Kaempferol Rhamnosides from Geranium sibiricum as Aldose Reductase Inhibitors and Their Content by HPLC Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Chemicals and Instruments
2.3. Extraction and Isolation of Flavonoids from G. sibiricum
2.4. Preparation of AR from Rat Lenses
2.5. Measurement of AR Inhibitory Activity
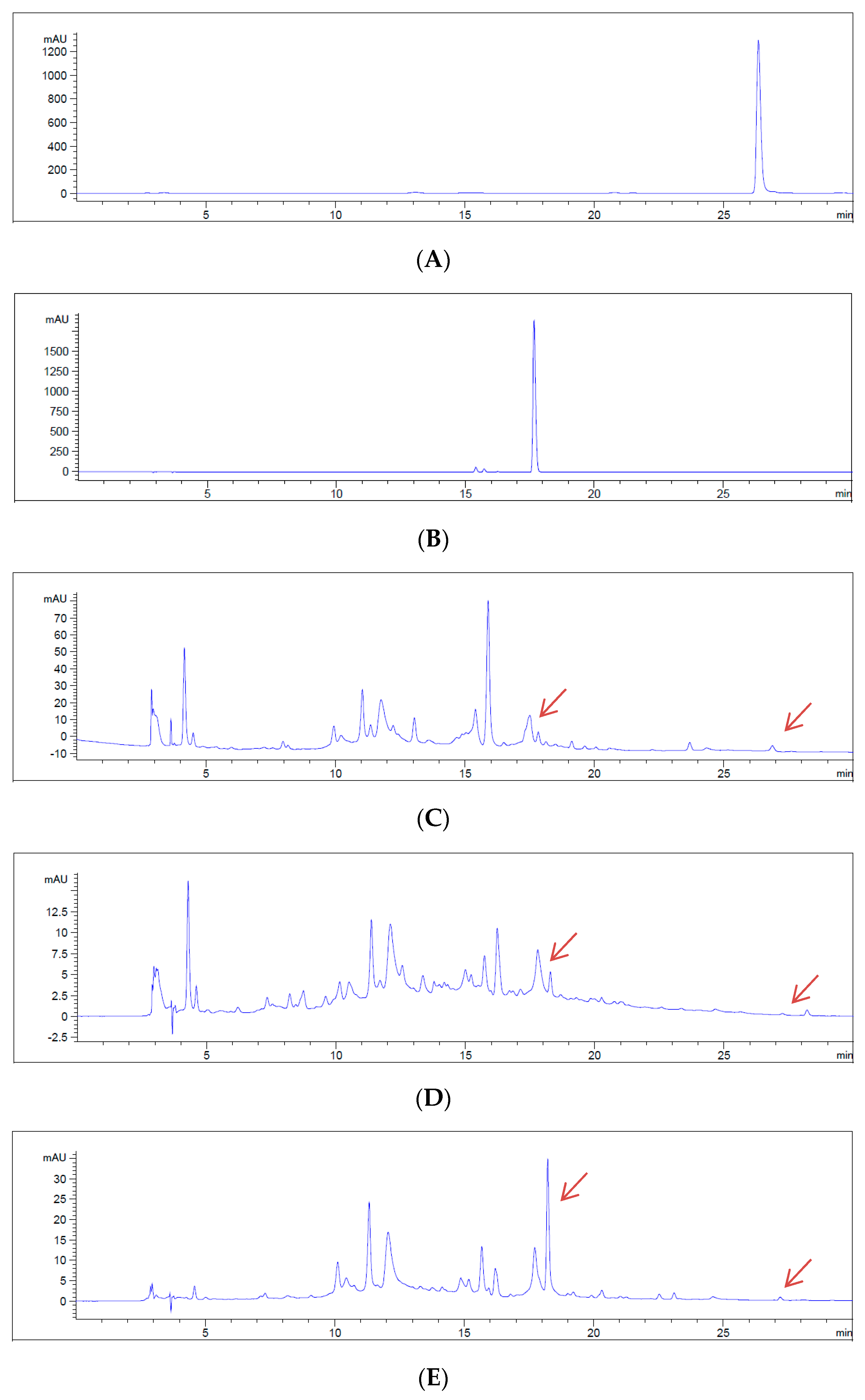
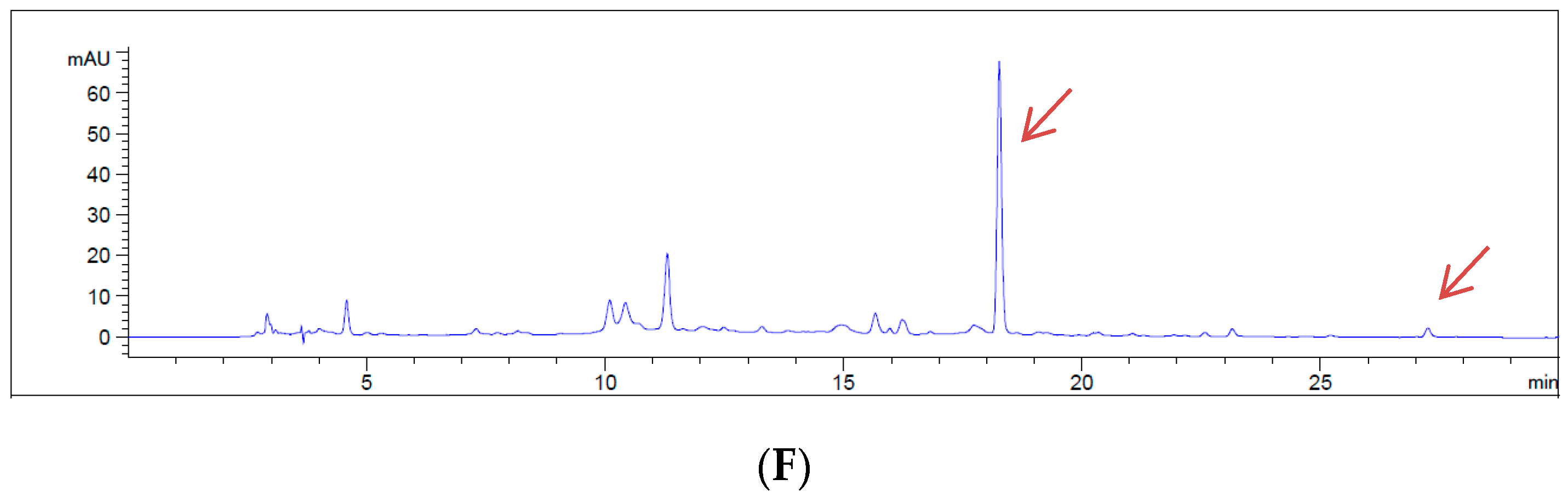
2.6. Sample Preparation and HPLC Conditions
2.7. Limit of Detection and Quantification (LOD and LOQ)
2.8. Calibration Curves
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Dunlop, M.E. Aldose reductase and the role of the polyol pathway in diabetic nephropathy. Kidney Int. 2000, 58, S3–S12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, W.H.; Martin, K.A.; Hwa, J. Aldose reductase, oxidative stress, and diabetic mellitus. Front. Pharmacol. 2012, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engerman, R.L.; Kern, T.S.; Larson, M.E. Nerve conduction and aldose reductase inhibition during 5 years of diabetes or galactosemia in dogs. Diabetologia 1994, 37, 141–144. [Google Scholar] [CrossRef]
- Hotta, N.; Akanuma, Y.; Kawamori, R.; Matsuoka, K.; Oka, Y.; Shichiri, M.; Toyota, T.; Nakashima, M.; Yoshimura, I.; Sakamoto, N.; et al. Long-term clinical effects of epalrestat, an aldose reductase inhibitor, on diabetic peripheral neuropathy: The 3-year, multicenter, comparative aldose reductase inhibitor-diabetes complications trial. Diabetes Care 2006, 29, 1538–1544. [Google Scholar] [CrossRef] [Green Version]
- Drel, V.; Pacher, P.; Ali, T.K.; Shin, J.; Julius, U.; El-Remessy, A.B.; Obrosova, I.G. Aldose reductase inhibitor fidarestat counteracts diabetes-associated cataract formation, retinal oxidative-nitrosative stress, glial activation, and apoptosis. Int. J. Mol. Med. 2008, 21, 667–676. [Google Scholar] [CrossRef] [Green Version]
