Sustainable Catalytic Processes Driven by Graphene-Based Materials
Abstract
1. Introduction
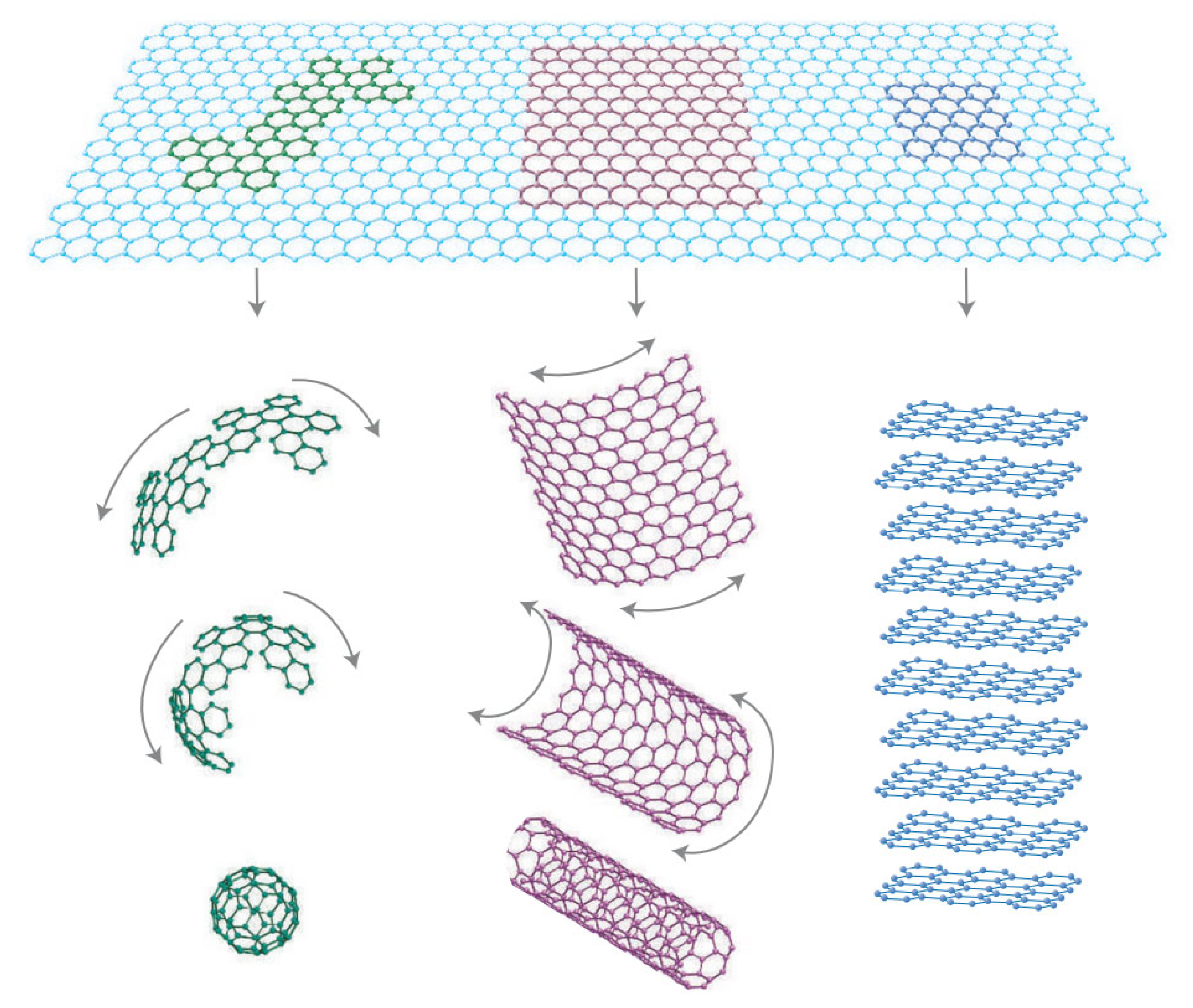
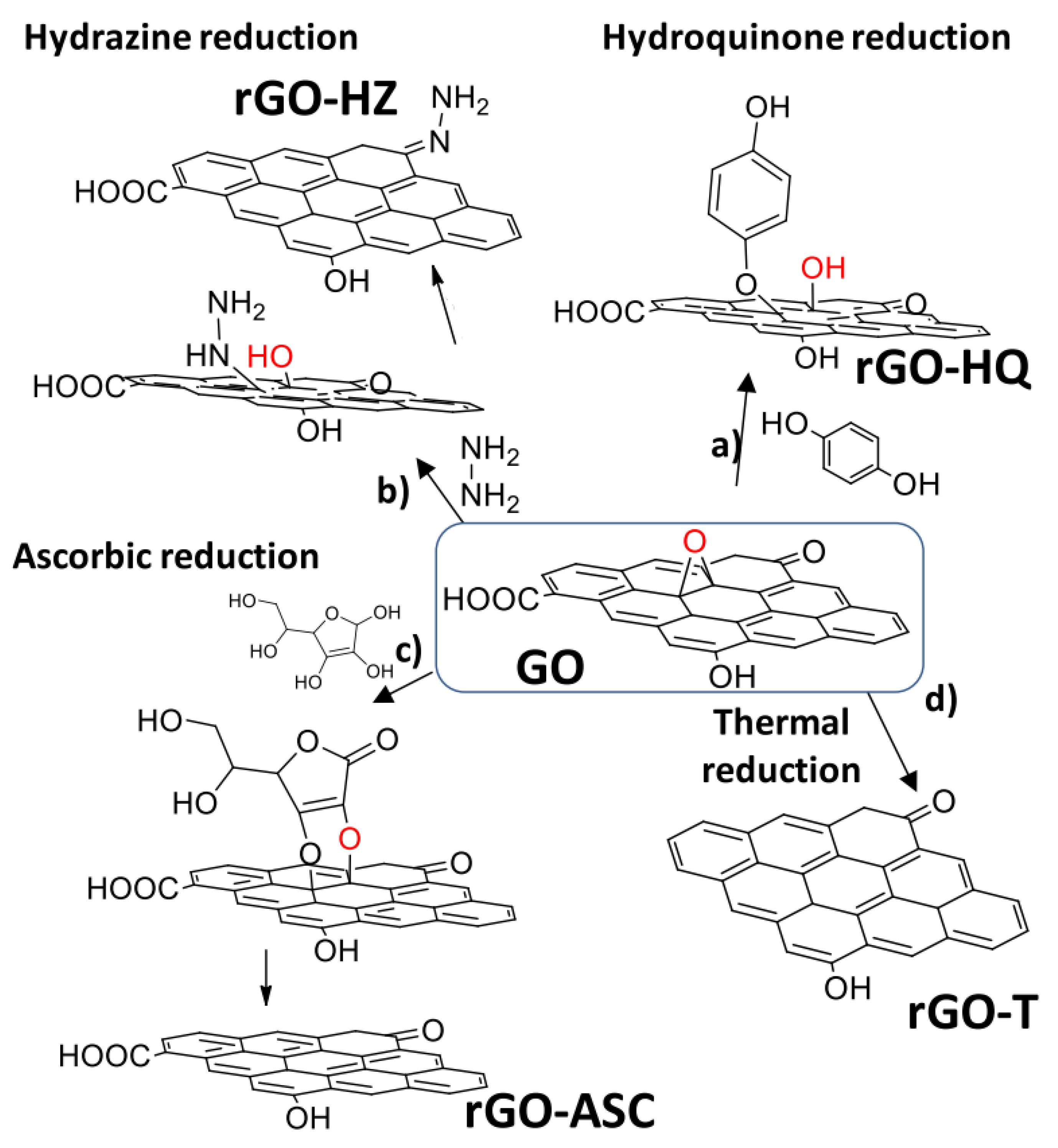
2. Active Sites in Graphene
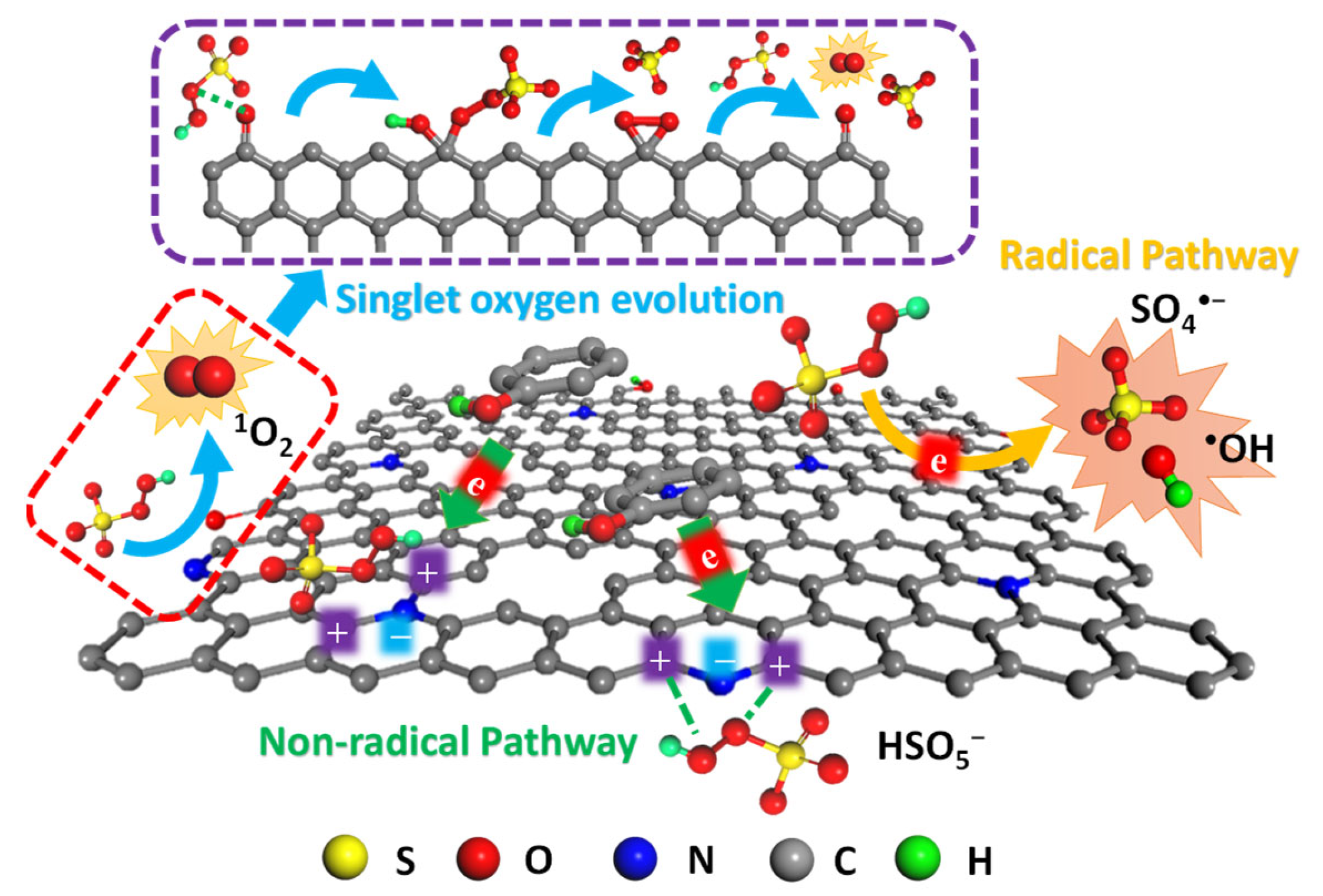
3. Advanced Oxidation Processes
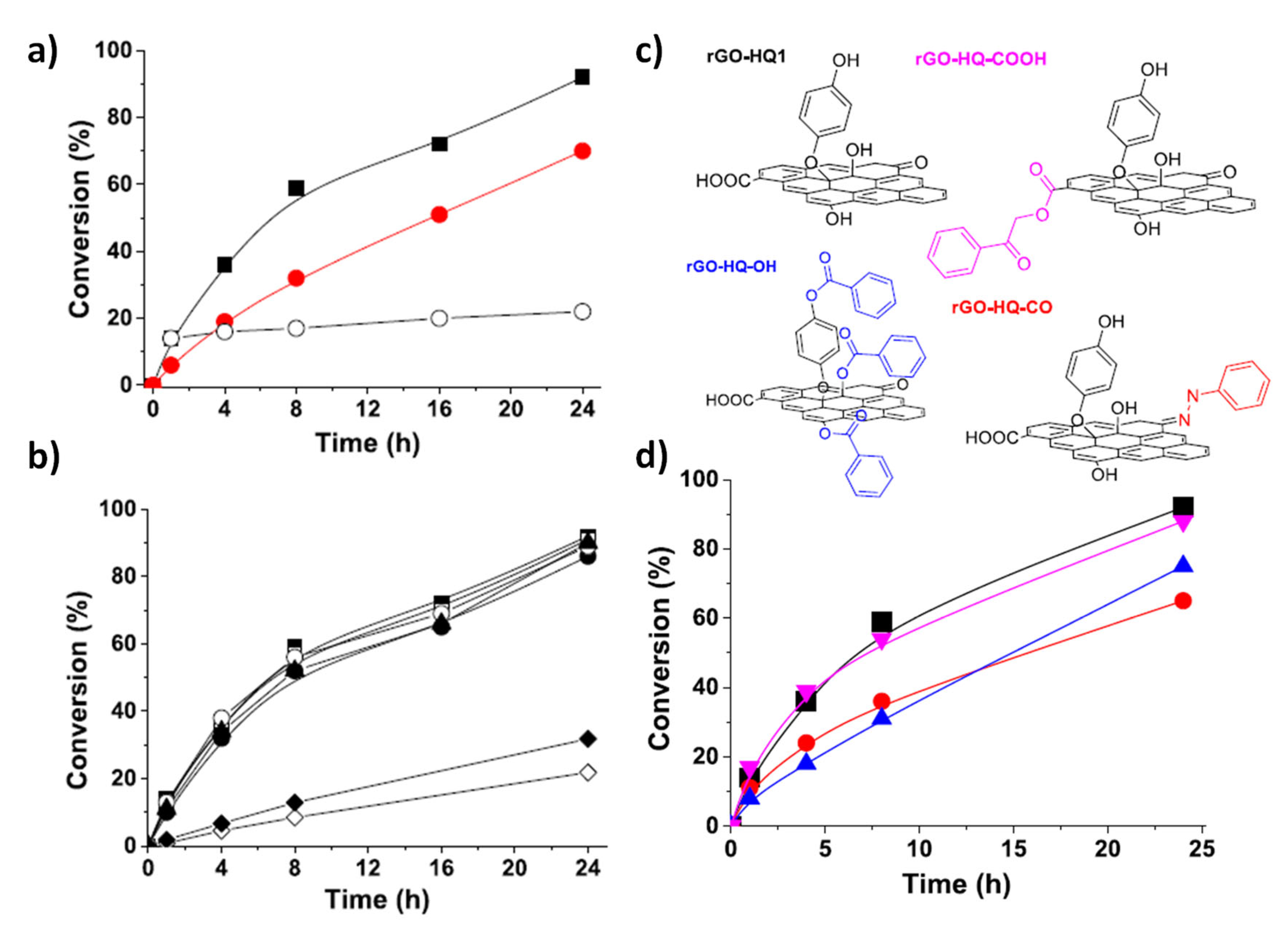
4. Chemical Synthesis and Green Catalysis
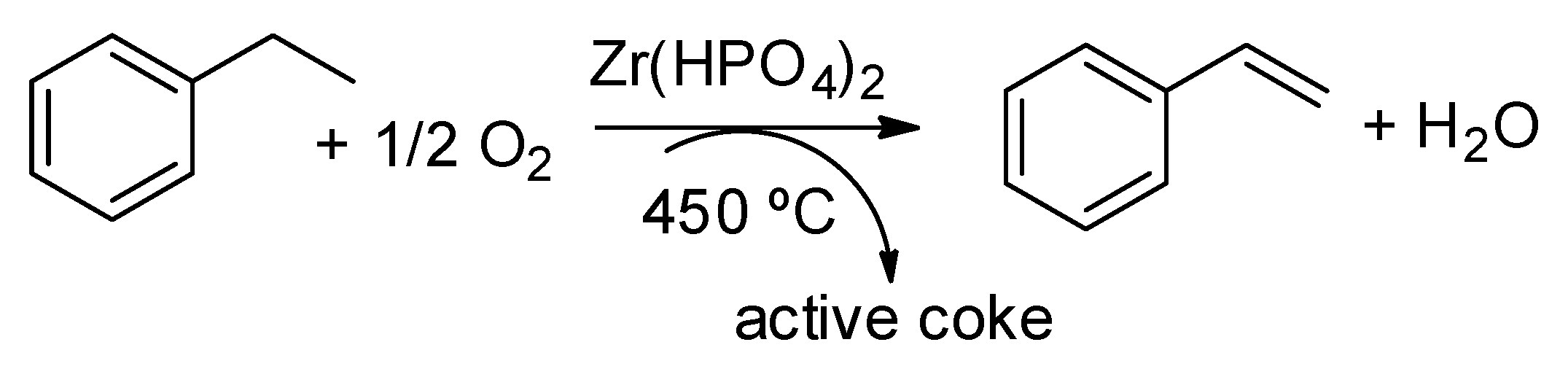
5. Oxidation Reactions
6. Hydrogenation Reactions
7. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Lim, B.; Jiang, M.J.; Camargo, P.H.C.; Cho, E.C.; Tao, J.; Lu, X.M.; Zhu, Y.M.; Xia, Y.N. Pd-Pt bimetallic nanodendrites with high activity for oxygen reduction. Science 2009, 324, 1302–1305. [Google Scholar] [CrossRef]
- Wang, Y.G.; Cantu, D.C.; Lee, M.S.; Li, J.; Glezakou, V.A.; Rousseau, R. CO Oxidation on Au/TiO2: Condition-dependent active sites and mechanistic pathways. J. Am. Chem. Soc. 2016, 138, 10467–10476. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.S.; Robinson, P.R. Gasoline production. In Petroleum Science and Technology; Hsu, C.S., Robinson, P.R., Eds.; Springer International Publishing: Cham, Switzerland, 2019. [Google Scholar]
- Hill, C.K.; Hartwig, J.F. Site-selective oxidation, amination and epimerization reactions of complex polyols enabled by transfer hydrogenation. Nat. Chem. 2017, 9, 1213–1221. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Cui, X.J.; Junge, K.; Surkus, A.E.; Kreyenschulte, C.; Bartling, S.; Beller, M. General and Chemoselective Copper Oxide Catalysts for Hydrogenation Reactions. ACS Catal. 2019, 9, 4302–4307. [Google Scholar] [CrossRef]
- Lee, S.; Halder, A.; Ferguson, G.A.; Seifert, S.; Winans, R.E.; Teschner, D.; Schlogl, R.; Papaefthimiou, V.; Greeley, J.; Curtiss, L.A.; et al. Subnanometer cobalt oxide clusters as selective low temperature oxidative dehydrogenation catalysts. Nat. Commun. 2019, 10, 954. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Zheng, Y.; Jaroniec, M.T.; Qiao, S.Z. Design of electrocatalysts for oxygen- and hydrogen-involving energy conversion reactions. Chem. Soc. Rev. 2015, 44, 2060–2086. [Google Scholar] [CrossRef] [PubMed]
- Su, D.S.; Zhang, J.; Frank, B.; Thomas, A.; Wang, X.C.; Paraknowitsch, J.; Schlogl, R. Metal-free heterogeneous catalysis for sustainable chemistry. ChemSusChem 2010, 3, 169–180. [Google Scholar] [CrossRef]
- Navalon, S.; Dhakshinamoorthy, A.; Alvaro, M.; Garcia, H. Carbocatalysis by graphene-based materials. Chem. Rev. 2014, 114, 6179–6212. [Google Scholar] [CrossRef]
- Park, S.; Ruoff, R.S. Chemical methods for the production of graphenes. Nat. Nanotechnol. 2009, 4, 217–224. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Fal’ko, V.I.; Colombo, L.; Gellert, P.R.; Schwab, M.G.; Kim, K. A roadmap for graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Bielawski, C.W. Carbocatalysis: Heterogeneous carbons finding utility in synthetic chemistry. Chem. Sci. 2011, 2, 1233–1240. [Google Scholar] [CrossRef]
- Primo, A.; Parvulescu, V.I.; Garcia, H. Graphenes as metal-free catalysts with engineered active sites. J. Phys. Chem. Lett. 2016, 8, 264–278. [Google Scholar] [CrossRef] [PubMed]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Chen, J.; Yao, B.; Li, C.; Shi, G. An improved Hummers method for eco-friendly synthesis of graphene oxide. Carbon 2013, 64, 225–229. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef]
