Thermophysical Properties of Newly Synthesized Ammonium-Based Protic Ionic Liquids: Effect of Temperature, Anion and Alkyl Chain Length
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of PILs
2.3. Structural Characterization and Water Content
2.4. Thermophysical Properties Characterization
3. Results and Discussion
3.1. Structural Characterization and Water Content Analysis
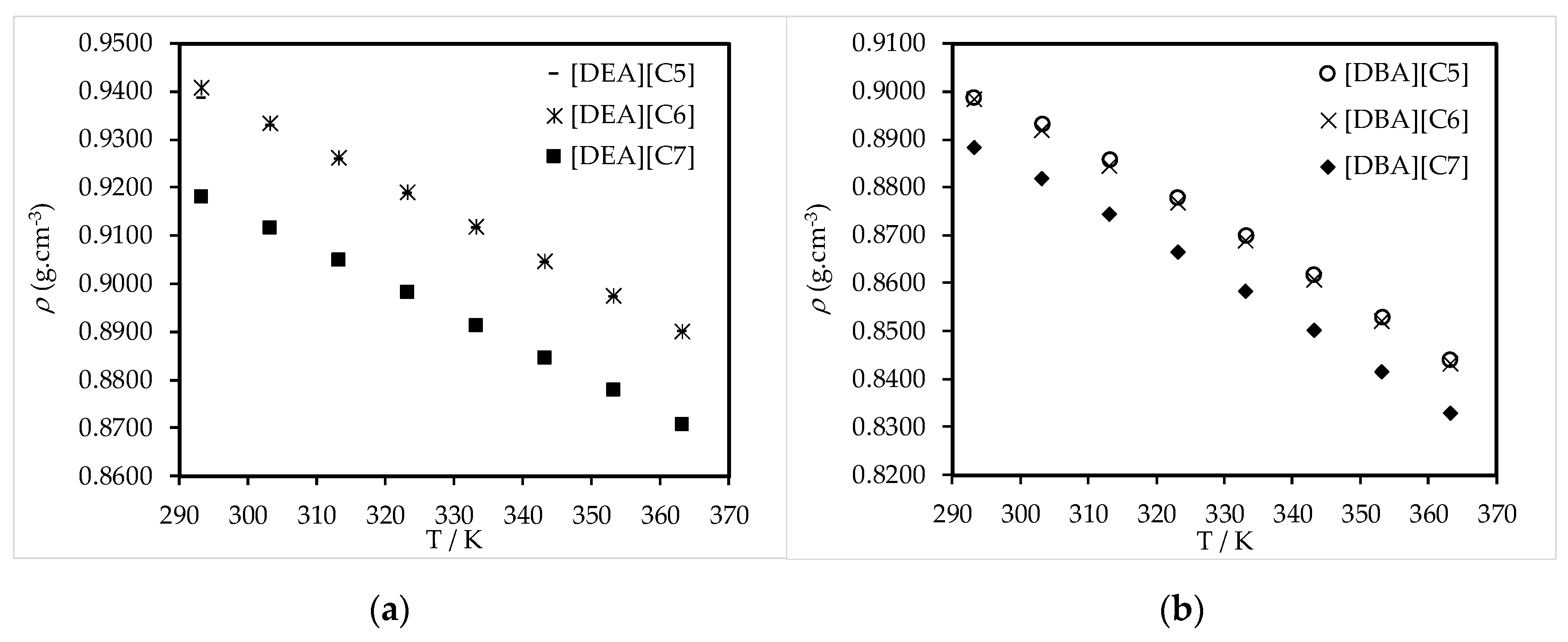
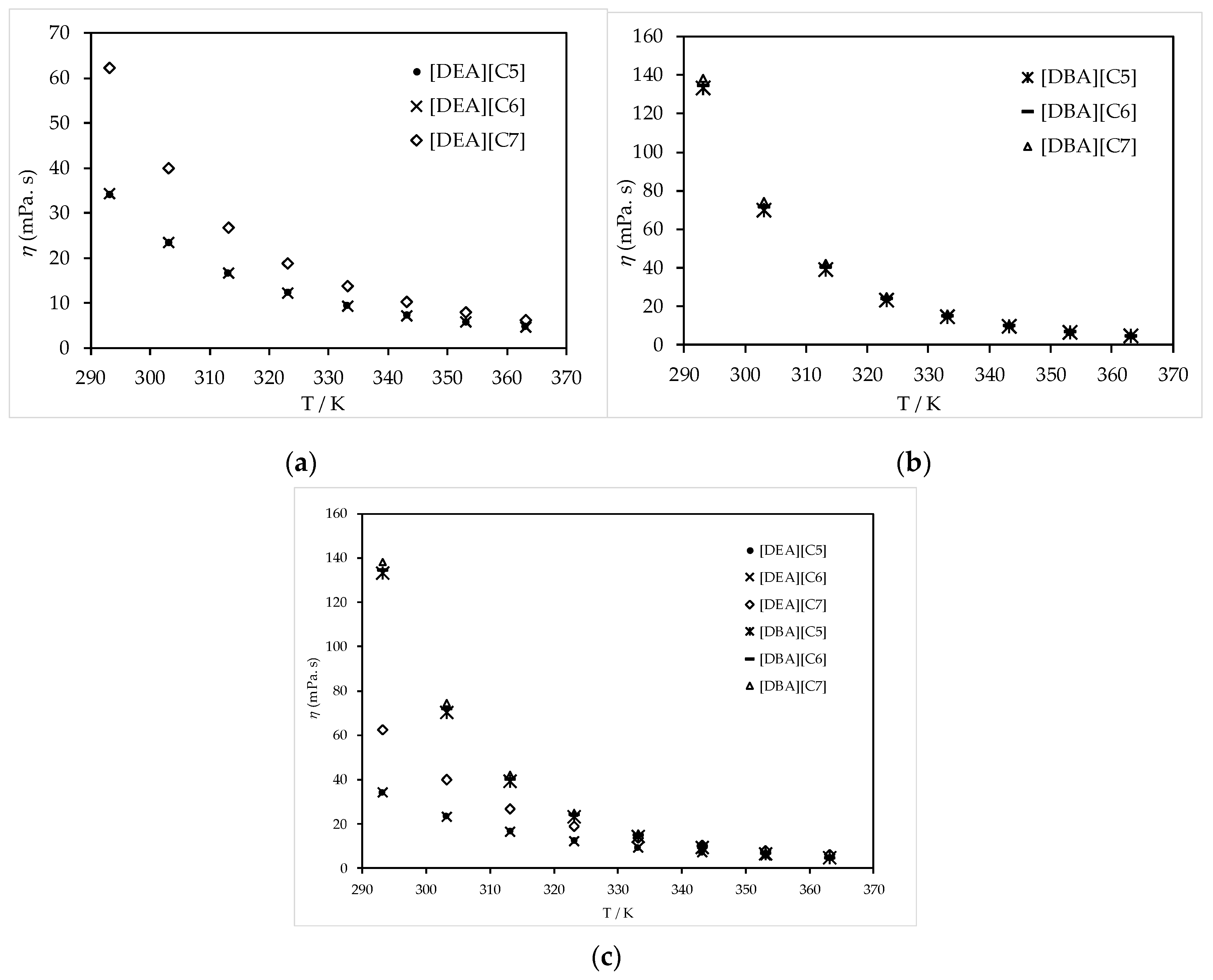
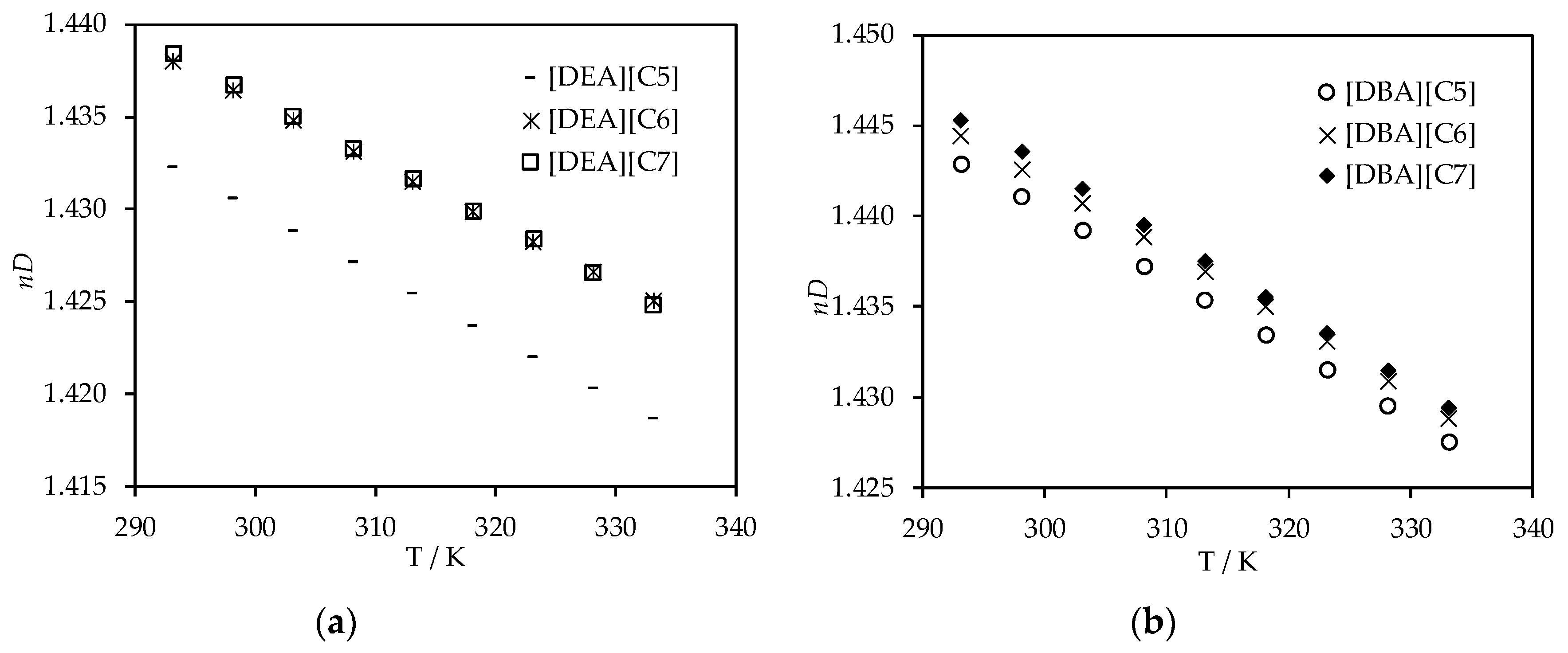
3.2. Thermophysical Properties Analysis
3.3. Thermophysical Properties Correlations
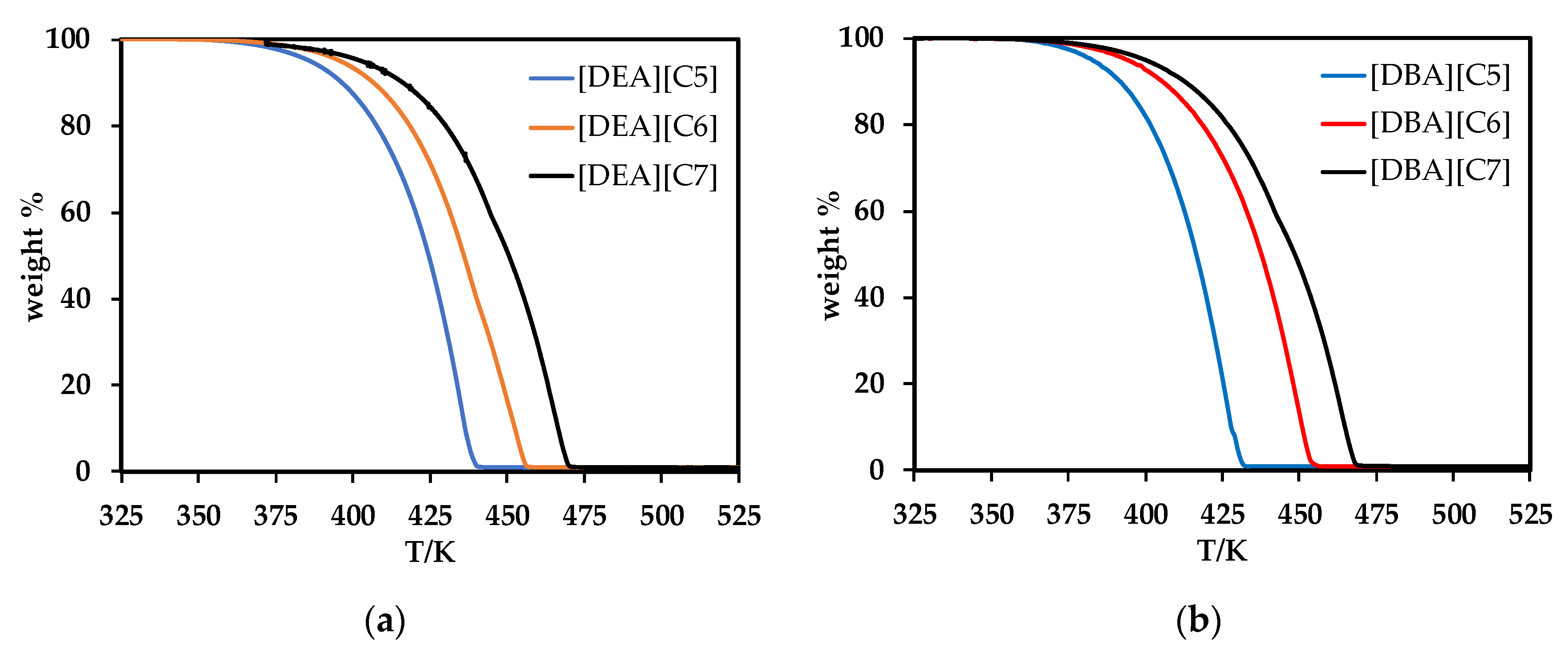
3.4. Thermal Expansion Coefficient, Standard Entropy, Lattice Potential Energy, Water Content and Thermal Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Endo, T.; Murata, H.; Imanari, M.; Mizushima, N.; Seki, H.; Nishikawa, K. NMR Study of cation dynamics in three crystalline states of 1-butyl-3-methylimidazolium hexafluorophosphate exhibiting crystal polymorphism. J. Phys. Chem. B 2012, 116, 3780–3788. [Google Scholar] [CrossRef]
- Smith, J.A.; Webber, G.B.; Warr, G.G.; Atkin, R. Rheology of protic ionic liquids and their mixtures. J. Phys. Chem. B 2013, 117, 13930–13935. [Google Scholar] [CrossRef]
- Patra, R.N.; Gardas, R.L. Effect of nitro groups on desulfurization efficiency of benzyl-substituted imidiazolium-based ionic liquids: Experimental and computational approach. Energy Fuels 2019, 33, 7659–7666. [Google Scholar] [CrossRef]
- Singh, V.; Sharma, G.; Gardas, R.L. Thermodynamic and ultrasonic properties of ascorbic acid in aqueous protic ionic liquid solutions. PLoS ONE 2015, 10, e0126091. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Sharma, G.; Singh, D.; Gardas, R.L. Effect of anion on thermophysical properties of N,N-diethanolammonium based protic ionic liquids. J. Mol. Liq. 2017, 242, 249–254. [Google Scholar] [CrossRef]
- Baicha, Z.; Salar-García, M.J.; Ortiz-Martínez, V.M.; Hernández-Fernández, F.J.; de los Ríos, A.P.; Maqueda Marín, D.P.; Collado, J.A.; Tomás-Alonso, F.; El Mahi, M. On the selective transport of nutrients through polymer inclusion membranes based on ionic liquids. Processes 2019, 7, 544. [Google Scholar] [CrossRef]
