Liquid Marbles as Miniature Reactors for Chemical and Biological Applications
Abstract
1. Introduction
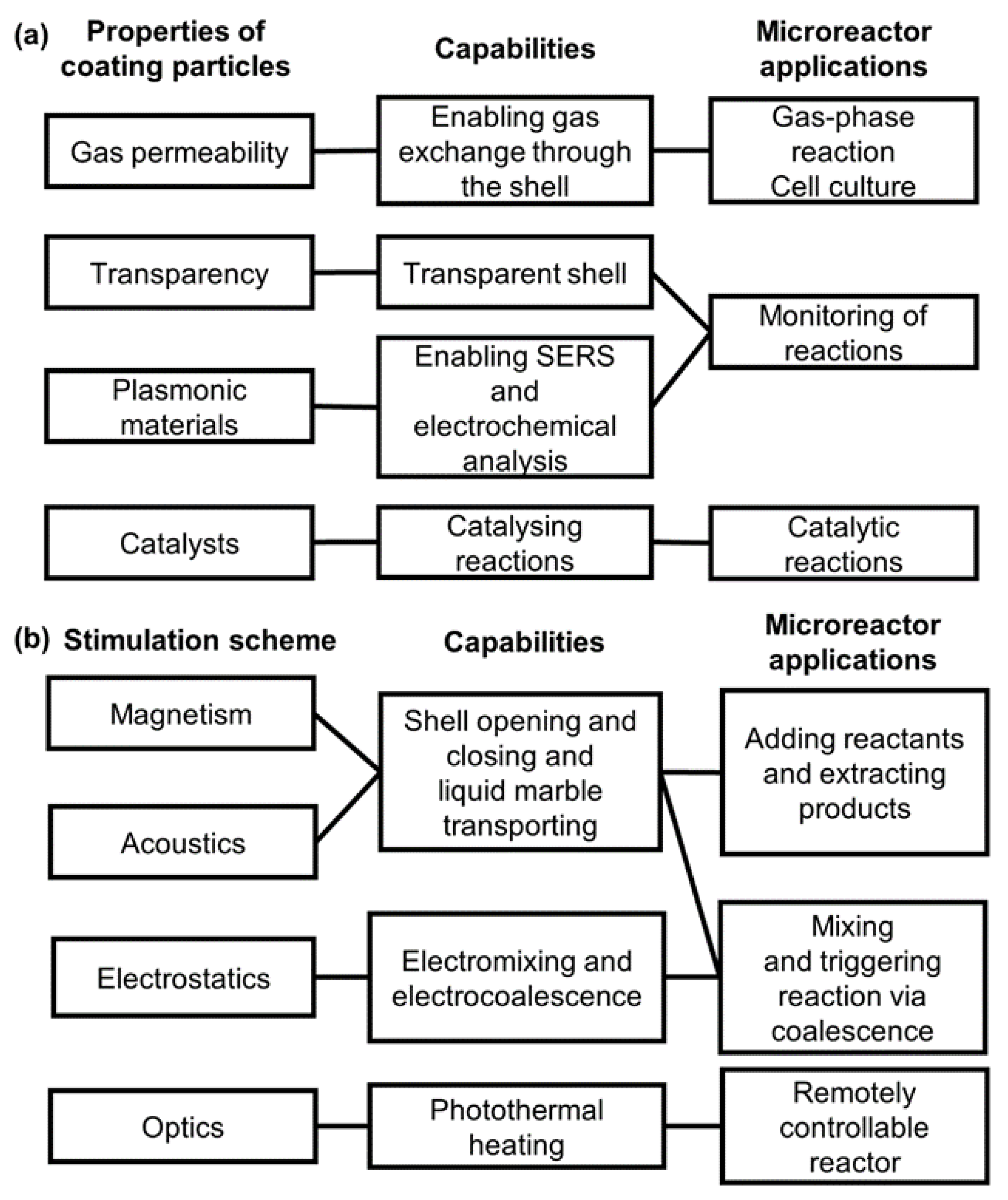
2. Liquid Marble-Based Microreactors
2.1. Formation of Liquid Marbles
2.2. Manipulation of Liquid Marbles for Microreactions
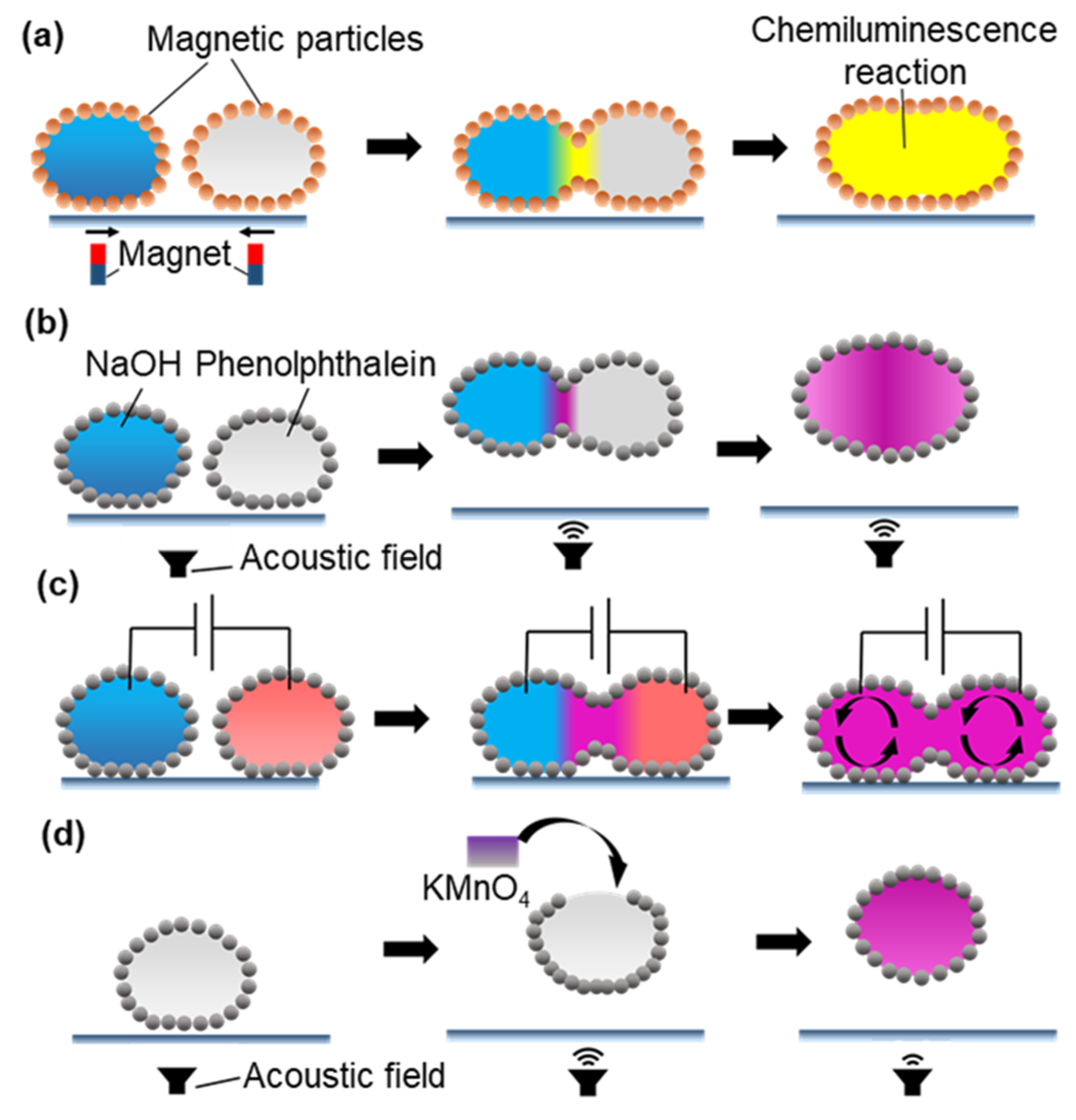
2.2.1. Manipulation for Coalescence
2.2.2. Manipulation for Mixing
2.2.3. Manipulation for Opening and Closing the Liquid Marbles
2.3. Lifetime of Liquid Marbles
3. Liquid Marbles as Microreactors for Chemical Applications
3.1. Advantages of Liquid Marble-Based Chemical Reactors
3.2. Practical Applications of Liquid Marble-Based Chemical Reactors
3.2.1. Microreactors for Gas-Phase Reactions
3.2.2. Microreactors for Solid Material Synthesis
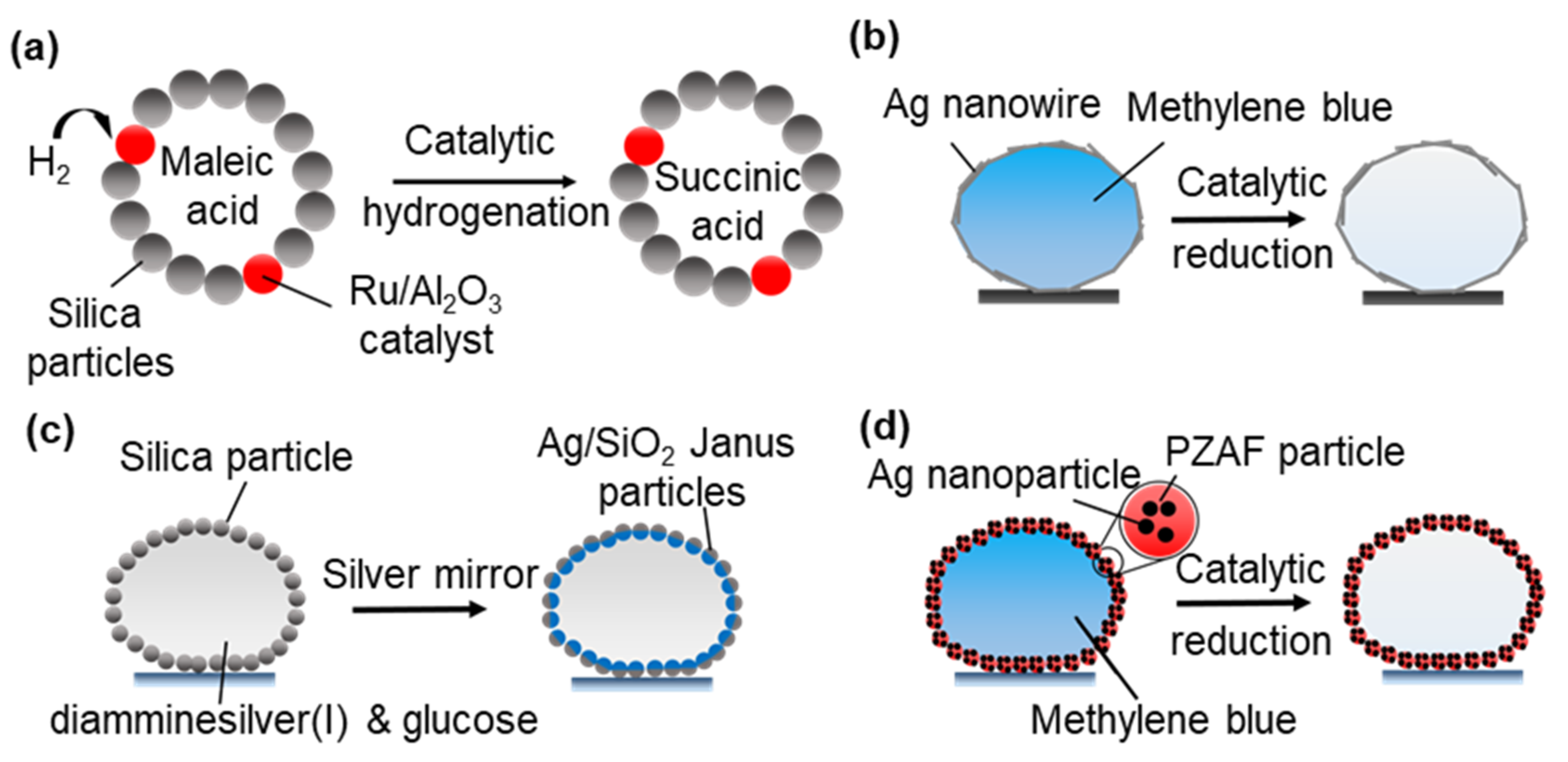
3.2.3. Microreactors for Heterogeneous Catalytic Reactions
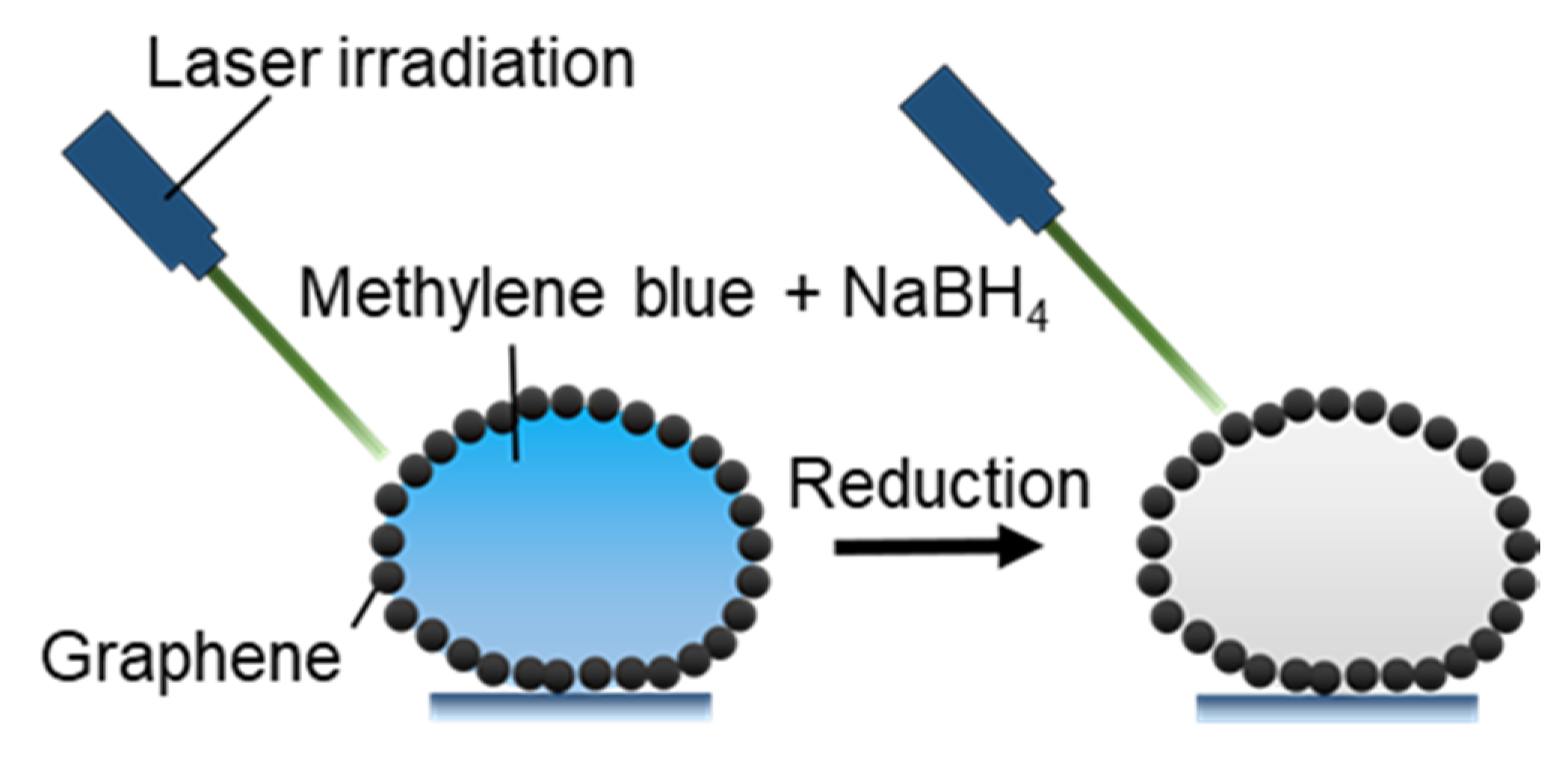
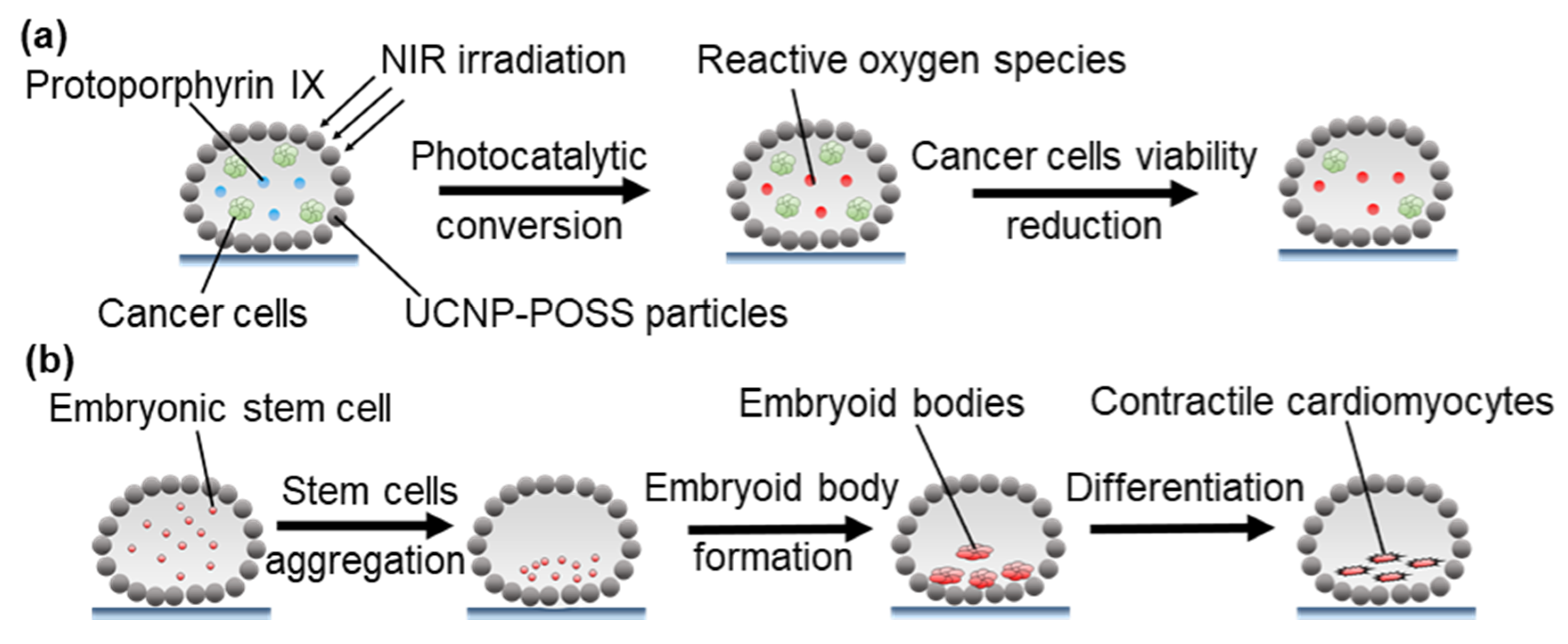
3.2.4. Photothermal Microreactors
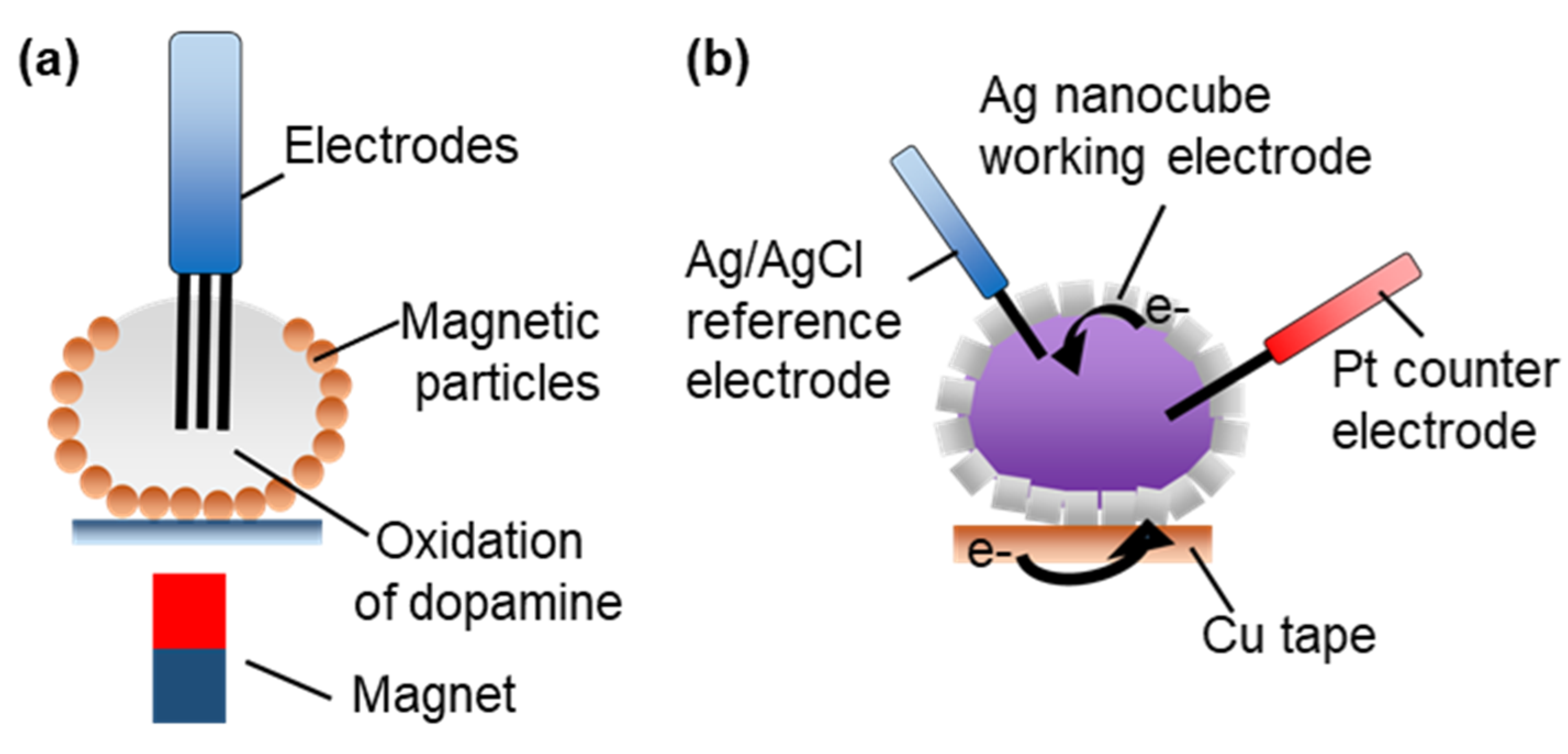
3.2.5. Electrochemical Microreactors
3.2.6. Other Applications
4. Liquid Marbles as Microreactors for Biological Applications
4.1. Advantages of Liquid Marble-Based Microbioreactors
4.2. Practical Application of Liquid Marble-Based Microbioreactors
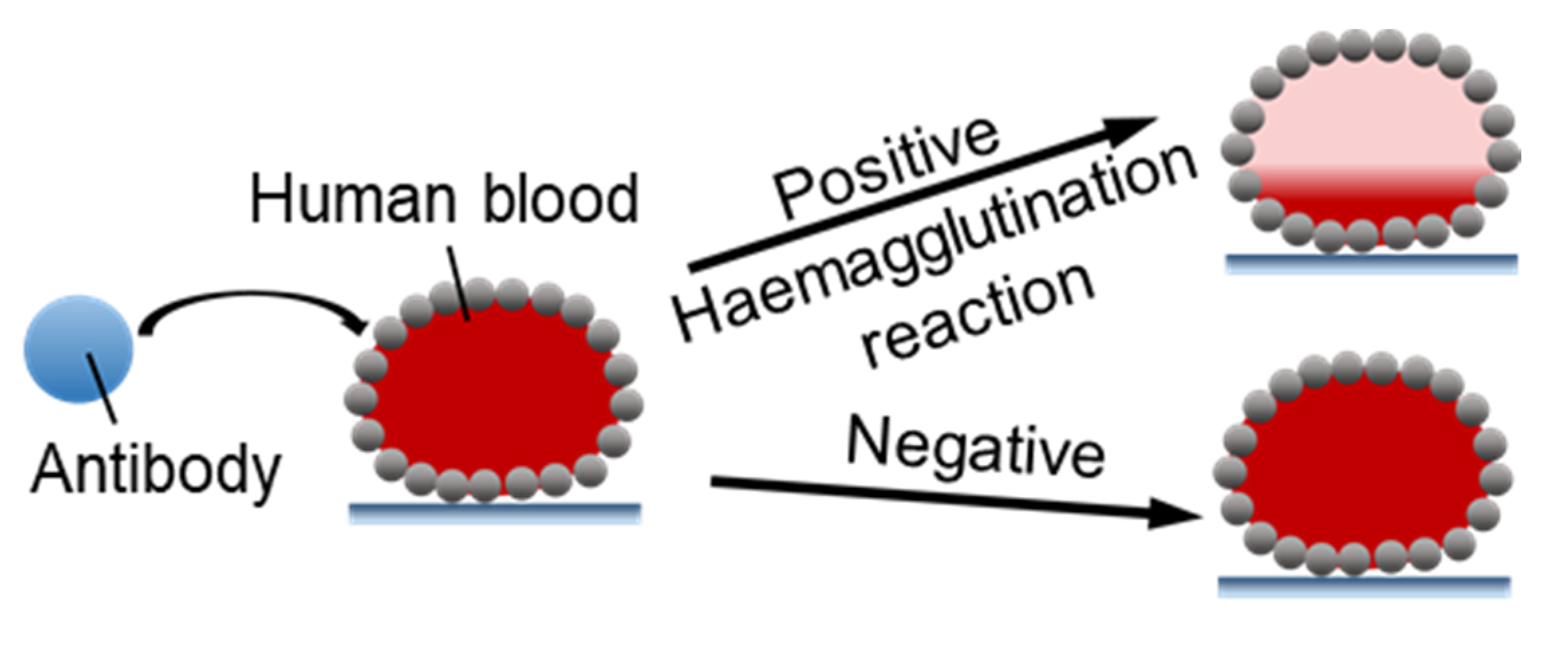
4.2.1. Microbioreactors for Blood Typing
4.2.2. Microbioreactors for Cell Culture and Treatment
4.2.3. Microbioreactors for Polymerase Chain Reaction
5. Future Perspectives
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kothare, M.V. Dynamics and control of integrated microchemical systems with application to micro-scale fuel processing. Comput. Chem. Eng. 2006, 30, 1725–1734. [Google Scholar] [CrossRef]
- Mills, P.L.; Quiram, D.J.; Ryley, J.F. Microreactor technology and process miniaturization for catalytic reactions—A perspective on recent developments and emerging technologies. Chem. Eng. Sci. 2007, 62, 6992–7010. [Google Scholar] [CrossRef]
- Tanimu, A.; Jaenicke, S.; Alhooshani, K. Heterogeneous catalysis in continuous flow microreactors: A review of methods and applications. Chem. Eng. J. 2017, 327, 792–821. [Google Scholar] [CrossRef]
- Watts, P.; Haswell, S.J. The application of micro reactors for organic synthesis. Chem. Soc. Rev. 2005, 34, 235–246. [Google Scholar] [CrossRef]
- Yoshida, J.-I.; Nagaki, A.; Yamada, T. Flash Chemistry: Fast Chemical Synthesis by Using Microreactors. Chem. A Eur. J. 2008, 14, 7450–7459. [Google Scholar] [CrossRef]
- Jiao, Z.; Huang, X.; Nguyen, N.T.; Abgrall, P. Thermocapillary actuation of droplet in a planar microchannel. Microfluid. Nanofluid. 2007, 5, 205–214. [Google Scholar] [CrossRef]
- Luong, T.D.; Nguyen, N.T. Surface Acoustic Wave Driven Microfluidics—A Review. Micro Nanosyst. 2010, 2, 217–225. [Google Scholar] [CrossRef]
- Murshed, S.M.S.; Tan, S.H.; Nguyen, N.T.; Wong, T.N.; Yobas, L. Microdroplet formation of water and nanofluids in heat-induced microfluidic T-junction. Microfluid. Nanofluid. 2008, 6, 253–259. [Google Scholar] [CrossRef]
- Song, C.; Nguyen, N.T.; Tan, S.H.; Asundi, A. Modelling and optimization of micro optofluidic lenses. Lab Chip 2009, 9, 1178–1184. [Google Scholar] [CrossRef]
- Demello, A.J. Control and detection of chemical reactions in microfluidic systems. Nature 2006, 442, 394–402. [Google Scholar] [CrossRef]