- Fu, Q.; Bao, X.H. Surface chemistry and catalysis confined under two-dimensional materials. Chem. Soc. Rev. 2017, 46, 1842–1874. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Natur. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Chen, X.; Oh, W.D.; Lim, T.T. Graphene- and CNTs-based carbocatalysts in persulfates activation: Material design and catalytic mechanisms. Chem. Eng. J. 2018, 354, 941–976. [Google Scholar] [CrossRef]
- Frank, B.; Blume, R.; Rinaldi, A.; Trunschke, A.; Schlögl, R. Oxygen insertion catalysis by sp2 carbon. Angew. Chem. Int. Ed. 2011, 50, 10226–10230. [Google Scholar] [CrossRef]
- Kong, X.K.; Chen, C.L.; Chen, Q.W. Doped graphene for metal-free catalysis. Chem. Soc. Rev. 2014, 43, 2841–2857. [Google Scholar] [CrossRef]
- Navalon, S.; Dhakshinamoorthy, A.; Alvaro, M.; Antonietti, M.; García, H. Active sites on graphene-based materials as metal-free catalysts. Chem. Soc. Rev. 2017, 46, 4501–4529. [Google Scholar] [CrossRef] [PubMed]
- Su, D.S.; Wen, G.D.; Wu, S.C.; Peng, F.; Schlogl, R. Carbocatalysis in Liquid-Phase Reactions. Angew. Chem. Int. Edit 2017, 56, 936–964. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Chen, S.; Jaroniec, M.; Qiao, S.Z. Heteroatom-doped graphene-based materials for energy-relevant electrocatalytic processes. ACS Catal. 2015, 5, 5207–5234. [Google Scholar] [CrossRef]
- Jana, D.; Sun, C.-L.; Chen, L.-C.; Chen, K.-H. Effect of chemical doping of boron and nitrogen on the electronic, optical, and electrochemical properties of carbon nanotubes. Prog. Mater. Sci. 2013, 58, 565–635. [Google Scholar] [CrossRef]
- Wang, H.; Maiyalagan, T.; Wang, X. Review on recent progress in nitrogen-doped graphene: Synthesis, characterization, and its potential applications. ACS Catal. 2012, 2, 781–794. [Google Scholar] [CrossRef]
- Rao, C.N.R.; Gopalakrishnan, K.; Govindaraj, A. Synthesis, properties and applications of graphene doped with boron, nitrogen and other elements. Nano Today 2014, 9, 324–343. [Google Scholar] [CrossRef]
- Kim, K.S.; Zhao, Y.; Jang, H.; Lee, S.Y.; Kim, J.M.; Kim, K.S.; Ahn, J.H.; Kim, P.; Choi, J.Y.; Hong, B.H. Large-scale pattern growth of graphene films for stretchable transparent electrodes. Nature 2009, 457, 706–710. [Google Scholar] [CrossRef]
- Zhang, B.B.; Song, J.L.; Yang, G.Y.; Han, B.X. Large-scale production of high-quality graphene using glucose and ferric chloride. Chem. Sci. 2014, 5, 4656–4660. [Google Scholar] [CrossRef]
- Kashani, H.; Ito, Y.; Han, J.H.; Liu, P.; Chen, M.W. Extraordinary tensile strength and ductility of scalable nanoporous graphene. Sci. Adv. 2019, 5, eaat6951. [Google Scholar] [CrossRef]
- Liu, H.T.; Liu, Y.Q.; Zhu, D.B. Chemical doping of graphene. J. Mater. Chem. 2011, 21, 3335–3345. [Google Scholar] [CrossRef]
- Castro Neto, A.H.; Guinea, F.; Peres, N.M.R.; Novoselov, K.S.; Geim, A.K. The electronic properties of graphene. Rev. Mod. Phys. 2009, 81, 109–162. [Google Scholar] [CrossRef]
- Yang, X.F.; Wang, A.Q.; Qiao, B.T.; Li, J.; Liu, J.Y.; Zhang, T. Single-atom catalysts: A new frontier in heterogeneous catalysis. Accounts Chem. Res. 2013, 46, 1740–1748. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.B.; Liu, G.G.; Shi, L.; Ye, J.H. Single-atom catalysts: Emerging multifunctional materials in heterogeneous catalysis. Adv. Energy Mater. 2018, 8, 1701343. [Google Scholar] [CrossRef]