- Abejón, R.; Rabadán, J.; Lanza, S.; Abejón, A.; Garea, A.; Irabien, A. Supported ionic liquid membranes for separation of lignin aqueous solutions. Processes 2018, 6, 143. [Google Scholar] [CrossRef]
- Taimoor, A.A.; Al-Shahrani, S.; Muhammad, A. Ionic liquid (1-butyl-3-metylimidazolium methane sulphonate) corrosion and energy analysis for high pressure CO2 absorption process. Processes 2018, 6, 45. [Google Scholar] [CrossRef]
- Bogdanov, M.G.; Svinyarov, I. Distribution of N-methylimidazole in ionic liquids/organic solvents systems. Processes 2017, 5, 52. [Google Scholar] [CrossRef]
- Keremedchieva, R.; Svinyarov, I.; Bogdanov, M.G. Ionic Liquid-Based Aqueous Biphasic Systems—A facile approach for ionic liquid regeneration from crude plant extracts. Processes 2015, 3, 769–778. [Google Scholar] [CrossRef]
- Ahmat, X.; Ablajan, K.; Shinozaki, H. Furans-Maleimides Diels-Alder reactions in protic ionic liquid. Chem. Res. Chin. Univ. 2009, 25, 161–168. [Google Scholar]
- Mancini, P.M.E.; Ormachea, C.M.; Rosa, C.D.D.; Kneeteman, M.N.; Domingo, L.R. Protic and nonprotic ionic liquids in polar Diels-Alder reactions using properly substituted heterocycles and carbocycles as dienophiles. A DFT study. In Ionic Liquids: New Aspects for the Future; IntechOpen: London, UK, 2013; Volume 9, pp. 691–695. [Google Scholar]
- Rosa, C.D.; Ormachea, C.; Kneeteman, M.N.; Adam, C.; Mancini, P.M.E. Diels–Alder reactions of N-tosylpirroles developed in protic ionic liquids. Theoretical studies using DFT methods. Tetrahedron Lett. 2011, 52, 6754–6757. [Google Scholar] [CrossRef]
- Greaves, T.L.; Drummond, C.J. Protic Ionic Liquids: Evolving structure–property relationships and expanding applications. Chem. Rev. 2015, 115, 11379–11448. [Google Scholar] [CrossRef] [PubMed]
- Miran, M.S.; Yasuda, T.; Susan, M.A.B.H.; Dokko, K.; Watanabe, M. Binary protic ionic liquid mixtures as a proton conductor: High fuel cell reaction activity and facile proton transport. J. Phys. Chem. C 2014, 118, 27631–27639. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Ogawa, A.; Kanno, M.; Nakamoto, H.; Yasuda, T.; Watanabe, M. Nonhumidified intermediate temperature fuel cells using protic ionic liquids. J. Am. Chem. Soc. 2010, 132, 9764–9773. [Google Scholar] [CrossRef]
- Greaves, T.L.; Drummond, C.J. Protic ionic liquids: Properties and applications. Chem. Rev. 2008, 108, 206–237. [Google Scholar] [CrossRef]