- Su, B.; Wang, S.; Song, Y.; Jiang, L. A miniature droplet reactor built on nanoparticle-derived superhydrophobic pedestals. Nano Res. 2010, 4, 266–273. [Google Scholar] [CrossRef]
- Haswell, S.; Watts, P. Green chemistry: Synthesis in micro reactors. Green Chem. 2003, 5, 240–249. [Google Scholar] [CrossRef]
- Baraldi, P.T.; Hessel, V. Micro reactor and flow chemistry for industrial applications in drug discovery and development. Green Process. Synth. 2012, 1, 149–167. [Google Scholar] [CrossRef]
- Watts, P.; Wiles, C. Micro reactors, flow reactors and continuous flow synthesis. J. Chem. Res. 2012, 36, 181–193. [Google Scholar] [CrossRef]
- Pike, N.; Richard, D.; Foster, W.; Mahadevan, L. How aphids lose their marbles. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2002, 269, 1211–1215. [Google Scholar] [CrossRef]
- Aussillous, P.; Quéré, D. Liquid marbles. Nature 2001, 411, 924–927. [Google Scholar] [CrossRef]
- Draper, T.C.; Fullarton, C.; Phillips, N.; de Lacy Costello, B.P.; Adamatzky, A. Mechanical Sequential Counting with Liquid Marbles. In Proceedings of the International Conference on Unconventional Computation and Natural Computation, Fontainebleau, France, 25–29 June 2018; pp. 59–71. [Google Scholar]
- Nguyen, N.T.; Hejazian, M.; Ooi, C.H.; Kashaninejad, N. Recent Advances and Future Perspectives on Microfluidic Liquid Handling. Micromachines 2017, 8, 186. [Google Scholar] [CrossRef]
- Draper, T.C.; Fullarton, C.; Phillips, N.; de Lacy Costello, B.P.; Adamatzky, A. Liquid marble interaction gate for collision-based computing. Mater. Today 2017, 20, 561–568. [Google Scholar] [CrossRef]
- McHale, G.; Newton, M.I. Liquid marbles: Principles and applications. Soft Matter 2011, 7, 5473–5481. [Google Scholar] [CrossRef]
- Ooi, C.H.; Nguyen, N.T. Manipulation of liquid marbles. Microfluid. Nanofluid. 2015, 19, 483–495. [Google Scholar] [CrossRef]
- Wu, H.; Watanabe, H.; Ma, W.; Fujimoto, A.; Higuchi, T.; Uesugi, K.; Takeuchi, A.; Suzuki, Y.; Jinnai, H.; Takahara, A. Robust Liquid Marbles Stabilized with Surface-Modified Halloysite Nanotubes. Langmuir 2013, 29, 14971–14975. [Google Scholar] [CrossRef]
- Matsukuma, D.; Watanabe, H.; Minn, M.; Fujimoto, A.; Shinohara, T.; Jinnai, H.; Takahara, A. Preparation of poly(lactic-acid)-particle stabilized liquid marble and the improvement of its stability by uniform shell formation through solvent vapor exposure. RSC Adv. 2013, 3, 7862–7866. [Google Scholar] [CrossRef]
- Bhosale, P.S.; Stretz, H.A.; Panchagnula, M.V. Mechanically robust nanoparticle stabilized transparent liquid marbles. Appl. Phys. Lett. 2008, 93, 034109. [Google Scholar] [CrossRef]
- Dupin, D.; Thompson, K.L.; Armes, S.P. Preparation of stimulus-responsive liquid marbles using a polyacid-stabilised polystyrene latex. Soft Matter 2011, 7, 6797–6800. [Google Scholar] [CrossRef]
- Castro, J.O.; Neves, B.M.; Rezk, A.R.; Eshtiaghi, N.; Yeo, L.Y. Continuous Production of Janus and Composite Liquid Marbles with Tunable Coverage. ACS Appl. Mater. Interfaces 2016, 8, 17751–17756. [Google Scholar] [CrossRef] [PubMed]