- Wang, A.; Li, J.; Zhang, T. Heterogeneous single-atom catalysis. Nat. Rev. Chem. 2018, 2, 65–81. [Google Scholar] [CrossRef]
- Su, C.; Acik, M.; Takai, K.; Lu, J.; Hao, S.J.; Zheng, Y.; Wu, P.; Bao, Q.; Enoki, T.; Chabal, Y.J.; et al. Probing the catalytic activity of porous graphene oxide and the origin of this behaviour. Nat. Commun. 2012, 3, 1298. [Google Scholar] [CrossRef]
- Jiang, D.E.; Sumpter, B.G.; Dai, S. Unique chemical reactivity of a graphene nanoribbon's zigzag edge. J. Chem. Phys. 2007, 126, 134701. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Zhang, Q. Nanocarbon for oxygen reduction electrocatalysis: Dopants, edges, and defects. Adv. Mater. 2017, 29, 1604103. [Google Scholar] [CrossRef]
- Tang, C.; Wang, H.F.; Chen, X.; Li, B.Q.; Hou, T.Z.; Zhang, B.S.; Zhang, Q.; Titirici, M.M.; Wei, F. Topological defects in metal-free nanocarbon for oxygen electrocatalysis. Adv. Mater. 2016, 28, 6845–6851. [Google Scholar] [CrossRef]
- Tian, W.; Zhang, H.; Duan, X.; Sun, H.; Shao, G.; Wang, S. Porous carbons: Structure-oriented design and versatile applications. Adv. Funct. Mater. 2020, 30, 1909265. [Google Scholar] [CrossRef]
- Yang, Q.; Xiao, Z.; Kong, D.; Zhang, T.; Duan, X.; Zhou, S.; Niu, Y.; Shen, Y.; Sun, H.; Wang, S.; et al. New insight to the role of edges and heteroatoms in nanocarbons for oxygen reduction reaction. Nano Energ. 2019, 66, 104096. [Google Scholar] [CrossRef]
- Tian, Y.; Ye, Y.F.; Wang, X.J.; Peng, S.; Wei, Z.; Zhang, X.; Liu, W.M. Three-dimensional N-doped, plasma-etched graphene: Highly active metal-free catalyst for hydrogen evolution reaction. Appl. Catal. A-Gen. 2017, 529, 127–133. [Google Scholar] [CrossRef]
- Jia, Y.; Zhang, L.Z.; Du, A.J.; Gao, G.P.; Chen, J.; Yan, X.C.; Brown, C.L.; Yao, X.D. Defect graphene as a trifunctional catalyst for electrochemical reactions. Adv. Mater. 2016, 28, 9532–9538. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, J.M.; Scheffler, M. Structural, electronic, and chemical properties of nanoporous carbon. Phys. Rev. Lett. 2006, 96, 046806. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.-J.; Tian, Y.; Sobhani, Z.; Naidu, R.; Fang, C. Synergistic degradation of PFAS in water and soil by dual-frequency ultrasonic activated persulfate. Chem. Eng. J. 2020, 388, 124215. [Google Scholar] [CrossRef]
- Han, W.; Li, D.; Zhang, M.; Ximin, H.; Duan, X.; Liu, S.; Wang, S. Photocatalytic activation of peroxymonosulfate by surface-tailored carbon quantum dots. J. Hazard. Mater. 2020, 395, 122695. [Google Scholar] [CrossRef]
- Duan, X.; Indrawirawan, S.; Kang, J.; Tian, W.; Zhang, H.; Sun, H.; Wang, S. Temperature-dependent evolution of hydroxyl radicals from peroxymonosulfate activation over nitrogen-modified carbon nanotubes. Sustain. Mater. Technol. 2018, 18, e00082. [Google Scholar] [CrossRef]
- Duan, X.; Indrawirawan, S.; Kang, J.; Tian, W.; Zhang, H.; Duan, X.; Zhou, X.; Sun, H.; Wang, S. Synergy of carbocatalytic and heat activation of persulfate for evolution of reactive radicals toward metal-free oxidation. Catal. Today 2019. [Google Scholar] [CrossRef]