- Hirao, M.; Sugimoto, H.; Ohno, H. Preparation of novel room-temperature molten salts by neutralization of amines. J. Electrochem. Soc. 2000, 147, 4168–4172. [Google Scholar] [CrossRef]
- MacFarlane, D.R.; Forsyth, M.; Izgorodina, E.I.; Abbott, A.P.; Annat, G.; Fraser, K. On the concept of ionicity in ionic liquids. Phys. Chem. Chem. Phys. 2009, 11, 4962–4967. [Google Scholar] [CrossRef]
- Shen, M.; Zhang, Y.; Chen, K.; Che, S.; Yao, J.; Li, H. Ionicity if protic ionic liquid: Quantitative measurement by spectroscopic methods. J. Phys. Chem. B 2017, 121, 1372–1376. [Google Scholar] [CrossRef]
- MacFarlane, D.R.; Seddon, K.R. Ionic liquids–Progress on the fundamental issues. Aust. J. Chem. 2007, 60, 3–5. [Google Scholar] [CrossRef]
- Yunus, N.M.; Halim, N.H.; Wilfred, C.D.; Murugesan, T.; Lim, J.W.; Show, P.L. Thermophysical properties and CO2 absorption of ammonium-based protic ionic liquids containing acetate and butyrate anions. Processes 2019, 7, 820. [Google Scholar] [CrossRef]
- Petrović, V.P.; Simijonović, D.; Petrović, Z.D.; Marković, S. Formation of a vanillic Mannich base—Theoretical study. Chem. Pap. 2015, 69, 1244–1252. [Google Scholar] [CrossRef]
- Khan, A.; Lu, X.; Aldous, L.; Zhao, C. oxygen reduction reaction in room temperature protic ionic liquids. J. Phys. Chem. C 2013, 117, 18334–18342. [Google Scholar] [CrossRef]
- Kondratenko, Y.A.; Nyanikova, G.G.; Molchanova, K.V.; Kochina, T.A. Characteristics of protic ionic liquids based on triethanolammonium salts of biologically active carboxylic acids and their impact on the growth properties of the Rhizopus oryzae fungus. Glass Phys. Chem. 2017, 43, 445–451. [Google Scholar] [CrossRef]
- Yi, L.; Feng, J.; Li, W.-Y. Separation of phenolic compounds from coal liquefaction oil by choline chloride-glycerol deep eutectic solvents. Energy Procedia 2019, 158, 5169–5174. [Google Scholar] [CrossRef]
- Lu, J.-G.; Li, X.; Zhao, Y.-X.; Ma, H.-L.; Wang, L.-F.; Wang, X.-Y.; Yu, Y.-F.; Shen, T.-Y.; Xu, H.; Zhang, Y.-T. CO2 capture by ionic liquid membrane absorption for reduction of emissions of greenhouse gas. Environ. Chem. Lett. 2019, 17, 1031–1038. [Google Scholar] [CrossRef]