- Sreejith, K.R.; Ooi, C.H.; Jin, J.; Dao, D.V.; Nguyen, N.T. An automated on-demand liquid marble generator based on electrohydrodynamic pulling. Rev. Sci. Instrum. 2019, 90, 055102. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Chan, K.F.; Ji, F.; Wang, Q.; Chiu, P.W.Y.; Guo, Z.; Zhang, L. On-Demand Coalescence and Splitting of Liquid Marbles and Their Bioapplications. Adv. Sci. 2019, 6, 1802033. [Google Scholar] [CrossRef]
- Xin, Z.; Skrydstrup, T. Liquid Marbles: A Promising and Versatile Platform for Miniaturized Chemical Reactions. Angew. Chem. Int. Ed. 2019, 58, 11952–11954. [Google Scholar] [CrossRef]
- Luo, X.; Yin, H.; Li, X.; Su, X.; Feng, Y. CO2-Triggered microreactions in liquid marbles. Chem. Commun. 2018, 54, 9119–9122. [Google Scholar] [CrossRef]
- Jin, J.; Ooi, C.H.; Dao, D.V.; Nguyen, N.T. Coalescence Processes of Droplets and Liquid Marbles. Micromachines 2017, 8, 336. [Google Scholar] [CrossRef]
- Jin, J.; Ooi, C.H.; Dao, D.V.; Nguyen, N.T. Liquid marble coalescence via vertical collision. Soft Matter 2018, 14, 4160–4168. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Fu, X.; Binks, B.; Shum, H.C. Coalescence of Electrically Charged Liquid Marbles. Soft Matter 2017, 13, 119–124. [Google Scholar] [CrossRef]
- Xue, Y.; Wang, H.; Zhao, Y.; Dai, L.; Feng, L.; Wang, X.; Lin, T. Magnetic Liquid Marbles: A “Precise” Miniature Reactor. Adv. Mater. 2010, 22, 4814–4818. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zang, D.; Zhao, L.; Qu, M.; Li, X.; Li, X.; Li, L.; Geng, X. Liquid Marble Coalescence and Triggered Microreaction Driven by Acoustic Levitation. Langmuir 2017, 33, 6232–6239. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yang, T.; Huang, Y.; Liu, Y.; Chen, L.; Deng, L.; Shum, H.C.; Kong, T. Electrocontrolled Liquid Marbles for Rapid Miniaturized Organic Reactions. Adv. Funct. Mater. 2019, 29, 1901101. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, X.; Guo, W.; Deng, Y.; Binks, B.P.; Shum, H.C.; Binks, B. Electrocoalescence of liquid marbles driven by embedded electrodes for triggering bioreactions. Lab Chip 2019, 19, 3526–3534. [Google Scholar] [CrossRef]
- Zang, D.; Li, J.; Chen, Z.; Zhai, Z.; Geng, X.; Binks, B.P. Switchable Opening and Closing of a Liquid Marble via Ultrasonic Levitation. Langmuir 2015, 31, 11502–11507. [Google Scholar] [CrossRef] [PubMed]
- Ooi, C.H.; Jin, J.; Sreejith, K.R.; Nguyen, N.T.; Evans, G.M.; Nguyen, N.T. Manipulation of a floating liquid marble using dielectrophoresis. Lab Chip 2018, 18, 3770–3779. [Google Scholar] [CrossRef] [PubMed]
- Fujii, S.; Yusa, S.-I.; Nakamura, Y. Stimuli-Responsive Liquid Marbles: Controlling Structure, Shape, Stability, and Motion. Adv. Funct. Mater. 2016, 26, 7206–7223. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, Z.; Niu, H.; Wang, X.; Lin, T. Magnetic Liquid Marbles: Toward “Lab in a Droplet”. Adv. Funct. Mater. 2014, 25, 437–444. [Google Scholar] [CrossRef]
- Li, M.; Tian, J.; Li, L.; Liu, A.; Shen, W. Charge transport between liquid marbles. Chem. Eng. Sci. 2013, 97, 337–343. [Google Scholar] [CrossRef]
- Arbatan, T.; Al-Abboodi, A.; Sarvi, F.; Chan, P.P.Y.; Shen, W. Tumor Inside a Pearl Drop. Adv. Healthc. Mater. 2012, 1, 467–469. [Google Scholar] [CrossRef] [PubMed]
- Sarvi, F.; Jain, K.; Arbatan, T.; Verma, P.J.; Hourigan, K.; Thompson, M.C.; Shen, W.; Chan, P.P.Y. Cardiogenesis of Embryonic Stem Cells with Liquid Marble Micro-Bioreactor. Adv. Healthc. Mater. 2014, 4, 77–86. [Google Scholar] [CrossRef]
