The Evolution of Cell Free Biomanufacturing
Abstract
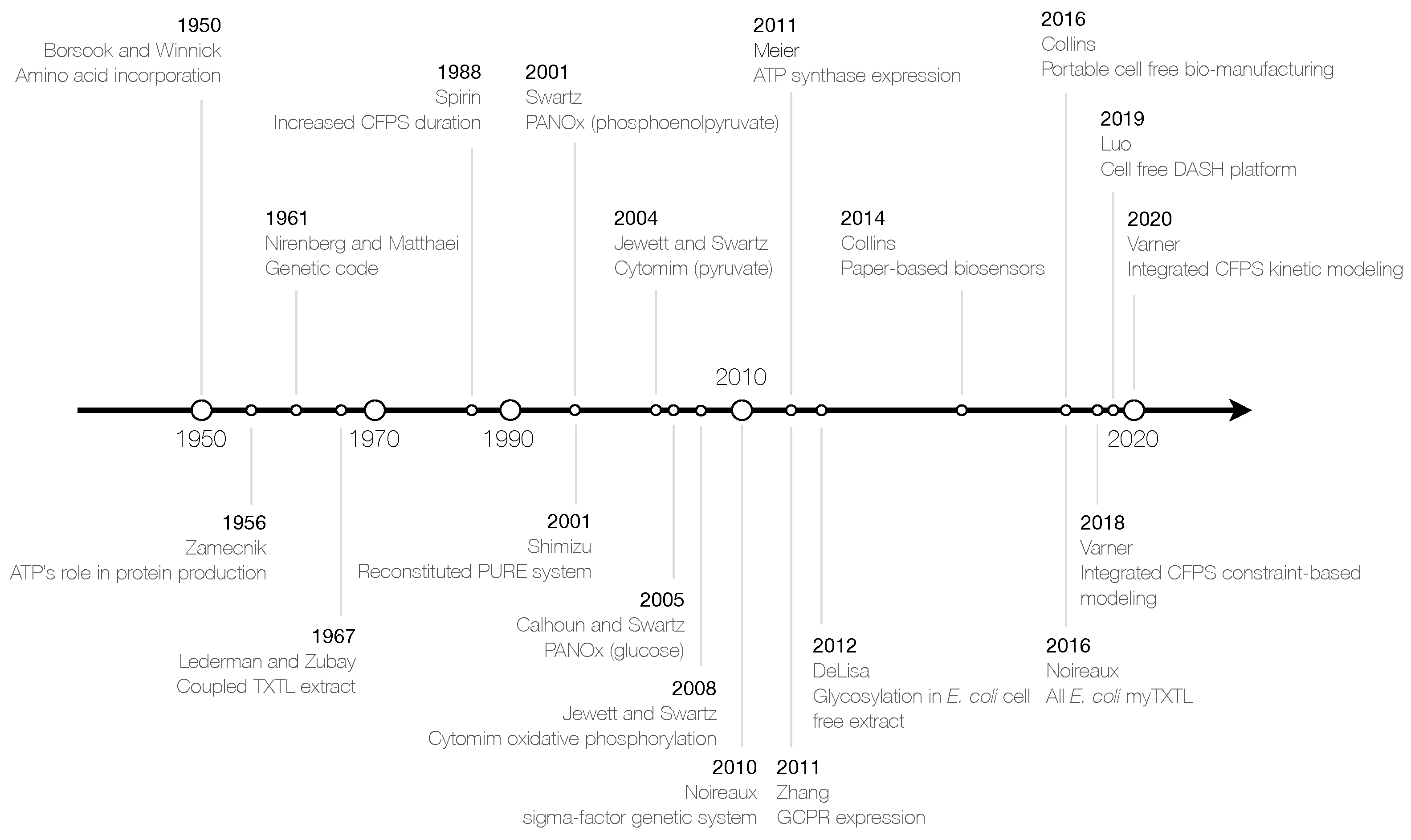
1. Introduction
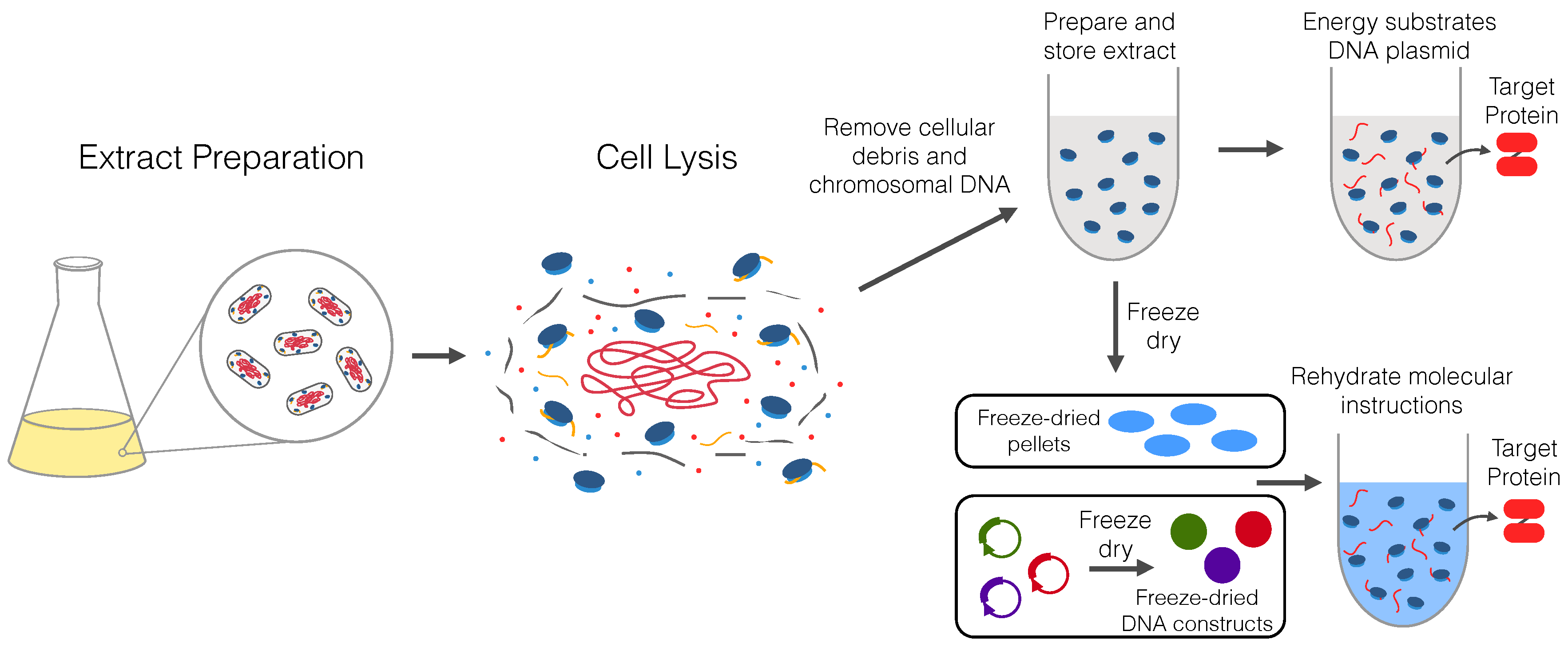
2. Origin and Preparation of Cell-Free Extracts
3. Applications of Cell-Free Technologies
3.1. Cell-Free Production of Biologics and Specialized Proteins
3.2. Cell-Free Systems in Synthetic Biology
3.3. Cell-Free Metabolic Engineering
4. Mathematical Modeling of Cell-Free Systems
4.1. Cell-Free Transcription and Translation Models
4.2. Metabolic Modeling Frameworks
4.3. Emergence of Integrated Cell-Free Models
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CFPS | cell-free protein synthesis |
| TXTL | transcription and translation |
| DNA | deoxyribonucleic acid |
| RNA | ribonucleic acid |
| NMP | nucleoside monophosphate |
| NTP | nucleoside triphosphate |
| ATP | adenosine triphosphate |
| GTP | guanosine triphosphate |
| NAD | nicotinamide adenine dinucleotide |
| CoA | coenzyme A |
| mRNA | messenger RNA |
| tRNA | transfer RNA |
| PURE | Protein synthesis Using Recombinant Elements |
| ODE | ordinary differential equation |
| CFME | cell-free metabolic engineering |
| FBA | flux balance analysis |
| MFA | metabolic flux analysis |
| ME | metabolic engineering |
| PCR | polymerase chain reaction |
References
- Albayrak, C.; Jones, K.; Swartz, J.R. Broadening horizons and teaching basic biology through cell-free synthesis of green fluorescent protein in a high school laboratory course. J. Sci. Educ. Technol. 2013, 22, 963–973. [Google Scholar] [CrossRef]
- Collias, D.; Marshall, R.; Collins, S.P.; Beisel, C.L.; Noireaux, V. An educational module to explore CRISPR technologies with a cell-free transcription-translation system. Synth. Biol. 2019, 4, ysz005. [Google Scholar] [CrossRef]
- Gregorio, N.E.; Levine, M.Z.; Oza, J.P. A user’s guide to cell-free protein synthesis. Methods Protoc. 2019, 2, 24. [Google Scholar] [CrossRef] [PubMed]
- Borsook, H. Protein turnover and incorporation of labeled amino acids into tissue proteins in vivo and in vitro. Physiol. Rev. 1950, 30, 206–219. [Google Scholar] [CrossRef]
- Winnick, T. Incorporation of labeled amino acids into the protein of embryonic and tumor tissue homogenates. Fed. Proc. 1950, 9, 247. [Google Scholar]
- Gale, E.F.; Folkes, J.P. Effect of nucleic acids on protein synthesis and amino-acid incorporation in disrupted staphylococcal cells. Nature 1954, 173, 1223–1227. [Google Scholar] [CrossRef]
- Hoagland, M.B.; Keller, E.B.; Zamecnik, P.C. Enzymatic carboxyl activation of amino acids. J. Biol. Chem. 1956, 218, 345–358. [Google Scholar]
- Matthaei, J.H.; Nirenberg, M.W. Characteristics and stabilization of DNAase-sensitive protein synthesis in E. coli extracts. Proc. Natl. Acad. Sci. USA 1961, 47, 1580. [Google Scholar] [CrossRef]
- Nirenberg, M.W.; Matthaei, J.H. The dependence of cell-free protein synthesis in E. coli upon naturally occurring or synthetic polyribonucleotides. Proc. Natl. Acad. Sci. USA 1961, 47, 1588–1602. [Google Scholar] [CrossRef]
- Lederman, M.; Zubay, G. DNA-directed peptide synthesis I. A comparison of T2 and Escherichia coli DNA-directed peptide synthesis in two cell-free systems. Biochim. Biophys. Acta (BBA) Nucleic Acids Protein Synth. 1967, 149, 253–258. [Google Scholar] [CrossRef]
- Spirin, A.; Baranov, V.; Ryabova, L.; Ovodov, S.; Alakhov, Y. A continuous cell-free translation system capable of producing polypeptides in high yield. Science 1988, 242, 1162–1164. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.M.; Swartz, J.R. Regeneration of adenosine triphosphate from glycolytic intermediates for cell-free protein synthesis. Biotechnol. Bioeng. 2001, 74, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Jewett, M.C.; Swartz, J.R. Mimicking the Escherichia coli cytoplasmic environment activates long-lived and efficient cell-free protein synthesis. Biotechnol. Bioeng. 2004, 86, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Jewett, M.C.; Swartz, J.R. Substrate replenishment extends protein synthesis with an in vitro translation system designed to mimic the cytoplasm. Biotechnol. Bioeng. 2004, 87, 465–471. [Google Scholar] [CrossRef]
- Jewett, M.C.; Calhoun, K.A.; Voloshin, A.; Wuu, J.J.; Swartz, J.R. An integrated cell-free metabolic platform for protein production and synthetic biology. Mol. Syst. Biol. 2008, 4, 220. [Google Scholar] [CrossRef]
- Calhoun, K.A.; Swartz, J.R. Energizing cell-free protein synthesis with glucose metabolism. Biotechnol. Bioeng. 2005, 90, 606–613. [Google Scholar] [CrossRef]