- Nie, C.Y.; Ao, Z.M.; Duan, X.G.; Wang, C.Y.; Wang, S.B.; An, T.C. Degradation of aniline by electrochemical activation of peroxydisulfate at MWCNT cathode: The proofed concept of nonradical oxidation process. Chemosphere 2018, 206, 432–438. [Google Scholar] [CrossRef]
- Anipsitakis, G.P.; Dionysiou, D.D. Radical generation by the interaction of transition metals with common oxidants. Environ. Sci. Technol. 2004, 38, 3705–3712. [Google Scholar] [CrossRef]
- Duan, X.G.; Sun, H.Q.; Wang, S.B. Metal-free carbocatalysis in advanced oxidation reactions. Accounts Chem. Res. 2018, 51, 678–687. [Google Scholar] [CrossRef]
- Espinosa, J.C.; Navalon, S.; Alvaro, M.; Garcia, H. Reduced graphene oxide as a metal-free catalyst for the light-assisted fenton-like reaction. Chemcatchem 2016, 8, 2642–2648. [Google Scholar] [CrossRef]
- Bernat-Quesada, F.; Espinosa, J.C.; Barbera, V.; Alvaro, M.; Galimberti, M.; Navalon, S.; Garcia, H. Catalytic ozonation using edge-hydroxylated graphite-based materials. ACS Sustain. Chem. Eng. 2019, 7, 17443–17452. [Google Scholar] [CrossRef]
- Sun, H.Q.; Liu, S.Z.; Zhou, G.L.; Ang, H.M.; Tade, M.O.; Wang, S.B. Reduced graphene oxide for catalytic oxidation of aqueous organic pollutants. ACS Appl. Mater. Interfaces 2012, 4, 5466–5471. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.G.; Sun, H.Q.; Ao, Z.M.; Zhou, L.; Wang, G.X.; Wang, S.B. Unveiling the active sites of graphene-catalyzed peroxymonosulfate activation. Carbon 2016, 107, 371–378. [Google Scholar] [CrossRef]
- Duan, X.G.; Ao, Z.M.; Zhou, L.; Sun, H.Q.; Wang, G.X.; Wang, S.B. Occurrence of radical and nonradical pathways from carbocatalysts for aqueous and nonaqueous catalytic oxidation. Appl. Catal. B-Environ. 2016, 188, 98–105. [Google Scholar] [CrossRef]
- Han, C.; Duan, X.G.; Zhang, M.J.; Fu, W.Z.; Duan, X.Z.; Ma, W.J.; Liu, S.M.; Wang, S.B.; Zhou, X.G. Role of electronic properties in partition of radical and nonradical processes of carbocatalysis toward peroxymonosulfate activation. Carbon 2019, 153, 73–80. [Google Scholar] [CrossRef]
- Cheng, X.; Guo, H.; Zhang, Y.; Wu, X.; Liu, Y. Non-photochemical production of singlet oxygen via activation of persulfate by carbon nanotubes. Water Res. 2017, 113, 80–88. [Google Scholar] [CrossRef]
- Li, D.; Duan, X.; Sun, H.; Kang, J.; Zhang, H.; Tade, M.O.; Wang, S. Facile synthesis of nitrogen-doped graphene via low-temperature pyrolysis: The effects of precursors and annealing ambience on metal-free catalytic oxidation. Carbon 2017, 115, 649–658. [Google Scholar] [CrossRef]
- Kang, J.; Duan, X.G.; Zhou, L.; Sun, H.Q.; Tade, M.O.; Wang, S.B. Carbocatalytic activation of persulfate for removal of antibiotics in water solutions. Chem. Eng. J. 2016, 288, 399–405. [Google Scholar] [CrossRef]
- Liang, P.; Zhang, C.; Duan, X.G.; Sun, H.Q.; Liu, S.M.; Tade, M.O.; Wang, S.B. N-Doped Graphene from Metal-Organic Frameworks for Catalytic Oxidation of p-HydroxylbenzoicL Acid: N-Functionality and Mechanism. ACS Sustain. Chem. Eng. 2017, 5, 2693–2701. [Google Scholar] [CrossRef]
- Liang, P.; Zhang, C.; Duan, X.; Sun, H.; Liu, S.; Tade, M.O.; Wang, S. An insight into metal organic framework derived N-doped graphene for the oxidative degradation of persistent contaminants: Formation mechanism and generation of singlet oxygen from peroxymonosulfate. Environ. Sci. Nano 2017, 4, 315–324. [Google Scholar] [CrossRef]