- Lin, K.; Chen, R.; Zhang, L.; Zang, D.; Geng, X.; Shen, W. Transparent Bioreactors Based on Nanoparticle-Coated Liquid Marbles for in Situ Observation of Suspending Embryonic Body Formation and Differentiation. ACS Appl. Mater. Interfaces 2018, 11, 8789–8796. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, N.; Correia, C.R.; Reis, R.L.; Mano, J.F. Liquid Marbles for High-Throughput Biological Screening of Anchorage-Dependent Cells. Adv. Healthc. Mater. 2014, 4, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Tosun, A.; Erbil, H.Y. Evaporation rate of PTFE liquid marbles. Appl. Surf. Sci. 2009, 256, 1278–1283. [Google Scholar] [CrossRef]
- Dandan, M.; Erbil, H.Y. Evaporation Rate of Graphite Liquid Marbles: Comparison with Water Droplets. Langmuir 2009, 25, 8362–8367. [Google Scholar] [CrossRef]
- Aberle, C.; Lewis, M.; Yu, G.; Lei, N.; Xu, J. Liquid marbles as thermally robust droplets: Coating-assisted Leidenfrost-like effect. Soft Matter 2011, 7, 11314–11318. [Google Scholar] [CrossRef]
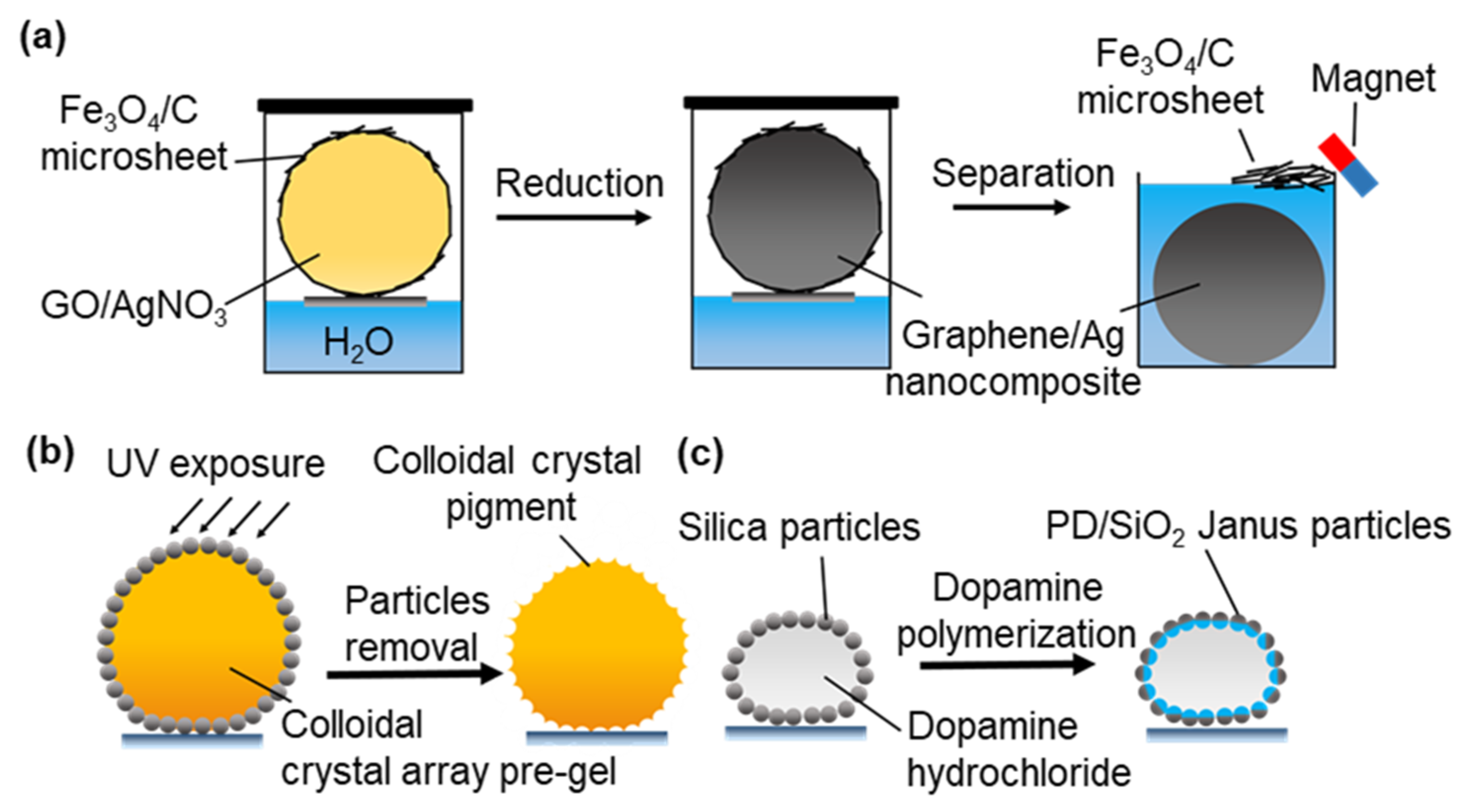
- Chu, Y.; Wang, Z.; Pan, Q. Constructing Robust Liquid Marbles for Miniaturized Synthesis of Graphene/Ag Nanocomposite. ACS Appl. Mater. Interfaces 2014, 6, 8378–8386. [Google Scholar] [CrossRef]
- Jeon, S.; Park, C.S.; Hackett, C.; Norbeck, J. Characteristics of steam hydrogasification of wood using a micro-batch reactor. Fuel 2007, 86, 2817–2823. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, H.; Chen, X.; Feng, X.; Yang, C. Effect of blending ratio on coke morphology and composition in co-coking of vacuum residue and bio-tar. J. Anal. Appl. Pyrolysis 2019, 141, 104629. [Google Scholar] [CrossRef]
- Ge, Z.; Jin, H.; Guo, L. Hydrogen production by catalytic gasification of coal in supercritical water with alkaline catalysts: Explore the way to complete gasification of coal. Int. J. Hygiene Energy 2014, 39, 19583–19592. [Google Scholar] [CrossRef]
- Wang, S.; Huang, X.; Yang, C. Mixing enhancement for high viscous fluids in a microfluidic chamber. Lab Chip 2011, 11, 2081. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Arbatan, T.; Li, X.; Shen, W. Liquid marble for gas sensing. Chem. Commun. 2010, 46, 4734–4736. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Arbatan, T.; Li, X.; Shen, W. Porous liquid marble shell offers possibilities for gas detection and gas reactions. Chem. Eng. J. 2010, 165, 347–353. [Google Scholar] [CrossRef]
- Li, X.; Shi, H.; Hu, Y. Rod-shaped liquid plasticine for gas diffusion detection. Soft Matter 2019, 15, 3085–3088. [Google Scholar] [CrossRef] [PubMed]
- Bormashenko, E.; Balter, R.; Aurbach, D. Use of liquid marbles as micro-reactors. Int. J. Chem. React. Eng. 2011, 9, S10. [Google Scholar] [CrossRef]
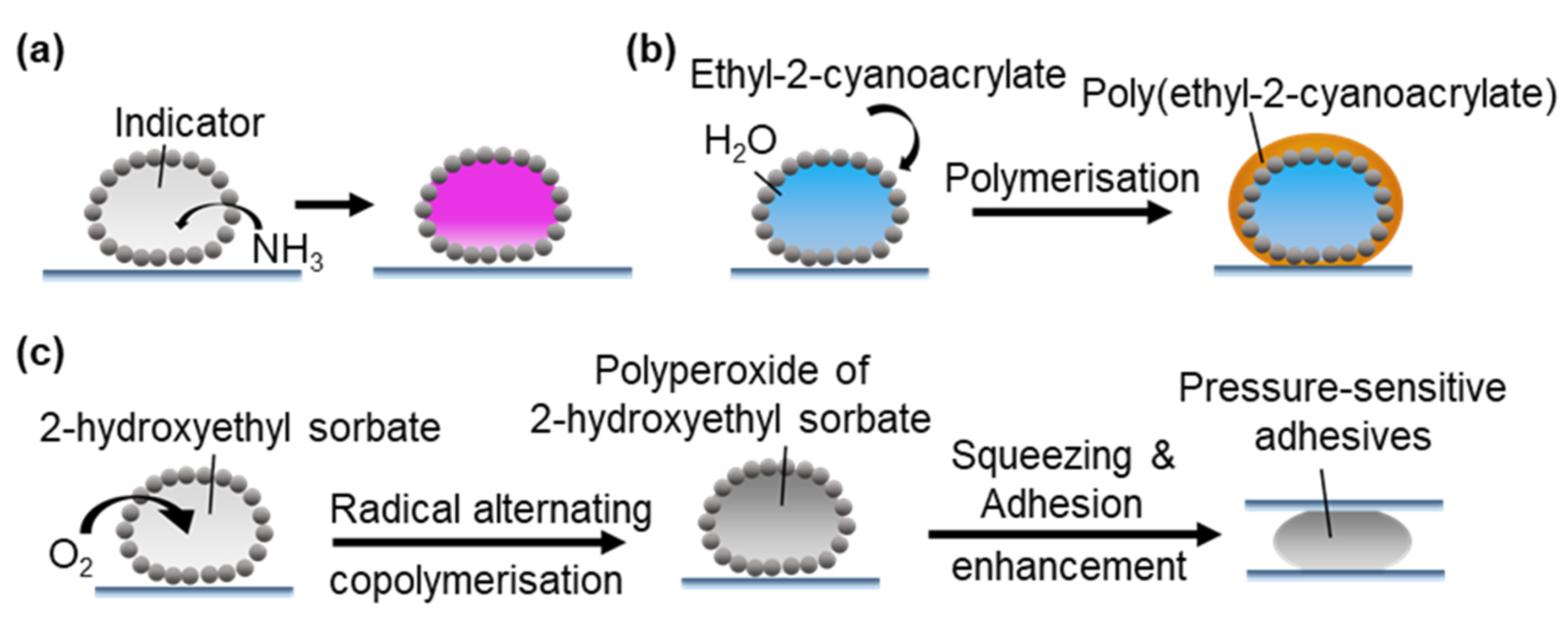
- Chin, J.M.; Reithofer, M.R.; Tan, T.T.Y.; Menon, A.G.; Chen, E.Y.; Chow, C.A.; Hor, A.; Xu, J. Supergluing MOF liquid marbles. Chem. Commun. 2013, 49, 493–495. [Google Scholar] [CrossRef]
- Sato, E.; Yuri, M.; Fujii, S.; Nishiyama, T.; Nakamura, Y.; Horibe, H. Liquid marbles as a micro-reactor for efficient radical alternating copolymerization of diene monomer and oxygen. Chem. Commun. 2015, 51, 17241–17244. [Google Scholar] [CrossRef]