- Liu, Q.; Mou, L.; Zheng, Q.; Xia, Q. Thermodynamic properties of ionic liquids. In Progress and Developments in Ionic Liquids; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Shen, Y.; Kennedy, D.F.; Greaves, T.L.; Weerawardena, A.; Mulder, R.J.; Kirby, N.; Song, G.; Drummond, C.J. Protic ionic liquids with fluorous anions: Physicochemical properties and self-assembly nanostructure. Phys. Chem. Chem. Phys. 2012, 14, 7981–7992. [Google Scholar] [CrossRef]
- Akbari, F.; Alavianmehr, M.M.; Behjatmanesh Ardakani, R.; Mohammad-Aghaie, D. Thermophysical properties of ionic liquids and their mixtures from a new equation of state. Ionics 2018, 24, 1357–1369. [Google Scholar] [CrossRef]
- Vijayraghavan, R.; Pas, S.J.; Izgorodina, E.I.; MacFarlane, D.R. Diamino protic ionic liquids for CO2 capture. Phys. Chem. Chem. Phys. 2013, 15, 19994–19999. [Google Scholar] [CrossRef]
- Kurnia, K.A.; Harris, F.; Wilfred, C.D.; Abdul Mutalib, M.I.; Murugesan, T. Thermodynamic properties of CO2 absorption in hydroxyl ammonium ionic liquids at pressures of (100–1600) kPa. J. Chem. Thermodyn. 2009, 41, 1069–1073. [Google Scholar] [CrossRef]
- Guo, H.; Smith, T.W.; Iglesias, P. The study of hexanoate-based protic ionic liquids used as lubricants in steel-steel contact. J. Mol. Liq. 2020, 299, 112208. [Google Scholar] [CrossRef]
- Kopczyńskia, K.; Gabryelczyka, A.; Baraniaka, M.; Łęgosza, B.; Pernaka, J.; Jankowskab, E.; Rzeszutekc, W.; Kędziorc, P.; Lota, G. Positive electrode material in lead-acid car battery modified by protic ammonium ionic liquid. J. Energy Storage 2019, 26, 100996. [Google Scholar] [CrossRef]
- Mayrand-Provencher, L.; Lin, S.; Lazzerini, D.; Rochefort, D. Pyridinium-based protic ionic liquids as electrolytes for RuO2 electrochemical capacitors. J. Power Sources 2010, 195, 5114–5121. [Google Scholar] [CrossRef]
- Iglesias, M.; Gonzalez-Olmosa, R.; Cota, I.; Medina, F. Brønsted ionic liquids: Study of physico-chemical properties and catalytic activity in aldol condensations. Chem. Eng. J. 2010, 162, 802–808. [Google Scholar] [CrossRef]
- Chen, Y.; Cao, Y.; Lu, X.; Zhao, C.; Yan, C.; Mu, T. Water sorption in protic ionic liquids: Correlation between hygroscopicity and polarity. New J. Chem. 2013, 37, 1959–1967. [Google Scholar] [CrossRef]
- Freire, M.G.; Santos, L.M.N.B.F.; Fernandes, A.M.; Coutinho, J.A.P.; Marrucho, I.M. An overview of the mutual solubilities of water–imidazolium-based ionic liquids systems. Fluid Phase Equilibria 2007, 261, 449–454. [Google Scholar] [CrossRef]