- Kang, J.; Zhou, L.; Duan, X.; Sun, H.; Wang, S. Catalytic degradation of antibiotics by metal-free catalysis over nitrogen-doped graphene. Catal. Today 2018. [Google Scholar] [CrossRef]
- Duan, X.G.; Ao, Z.M.; Sun, H.Q.; Indrawirawan, S.; Wang, Y.X.; Kang, J.; Liang, F.L.; Zhu, Z.H.; Wang, S.B. Nitrogen-doped graphene for generation and evolution of reactive radicals by metal-free catalysis. ACS Appl. Mater. Interfaces 2015, 7, 4169–4178. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.G.; Sun, H.Q.; Wang, Y.X.; Kang, J.; Wang, S.B. N-doping-induced nonradical reaction on single-walled carbon nanotubes for catalytic phenol oxidation. ACS Catal. 2015, 5, 553–559. [Google Scholar] [CrossRef]
- Wu, D.; Song, W.Y.; Chen, L.L.; Duan, X.G.; Xia, Q.; Fan, X.B.; Li, Y.; Zhang, F.B.; Peng, W.C.; Wang, S.B. High-performance porous graphene from synergetic nitrogen doping and physical activation for advanced nonradical oxidation. J. Hazard. Mater. 2020, 381, 121010. [Google Scholar] [CrossRef]
- Yang, Z.; Duan, X.; Wang, J.; Li, Y.; Fan, X.; Zhang, F.; Zhang, G.; Peng, W. Facile synthesis of high-performance nitrogen-doped hierarchically porous carbon for catalytic oxidation. ACS Sustain. Chem. Eng. 2020, 8, 4236–4243. [Google Scholar] [CrossRef]
- Zhang, W.; Li, Y.; Fan, X.B.; Zhang, F.B.; Zhang, G.L.; Zhu, Y.A.; Peng, W.C.; Wang, S.B.; Duan, X.G. Synergy of nitrogen doping and structural defects on hierarchically porous carbons toward catalytic oxidation via a non-radical pathway. Carbon 2019, 155, 268–278. [Google Scholar] [CrossRef]
- Duan, X.G.; O’Donnell, K.; Sun, H.Q.; Wang, Y.X.; Wang, S.B. Sulfur and nitrogen co-doped graphene for metal-free catalytic oxidation reactions. Small 2015, 11, 3036–3044. [Google Scholar] [CrossRef]
- Liu, H.; Sun, P.; Feng, M.B.; Liu, H.X.; Yang, S.G.; Wang, L.S.; Wang, Z.Y. Nitrogen and sulfur co-doped CNT-COOH as an efficient metal-free catalyst for the degradation of UV filter BP-4 based on sulfate radicals. Appl. Catal. B Environ. 2016, 187, 1–10. [Google Scholar] [CrossRef]
- Chen, X.; Duan, X.G.; Oh, W.D.; Zhang, P.H.; Guan, C.T.; Zhu, Y.A.; Lim, T.T. Insights into nitrogen and boron-co-doped graphene toward high-performance peroxymonosulfate activation: Maneuverable N-B bonding configurations and oxidation pathways. Appl. Catal. B Environ. 2019, 253, 419–432. [Google Scholar] [CrossRef]
- Sun, H.Q.; Wang, Y.X.; Liu, S.Z.; Ge, L.; Wang, L.; Zhu, Z.H.; Wang, S.B. Facile synthesis of nitrogen doped reduced graphene oxide as a superior metal-free catalyst for oxidation. Chem. Commun. 2013, 49, 9914–9916. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.G.; Tian, W.J.; Zhang, H.Y.; Sun, H.Q.; Ao, Z.M.; Shao, Z.P.; Wang, S.B. sp(2)/sp(3) framework from diamond nanocrystals: A key bridge of carbonaceous structure to carbocatalysis. ACS Catal. 2019, 9, 7494–7519. [Google Scholar] [CrossRef]
- Navalón, S.; Dhakshinamoorthy, A.; Álvaro, M.; García, H. Diamond nanoparticles in heterogeneous catalysis. Chem. Mater. 2020, 32, 4116–4143. [Google Scholar] [CrossRef]
- Duan, X.G.; Ao, Z.M.; Zhang, H.Y.; Saunders, M.; Sun, H.Q.; Shao, Z.P.; Wang, S.B. Nanodiamonds in sp2/sp3 configuration for radical to nonradical oxidation: Core-shell layer dependence. Appl. Catal. B Environ. 2018, 222, 176–181. [Google Scholar] [CrossRef]