- Sato, E.; Yuri, M.; Fujii, S.; Nishiyama, T.; Nakamura, Y.; Horibe, H. Liquid marble containing degradable polyperoxides for adhesion force-changeable pressure-sensitive adhesives. RSC Adv. 2016, 6, 56475–56481. [Google Scholar] [CrossRef]
- Gu, Z.; Ye, B.; Ding, H.; Liu, C.; Zhao, Y.; Gu, Z. Non-iridescent structural color pigments from liquid marbles. J. Mater. Chem. C 2015, 3, 6607–6612. [Google Scholar] [CrossRef]
- Sheng, Y.; Sun, G.; Ngai, T. Dopamine Polymerization in Liquid Marbles: A General Route to Janus Particle Synthesis. Langmuir 2016, 32, 3122–3129. [Google Scholar] [CrossRef] [PubMed]
- De Palma, R.; Peeters, S.; Van Bael, M.J.; Rul, H.V.D.; Bonroy, K.; Laureyn, W.; Mullens, J.; Borghs, G.; Maes, G. Silane Ligand Exchange to Make Hydrophobic Superparamagnetic Nanoparticles Water-Dispersible. Chem. Mater. 2007, 19, 1821–1831. [Google Scholar] [CrossRef]
- Jeong, Y.; Tonga, G.Y.; Duncan, B.; Yan, B.; Das, R.; Sahub, C.; Rotello, V. Solubilization of Hydrophobic Catalysts Using Nanoparticle Hosts. Small 2017, 14, 1702198. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Jiao, X.; Zou, H.; Yang, H.; Liu, J. Growing a hydrophilic nanoporous shell on a hydrophobic catalyst interface for aqueous reactions with high reaction efficiency and in situ catalyst recycling. J. Mater. Chem. A 2017, 5, 16162–16170. [Google Scholar] [CrossRef]
- Carter, B.O.; Adams, D.; Cooper, A.I. Pausing a stir: Heterogeneous catalysis in “dry water”. Green Chem. 2010, 12, 783–785. [Google Scholar] [CrossRef]
- Miao, Y.-E.; Lee, H.K.; Chew, W.S.; Phang, I.Y.; Liu, T.; Ling, X.Y. Catalytic liquid marbles: Ag nanowire-based miniature reactors for highly efficient degradation of methylene blue. Chem. Commun. 2014, 50, 5923–5926. [Google Scholar] [CrossRef]
- Sheng, Y.; Sun, G.; Wu, J.; Ma, G.; Ngai, T. Silica-Based Liquid Marbles as Microreactors for the Silver Mirror Reaction. Angew. Chem. 2015, 127, 7118–7123. [Google Scholar] [CrossRef]
- Pol, V.G.; Srivastava, D.N.; Palchik, O.; Palchik, V.; Slifkin, M.A.; Weiss, A.M.; Gedanken, A. Sonochemical Deposition of Silver Nanoparticles on Silica Spheres. Langmuir 2002, 18, 3352–3357. [Google Scholar] [CrossRef]
- Wei, W.; Lu, R.; Ye, W.; Sun, J.; Zhu, Y.; Luo, J.; Liu, X. Liquid Marbles Stabilized by Fluorine-Bearing Cyclomatrix Polyphosphazene Particles and Their Application as High-Efficiency Miniature Reactors. Langmuir 2016, 32, 1707–1715. [Google Scholar] [CrossRef]
- Wang, D.; Zhu, L.; Chen, J.-F.; Dai, L. Liquid Marbles Based on Magnetic Upconversion Nanoparticles as Magnetically and Optically Responsive Miniature Reactors for Photocatalysis and Photodynamic Therapy. Angew. Chem. Int. Ed. 2016, 55, 10795–10799. [Google Scholar] [CrossRef]
- Gao, W.; Lee, H.K.; Hobley, J.; Liu, T.; Phang, I.Y.; Ling, X.Y. Graphene Liquid Marbles as Photothermal Miniature Reactors for Reaction Kinetics Modulation. Angew. Chem. Int. Ed. 2015, 54, 3993–3996. [Google Scholar] [CrossRef]