- Schemmel, K.E.; Padiyara, R.S.; D’Souza, J.J. Aldose reductase inhibitors in the treatment of diabetic peripheral neuropathy: A review. J. Diabetes Its Complicat. 2010, 24, 354–360. [Google Scholar] [CrossRef]
- De la Fuente, J.A.; Manzanaro, S. Aldose reductase inhibitors from natural resources. Nat. Prod. Rep. 2003, 20, 243–251. [Google Scholar] [CrossRef]
- Lee, J.; Rodriguez, J.P.; Lee, K.H.; Park, J.Y.; Kang, K.; Hahm, D.-H.; Huh, C.K.; Lee, S.C.; Lee, S. Determination of flavonoids from Cirsium japonicum var. maackii and their inhibitory activities against aldose reductase. Appl. Biol. Chem. 2017, 60, 487–496. [Google Scholar] [CrossRef]
- Lee, J.; Rodriguez, J.P.; Quilantang, N.G.; Lee, M.-H.; Cho, E.J.; Jacinto, S.D.; Lee, S. Determination of flavonoids from Perilla frutescens var. japonica seeds and their inhibitory effect on aldose reductase. Appl. Biol. Chem. 2017, 60, 155–162. [Google Scholar] [CrossRef]
- Rodriguez, J.P.; Lee, Y.K.; Woo, D.G.; Shim, J.S.; Geraldino, P.J.L.; Jacinto, S.D. Flavonoids from Cirsium japonicum var. maackii pappus as inhibitors of aldose reductase and their simultaneous determination. Chem. Pap. 2017, 72, 81–88. [Google Scholar] [CrossRef]
- Alim, Z.; Kilinc, N.; Sengul, B.; Beydemir, S. Mechanism of capsaicin inhibition of aldose reductase activity. J. Biochem. Mol. Toxicol. 2017, 31, e21898. [Google Scholar] [CrossRef]
- Demir, Y.; Işık, M.; Gulçin, I.; Beydemir, Ş. Phenolic compounds inhibit the aldose reductase enzyme from the sheep kidney. J. Biochem. Mol. Toxicol. 2017, 31, e21936. [Google Scholar] [CrossRef]
- Demir, Y.; Taslimi, P.; Ozaslan, M.S.; Oztaskin, N.; Çetinkaya, Y.; Gulçin, I.; Beydemir, Ş.; Goksu, S. Antidiabetic potential: In vitro inhibition effects of bromophenol and diarylmethanones derivatives on metabolic enzymes. Arch. Pharm. Chem. Life Sci. 2018, 351, 1800263. [Google Scholar] [CrossRef] [PubMed]
- Fiz, O.; Vargas, P.; Alarcón, M.; Aedo, C.; García, J.L.; Aldasoro, J.J. Phylogeny and historical biogeography of Geraniaceae in relation to climate changes and pollination ecology. Syst. Bot. 2008, 33, 326–342. [Google Scholar] [CrossRef] [Green Version]