- Duan, X.G.; Ao, Z.M.; Li, D.G.; Sun, H.Q.; Zhou, L.; Suvorova, A.; Saunders, M.; Wang, G.X.; Wang, S.B. Surface-tailored nanodiamonds as excellent metal-free catalysts for organic oxidation. Carbon 2016, 103, 404–411. [Google Scholar] [CrossRef]
- Duan, X.G.; Su, C.; Zhou, L.; Sun, H.Q.; Suvorova, A.; Odedairo, T.; Zhu, Z.H.; Shao, Z.P.; Wang, S.B. Surface controlled generation of reactive radicals from persulfate by carbocatalysis on nanodiamonds. Appl. Catal. B Environ. 2016, 194, 7–15. [Google Scholar] [CrossRef]
- Shao, P.; Tian, J.; Yang, F.; Duan, X.; Gao, S.; Shi, W.; Luo, X.; Cui, F.; Luo, S.; Wang, S. Identification and regulation of active sites on nanodiamonds: Establishing a highly efficient catalytic system for oxidation of organic contaminants. Adv. Funct. Mater. 2018, 28, 1705295. [Google Scholar] [CrossRef]
- Li, H.R.; Tian, J.Y.; Zhu, Z.G.; Cui, F.Y.; Zhu, Y.A.; Duan, X.G.; Wang, S.B. Magnetic nitrogen-doped nanocarbons for enhanced metal-free catalytic oxidation: Integrated experimental and theoretical investigations for mechanism and application. Chem. Eng. J. 2018, 354, 507–516. [Google Scholar] [CrossRef]
- Kang, J.; Zhang, H.Y.; Duan, X.G.; Sun, H.Q.; Tan, X.Y.; Liu, S.M.; Wang, S.B. Magnetic Ni-Co alloy encapsulated N-doped carbon nanotubes for catalytic membrane degradation of emerging contaminants. Chem. Eng. J. 2019, 362, 251–261. [Google Scholar] [CrossRef]
- Zhou, H.; Wu, S.K.; Zhou, Y.Y.; Yang, Y.; Zhang, J.C.; Luo, L.; Duan, X.G.; Wang, S.B.; Wang, L.; Tsang, D.C.W. Insights into the oxidation of organic contaminants by iron nanoparticles encapsulated within boron and nitrogen co-doped carbon nanoshell: Catalyzed Fenton-like reaction at natural pH. Environ. Int. 2019, 128, 77–88. [Google Scholar] [CrossRef]
- Wang, Y.X.; Sun, H.Q.; Duan, X.G.; Ang, H.M.; Tade, M.O.; Wang, S.B. A new magnetic nano zero-valent iron encapsulated in carbon spheres for oxidative degradation of phenol. Appl. Catal. B Environ. 2015, 172, 73–81. [Google Scholar] [CrossRef]
- Kang, J.; Zhou, L.; Duan, X.G.; Sun, H.Q.; Ao, Z.M.; Wang, S.B. Degradation of cosmetic microplastics via functionalized carbon nanosprings. Matter 2019, 1, 745–758. [Google Scholar] [CrossRef]
- Kang, J.; Duan, X.; Wang, C.; Sun, H.; Tan, X.; Tade, M.O.; Wang, S. Nitrogen-doped bamboo-like carbon nanotubes with Ni encapsulation for persulfate activation to remove emerging contaminants with excellent catalytic stability. Chem. Eng. J. 2018, 332, 398–408. [Google Scholar] [CrossRef]
- Kang, J.; Zhang, H.; Duan, X.; Sun, H.; Tan, X.; Wang, S. Nickel in hierarchically structured nitrogen-doped graphene for robust and promoted degradation of antibiotics. J. Cleaner Prod. 2019, 218, 202–211. [Google Scholar] [CrossRef]
- Duan, X.G.; Kang, J.; Tian, W.J.; Zhang, H.Y.; Ho, S.H.; Zhu, Y.A.; Ao, Z.M.; Sun, H.Q.; Wang, S.B. Interfacial-engineered cobalt@carbon hybrids for synergistically boosted evolution of sulfate radicals toward green oxidation. Appl. Catal. B Environ. 2019, 256. [Google Scholar] [CrossRef]
- Zhu, S.; Jin, C.; Duan, X.; Wang, S.; Ho, S.-H. Nonradical oxidation in persulfate activation by graphene-like nanosheets (GNS): Differentiating the contributions of singlet oxygen (1O2) and sorption-dependent electron transfer. Chem. Eng. J. 2020, 393, 124725. [Google Scholar] [CrossRef]