- Koh, C.S.L.; Lee, H.K.; Han, X.; Lee, M.R.; Yang, Z.; Ling, X.Y.; Phan-Quang, G.C. SERS- and Electrochemically Active 3D Plasmonic Liquid Marbles for Molecular-Level Spectroelectrochemical Investigation of Microliter Reactions. Angew. Chem. Int. Ed. 2017, 56, 8813–8817. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Lee, H.K.; Lee, Y.H.; Hao, W.; Liu, Y.; Phang, I.Y.; Li, S.; Ling, X.Y. Identifying Enclosed Chemical Reaction and Dynamics at the Molecular Level Using Shell-Isolated Miniaturized Plasmonic Liquid Marble. J. Phys. Chem. Lett. 2016, 7, 1501–1506. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Koh, C.S.L.; Lee, H.K.; Chew, W.S.; Ling, X.Y. Microchemical Plant in a Liquid Droplet: Plasmonic Liquid Marble for Sequential Reactions and Attomole Detection of Toxin at Microliter Scale. ACS Appl. Mater. Interfaces 2017, 9, 39635–39640. [Google Scholar] [CrossRef]
- Tyowua, A.T.; Ahor, F.; Yiase, S.G.; Binks, B.P. Liquid marbles as microreactors for qualitative and quantitative inorganic analyses. SN Appl. Sci. 2020, 2, 345. [Google Scholar] [CrossRef]
- Fullarton, C.; Draper, T.C.; Phillips, N.; de Lacy Costello, B.P.; Adamatzky, A. Belousov–Zhabotinsky reaction in liquid marbles. J. Phys. Mater. 2019, 2, 015005. [Google Scholar] [CrossRef]
- Tsompanas, M.-A.; Fullarton, C.; Adamatzky, A. Belousov-Zhabotinsky liquid marbles in robot control. Sens. Actuators B Chem. 2019, 295, 194–203. [Google Scholar] [CrossRef]
- Adamatzky, A.; Tsomapanas, M.-A.; Draper, T.C.; Fullarton, C.; Mayne, R. Liquid Marble Photosensor. ChemPhysChem 2019, 21, 90–98. [Google Scholar] [CrossRef]
- Oliveira, N.; Reis, R.L.; Mano, J.F. The Potential of Liquid Marbles for Biomedical Applications: A Critical Review. Adv. Healthc. Mater. 2017, 6, 1700192. [Google Scholar] [CrossRef]
- Avrămescu, R.-E.; Ghica, M.-V.; Dinu-Pirvu, C.; Udeanu, D.I.; Popa, L. Liquid Marbles: From Industrial to Medical Applications. Molecules 2018, 23, 1120. [Google Scholar] [CrossRef]
- Arbatan, T.; Li, L.; Tian, J.; Shen, W. Liquid Marbles as Micro-bioreactors for Rapid Blood Typing. Adv. Healthc. Mater. 2011, 1, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.; Yan, X.; Zhao, L.; Liao, Q.; Zhao, K.; Du, H.; Zhang, X.; Zhang, X.; Zhang, Y. Gold nanoparticle/ZnO nanorod hybrids for enhanced reactive oxygen species generation and photodynamic therapy. Nano Res. 2015, 8, 2004–2014. [Google Scholar] [CrossRef]
- Sreejith, K.R.; Gorgannezhad, L.; Jin, J.; Ooi, C.H.; Stratton, H.; Dao, D.V.; Nguyen, N.T. Liquid marbles as biochemical reactors for the polymerase chain reaction. Lab Chip 2019, 19, 3220–3227. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, P.; Kaur, G.; Yao, X.; Yang, M. Transparent and Gas-Permeable Liquid Marbles for Culturing and Drug Sensitivity Test of Tumor Spheroids. Adv. Healthc. Mater. 2017, 6, 1700185. [Google Scholar] [CrossRef] [PubMed]
- Sarvi, F.; Arbatan, T.; Chan, P.P.Y.; Shen, W. A novel technique for the formation of embryoid bodies inside liquid marbles. RSC Adv. 2013, 3, 14501–14508. [Google Scholar] [CrossRef]
- Ledda, S.; Idda, A.; Kelly, J.; Ariu, F.; Bogliolo, L.; Bebbere, D. A novel technique for in vitro maturation of sheep oocytes in a liquid marble microbioreactor. J. Assist. Reprod. Genet. 2016, 33, 513–518. [Google Scholar] [CrossRef][Green Version]
- Vadivelu, R.K.; Ooi, C.H.; Yao, R.-Q.; Velasquez, J.T.; Pastrana, E.; Díaz-Nido, J.; Lim, F.; Ekberg, J.A.K.; Nguyen, N.T.; John, J.S. Generation of three-dimensional multiple spheroid model of olfactory ensheathing cells using floating liquid marbles. Sci. Rep. 2015, 5, 15083. [Google Scholar] [CrossRef]