- Garamella, J.; Marshall, R.; Rustad, M.; Noireaux, V. The All E. coli TX-TL Toolbox 2.0: A Platform for Cell-Free Synthetic Biology. ACS Synth. Biol. 2016, 5, 344–355. [Google Scholar] [CrossRef]
- Shin, J.; Noireaux, V. Efficient cell-free expression with the endogenous E. coli RNA polymerase and sigma factor 70. J. Biol. Eng. 2010, 4, 8. [Google Scholar] [CrossRef]
- Shin, J.; Noireaux, V. An E. coli cell-free expression toolbox: Application to synthetic gene circuits and artificial cells. ACS Synth. Biol. 2012, 1, 29–41. [Google Scholar] [CrossRef]
- Rosenblum, G.; Cooperman, B.S. Engine out of the chassis: Cell-free protein synthesis and its uses. FEBS Lett. 2014, 588, 261–268. [Google Scholar] [CrossRef]
- Shimizu, Y.; Inoue, A.; Tomari, Y.; Suzuki, T.; Yokogawa, T.; Nishikawa, K.; Ueda, T. Cell-free translation reconstituted with purified components. Nat. Biotechnol. 2001, 19, 751. [Google Scholar] [CrossRef]
- Zhou, Y.; Asahara, H.; Gaucher, E.A.; Chong, S. Reconstitution of translation from Thermus thermophilus reveals a minimal set of components sufficient for protein synthesis at high temperatures and functional conservation of modern and ancient translation components. Nucleic Acids Res. 2012, 40, 7932–7945. [Google Scholar] [CrossRef] [PubMed]
- Jackson, K.; Jin, S.; Fan, Z.H. Optimization of a miniaturized fluid array device for cell-free protein synthesis. Biotechnol. Bioeng. 2015, 112, 2459–2467. [Google Scholar] [CrossRef] [PubMed]
- Niwa, T.; Sasaki, Y.; Uemura, E.; Nakamura, S.; Akiyama, M.; Ando, M.; Sawada, S.; Mukai, S.A.; Ueda, T.; Taguchi, H.; et al. Comprehensive study of liposome-assisted synthesis of membrane proteins using a reconstituted cell-free translation system. Sci. Rep. 2015, 5, 18025. [Google Scholar] [CrossRef] [PubMed]
- Murakami, S.; Matsumoto, R.; Kanamori, T. Constructive approach for synthesis of a functional IgG using a reconstituted cell-free protein synthesis system. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Gessesse, B.; Nagaike, T.; Nagata, K.; Shimizu, Y.; Ueda, T. G-protein coupled receptor protein synthesis on a lipid bilayer using a reconstituted cell-free protein synthesis system. Life 2018, 8, 54. [Google Scholar] [CrossRef]
- Li, J.; Gu, L.; Aach, J.; Church, G.M. Improved cell-free RNA and protein synthesis system. PLoS ONE 2014, 9, e106232. [Google Scholar] [CrossRef]
- Swartz, J.R. Expanding biological applications using cell-free metabolic engineering: An overview. Metab. Eng. 2018, 50, 156–172. [Google Scholar] [CrossRef]
- Hillebrecht, J.R.; Chong, S. A comparative study of protein synthesis in in vitro systems: From the prokaryotic reconstituted to the eukaryotic extract-based. BMC Biotechnol. 2008, 8, 58. [Google Scholar] [CrossRef]
- Shrestha, P.; Holland, T.M.; Bundy, B.C. Streamlined extract preparation for Escherichia coli-based cell-free protein synthesis by sonication or bead vortex mixing. Biotechniques 2012, 53, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Zubay, G. In vitro synthesis of protein in microbial systems. Annu. Rev. Genet. 1973, 7, 267–287. [Google Scholar] [CrossRef] [PubMed]
- Pratt, J. Coupled transcription-translation in prokaryotic cell-free systems. In Transcription and Translation: A Practical Approach; IRL Press: New York, NY, USA, 1984; pp. 179–209. [Google Scholar]
- Kigawa, T.; Yabuki, T.; Matsuda, N.; Matsuda, T.; Nakajima, R.; Tanaka, A.; Yokoyama, S. Preparation of Escherichia coli cell extract for highly productive cell-free protein expression. J. Struct. Funct. Genom. 2004, 5, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.V.; Zawada, J.F.; Swartz, J.R. Streamlining Escherichia coli S30 extract preparation for economical cell-free protein synthesis. Biotechnol. Prog. 2005, 21, 460–465. [Google Scholar] [CrossRef]
- Kim, T.W.; Keum, J.W.; Oh, I.S.; Choi, C.Y.; Park, C.G.; Kim, D.M. Simple procedures for the construction of a robust and cost-effective cell-free protein synthesis system. J. Biotechnol. 2006, 126, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Didovyk, A.; Tonooka, T.; Tsimring, L.; Hasty, J. Rapid and scalable preparation of bacterial lysates for cell-free gene expression. ACS Synth. Biol. 2017, 6, 2198–2208. [Google Scholar] [CrossRef] [PubMed]