- Seddon, K.; Stark, A.; Torres, M.-J. Influence of Chloride, Water, and Organic Solvents on the Physical Properties of Ionic Liquids. Pure Appl. Chem. 2000, 72, 2275–2287. [Google Scholar] [CrossRef]
- Yunus, N.M.; Abdul Mutalib, M.I.; Man, Z.; Bustam, M.A.; Murugesan, T. Thermophysical properties of 1-alkylpyridinum bis(trifluoromethylsulfonyl)imide ionic liquids. J. Chem. Thermodyn. 2010, 42, 491–495. [Google Scholar] [CrossRef]
- Gusain, R.; Panda, S.; Bakshi, P.S.; Gardas, R.L.; Khatri, O.P. Thermophysical properties of trioctylalkylammonium bis(salicylato)borate ionic liquids: Effect of alkyl chain length. J. Mol. Liq. 2018, 269, 540–546. [Google Scholar] [CrossRef]
- Wu, B.; Yamashita, Y.; Endo, T.; Takahashi, K.; Castner, E.W. Structure and dynamics of ionic liquids: Trimethylsilylpropyl-substituted cations and bis(sulfonyl)amide anions. J. Chem. Phys. 2016, 145, 244506. [Google Scholar] [CrossRef]
- Pinto, R.R.; Mattedi, S.; Aznar, M. Synthesis and physical properties of three protic ionic liquids with the ethylammonium cation. Chem. Eng. Trans. 2015, 43, 1165–1170. [Google Scholar]
- Chennuri, B.K.; Gardas, R.L. Measurement and correlation for the thermophysical properties of hydroxyethyl ammonium based protic ionic liquids: Effect of temperature and alkyl chain length on anion. Fluid Phase Equilibria 2016, 427, 282–290. [Google Scholar] [CrossRef]
- Keshapolla, D.; Srinivasarao, K.; Gardas, R.L. Influence of temperature and alkyl chain length on physicochemical properties of trihexyl- and trioctylammonium based protic ionic liquids. J. Chem. Thermodyn. 2019, 133, 170–180. [Google Scholar] [CrossRef]
- Zhang, X.U.; Faber, D.J.; Post, A.L.; van Leeuwen, T.G.; Sterenborg, H.J.C.M. Refractive index measurement using single fiber reflectance spectroscopy. J. Biophotonics 2019, 12, 701–713. [Google Scholar] [CrossRef] [PubMed]
- Soave, G. Equilibrium constants from a modified Redlich-Kwong equation of state. Chem. Eng. Sci. 1972, 27, 1197–1203. [Google Scholar] [CrossRef]
- Singh, D.; Gardas, R.L. Influence of cation size on the ionicity, fluidity, and physiochemical properties of 1,2,4-triazolium based ionic liquids. J. Phys. Chem. B 2016, 120, 4834–4842. [Google Scholar] [CrossRef]