- Abraham, Y.; Elbaum, R. Hygroscopic movements in Geraniaceae: The structural variations that are responsible for coiling or bending. New Phytol. 2013, 199, 584–594. [Google Scholar] [CrossRef]
- Ávila, M.B.; De Lúcio, J.A.G.; Mendoza, N.V.; González, C.V.; De la Arciniega, M.O.; Vargas, G.A. Chapter 5. Geranium Species as Antioxidants. In Oxidative Stress and Chronic Degenerative Diseases—A Role for Antioxidants; IntechOpen: London, UK, 2013; pp. 113–129. [Google Scholar]
- Hayman, S.; Kinoshita, J.H. Isolation and properties of lens aldose reductase. J. Biol. Chem. 1965, 240, 877–882. [Google Scholar] [PubMed]
- Kuçukislamoglu, M.; Yayli, N.; Senturk, H.B.; Genc, H. Flavonol glycosides from Consolida armeniaca. Turk. J. Chem. 2000, 24, 191–197. [Google Scholar]
- Valente, L.M.M.; Bizarri, C.; Liechocki, S.; Barboza, R.; Da Paixão, D.; Almeida, M.B.S.; Benevides, P.J.C.; Magalhães, A.; Siani, A.C. Kaempferitrin from Uncaria guianensis (Rubiaceae) and its potential as a chemical marker for the species. J. Braz. Chem. Soc. 2009, 20, 1041–1045. [Google Scholar] [CrossRef]
- Serkedjieva, J.; Ivancheva, S. Antiherpes virus activity of extracts from the medicinal plant Geranium sanguineum L. J. Ethnopharmacol. 1998, 64, 59–68. [Google Scholar] [CrossRef]
- Ben Jemia, M.; Wannes, W.A.; Ouchikh, O.; Bruno, M.; Kchouck, M.E. Antioxidant activity of Tunisian Geranium robertianum L. (Geraniaceae). Nat. Prod. Res. 2013, 2013, 2076–2083. [Google Scholar] [CrossRef]
- Graça, V.C.; Barros, L.; Calhelha, R.C.; Dias, M.I.; Carvalho, A.M.; Santos-Buelga, C.; Santos, P.F.; Ferreira, I.C.F.R. Chemical characterization and bioactive properties of aqueous and organic extracts of Geranium robertianum L. Food Funct. 2016, 7, 3807–3814. [Google Scholar] [CrossRef] [PubMed]