- Calhoun, K.A.; Swartz, J.R. Total amino acid stabilization during cell-free protein synthesis reactions. J. Biotechnol. 2006, 123, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Michel-Reydellet, N.; Calhoun, K.; Swartz, J. Amino acid stabilization for cell-free protein synthesis by modification of the Escherichia coli genome. Metab. Eng. 2004, 6, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Dudley, Q.M.; Anderson, K.C.; Jewett, M.C. Cell-free mixing of Escherichia coli crude extracts to prototype and rationally engineer high-titer mevalonate synthesis. ACS Synth. Biol. 2016, 5, 1578–1588. [Google Scholar] [CrossRef]
- Silverman, A.D.; Kelley-Loughnane, N.; Lucks, J.B.; Jewett, M.C. Deconstructing cell-free extract preparation for in vitro activation of transcriptional genetic circuitry. ACS Synth. Biol. 2018, 8, 403–414. [Google Scholar] [CrossRef]
- Pardee, K.; Slomovic, S.; Nguyen, P.Q.; Lee, J.W.; Donghia, N.; Burrill, D.; Ferrante, T.; McSorley, F.R.; Furuta, Y.; Vernet, A.; et al. Portable, On-Demand Biomolecular Manufacturing. Cell 2016, 167, 248–259. [Google Scholar] [CrossRef]
- Carlson, E.D.; Gan, R.; Hodgman, C.E.; Jewett, M.C. Cell-free protein synthesis: Applications come of age. Biotechnol. Adv. 2012, 30, 1185–1194. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Welsh, J.P.; Swartz, J.R. Production and stabilization of the trimeric influenza hemagglutinin stem domain for potentially broadly protective influenza vaccines. Proc. Natl. Acad. Sci. USA 2014, 111, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Goerke, A.R.; Swartz, J.R. Development of cell-free protein synthesis platforms for disulfide bonded proteins. Biotechnol. Bioeng. 2008, 99, 351–367. [Google Scholar] [CrossRef] [PubMed]
- Ng, P.P.; Jia, M.; Patel, K.G.; Brody, J.D.; Swartz, J.R.; Levy, S.; Levy, R. A vaccine directed to B cells and produced by cell-free protein synthesis generates potent antilymphoma immunity. Proc. Natl. Acad. Sci. USA 2012, 109, 14526–14531. [Google Scholar] [CrossRef]
- Takai, K.; Sawasaki, T.; Endo, Y. Practical cell-free protein synthesis system using purified wheat embryos. Nat. Protoc. 2001, 5, 227. [Google Scholar] [CrossRef]
- Guarino, C.; DeLisa, M.P. A prokaryote-based cell-free translation system that efficiently synthesizes glycoproteins. Glycobiology 2012, 22, 596–601. [Google Scholar] [CrossRef]
- Jaroentomeechai, T.; Stark, J.C.; Natarajan, A.; Glasscock, C.J.; Yates, L.E.; Hsu, K.J.; Mrksich, M.; Jewett, M.C.; DeLisa, M.P. Single-pot glycoprotein biosynthesis using a cell-free transcription-translation system enriched with glycosylation machinery. Nat. Commun. 2018, 9, 2686. [Google Scholar] [CrossRef]
- Perez, J.G.; Stark, J.C.; Jewett, M.C. Cell-free synthetic biology: Engineering beyond the cell. Cold Spring Harb. Perspect. Biol. 2016, 8, a023853. [Google Scholar] [CrossRef]
- Matthies, D.; Haberstock, S.; Joos, F.; Dötsch, V.; Vonck, J.; Bernhard, F.; Meier, T. Cell-free expression and assembly of ATP synthase. J. Mol. Biol. 2011, 413, 593–603. [Google Scholar] [CrossRef]
- Wang, X.; Corin, K.; Baaske, P.; Wienken, C.J.; Jerabek-Willemsen, M.; Duhr, S.; Braun, D.; Zhang, S. Peptide surfactants for cell-free production of functional G protein-coupled receptors. Proc. Natl. Acad. Sci. USA 2011, 108, 9049–9054. [Google Scholar] [CrossRef]
- Shinoda, T.; Shinya, N.; Ito, K.; Ishizuka-Katsura, Y.; Ohsawa, N.; Terada, T.; Hirata, K.; Kawano, Y.; Yamamoto, M.; Tomita, T.; et al. Cell-free methods to produce structurally intact mammalian membrane proteins. Sci. Rep. 2016, 6, 30442. [Google Scholar] [CrossRef] [PubMed]
- Stark, J.C.; Jaroentomeechai, T.; Moeller, T.D.; Dubner, R.S.; Hsu, K.J.; Stevenson, T.C.; DeLisa, M.P.; Jewett, M.C. On-demand, cell-free biomanufacturing of conjugate vaccines at the point-of-care. bioRxiv 2019, 681841. [Google Scholar] [CrossRef]
- Jewett, M.C.; Fritz, B.R.; Timmerman, L.E.; Church, G.M. In vitro integration of ribosomal RNA synthesis, ribosome assembly, and translation. Mol. Syst. Biol. 2013, 9, 678. [Google Scholar] [CrossRef] [PubMed]
- Fritz, B.R.; Jewett, M.C. The impact of transcriptional tuning on in vitro integrated rRNA transcription and ribosome construction. Nucleic Acids Res. 2014, 42, 6774–6785. [Google Scholar] [CrossRef] [PubMed]
- Du, D.; van Veen, H.W.; Luisi, B.F. Assembly and operation of bacterial tripartite multidrug efflux pumps. Trends Microbiol. 2015, 23, 311–319. [Google Scholar] [CrossRef]
- Albayrak, C.; Swartz, J.R. Direct polymerization of proteins. ACS Synth. Biol. 2013, 3, 353–362. [Google Scholar] [CrossRef]
