello, F.; Tabanelli, T. A Short Overview on the Hydrogen Production Via Aqueous Phase Reforming (APR) of Cellulose, C6-C5 Sugars and Polyols. Catalysts 2019, 9, 917. [Google Scholar] [CrossRef]
- Deng, W.; Zhang, Q.; Wang, Y. Catalytic transformations of cellulose and cellulose-derived carbohydrates into organic acids. Catal. Today 2014, 234, 31–41. [Google Scholar] [CrossRef]
- Signoretto, M.; Taghavi, S.; Ghedini, E.; Menegazzo, F. Catalytic Production of Levulinic Acid (LA) from Actual Biomass. Molecules 2019, 24, 2760. [Google Scholar] [CrossRef] [PubMed]
- Sudarsanam, P.; Zhong, R.; Bosch, S.V.D.; Coman, S.M.; Parvulescu, V.I.; Sels, B.F. Functionalised heterogeneous catalysts for sustainable biomass valorisation. Chem. Soc. Rev. 2018, 47, 8349–8402. [Google Scholar] [CrossRef]
- Sarkar, J.; Bhattacharyya, S. Application of Graphene and Graphene-Based Materials in Clean Energy-Related Devices Minghui. Arch. Thermodyn. 2012, 33, 23–40. [Google Scholar] [CrossRef]
- Lam, E.; Luong, J.H. Carbon Materials as Catalyst Supports and Catalysts in the Transformation of Biomass to Fuels and Chemicals. ACS Catal. 2014, 4, 3393–3410. [Google Scholar] [CrossRef]
- Taghavi, S.; Norouzi, O.; Tavasoli, A.; di Maria, F.; Signoretto, M.; Menegazzo, F.; di Michele, A. Catalytic conversion of Venice lagoon brown marine algae for producing hydrogen-rich gas and valuable biochemical using algal biochar and Ni/SBA-15 catalyst. Int. J. Hydrogen Energy 2018, 43, 19918–19929. [Google Scholar] [CrossRef]
- Xu, Q.; Zhu, Z.; Tian, Y.; Deng, J.; Shi, J.; Fu, Y. Sn-MCM-41 as Efficient Catalyst for the Conversion of Glucose into 5-Hydroxymethylfurfural in Ionic Liquids. BioResources 2013, 9, 303–315. [Google Scholar] [CrossRef]
- Ya’Aini, N.; Amin, N.A.S.; Endud, S. Characterization and performance of hybrid catalysts for levulinic acid production from glucose. Microporous Mesoporous Mater. 2013, 171, 14–23. [Google Scholar] [CrossRef]
- Ya’Aini, N.; Amin, N.A.S.; Asmadi, M. Optimization of levulinic acid from lignocellulosic biomass using a new hybrid catalyst. Bioresour. Technol. 2012, 116, 58–65. [Google Scholar] [CrossRef]
- Cao, X.; Peng, X.; Sun, S.; Zhong, L.; Chen, W.; Wang, S.; Sun, S. Hydrothermal conversion of xylose, glucose, and cellulose under the catalysis of transition metal sulfates. Carbohydr. Polym. 2015, 118, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Ghedini, E.; Menegazzo, F.; Signoretto, M.; Manzoli, M.; Pinna, F.; Strukul, G. Mesoporous silica as supports for Pd-catalyzed H2O2 direct synthesis: Effect of the textural properties of the support on the activity and selectivity. J. Catal. 