- Duan, X.G.; Sun, H.Q.; Kang, J.; Wang, Y.X.; Indrawirawan, S.; Wang, S.B. Insights into heterogeneous catalysis of persulfate activation on dimensional-structured nanocarbons. ACS Catal. 2015, 5, 4629–4636. [Google Scholar] [CrossRef]
- Duan, X.G.; Sun, H.Q.; Shao, Z.P.; Wang, S.B. Nonradical reactions in environmental remediation processes: Uncertainty and challenges. Appl. Catal. B Environ. 2018, 224, 973–982. [Google Scholar] [CrossRef]
- Cheng, X.; Guo, H.; Zhang, Y.; Korshin, G.V.; Yang, B. Insights into the mechanism of nonradical reactions of persulfate activated by carbon nanotubes: Activation performance and structure-function relationship. Water Res. 2019, 157, 406–414. [Google Scholar] [CrossRef]
- Ren, W.; Xiong, L.L.; Nie, G.; Zhang, H.; Duan, X.G.; Wang, S.B. Insights into the electron-transfer regime of peroxydisulfate activation on carbon nanotubes: The role of oxygen functional groups. Environ. Sci. Technol. 2020, 54, 1267–1275. [Google Scholar] [CrossRef]
- Ren, W.; Xiong, L.; Yuan, X.; Yu, Z.; Zhang, H.; Duan, X.; Wang, S. Activation of peroxydisulfate on carbon nanotubes: Electron-transfer mechanism. Environ. Sci. Technol. 2019, 53, 14595–14603. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Wei, Y.; Qu, X.; Yu, C.; Li, Q.; Alvarez, P.J.J.; Long, M. Mechanistic inference on the reaction kinetics of phenols and anilines in carbon nanotubes-activated peroxydisulfate systems: pp-LFERs and QSARs analyses. Chem. Eng. J. 2020, 385, 123923. [Google Scholar] [CrossRef]
- Sun, H.Q.; Kwan, C.; Suvorova, A.; Ang, H.M.; Tade, M.O.; Wang, S.B. Catalytic oxidation of organic pollutants on pristine and surface nitrogen-modified carbon nanotubes with sulfate radicals. Appl. Catal. B Environ. 2014, 154, 134–141. [Google Scholar] [CrossRef]
- Ren, W.; Nie, G.; Zhou, P.; Zhang, H.; Duan, X.; Wang, S. The Intrinsic nature of persulfate activation and N-doping in carbocatalysis. Environ. Sci. Technol. 2020, 54, 6438–6447. [Google Scholar] [CrossRef]
- Duan, X.G.; Ao, Z.M.; Sun, H.Q.; Zhou, L.; Wang, G.X.; Wang, S.B. Insights into N-doping in single-walled carbon nanotubes for enhanced activation of superoxides: A mechanistic study. Chem. Commun. 2015, 51, 15249–15252. [Google Scholar] [CrossRef]
- Tian, W.J.; Zhang, H.Y.; Duan, X.G.; Sun, H.Q.; Tade, M.O.; Ang, H.M.; Wang, S.B. Nitrogen- and sulfur-codoped hierarchically porous carbon for adsorptive and oxidative removal of pharmaceutical contaminants. ACS Appl. Mater. Interfaces 2016, 8, 7184–7193. [Google Scholar] [CrossRef]
- Wang, J.; Duan, X.; Dong, Q.; Meng, F.; Tan, X.; Liu, S.; Wang, S. Facile synthesis of N-doped 3D graphene aerogel and its excellent performance in catalytic degradation of antibiotic contaminants in water. Carbon 2019, 144, 781–790. [Google Scholar] [CrossRef]
- Indrawirawan, S.; Sun, H.Q.; Duan, X.G.; Wang, S.B. Nanocarbons in different structural dimensions (0-3D) for phenol adsorption and metal-free catalytic oxidation. Appl. Catal. B Environ. 2015, 179, 352–362. [Google Scholar] [CrossRef]
- Ertl, G.; Freund, H.J. Catalysis and surface science. Phys. Today 1999, 52, 32–38. [Google Scholar] [CrossRef]
- Schlögl, R. Heterogeneous catalysis—still magic or already science? Angew. Chem. Int. Ed.
- Somorjai, G.A. The surface science of heterogeneous catalysis. Surf. Sci.