- Albayrak, C.; Swartz, J.R. Cell-free co-production of an orthogonal transfer RNA activates efficient site-specific non-natural amino acid incorporation. Nucleic Acids Res. 2013, 41, 5949–5963. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.W.; Des Soye, B.J.; Kwon, Y.C.; Kay, J.; Davis, R.G.; Thomas, P.M.; Majewska, N.I.; Chen, C.X.; Marcum, R.D.; Weiss, M.G.; et al. Cell-free protein synthesis from genomically recoded bacteria enables multisite incorporation of noncanonical amino acids. Nat. Commun. 2018, 9, 1203. [Google Scholar] [CrossRef]
- Qiu, J.; Swartz, J.R.; Georgiou, G. Expression of active human tissue-type plasminogen activator in Escherichia coli. Appl. Environ. Microbiol. 1998, 64, 4891–4896. [Google Scholar] [CrossRef]
- Yin, G.; Swartz, J.R. Enhancing multiple disulfide bonded protein folding in a cell-free system. Biotechnol. Bioeng. 2004, 86, 188–195. [Google Scholar] [CrossRef]
- Min, S.E.; Lee, K.H.; Park, S.W.; Yoo, T.H.; Oh, C.H.; Park, J.H.; Yang, S.Y.; Kim, Y.S.; Kim, D.M. Cell-free production and streamlined assay of cytosol-penetrating antibodies. Biotechnol. Bioeng. 2016, 113, 2107–2112. [Google Scholar] [CrossRef] [PubMed]
- Salehi, A.S.M.; Smith, M.T.; Bennett, A.M.; Williams, J.B.; Pitt, W.G.; Bundy, B.C. Cell-free protein synthesis of a cytotoxic cancer therapeutic: Onconase production and a just-add-water cell-free system. Biotechnol. J. 2015, 11, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Hartman, R.L.; McMullen, J.P.; Jensen, K.F. Deciding Whether To Go with the Flow: Evaluating the Merits of Flow Reactors for Synthesis. Angew. Chem. Int. Ed. 2011, 50, 7502–7519. [Google Scholar] [CrossRef] [PubMed]
- McQuade, D.T.; Seeberger, P.H. Applying Flow Chemistry: Methods, Materials, and Multistep Synthesis. J. Org. Chem. 2013, 78, 6384–6389. [Google Scholar] [CrossRef]
- Georgi, V.; Georgi, L.; Blechert, M.; Bergmeister, M.; Zwanzig, M.; Wüstenhagen, D.A.; Bier, F.F.; Jung, E.; Kubick, S. On-chip automation of cell-free protein synthesis: New opportunities due to a novel reaction mode. Lab Chip 2016, 16, 269–281. [Google Scholar] [CrossRef]
- Nge, P.N.; Rogers, C.I.; Woolley, A.T. Advances in Microfluidic Materials, Functions, Integration, and Applications. Chem. Rev. 2013, 113, 2550–2583. [Google Scholar] [CrossRef]
- Murphy, T.W.; Sheng, J.; Naler, L.B.; Feng, X.; Lu, C. On-chip manufacturing of synthetic proteins for point-of-care therapeutics. Microsyst. Nanoeng. 2019, 5, 1–12. [Google Scholar] [CrossRef]
- Timm, A.C.; Shankles, P.G.; Foster, C.M.; Doktycz, M.J.; Retterer, S.T. Toward microfluidic reactors for cell-free protein synthesis at the point-of-care. Small 2016, 12, 810–817. [Google Scholar] [CrossRef]
- Silverman, A.D.; Karim, A.S.; Jewett, M.C. Cell-free gene expression: An expanded repertoire of applications. Nat. Rev. Genet. 2019, 21, 151–170. [Google Scholar] [CrossRef]
- Voloshin, A.M.; Swartz, J.R. Efficient and scalable method for scaling up cell-free protein synthesis in batch mode. Biotechnol. Bioeng. 2005, 91, 516–521. [Google Scholar] [CrossRef]
- Swartz, J. Developing cell-free biology for industrial applications. J. Ind. Microbiol. Biotechnol. 2006, 33, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Zawada, J.F.; Yin, G.; Steiner, A.R.; Yang, J.; Naresh, A.; Roy, S.M.; Gold, D.S.; Heinsohn, H.G.; Murray, C.J. Microscale to manufacturing scale-up of cell-free cytokine production–a new approach for shortening protein production development timelines. Biotechnol. Bioeng. 2011, 108, 1570–1578. [Google Scholar] [CrossRef]
- Ogonah, O.W.; Polizzi, K.M.; Bracewell, D.G. Cell-free protein synthesis: A viable option for stratified medicines manufacturing? Curr. Opin. Chem. Eng. 2017, 18, 77–83. [Google Scholar] [CrossRef]
- Yin, G.; Garces, E.D.; Yang, J.; Zhang, J.; Tran, C.; Steiner, A.R.; Roos, C.; Bajad, S.; Hudak, S.; Penta, K.; et al. Aglycosylated antibodies and antibody fragments produced in a scalable in vitro transcription-translation system. MAbs 2012, 4, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Shen, L.; Wu, K.; Diehnelt, C.W.; Green, A.A. Low-cost detection of norovirus using paper-based cell-free systems and synbody-based viral enrichment. Synth. Biol. 2018, 3, ysy018. [Google Scholar] [CrossRef]
- Pardee, K.; Green, A.A.; Ferrante, T.; Cameron, D.E.; DaleyKeyser, A.; Yin, P.; Collins, J.J. Paper-based synthetic gene networks. Cell 2014, 159, 940–954. [Google Scholar] [CrossRef]
- Pardee, K.; Green, A.A.; Takahashi, M.K.; Braff, D.; Lambert, G.; Lee, J.W.; Ferrante, T.; Ma, D.; Donghia, N.; Fan, M.; et al. Rapid, low-cost detection of Zika virus using programmable biomolecular components. Cell 2016, 165, 1255–1266. [Google Scholar] [CrossRef]