- Vadivelu, R.K.; Kamble, H.; Munaz, A.; Nguyen, N.-T. Liquid marbles as bioreactors for the study of three-dimensional cell interactions. Biomed. Microdevices 2017, 19, 31. [Google Scholar] [CrossRef]
- Tian, J.; Fu, N.; Chen, X.D.; Shen, W. Respirable liquid marble for the cultivation of microorganisms. Colloids Surf. B Biointerfaces 2013, 106, 187–190. [Google Scholar] [CrossRef]
- Sreejith, K.R.; Ooi, C.H.; Jin, J.; Dao, D.V.; Nguyen, N.T. Digital polymerase chain reaction technology—recent advances and future perspectives. Lab Chip 2018, 18, 3717–3732. [Google Scholar] [CrossRef] [PubMed]
- Sreejith, K.R.; Gorgannezhad, L.; Jin, J.; Ooi, C.H.; Takei, T.; Hayase, G.; Stratton, H.; Lamb, K.; Shiddiky, M.J.A.; Dao, D.V.; et al. Core-Shell Beads Made by Composite Liquid Marble Technology as A Versatile Microreactor for Polymerase Chain Reaction. Micromachines 2020, 11, 242. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Fan, L.; Mu, B.; Wang, A.; Zhang, J. Palygorskite@Fe3O4 @polyperfluoroalkylsilane nanocomposites for superoleophobic coatings and magnetic liquid marbles. J. Mater. Chem. A 2016, 4, 5859–5868. [Google Scholar] [CrossRef]
- Liu, L.; Pan, Y.; Bhushan, B.; Li, F.; Zhao, X. Core-shell magnetic nanoparticles for substrate-Independent super-amphiphobic surfaces and mechanochemically robust liquid marbles. Chem. Eng. J. 2019, 391, 123523. [Google Scholar] [CrossRef]
- Gao, L.; McCarthy, T.J. Ionic Liquid Marbles. Langmuir 2007, 23, 10445–10447. [Google Scholar] [CrossRef] [PubMed]
- Marsh, K.N.; Deev, A.; Wu, A.C.-T.; Tran, E.; Klamt, A. Room temperature ionic liquids as replacements for conventional solvents—A review. Korean J. Chem. Eng. 2002, 19, 357–362. [Google Scholar] [CrossRef]
- Zhao, D.; Wu, M.; Kou, Y.; Min, E. Ionic liquids: Applications in catalysis. Catal. Today 2002, 74, 157–189. [Google Scholar] [CrossRef]
- Huddleston, J.G.; Rogers, R.D. Room temperature ionic liquids as novel media for ‘clean’ liquid–liquid extraction. Chem. Commun. 1998, 1765–1766. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Choi, Y.-K.; Park, J.; Lee, S.; Yang, Y.-H.; Kim, H.; Park, T.-J.; Kim, Y.H.; Lee, S.H. Ionic liquid-mediated extraction of lipids from algal biomass. Bioresour. Technol. 2012, 109, 312–315. [Google Scholar] [CrossRef] [PubMed]
- Achinivu, E.C.; Howard, R.M.; Li, G.; Gracz, H.; Henderson, W.A. Lignin extraction from biomass with protic ionic liquids. Green Chem. 2014, 16, 1114–1119. [Google Scholar] [CrossRef]
- Passos, H.; Freire, M.G.; Coutinho, J.A.P. Ionic liquid solutions as extractive solvents for value-added compounds from biomass. Green Chem. 2014, 16, 4786–4815. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, S.; Deng, Y. Recent advances in ionic liquid catalysis. Green Chem. 2011, 13, 2619. [Google Scholar] [CrossRef]
- Vekariya, R.L. A review of ionic liquids: Applications towards catalytic organic transformations. J. Mol. Liq. 2017, 227, 44–60. [Google Scholar] [CrossRef]
- Zhang, M.; Ettelaie, R.; Yan, T.; Zhang, S.-J.; Cheng, F.; Binks, B.P.; Yang, H. Ionic Liquid Droplet Microreactor for Catalysis Reactions Not at Equilibrium. J. Am. Chem. Soc. 2017, 139, 17387–17396. [Google Scholar] [CrossRef]