- Zec, N.; Vraneš, M.; Bešter-Rogač, M.; Trtić-Petrović, T.; Dimitrijević, A.; Čobanov, I.; Gadžurić, S. Influence of the alkyl chain length on densities and volumetric properties of 1,3-dialkylimidazolium bromide ionic liquids and their aqueous solutions. J. Chem. Thermodyn. 2018, 121, 72–78. [Google Scholar] [CrossRef]
- Tariq, M.; Forte, P.A.S.; Gomes, M.F.C.; Lopes, J.N.C.; Rebelo, L.P.N. Densities and refractive indices of imidazolium- and phosphonium-based ionic liquids: Effect of temperature, alkyl chain length, and anion. J. Chem. Thermodyn. 2009, 41, 790–798. [Google Scholar] [CrossRef]
- Liu, Q.-S.; Yang, M.; Li, P.-P.; Sun, S.-S.; Welz-Biermann, U.; Tan, Z.-C.; Zhang, Q.-G. Physicochemical properties of ionic liquids [C3py][NTf2] and [C6py][NTf2]. J. Chem. Eng. Data 2011, 56, 4094–4101. [Google Scholar] [CrossRef]
- Liu, Q.-S.; Li, P.-P.; Welz-Biermann, U.; Chen, J.; Liu, X.-X. Density, dynamic viscosity, and electrical conductivity of pyridinium-based hydrophobic ionic liquids. J. Chem. Thermodyn. 2013, 66, 88–94. [Google Scholar] [CrossRef]
- Liu, Q.-S.; Li, Z.; Welz-Biermann, U.; Li, C.-P.; Liu, X.-X. Thermodynamic properties of a new hydrophobic amide-based task-specific ionic liquid [EimCH2CONHBu][NTf2]. J. Chem. Eng. Data 2013, 58, 93–98. [Google Scholar] [CrossRef]
- Glasser, L. Lattice and phase transition thermodynamics of ionic liquids. Thermochim. Acta 2004, 421, 87–93. [Google Scholar] [CrossRef]
- Bandrés, I.; Royo, F.M.; Gascón, I.; Castro, M.; Lafuente, C. Anion influence on thermophysical properties of ionic liquids: 1-butylpyridinium tetrafluoroborate and 1-butylpyridinium triflate. J. Phys. Chem. B 2010, 114, 3601–3607. [Google Scholar] [CrossRef] [PubMed]
- Greaves, T.L.; Weerawardena, A.; Fong, C.; Krodkiewska, I.; Drummond, C.J. Protic ionic liquids: solvents with tunable phase behavior and physicochemical properties. J. Phys. Chem. B 2006, 110, 22479–22487. [Google Scholar] [CrossRef]
- Cai, G.; Yang, S.; Zhou, Q.; Liu, L.; Lu, X.; Xu, J.; Zhang, S. Physicochemical properties of various 2-hydroxyethylammonium sulfonate -based protic ionic liquids and their potential application in hydrodeoxygenation. Front. Chem. 2019, 7, 196. [Google Scholar] [CrossRef]