- Gräwe, A.; Dreyer, A.; Vornholt, T.; Barteczko, U.; Buchholz, L.; Drews, G.; Ho, U.L.; Jackowski, M.E.; Kracht, M.; Lüders, J.; et al. A paper-based, cell-free biosensor system for the detection of heavy metals and date rape drugs. PLoS ONE 2019, 14, e0210940. [Google Scholar] [CrossRef]
- Morowitz, H.J. The completeness of molecular biology. ISR J. Med. Sci. 1984, 20, 750–753. [Google Scholar]
- Garenne, D.; Noireaux, V. Cell-free transcription–translation: Engineering biology from the nanometer to the millimeter scale. Curr. Opin. Biotechnol. 2019, 58, 19–27. [Google Scholar] [CrossRef]
- Ishikawa, K.; Sato, K.; Shima, Y.; Urabe, I.; Yomo, T. Expression of a cascading genetic network within liposomes. FEBS Lett. 2004, 576, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Noireaux, V.; Libchaber, A. A vesicle bioreactor as a step toward an artificial cell assembly. Proc. Natl. Acad. Sci. USA 2004, 101, 17669–17674. [Google Scholar] [CrossRef] [PubMed]
- Karzbrun, E.; Tayar, A.M.; Noireaux, V.; Bar-Ziv, R.H. Programmable on-chip DNA compartments as artificial cells. Science 2014, 345, 829–832. [Google Scholar] [CrossRef] [PubMed]
- Pereira de Souza, T.; Stano, P.; Luisi, P.L. The minimal size of liposome-based model cells brings about a remarkably enhanced entrapment and protein synthesis. ChemBioChem 2009, 10, 1056–1063. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wu, H.; Cui, M.; Lai, S.N.; Zheng, B. Long-lived protein expression in hydrogel particles: Towards artificial cells. Chem. Sci. 2018, 9, 4275–4279. [Google Scholar] [CrossRef] [PubMed]
- Tayar, A.M.; Karzbrun, E.; Noireaux, V.; Bar-Ziv, R.H. Synchrony and pattern formation of coupled genetic oscillators on a chip of artificial cells. Proc. Natl. Acad. Sci. USA 2017, 114, 11609–11614. [Google Scholar] [CrossRef]
- Niederholtmeyer, H.; Sun, Z.Z.; Hori, Y.; Yeung, E.; Verpoorte, A.; Murray, R.M.; Maerkl, S.J. Rapid cell-free forward engineering of novel genetic ring oscillators. Elife 2015, 4, e09771. [Google Scholar] [CrossRef]
- Karig, D.K.; Iyer, S.; Simpson, M.L.; Doktycz, M.J. Expression optimization and synthetic gene networks in cell-free systems. Nucleic Acids Res. 2011, 40, 3763–3774. [Google Scholar] [CrossRef]
- Martini, L.; Mansy, S.S. Measuring Riboswitch Activity In Vitro and in Artificial Cells with Purified Transcription–Translation Machinery. In Artificial Riboswitches; Humana Press: Totowa, NJ, USA, 2014; pp. 153–164. [Google Scholar]
- Adamala, K.P.; Martin-Alarcon, D.A.; Guthrie-Honea, K.R.; Boyden, E.S. Engineering genetic circuit interactions within and between synthetic minimal cells. Nat. Chem. 2017, 9, 431. [Google Scholar] [CrossRef]
- Hamada, S.; Yancey, K.G.; Pardo, Y.; Gan, M.; Vanatta, M.; An, D.; Hu, Y.; Derrien, T.L.; Ruiz, R.; Liu, P.; et al. Dynamic DNA material with emergent locomotion behavior powered by artificial metabolism. Sci. Robot. 2019, 4, eaaw3512. [Google Scholar] [CrossRef]
- Moore, S.J.; MacDonald, J.T.; Wienecke, S.; Ishwarbhai, A.; Tsipa, A.; Aw, R.; Kylilis, N.; Bell, D.J.; McClymont, D.W.; Jensen, K.; et al. Rapid acquisition and model-based analysis of cell-free transcription–translation reactions from nonmodel bacteria. Proc. Natl. Acad. Sci. USA 2018, 115, E4340–E4349. [Google Scholar] [CrossRef] [PubMed]
- Niederholtmeyer, H.; Xu, L.; Maerkl, S.J. Real-time mRNA measurement during an in vitro transcription and translation reaction using binary probes. ACS Synth. Biol. 2012, 2, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Damiati, S.; Mhanna, R.; Kodzius, R.; Ehmoser, E.K. Cell-free approaches in synthetic biology utilizing microfluidics. Genes 2018, 9, 144. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, R.; Noireaux, V.; Maeda, Y.T. Anomalous scaling of gene expression in confined cell-free reactions. Sci. Rep. 2018, 8, 7364. [Google Scholar] [CrossRef]
- Guo, W.; Sheng, J.; Feng, X. Mini-review: In vitro Metabolic Engineering for Biomanufacturing of High-value Products. Comput. Struct. Biotechnol. J. 2017, 15, 161–167. [Google Scholar] [CrossRef]
- Dudley, Q.M.; Karim, A.S.; Jewett, M.C. Cell-free metabolic engineering: Biomanufacturing beyond the cell. Biotechnol. J. 2015, 10, 69–82. [Google Scholar] [CrossRef]
- Opgenorth, P.H.; Korman, T.P.; Bowie, J.U. A synthetic biochemistry molecular purge valve module that maintains redox balance. Nat. Commun. 2014, 5, 4113. [Google Scholar] [CrossRef]