- Neagu, E.; Paun, G.; Constantin, D.; Radu, G.L. Cytostatic activity of Geranium robertianum L. extracts processed by membrane procedures. Arab. J. Chem. 2017, 10, S2547–S2553. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Lee, D.G.; Rodriguez, J.P.; Park, J.Y.; Cho, E.J.; Jacinto, S.D.; Lee, S. Determination of flavonoids in Acer okamotoanum and their aldose reductase inhibitory activities. Hortic. Environ. Biotechnol. 2018, 59, 131–137. [Google Scholar] [CrossRef]
- Quilantang, N.G.; Ryu, S.H.; Park, S.H.; Byun, J.S.; Chun, J.S.; Lee, J.S.; Rodriguez, J.P.; Yun, Y.-S.; Jacinto, S.D.; Lee, S. Inhibitory activity of methanol extracts from different colored flowers on aldose reductase and HPLC—UV analysis of quercetin. Hortic. Environ. Biotechnol. 2018, 59, 899–907. [Google Scholar] [CrossRef]
- Shim, J.-U.; Lim, K.-T. Anti-oxidative and anti-proliferative character of glycoprotein isolated from Geranium sibiricum Linne in Chang liver cells. Environ. Toxicol. Pharmacol. 2008, 26, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.-U.; Oh, P.-S.; Lim, K.-T. Anti-inflammatory activity of ethanol extract from Geranium sibiricum Linne. J. Ethnopharmacol. 2009, 126, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Zu, Y.; Fu, Y.-J.; Kong, Y.; Zhao, J.; Li, X.; Li, J.; Wink, M.; Efferth, T. Antioxidant activities and xanthine oxidase inhibitory effects of extracts and main polyphenolic compounds obtained from Geranium sibiricum L. J. Agric. Food Chem. 2010, 58, 4737–4743. [Google Scholar] [CrossRef]
- Boisvert, W.A.; Yu, M.; Choi, Y.; Jeong, G.H.; Zhang, Y.-L.; Cho, S.; Choi, C.; Lee, S.; Lee, B.-H. Hair growth-promoting effect of Geranium sibiricum extract in human dermal papilla cells and C57BL/6 mice. BMC Complement. Altern. Med. 2017, 17, 109. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.H.; Li, Y.Y.; Li, L.J.; Liang, L.Y.; Shen, Y.M. Anti-inflammatory activities of fractions from Geranium nepalense and related polyphenols. Drug Discov. Ther. 2012, 6, 194–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