- Dharaskar, S.A.; Wasewar, K.L.; Varma, M.N.; Shende, D.Z.; Yoo, C.K. Synthesis, characterization and application of 1-butyl-3-methylimidazolium tetrafluoroborate for extractive desulfurization of liquid fuel. Arab. J. Chem. 2016, 9, 578–587. [Google Scholar] [CrossRef]
- Efimova, A.; Hubrig, G.; Schmidt, P. Thermal stability and crystallization behavior of imidazolium halide ionic liquids. Thermochim. Acta 2013, 573, 162–169. [Google Scholar] [CrossRef]
- Dharaskar, S.A.; Wasewar, K.L.; Varma, M.N.; Shende, D.Z.; Yoo, C.K. Deep removal of sulfur from model liquid fuels using 1-butyl-3-methylimidazolium chloride. Procedia Eng. 2013, 51, 416–422. [Google Scholar] [CrossRef]
| Base | Acid | Name of Synthesized PILs | Abbreviation |
|---|---|---|---|
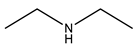 Diethylamine |  Pentanoic acid | 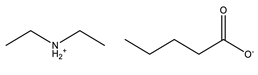 Diethylammonium pentanoate | [DEA][C5] |
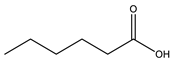 Hexanoic acid | 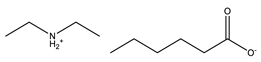 Diethylammonium hexanonate | [DEA][C6] | |
 Heptanoic acid |  Diethylammonium heptanoate | [DEA][C7] | |
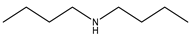 Dibutylamine |  Pentanoic acid | 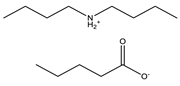 Dibutylammonium pentanoate | [DBA][C5] |
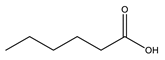 Hexanoic acid | 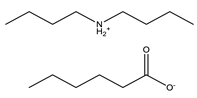 Dibutylammonium hexanoate | [DBA][C6] | |
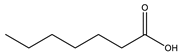 Heptanoic acid | 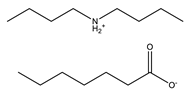 Dibutylammonium heptanoate | [DBA][C7] |
| Ionic Liquids | A1 | A2 | SD |
|---|---|---|---|
| [DEA][C5] | 1.1456 | −0.0007 | 0.0010 |
| [DEA][C6] | 1.1519 | −0.0007 | 0.0068 |
| [DEA][C7] | 1.1168 | −0.0007 | 0.0076 |
| [DBA][C5] | 1.1331 | −0.0008 | 0.0027 |
| [DBA][C6] | 1.1345 | −0.0008 | 0.0027 |
| [DBA][C7] | 1.1269 | −0.0008 | 0.0008 |
| Ionic Liquid | A3 | A4 | SD |
|---|---|---|---|
| [DEA][C5] | −2.9587 | 1312.0 | 0.0106 |
| [DEA][C6] | −2.9597 | 1312.4 | 0.0108 |
| [DEA][C7] | −3.4058 | 1518.0 | 0.0133 |
| [DBA][C5] | −5.4771 | 2219.3 | 0.0207 |
| [DBA][C6] | −5.4156 | 2203.9 | 0.0179 |
| [DBA][C7] | −5.3421 | 2186.0 | 0.0176 |
| Ionic Liquid | A5 | A6 | SD |
|---|---|---|---|
| [DEA][C5] | 1.5326 | −0.0003 | 0.0132 |
| [DEA][C6] | 1.5335 | −0.0003 | 0.0081 |
| [DEA][C7] | 1.5376 | −0.0003 | 0.0120 |
| [DBA][C5] | 1.5557 | −0.0004 | 0.0265 |
| [DBA][C6] | 1.5583 | −0.0004 | 0.0276 |