- Opgenorth, P.H.; Korman, T.P.; Bowie, J.U. A synthetic biochemistry module for production of bio-based chemicals from glucose. Nat. Chem. Biol. 2016, 12, 393–395. [Google Scholar] [CrossRef]
- Valliere, M.A.; Korman, T.P.; Woodall, N.B.; Khitrov, G.A.; Taylor, R.E.; Baker, D.; Bowie, J.U. A cell-free platform for the prenylation of natural products and application to cannabinoid production. Nat. Commun. 2019, 10, 565. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, C.; Zou, R.; Stephanopoulos, G.; Too, H.P. In Vitro Metabolic Engineering of Amorpha-4,11-diene Biosynthesis at Enhanced Rate and Specific Yield of Production. ACS Synth. Biol. 2017, 6, 1691–1700. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, C.; Zou, R.; Zhou, K.; Stephanopoulos, G.; Too, H.P. Statistical experimental design guided optimization of a one-pot biphasic multienzyme total synthesis of amorpha-4,11-diene. PLoS ONE 2013, 8, e79650. [Google Scholar] [CrossRef] [PubMed]
- Guterl, J.K.; Garbe, D.; Carsten, J.; Steffler, F.; Sommer, B.; Reiße, S.; Philipp, A.; Haack, M.; Rühmann, B.; Koltermann, A.; et al. Cell-free metabolic engineering: Production of chemicals by minimized reaction cascades. ChemSusChem 2012, 5, 2165–2172. [Google Scholar] [CrossRef] [PubMed]
- Krutsakorn, B.; Honda, K.; Ye, X.; Imagawa, T.; Bei, X.; Okano, K.; Ohtake, H. In vitro production of n-butanol from glucose. Metab. Eng. 2013, 20, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Bechtold, M.; Brenna, E.; Femmer, C.; Gatti, F.G.; Panke, S.; Parmeggiani, F.; Sacchetti, A. Biotechnological development of a practical synthesis of ethyl (S)-2-ethoxy-3-(p-methoxyphenyl) propanoate (EEHP): Over 100-fold productivity increase from yeast whole cells to recombinant isolated enzymes. Org. Process Res. Dev. 2011, 16, 269–276. [Google Scholar] [CrossRef]
- Zhu, Z.; Tam, T.K.; Sun, F.; You, C.; Zhang, Y.H.P. A high-energy-density sugar biobattery based on a synthetic enzymatic pathway. Nat. Commun. 2014, 5, 3026. [Google Scholar] [CrossRef]
- Dudley, Q.M.; Nash, C.J.; Jewett, M.C. Cell-free biosynthesis of limonene using enzyme-enriched Escherichia coli lysates. Synth. Biol. 2019, 4, ysz003. [Google Scholar] [CrossRef]
- Alonso-Gutierrez, J.; Kim, E.M.; Batth, T.S.; Cho, N.; Hu, Q.; Chan, L.J.G.; Petzold, C.J.; Hillson, N.J.; Adams, P.D.; Keasling, J.D.; et al. Principal component analysis of proteomics (PCAP) as a tool to direct metabolic engineering. Metab. Eng. 2015, 28, 123–133. [Google Scholar] [CrossRef]
- Yi, T.; Lim, H.J.; Lee, S.J.; Lee, K.H.; Kim, D.M. Synthesis of (R,R)-2,3-butanediol from starch in a hybrid cell-free reaction system. J. Ind. Eng. Chem. 2018, 67, 231–235. [Google Scholar] [CrossRef]
- Calhoun, K.A.; Swartz, J.R. An Economical Method for Cell-Free Protein Synthesis using Glucose and Nucleoside Monophosphates. Biotechnol. Prog. 2005, 21, 1146–1153. [Google Scholar] [CrossRef]
- Welsh, J.P.; Lu, Y.; He, X.S.; Greenberg, H.B.; Swartz, J.R. Cell-free production of trimeric influenza hemagglutinin head domain proteins as vaccine antigens. Biotechnol. Bioeng. 2012, 109, 2962–2969. [Google Scholar] [CrossRef]
- Boyer, M.E.; Stapleton, J.A.; Kuchenreuther, J.M.; Wang, C.W.; Swartz, J.R. Cell-free synthesis and maturation of [FeFe] hydrogenases. Biotechnol. Bioeng. 2008, 99, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Bogorad, I.W.; Lin, T.S.; Liao, J.C. Synthetic non-oxidative glycolysis enables complete carbon conservation. Nature 2013, 502, 693. [Google Scholar] [CrossRef]
- Bailey, J. Toward a science of metabolic engineering. Science 1991, 252, 1668–1675. [Google Scholar] [CrossRef] [PubMed]
- Karzbrun, E.; Shin, J.; Bar-Ziv, R.H.; Noireaux, V. Coarse-Grained Dynamics of Protein Synthesis in a Cell-Free System. Phys. Rev. Lett. 2011, 106. [Google Scholar] [CrossRef] [PubMed]
- Stögbauer, T.; Windhager, L.; Zimmer, R.; Rädler, J.O. Experiment and mathematical modeling of gene expression dynamics in a cell-free system. Integr. Biol. 2012, 4, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Nieß, A.; Failmezger, J.; Kuschel, M.; Siemann-Herzberg, M.; Takors, R. Experimentally Validated Model Enables Debottlenecking of in Vitro Protein Synthesis and Identifies a Control Shift under in Vivo Conditions. ACS Synth. Biol. 2017, 6, 1913–1921. [Google Scholar] [CrossRef]
- Gyorgy, A.; Murray, R.M. Quantifying resource competition and its effects in the TX-TL system. In Proceedings of the 2016 IEEE 55th Conference on Decision and Control (CDC), Las Vegas, NV, USA, 12–14 December 2016; pp. 3363–3368. [Google Scholar] [CrossRef]