2010, 273, 266–273. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H. The Use of Low Temperature van der Waals Adsorption Isotherms in Determining the Surface Areas of Various Adsorbents. J. Am. Chem. Soc. 1937, 59, 2682–2689. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Gregg, S.J.; Sing, K.S.W. Adsorption, Surface Area end Porosity; Academic Press: New York, NY, USA, 1967. [Google Scholar]
- Union, I.; Pure, O.F.; Chemistry, A. International union of pure commission on colloid and syrface chemistry including catalysis reporting physisorption data for gas/solid systems with Special Reference to the Determination of Surface Area and Porosity. Area 1985, 57, 603–619. [Google Scholar]
- Ungár, T. Microstructural parameters from X-ray diffraction peak broadening. Scr. Mater. 2004, 51, 777–781. [Google Scholar] [CrossRef]
- Hadjiev, V.G.; Iliev, M.; Vergilov, I.V. The Raman spectra of Co3O4. J. Phys. C Solid State Phys. 1988, 21, L199–L201. [Google Scholar] [CrossRef]
- Rivas-Murias, B.; Salgueiriño, V. Thermodynamic CoO-Co3O4 crossover using Raman spectroscopy in magnetic octahedron-shaped nanocrystals. J. Raman Spectrosc. 2017, 14, 640–841. [Google Scholar] [CrossRef]
- Gouadec, G.; Colomban, P. Raman Spectroscopy of nanomaterials: How spectra relate to disorder, particle size and mechanical properties. Prog. Cryst. Growth Charact. Mater. 2007, 53, 1–56. [Google Scholar] [CrossRef]
- Sharma, R.K.; Reddy, G.B. Synthesis, and characterization ofα-MoO3microspheres packed with nanoflakes. J. Phys. D Appl. Phys. 2014, 47, 65305. [Google Scholar] [CrossRef]
- Cherian, C.T.; Reddy, M.V.; Haur, S.C.; Chowdari, B.V.R. Interconnected Network of CoMoO4 Submicrometer Particles As High Capacity Anode Material for Lithium Ion Batteries. ACS Appl. Mater. Interfaces 2013, 5, 918–923. [Google Scholar] [CrossRef] [PubMed]
- Julien, C.; Massot, M.; Poinsignon, C. Lattice vibrations of manganese oxides. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2004, 60, 689–700. [Google Scholar] [CrossRef]
- Lorite, I.; Romero, J.J.; Fernández, J.F. Effects of the agglomeration state on the Raman properties of Co3 O4 nanoparticles. J. Raman Spectrosc. 2012, 43, 1443–1448. [Google Scholar] [CrossRef]
- Klissurski, D.G.; Uzunova, E. Cation-deficient nano-dimensional particle size cobalt–manganese spinel mixed oxides. Appl. Surf. Sci. 2003, 214, 370–374. [Google Scholar] [CrossRef]