| [DBA [C7] | 1.5623 | −0.0004 | 0.0309 |
| T/K | 10−4 α/K−1 | |||||
|---|---|---|---|---|---|---|
| [DEA][C5] | [DEA][C6] | [DEA][C7] | [DBA][C5] | [DBA][C6] | [DBA][C7] | |
| 293.15 | 7.4 | 7.4 | 7.7 | 8.9 | 8.9 | 9.0 |
| 303.15 | 7.5 | 7.4 | 7.7 | 9.0 | 9.0 | 9.1 |
| 313.15 | 7.6 | 7.5 | 7.8 | 9.1 | 9.1 | 9.2 |
| 323.15 | 7.6 | 7.6 | 7.9 | 9.1 | 9.2 | 9.2 |
| 333.15 | 7.7 | 7.6 | 7.9 | 9.2 | 9.2 | 9.3 |
| 343.15 | 7.7 | 7.7 | 8.0 | 9.3 | 9.3 | 9.4 |
| 353.15 | 7.8 | 7.7 | 8.0 | 9.4 | 9.4 | 9.5 |
| 363.15 | 7.9 | 7.8 | 8.1 | 9.5 | 9.5 | 9.6 |
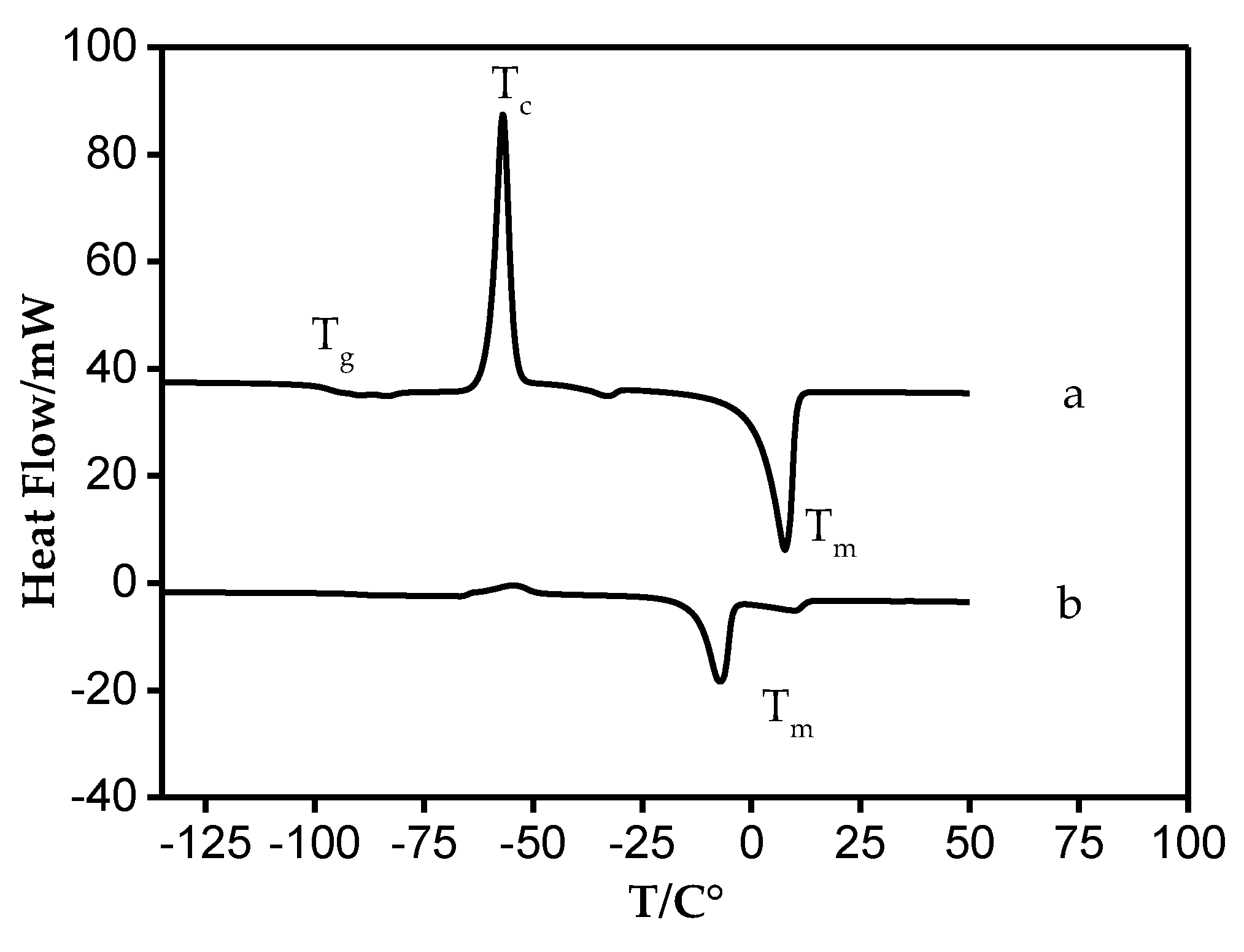
| Ionic Liquids | Vm | S° | Upot | Td | Tg | Tm | Tc |
|---|---|---|---|---|---|---|---|
| nm3 | J·K−1·mol−1 | kJ·mol−1 | K | °C | °C | °C | |
| [DEA][C5] | 0.3120 | 418.4 | 449.8 | 412.82 | −100.4 | −9.7 | −58.8 |
| [DEA][C6] | 0.3369 | 449.4 | 441.1 | 419.24 | −99.5 | −6.3 | −62.8 |
| [DEA][C7] | 0.3704 | 491.2 | 430.6 | 442.00 | −99.6 | 0.8 | −60.2 |
| [DBA][C5] | 0.4302 | 565.8 | 414.7 | 404.78 | - | −20.9 | - |
| [DBA][C6] | 0.4570 | 599.1 | 408.5 | 425.33 | - | −12.8 | - |
| [DBA][C7] | 0.4886 | 638.5 | 401.8 | 435.65 | - | −8.1 | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Othman Zailani, N.H.Z.; Yunus, N.M.; Ab Rahim, A.H.; Bustam, M.A. Thermophysical Properties of Newly Synthesized Ammonium-Based Protic Ionic Liquids: Effect of Temperature, Anion and Alkyl Chain Length. Processes 2020, 8, 742. https://doi.org/10.3390/pr8060742
Othman Zailani NHZ, Yunus NM, Ab Rahim AH, Bustam MA. Thermophysical Properties of Newly Synthesized Ammonium-Based Protic Ionic Liquids: Effect of Temperature, Anion and Alkyl Chain Length. Processes. 2020; 8(6):742. https://doi.org/10.3390/pr8060742
Chicago/Turabian StyleOthman Zailani, Nur Hidayah Zulaikha, Normawati M. Yunus, Asyraf Hanim Ab Rahim, and Mohamad Azmi Bustam. 2020. "Thermophysical Properties of Newly Synthesized Ammonium-Based Protic Ionic Liquids: Effect of Temperature, Anion and Alkyl Chain Length" Processes 8, no. 6: 742. https://doi.org/10.3390/pr8060742
APA StyleOthman Zailani, N. H. Z., Yunus, N. M., Ab Rahim, A. H., & Bustam, M. A. (2020). Thermophysical Properties of Newly Synthesized Ammonium-Based Protic Ionic Liquids: Effect of Temperature, Anion and Alkyl Chain Length. Processes, 8(6), 742. https://doi.org/10.3390/pr8060742