- Lucks, J.B.; Qi, L.; Mutalik, V.K.; Wang, D.; Arkin, A.P. Versatile RNA-sensing transcriptional regulators for engineering genetic networks. Proc. Natl. Acad. Sci. USA 2011, 108, 8617–8622. [Google Scholar] [CrossRef]
- Brantl, S.; Wagner, E.G.H. Antisense RNA-mediated transcriptional attenuation: An in vitro study of plasmid pT181. Mol. Microbiol. 2000, 35, 1469–1482. [Google Scholar] [CrossRef]
- Chappell, J.; Takahashi, M.K.; Lucks, J.B. Creating small transcription activating RNAs. Nat. Chem. Biol. 2015, 11, 214–220. [Google Scholar] [CrossRef]
- Hu, C.Y.; Varner, J.D.; Lucks, J.B. Generating Effective Models and Parameters for RNA Genetic Circuits. ACS Synth. Biol. 2015, 4, 914–926. [Google Scholar] [CrossRef]
- Fredrickson, A.G. Formulation of structured growth models. Biotechnol. Bioeng. 1976, 18, 1481–1486. [Google Scholar] [CrossRef] [PubMed]
- Domach, M.M.; Leung, S.K.; Cahn, R.E.; Cocks, G.G.; Shuler, M.L. Computer model for glucose-limited growth of a single cell of Escherichia coli B/r-A. Biotechnol. Bioeng. 1984, 26, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Steinmeyer, D.; Shuler, M. Structured model for Saccharomyces cerevisiae. Chem. Eng. Sci. 1989, 44, 2017–2030. [Google Scholar] [CrossRef]
- Wu, P.; Ray, N.G.; Shuler, M.L. A single-cell model for CHO cells. Ann. N. Y. Acad. Sci. 1992, 665, 152–187. [Google Scholar] [CrossRef] [PubMed]
- Castellanos, M.; Wilson, D.B.; Shuler, M.L. A modular minimal cell model: Purine and pyrimidine transport and metabolism. Proc. Natl. Acad. Sci. USA 2004, 101, 6681–6686. [Google Scholar] [CrossRef]
- Atlas, J.C.; Nikolaev, E.V.; Browning, S.T.; Shuler, M.L. Incorporating genome-wide DNA sequence information into a dynamic whole-cell model of Escherichia coli: Application to DNA replication. IET Syst. Biol. 2008, 2, 369–382. [Google Scholar] [CrossRef]
- Tomita, M.; Hashimoto, K.; Takahashi, K.; Shimizu, T.S.; Matsuzaki, Y.; Miyoshi, F.; Saito, K.; Tanida, S.; Yugi, K.; Venter, J.C.; et al. E-CELL: Software environment for whole-cell simulation. Bioinformatics 1999, 15, 72–84. [Google Scholar] [CrossRef]
- Karr, J.R.; Sanghvi, J.C.; Macklin, D.N.; Gutschow, M.V.; Jacobs, J.M.; Bolival, B.; Assad-Garcia, N.; Glass, J.I.; Covert, M.W. A whole-cell computational model predicts phenotype from genotype. Cell 2012, 150, 389–401. [Google Scholar] [CrossRef]
- Varma, A.; Palsson, B.O. Stoichiometric flux balance models quantitatively predict growth and metabolic by-product secretion in wild-type Escherichia coli W3110. Appl. Environ. Microbiol. 1994, 60, 3724–3731. [Google Scholar] [CrossRef]
- Lewis, N.E.; Nagarajan, H.; Palsson, B.Ø. Constraining the metabolic genotype-phenotype relationship using a phylogeny of in silico methods. Nat. Rev. Microbiol. 2012, 10, 291–305. [Google Scholar] [CrossRef]
- Wiechert, W. 13C Metabolic Flux Analysis. Metabol. Eng. 2001, 3, 195–206. [Google Scholar] [CrossRef]
- Schuster, S.; Fell, D.A.; Dandekar, T. A general definition of metabolic pathways useful for systematic organization and analysis of complex metabolic networks. Nat. Biotechnol. 2000, 18, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Schilling, C.H.; Letscher, D.; Palsson, B.O. Theory for the systemic definition of metabolic pathways and their use in interpreting metabolic function from a pathway-oriented perspective. J. Theor. Biol. 2000, 203, 229–248. [Google Scholar] [CrossRef]
- Henry, C.S.; Broadbelt, L.J.; Hatzimanikatis, V. Thermodynamics-Based Metabolic Flux Analysis. Biophys. J. 2006, 92, 1792–1805. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.J.; Dwivedi, V.; Reed, J.L. Quantitative Assessment of Thermodynamic Constraints on the Solution Space of Genome-Scale Metabolic Models. Biophys. J. 2013, 105, 512–522. [Google Scholar] [CrossRef] [PubMed]
- Covert, M.W.; Knight, E.M.; Reed, J.L.; Herrgard, M.J.; Palsson, B.O. Integrating high-throughput and computational data elucidates bacterial networks. Nature 2004, 429, 92–96. [Google Scholar] [CrossRef]
- Sánchez, C.; Quintero, J.C.; Ochoa, S. Flux balance analysis in the production of clavulanic acid by Streptomyces clavuligerus. Biotechnol. Prog. 2015, 31, 1226–1236. [Google Scholar] [CrossRef]
- Edwards, J.S.; Palsson, B.O. Metabolic flux balance analysis and the in silico analysis of Escherichia coli K-12 gene deletions. BMC Bioinform. 2000, 1, 1. [Google Scholar] [CrossRef]