- Kovanda, F.; Rojka, T.; Dobešová, J.; Machovič, V.; Bezdička, P.; Obalová, L.; Jirátová, K.; Grygar, T.M. Mixed oxides obtained from Co and Mn containing layered double hydroxides: Preparation, characterization, and catalytic properties. J. Solid State Chem. 2006, 179, 812–823. [Google Scholar] [CrossRef]
- Liu, B.; Ouyang, B.; Zhang, Y.; Lv, K.; Li, Q.; Ding, Y.; Li, J. Effects of mesoporous structure and Pt promoter on the activity of Co-based catalysts in low-temperature CO2 hydrogenation for higher alcohol synthesis. J. Catal. 2018, 366, 91–97. [Google Scholar] [CrossRef]
- Griboval, A.; Butel, A.; Ordomsky, V.V.; Chernavskii, P.A.; Khodakov, A.Y. Cobalt and iron species in alumina supported bimetallic catalysts for Fischer–Tropsch reaction. Appl. Catal. A Gen. 2014, 481, 116–126. [Google Scholar] [CrossRef]
- Tavasoli, A.; Trépanier, M.; Abbaslou, R.M.M.; Dalai, A.K.; Abatzoglou, N. Fischer–Tropsch synthesis on mono- and bimetallic Co and Fe catalysts supported on carbon nanotubes. Fuel Process. Technol. 2009, 90, 1486–1494. [Google Scholar] [CrossRef]
- Mai, K.; Elder, T.; Groom, L.H.; Spivey, J.J. Fe-based Fischer Tropsch synthesis of biomass-derived syngas: Effect of synthesis method. Catal. Commun. 2015, 65, 76–80. [Google Scholar] [CrossRef]
- Kukushkin, R.; Bulavchenko, O.; Kaichev, V.; Yakovlev, V. Influence of Mo on catalytic activity of Ni-based catalysts in hydrodeoxygenation of esters. Appl. Catal. B Environ. 2015, 163, 531–538. [Google Scholar] [CrossRef]
- Saghafi, M.; Heshmati-Manesh, S.; Ataie, A.; Khodadadi, A.A. Synthesis of nanocrystalline molybdenum by hydrogen reduction of mechanically activated MoO3. Int. J. Refract. Met. Hard Mater. 2012, 30, 128–132. [Google Scholar] [CrossRef]
- Rodríguez, J.A.; Chaturvedi, S.; Hanson, J.C.; Brito, J.L. Reaction of H2and H2S with CoMoO4and NiMoO4: TPR, XANES, Time-Resolved XRD, and Molecular-Orbital Studies. J. Phys. Chem. B 1999, 103, 770–781. [Google Scholar] [CrossRef]
- Khodakov, A.Y.; Chu, W.; Fongarland, P. Advances in the Development of Novel Cobalt Fischer−Tropsch Catalysts for Synthesis of Long-Chain Hydrocarbons and Clean Fuels. Chem. Rev. 2007, 107, 1692–1744. [Google Scholar] [CrossRef] [PubMed]
- Bragança, L.; Ojeda, M.; Fierro, J.L.G.; da Silva, M.P. Bimetallic Co-Fe nanocrystals deposited on SBA-15 and HMS mesoporous silicas as catalysts for Fischer–Tropsch synthesis. Appl. Catal. A Gen. 2012, 423, 146–153. [Google Scholar] [CrossRef]
- Srivastava, S.; Jadeja, G.C.; Parikh, J.K. A versatile bi-metallic copper–cobalt catalyst for liquid phase hydrogenation of furfural to 2-methylfuran. RSC Adv. 2016, 6, 1649–1658. [Google Scholar] [CrossRef]