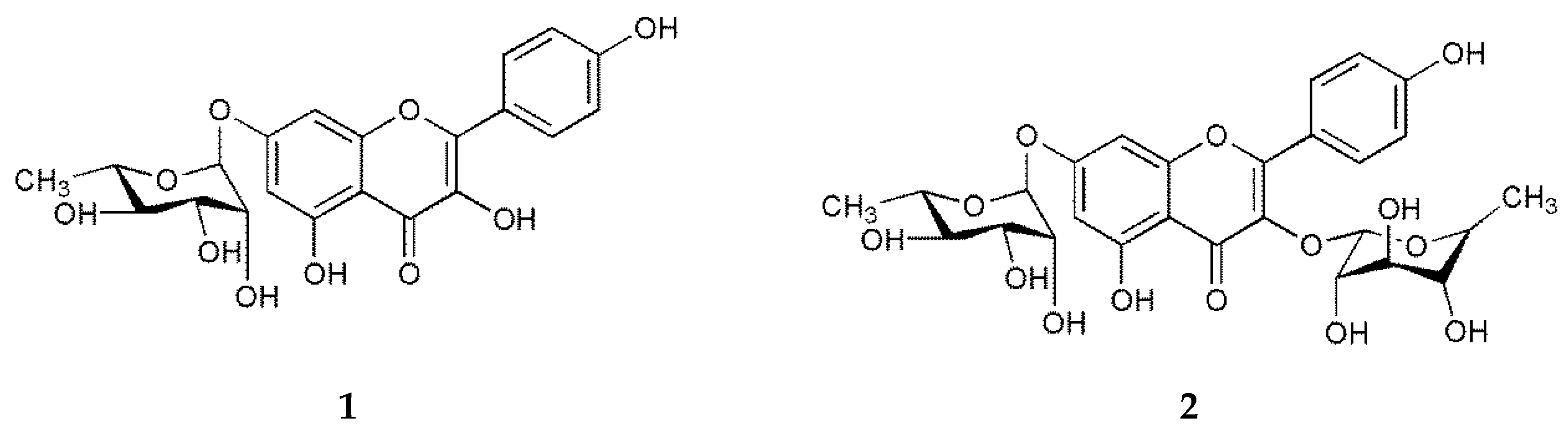
| No. | 1 | 2 | ||
|---|---|---|---|---|
| δH | δc | δH | δc | |
| 2 | - | 155.7 | - | 157.5 |
| 3 | - | 136.0 | - | 134.4 |
| 4 | - | 176.1 | - | 178.4 |
| 5 | - | 160.4 | - | 161.9 |
| 6 | 6.42 | 98.8 | 6.35 | 100.8 |
| 7 | - | 161.4 | - | 162.5 |
| 8 | 6.83 | 94.3 | 6.67 | 95.2 |
| 9 | - | 147.5 | - | 156.1 |
| 10 | - | 104.7 | - | 107.2 |
| 1’ | - | 121.5 | - | 121.3 |
| 2’,6’ | 8.09 | 129.6 | 7.78 | 131.4 |
| 3’,5’ | 6.93 | 115.5 | 6.93 | 116.5 |
| 4’ | - | 159.4 | - | 161.4 |
| 3-O-Rh 1’’ | - | - | 5.32 | 100.7 |
| 2’’ | - | - | 3.87 | 70.9 |
| 3’’ | - | - | 3.62 | 71.2 |
| 4’’ | - | - | 3.55 | 72.8 |
| 5’’ | - | - | 3.18 | 70.7 |
| 6’’ | - | - | 0.82 | 18.9 |
| 7-O-Rh 1’’’ | 5.54 | 98.4 | 5.55 | 99.2 |
| 2’’’ | - | 70.1 | 3.87 | 70.6 |
| 3’’’ | - | 70.2 | 3.65 | 71.3 |
| 4’’’ | - | 71.6 | 3.32 | 71.9 |
| 5’’’ | - | 69.8 | 3.45 | 70.4 |
| 6’’’ | 1.13 | 17.9 | 1.15 | 18.4 |
| Compound | Linear Range (µg/mL) | Linear Regression Equation a | Correlation Coefficient (r2 b) | LOD (µg/mL) | LOQ (µg/mL) | |
|---|---|---|---|---|---|---|
| Y = ax − b | ||||||
| S (a) | σ (b) | |||||
| 1 | 0.01–1.00 | 16,047.7 | 45.833 | 0.9990 | 0.001 | 0.003 |
| 2 | 0.01–1.00 | 1,652.4 | 83.422 | 0.9992 | 0.001 | 0.003 |
| Species | Conc. (µg/mL) | Inhibition a (%) | IC50 b (µg/mL) |
|---|---|---|---|
| Geranium eriostemon var. reinii | 10 | 87.2 | 3.4 ± 0.3 |
| 1 | 40.1 | ||
| 0.1 | 27.8 | ||
| G. koreanum | 10 | 45.9 | >10 |
| G. kunthii | 10 | 84.5 | 4.2 ± 0.3 |
| 1 | 32.6 | ||
| 0.1 | 24.6 | ||
| G. nepalense subsp. thunbergii | 10 | 85.6 | 2.7 ± 0.1 |
| 1 | 44.9 | ||
| 0.1 | 34.2 | ||
| G. sibiricum | 10 | 70.6 | 2.4 ± 0.1 |
| 1 | 34.8 | ||
| 0.1 | 11.8 | ||
| G. wilfordii | 10 | 33.7 | >10 |
| TMG c | 10 | 92 | 0.25 ± 0.0 |
| 1 | 67.4 | ||
| 0.1 | 31 |
| Fractions | Conc. (µg/mL) | Inhibition a (%) | IC50 b (µg/mL) |
|---|---|---|---|
| n-hexane fraction | 10 | 46 | >10 |