- O’Brien, E.J.; Lerman, J.A.; Chang, R.L.; Hyduke, D.R.; Palsson, B.Ø. Genome-scale models of metabolism and gene expression extend and refine growth phenotype prediction. Mol. Syst. Biol. 2013, 9, 693. [Google Scholar] [CrossRef]
- Atsumi, S.; Hanai, T.; Liao, J.C. Non-fermentative pathways for synthesis of branched-chain higher alcohols as biofuels. Nature 2008, 451, 86–89. [Google Scholar] [CrossRef]
- Nakamura, C.E.; Whited, G.M. Metabolic engineering for the microbial production of 1,3-propanediol. Curr. Opin. Biotechnol. 2003, 14, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Lee, S.Y. Production of succinic acid by bacterial fermentation. Enzym. Microb. Technol. 2006, 39, 352–361. [Google Scholar] [CrossRef]
- Yim, H.; Haselbeck, R.; Niu, W.; Pujol-Baxley, C.; Burgard, A.; Boldt, J.; Khandurina, J.; Trawick, J.D.; Osterhout, R.E.; Stephen, R.; et al. Metabolic engineering of Escherichia coli for direct production of 1,4-butanediol. Nat. Chem. Biol. 2011, 7, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Ro, D.K.; Paradise, E.M.; Ouellet, M.; Fisher, K.J.; Newman, K.L.; Ndungu, J.M.; Ho, K.A.; Eachus, R.A.; Ham, T.S.; Kirby, J.; et al. Production of the antimalarial drug precursor artemisinic acid in engineered yeast. Nature 2006, 440, 940–943. [Google Scholar] [CrossRef]
- Fossati, E.; Ekins, A.; Narcross, L.; Zhu, Y.; Falgueyret, J.P.; Beaudoin, G.A.W.; Facchini, P.J.; Martin, V.J.J. Reconstitution of a 10-gene pathway for synthesis of the plant alkaloid dihydrosanguinarine in Saccharomyces cerevisiae. Nat. Commun. 2014, 5, 1–11. [Google Scholar] [CrossRef]
- Allen, T.E.; Palsson, B.Ø. Sequence-based analysis of metabolic demands for protein synthesis in prokaryotes. J. Theor. Biol. 2003, 220, 1–18. [Google Scholar] [CrossRef]
- Thiele, I.; Jamshidi, N.; Fleming, R.M.T.; Palsson, B.O. Genome-Scale Reconstruction of Escherichia coli’s Transcriptional and Translational Machinery: A Knowledge Base, Its Mathematical Formulation, and Its Functional Characterization. PLoS Comput. Biol. 2009, 5, 1–13. [Google Scholar] [CrossRef]
- Zhang, Y.; Thiele, I.; Weekes, D.; Li, Z.; Jaroszewski, L.; Ginalski, K.; Deacon, A.M.; Wooley, J.; Lesley, S.A.; Wilson, I.A.; et al. Three-Dimensional Structural View of the Central Metabolic Network of Thermotoga maritima. Science 2009, 325, 1544–1549. [Google Scholar] [CrossRef]
- Chang, R.L.; Andrews, K.; Kim, D.; Li, Z.; Godzik, A.; Palsson, B.O. Structural Systems Biology Evaluation of Metabolic Thermotolerance in Escherichia coli. Science 2013, 340, 1220–1223. [Google Scholar] [CrossRef]
- Dai, D.; Horvath, N.; Varner, J. Dynamic Sequence Specific Constraint-Based Modeling of Cell-Free Protein Synthesis. Processes 2018, 6, 132. [Google Scholar] [CrossRef]
- Vilkhovoy, M.; Horvath, N.; Shih, C.H.; Wayman, J.A.; Calhoun, K.; Swartz, J.; Varner, J.D. Sequence Specific Modeling of E. coli Cell-Free Protein Synthesis. ACS Synth. Biol. 2018, 7, 1844–1857. [Google Scholar] [CrossRef] [PubMed]
- Horvath, N.; Vilkhovoy, M.; Wayman, J.A.; Calhoun, K.; Swartz, J.; Varner, J.D. Toward a Genome Scale Sequence Specific Dynamic Model of Cell-Free Protein Synthesis in Escherichia coli. Metab. Eng. Commun. 2020, 10, e00113. [Google Scholar] [CrossRef] [PubMed]
- Wayman, J.A.; Sagar, A.; Varner, J.D. Dynamic Modeling of Cell-Free Biochemical Networks Using Effective Kinetic Models. Processes 2015, 3, 138. [Google Scholar] [CrossRef]
- Vilkhovoy, M.; Dai, D.; Vadhin, S.; Adhikari, A.; Varner, J.D. Absolute Quantification of Cell-Free Protein Synthesis Metabolism by Reversed-Phase Liquid Chromatography-Mass Spectrometry. J. Vis. Exp. 2019, 25, e60329. [Google Scholar] [CrossRef]
- Smith, M.T.; Bennett, A.M.; Hunt, J.M.; Bundy, B.C. Creating a completely “cell-free” system for protein synthesis. Biotechnol. Prog. 2015, 31, 1716–1719. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilkhovoy, M.; Adhikari, A.; Vadhin, S.; Varner, J.D. The Evolution of Cell Free Biomanufacturing. Processes 2020, 8, 675. https://doi.org/10.3390/pr8060675
Vilkhovoy M, Adhikari A, Vadhin S, Varner JD. The Evolution of Cell Free Biomanufacturing. Processes. 2020; 8(6):675. https://doi.org/10.3390/pr8060675
Chicago/Turabian StyleVilkhovoy, Michael, Abhinav Adhikari, Sandra Vadhin, and Jeffrey D. Varner. 2020. "The Evolution of Cell Free Biomanufacturing" Processes 8, no. 6: 675. https://doi.org/10.3390/pr8060675
APA StyleVilkhovoy, M., Adhikari, A., Vadhin, S., & Varner, J. D. (2020). The Evolution of Cell Free Biomanufacturing. Processes, 8(6), 675. https://doi.org/10.3390/pr8060675