- Peng, Y.; Chang, H.; Dai, Y.; Li, J. Structural and Surface Effect of MnO2 for Low Temperature Selective Catalytic Reduction of NO with NH3. Procedia Environ. Sci. 2013, 18, 384–390. [Google Scholar] [CrossRef]
- Mosallanejad, S.; Dlugogorski, B.Z.; Kennedy, E.; Stockenhuber, M. On the Chemistry of Iron Oxide Supported on γ-Alumina and Silica Catalysts. ACS Omega 2018, 3, 5362–5374. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, Z.; Zhai, X.; Wang, X.; Gui, J.Z.; Zhang, C.; Zhu, Y.; Li, Y. Synergistic effect between copper and different metal oxides in the selective hydrogenolysis of glucose. New J. Chem. 2019, 43, 3733–3742. [Google Scholar] [CrossRef]
- Watanabe, M.; Bayer, F.; Kruse, A. Oil formation from glucose with formic acid and cobalt catalyst in hot-compressed water. Carbohydr. Res. 2006, 341, 2891–2900. [Google Scholar] [CrossRef]
- Aman, D.; Radwan, D.; Ebaid, M.; Mikhail, S.; van Steen, E. Comparing nickel and cobalt perovskites for steam reforming of glycerol. Mol. Catal. 2018, 452, 60–67. [Google Scholar] [CrossRef]
- Cheng, Z.; Everhart, J.L.; Tsilomelekis, G.; Nikolakis, V.; Saha, B.; Vlachos, D.G.; Vlachos, D. Structural analysis of humins formed in the Brønsted acid catalyzed dehydration of fructose. Green Chem. 2018, 20, 997–1006. [Google Scholar] [CrossRef]
- Swift, T.D.; Nguyen, H.; Anderko, A.; Nikolakis, V.; Vlachos, D.G. Tandem Lewis/Brønsted homogeneous acid catalysis: Conversion of glucose to 5-hydoxymethylfurfural in an aqueous chromium(iii) chloride and hydrochloric acid solution. Green Chem. 2015, 17, 4725–4735. [Google Scholar] [CrossRef]
- Kuninobu, Y.; Uesugi, T.; Kawata, A.; Takai, K. Manganese-Catalyzed Cleavage of a Carbon-Carbon Single Bond between Carbonyl Carbon and α-Carbon Atoms of Ketones. Angew. Chem. Int. Ed. 2011, 50, 10406–10408. [Google Scholar] [CrossRef]

| Catalyst. | Specific Surface Area (m2/g) | Average Pore Diameter (nm) | Pore Volume (cm3/g) |
|---|---|---|---|
| SiO2 | 1000 | - | 0.40 |
| 20CoSiO2 | 460 | 1.6 | 0.32 |
| 10Fe10CoSiO2 | 510 | 1.5 | 0.33 |
| 10Mn10CoSiO2 | 403 | 1.6 | 0.30 |
| 10Mo10CoSiO2 | 185 | 2.9 | 0.23 |
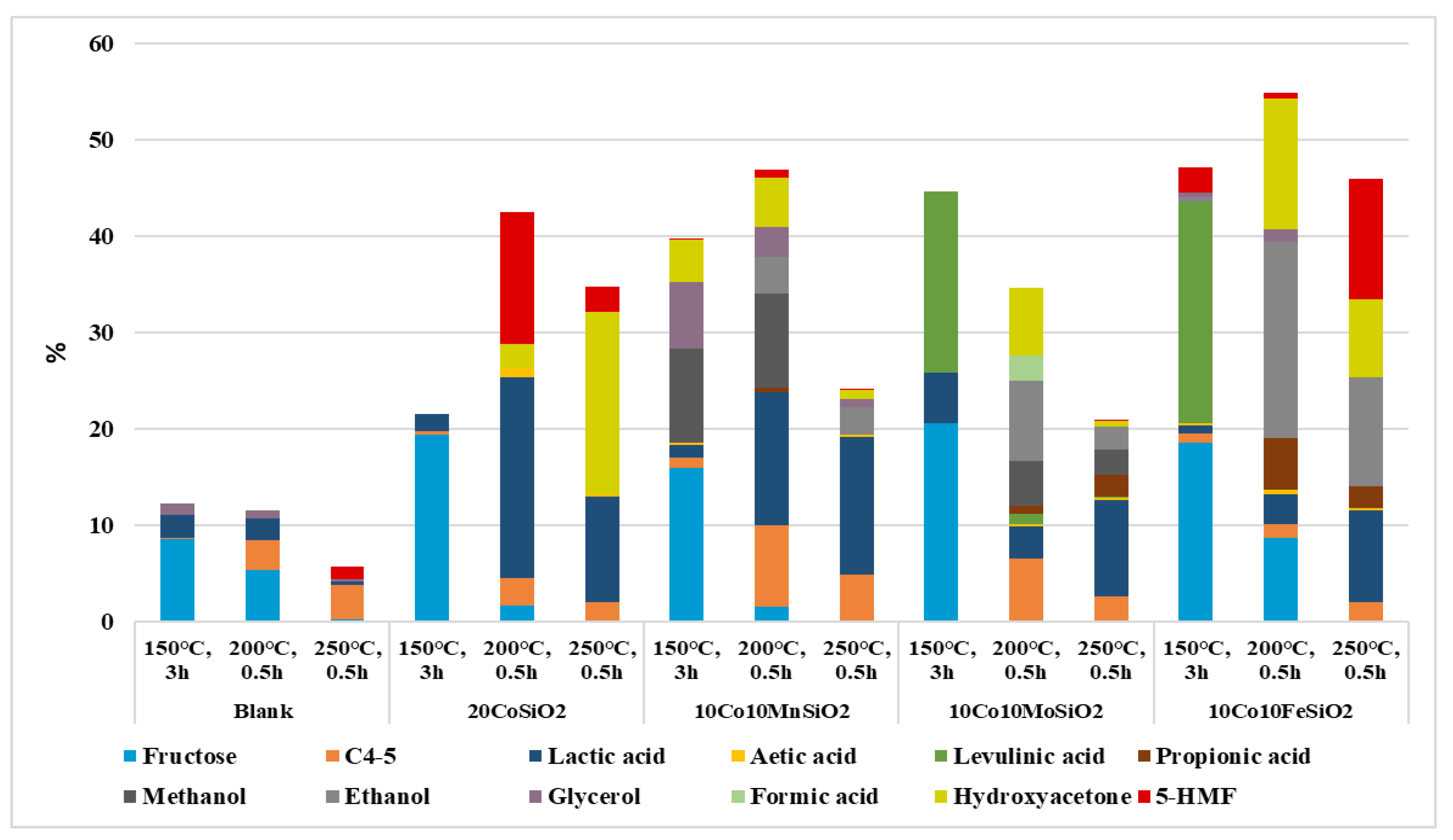
| Catalyst | Reaction Condtions | Conversion, % | Selectivity % | Carbon Balance*, % | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fructose | C4-5 | Lactic Acid | Acetic Acid | Levulinic Acid | Propiionic Acid | Methanol | Etanol | Glcyerol | Formic Acid | Hydroxyacetone | 5-HMF | ||||
| No cat | 150° C, 3h | 22 | 40 | <1 | 11 | − | − | − | − | − | 5 | − | − | − | 91 |