| CHCl3 fraction | 10 | 48.1 | >10 |
| EtOAc fraction | 10 | 71.7 | 0.41 ± 0.04 |
| 1 | 67.4 | ||
| 0.1 | 33.2 | ||
| n-BuOH fraction | 10 | 81.8 | 0.79 ± 0.06 |
| 1 | 51.9 | ||
| 0.1 | 25.1 | ||
| TMG c | 10 | 92 | 0.25 ± 0.03 |
| 1 | 67.4 | ||
| 0.1 | 31 |
| Compound | Conc. (μg/mL) | AR inhibition a (%) | IC50 b | |
|---|---|---|---|---|
| (μg/mL) | (μM) | |||
| KR | 10 | 80.37 | 0.53 ± 0.0 | 1.23 ± 0.0 |
| 1 | 68.1 | |||
| 0.1 | 24.54 | |||
| KD | 10 | 96.93 | 0.32 ± 0.0 | 0.55 ± 0.1 |
| 1 | 60.74 | |||
| 0.1 | 36.81 | |||
| TMG c | 10 | 92 | 0.25 ± 0.0 | 0.74 ± 0.0 |
| 1 | 67.4 | |||
| 0.1 | 31 | |||
| Species | Content (mg/g Extract) | |
|---|---|---|
| KR | KD | |
| Geranium eriostemon var. reinii | 4.78 ± 0.05 | 0.04 ± 0.02 |
| G. koreanum | 1.33 ± 0.07 | 1.50 ± 0.10 |
| G. kunthii | ND | ND |
| G. nepalense subsp. thunbergii | 23.90 ± 0.50 | 0.14 ± 0.05 |
| G. sibiricum | 28.10 ± 0.40 | 2.20 ± 0.20 |
| G. wilfordii | ND | ND |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quilantang, N.G.; Choi, K.; Lee, B.-H.; Lee, S. Kaempferol Rhamnosides from Geranium sibiricum as Aldose Reductase Inhibitors and Their Content by HPLC Analysis. Processes 2020, 8, 694. https://doi.org/10.3390/pr8060694
Quilantang NG, Choi K, Lee B-H, Lee S. Kaempferol Rhamnosides from Geranium sibiricum as Aldose Reductase Inhibitors and Their Content by HPLC Analysis. Processes. 2020; 8(6):694. https://doi.org/10.3390/pr8060694
Chicago/Turabian StyleQuilantang, Norman G., Kyung Choi, Bog-Hieu Lee, and Sanghyun Lee. 2020. "Kaempferol Rhamnosides from Geranium sibiricum as Aldose Reductase Inhibitors and Their Content by HPLC Analysis" Processes 8, no. 6: 694. https://doi.org/10.3390/pr8060694
APA StyleQuilantang, N. G., Choi, K., Lee, B.-H., & Lee, S. (2020). Kaempferol Rhamnosides from Geranium sibiricum as Aldose Reductase Inhibitors and Their Content by HPLC Analysis. Processes, 8(6), 694. https://doi.org/10.3390/pr8060694