- Dickey, M.D. Stretchable and Soft Electronics using Liquid Metals. Adv. Mater. 2017, 29, 1606425. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Rydberg, A.; Hjort, K.; Wu, Z. Liquid metal stretchable unbalanced loop antenna. Appl. Phys. Lett. 2009, 94, 144103. [Google Scholar] [CrossRef]
- Sen, P.; Kim, C.-J. A Fast Liquid-Metal Droplet Microswitch Using EWOD-Driven Contact-Line Sliding. J. Microelectromech. Syst. 2008, 18, 174–185. [Google Scholar] [CrossRef]
- Daeneke, T.; Khoshmanesh, K.; Mahmood, N.; De Castro, I.A.; Esrafilzadeh, D.; Barrow, S.J.; Dickey, M.D.; Kalantar-Zadeh, K. Liquid metals: Fundamentals and applications in chemistry. Chem. Soc. Rev. 2018, 47, 4073–4111. [Google Scholar] [CrossRef] [PubMed]
- Bobev, S.; Merz, J.; Lima, A.; Fritsch, V.; Thompson, J.D.; Sarrao, J.L.; Gillessen, M.; Dronskowski, R. Unusual Mn—Mn spin coupling in the polar intermetallic compounds CaMn2Sb2 and SrMn2Sb2. Inorg. Chem. 2006, 45, 4047–4054. [Google Scholar]
- Benbow, E.M.; Dalal, N.S.; Latturner, S.E. Crystal growth and magnetic behavior of R6T13− xAlxMy phases (R= La, Nd; T= Mn, Fe; M= main group) grown from lanthanide-rich eutectic fluxes. J. Solid State Chem. 2009, 182, 3055–3062. [Google Scholar] [CrossRef]
- Yazdanpanah, M.M.; Harfenist, S.A.; Safir, A.; Cohn, R.W. Selective self-assembly at room temperature of individual freestanding Ag2Ga alloy nanoneedles. J. Appl. Phys. 2005, 98, 73510. [Google Scholar] [CrossRef]
- Gille, P.; Ziemer, T.; Schmidt, M.; Kovnir, K.; Burkhardt, U.; Armbrüster, M. Growth of large PdGa single crystals from the melt. Intermetallics 2010, 18, 1663–1668. [Google Scholar] [CrossRef]
- Wang, W.; Chen, W.; Zhao, X.; Chen, D.; Yang, K. Effect of composition on the reactivity of Al-rich alloys with water. Int. J. Hydrogen Energy 2012, 37, 18672–18678. [Google Scholar] [CrossRef]
- Xu, S.; Zhao, X.; Liu, J. Liquid metal activated aluminum-water reaction for direct hydrogen generation at room temperature. Renew. Sustain. Energy Rev. 2018, 92, 17–37. [Google Scholar] [CrossRef]
- Sivan, V.; Tang, S.; O’Mullane, A.P.; Petersen, P.; Eshtiaghi, N.; Kalantar-Zadeh, K.; Mitchell, A. Liquid Metal Marbles. Adv. Funct. Mater. 2012, 23, 144–152. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, N.-K.; Ooi, C.H.; Singha, P.; Jin, J.; Sreejith, K.R.; Phan, H.-P.; Nguyen, N.-T. Liquid Marbles as Miniature Reactors for Chemical and Biological Applications. Processes 2020, 8, 793. https://doi.org/10.3390/pr8070793
Nguyen N-K, Ooi CH, Singha P, Jin J, Sreejith KR, Phan H-P, Nguyen N-T. Liquid Marbles as Miniature Reactors for Chemical and Biological Applications. Processes. 2020; 8(7):793. https://doi.org/10.3390/pr8070793
Chicago/Turabian StyleNguyen, Nhat-Khuong, Chin Hong Ooi, Pradip Singha, Jing Jin, Kamalalayam Rajan Sreejith, Hoang-Phuong Phan, and Nam-Trung Nguyen. 2020. "Liquid Marbles as Miniature Reactors for Chemical and Biological Applications" Processes 8, no. 7: 793. https://doi.org/10.3390/pr8070793
APA StyleNguyen, N.-K., Ooi, C. H., Singha, P., Jin, J., Sreejith, K. R., Phan, H.-P., & Nguyen, N.-T. (2020). Liquid Marbles as Miniature Reactors for Chemical and Biological Applications. Processes, 8(7), 793. https://doi.org/10.3390/pr8070793