| 200° C, 0.5h | 98 | 5 | 3 | 2 | − | − | − | − | − | <1 | − | − | − | 13 | |
| 250 °C, 0.5h | 99 | <1 | 4 | <1 | 0.0 | − | − | - | − | <1 | − | − | 1 | 9 | |
| Co | 150 °C, 3 h | 56 | 35 | <1 | 3 | − | − | − | − | − | − | − | − | − | 79 |
| 200 °C, 0.5h | 95 | 2 | 3 | 22 | 1 | − | − | − | − | − | − | 3 | 14 | 51 | |
| 250 °C, 0.5 h | 100 | − | 2 | 11 | <1 | − | − | − | − | − | − | 19 | 3 | 35 | |
| Co-Mn | 150 °C, 3 h | 64 | 25 | 2 | 2 | <1 | − | − | 15 | − | 11 | − | 7 | <1 | 76 |
| 200 °C, 0.5 h | 95 | 2 | 9 | 15 | − | − | <1 | 10 | 4 | 3 | − | 5 | <1 | 52 | |
| 250 °C, 0.5h | 99 | − | 5 | 14 | <1 | − | − | − | 3 | <1 | − | <1 | <1 | 27 | |
| Co-Mo | 150 °C, 3 h | 77 | 27 | − | 7 | − | 25 | − | − | − | − | − | − | − | − |
| 200 °C, 0.5h | 99 | − | 6 | 3 | <1 | 1 | <1 | 5 | 9 | − | 3 | 7 | − | − | |
| 250 °C, 0.5h | 100 | − | 2 | 10 | <1 | <1 | 2 | 3 | 2 | − | − | <1 | − | − | |
| Co-Fe | 150 °C, 3 h | 48 | 39 | 2 | 2 | <1 | 48 | − | − | 1 | <1 | − | − | 5 | 100 |
| 200 °C, 0.5h | 100 | 9 | 1 | 3 | <1 | − | 5 | − | 20 | 1 | - | 14 | <1 | 55 | |
| 250 °C, 0.5 h | 100 | − | 20 | 9 | <1 | − | 2 | − | 11 | − | − | 8 | 12 | 46 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taghavi, S.; Ghedini, E.; Menegazzo, F.; Signoretto, M.; Gazzoli, D.; Pietrogiacomi, D.; Matayeva, A.; Fasolini, A.; Vaccari, A.; Basile, F.; et al. MCM-41 Supported Co-Based Bimetallic Catalysts for Aqueous Phase Transformation of Glucose to Biochemicals. Processes 2020, 8, 843. https://doi.org/10.3390/pr8070843
Taghavi S, Ghedini E, Menegazzo F, Signoretto M, Gazzoli D, Pietrogiacomi D, Matayeva A, Fasolini A, Vaccari A, Basile F, et al. MCM-41 Supported Co-Based Bimetallic Catalysts for Aqueous Phase Transformation of Glucose to Biochemicals. Processes. 2020; 8(7):843. https://doi.org/10.3390/pr8070843
Chicago/Turabian StyleTaghavi, Somayeh, Elena Ghedini, Federica Menegazzo, Michela Signoretto, Delia Gazzoli, Daniela Pietrogiacomi, Aisha Matayeva, Andrea Fasolini, Angelo Vaccari, Francesco Basile, and et al. 2020. "MCM-41 Supported Co-Based Bimetallic Catalysts for Aqueous Phase Transformation of Glucose to Biochemicals" Processes 8, no. 7: 843. https://doi.org/10.3390/pr8070843
APA StyleTaghavi, S., Ghedini, E., Menegazzo, F., Signoretto, M., Gazzoli, D., Pietrogiacomi, D., Matayeva, A., Fasolini, A., Vaccari, A., Basile, F., & Fornasari, G. (2020). MCM-41 Supported Co-Based Bimetallic Catalysts for Aqueous Phase Transformation of Glucose to Biochemicals. Processes, 8(7), 843. https://doi.org/10.3390/pr8070843