- Chua, C.K.; Pumera, M. Carbocatalysis: The State of metal-free catalysis. Chem. Eur. J. 2015, 21, 12550–12562. [Google Scholar] [CrossRef]
- Liu, L.; Zhu, Y.P.; Su, M.; Yuan, Z.Y. Metal-free carbonaceous materials as promising heterogeneous catalysts. ChemCatChem 2015, 7, 2765–2787. [Google Scholar] [CrossRef]
- Navalón, S.; Herance, J.R.; Alvaro, M.; García, H. General aspects in the use of graphenes in catalysis. Mater. Horiz. 2018, 5, 363–378. [Google Scholar] [CrossRef]
- Navalón, S.; Herance, J.R.; lvaro, M.; García, H. Covalently modified graphenes in catalysis, electrocatalysis and photoresponsive materials. Chem. Eur. J. 2017, 23, 15244–15275. [Google Scholar] [CrossRef] [PubMed]
- Su, D.S.; Perathoner, S.; Centi, G. Nanocarbons for the development of advanced catalysts. Chem. Rev. 2013, 113, 5782–5816. [Google Scholar] [CrossRef]
- Tang, P.; Hu, G.; Li, M.; Ma, D. Graphene-based metal-free catalysts for catalytic reactions in the liquid phase. ACS Catal. 2016, 6, 6948–6958. [Google Scholar] [CrossRef]
- Thomas, A.; Fischer, A.; Goettmann, F.; Antonietti, M.; Mueller, J.-O.; Schloegl, R.; Carlsson, J.M. Graphitic carbon nitride materials: Variation of structure and morphology and their use as metal-free catalysts. J. Mater. Chem. 2008, 18, 4893–4908. [Google Scholar] [CrossRef]
- Wang, X.; Sun, G.; Routh, P.; Kim, D.-H.; Huang, W.; Chen, P. Heteroatom-doped graphene materials: Syntheses, properties and applications. Chem. Soc. Rev. 2014, 43, 7067–7098. [Google Scholar] [CrossRef]
- Zhai, Y.; Zhu, Z.; Dong, S. Carbon-based nanostructures for advanced catalysis. ChemCatChem 2015, 7, 2806–2815. [Google Scholar] [CrossRef]
- Espinosa, J.C.; Navalon, S.; Alvaro, M.; Dhakshinamoorthy, A.; Garcia, H. Reduction of C═C double bonds by hydrazine using active carbons as metal-free catalysts. ACS Sustain. Chem. Eng. 2018, 6, 5607–5614. [Google Scholar] [CrossRef]
- Blandez, J.F.; Navalon, S.; Alvaro, M.; Garcia, H. Graphenes as metal-free catalysts for the oxidative depolymerization of lignin models. ChemCatChem 2015, 7, 3020–3026. [Google Scholar] [CrossRef]
- Primo, A.; Navalon, S.; Asiri, A.M.; Garcia, H. Chitosan-templated synthesis of few-layers boron nitride and its unforeseen activity as a fenton catalyst. Chem. Eur. J. 2015, 21, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Chen, Y.; Duan, X.; Zhou, S.; Niu, Y.; Sun, H.; Zhi, L.; Wang, S. Unzipping carbon nanotubes to nanoribbons for revealing the mechanism of nonradical oxidation by carbocatalysis. Appl. Catal. B Environ. 2020, 276, 119146. [Google Scholar] [CrossRef]
- Wang, Y.; Xi, J.; Duan, X.; Lv, W.; Cao, H.; Chen, C.; Guo, Z.; Xie, Y.; Wang, S. The duet of surface and radical-based carbocatalysis for oxidative destructions of aqueous contaminants over built-in nanotubes of graphite. J. Hazard. Mater. 2020, 384, 121486. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, L.; Chen, C.; Xi, J.; Cao, H.; Duan, X.; Xie, Y.; Song, W.; Wang, S. Occurrence of both hydroxyl radical and surface oxidation pathways in N-doped layered nanocarbons for aqueous catalytic ozonation. Appl. Catal. B Environ. 2019, 254, 283–291. [Google Scholar] [CrossRef]
- Cadus, L.E.; Arrua, L.A.; Gorriz, O.F.; Rivarola, J.B. Action of activated coke as a catalyst: Oxydehydrogenation of ethylbenzene to styrene. Ind. Eng. Chem. Res. 1988, 27, 2241–2246. [Google Scholar] [CrossRef]
- Emig, G.; Hofmann, H. Action of zirconium phosphate as a catalyst for the oxydehydrogenation of ethylbenzene to styrene. J. Catal. 1983, 84, 15–26. [Google Scholar] [CrossRef]
- Schraut, A.; Emig, G.; Sockel, H.G. Composition and structure of active coke in the oxydehydrogenation of ethylbenzene. Appl. Catal. 1987, 29, 311–326. [Google Scholar] [CrossRef]
- Vrieland, G.E. Oxydehydrogenation of ethylbenzene to styrene over metal pyrophosphates: 1. Catalyst composition and reaction variables. J. Catal. 1988, 111, 1–13. [Google Scholar] [CrossRef]
- Liu, X.; Dai, L. Carbon-based metal-free catalysts. Nat. Rev. Mater. 2016, 16064. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Jia, H.P.; Bielawski, C.W. Graphene Oxide: A convenient carbocatalyst for facilitating oxidation and hydration reactions. Angew. Chem. Int. Ed. 2010, 49, 6813–6816. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Chua, C.K.; Pumera, M. Chemical reduction of graphene oxide: A synthetic chemistry viewpoint. Chem. Soc. Rev. 2014, 43, 291–312. [Google Scholar] [CrossRef] [PubMed]
- Corma, A.; Garcia, H. Supported gold nanoparticles as catalysts for organic reactions. Chem. Soc. Rev. 2008, 37, 2096–2126. [Google Scholar] [CrossRef] [PubMed]
- Haruta, M.; Yamada, N.; Kobayashi, T.; Iijima, S. Gold catalysts prepared by coprecipitation for low-temperature oxidation of hydrogen and of carbon monoxide. J. Catal. 1989, 115, 301–309. [Google Scholar] [CrossRef]
- Hashmi, A.S.K.; Hutchings, G.J. Gold catalysis. Angew. Chem. Int. Ed. 2006, 45, 7896–7936. [Google Scholar] [CrossRef]
- Punniyamurthy, T.; Velusamy, S.; Iqbal, J. Recent advances in transition metal catalyzed oxidation of organic substrates with molecular oxygen. Chem. Rev. 2005, 105, 2329–2363. [Google Scholar] [CrossRef]
- Boukhvalov, D.W.; Dreyer, D.R.; Bielawski, C.W.; Son, Y.-W. A computational investigation of the catalytic properties of graphene oxide: Exploring mechanisms by using DFT methods. ChemCatChem 2012, 4, 1844–1849. [Google Scholar] [CrossRef]
- Espinosa, J.C.; Álvaro, M.; Dhakshinamoorthy, A.; Navalón, S.; García, H. Engineering active sites in reduced graphene oxide: Tuning the catalytic activity for aerobic oxidation. ACS Sustainable Chem. Eng. 2019, 7, 15948–15956. [Google Scholar] [CrossRef]
- Lam, J.; Szkop, K.M.; Mosaferi, E.; Stephan, D.W. FLP catalysis: Main group hydrogenations of organic unsaturated substrates. Chem. Soc. Rev. 2019, 48, 3592–3612. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, M.; Wang, A.; Zhang, T. Selective hydrogenation over supported metal catalysts: From nanoparticles to single atoms. Chem. Rev. 2019, 120, 683–733. [Google Scholar] [CrossRef]
- Bridier, B.; Lopez, N.; Perez-Ramirez, J. Molecular understanding of alkyne hydrogenation for the design of selective catalysts. Dalton Trans. 2010, 39, 8412–8419. [Google Scholar] [CrossRef]
- Teschner, D.; Borsodi, J.; Wootsch, A.; Révay, Z.; Hävecker, M.; Knop-Gericke, A.; Jackson, S.D.; Schlögl, R. The roles of subsurface carbon and hydrogen in palladium catalyzed alkyne hydrogenation. Science 2008, 320, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Navalón, S.; Álvaro, M.; Dhakshinamoorthy, A.; García, H. Encapsulation of metal nanoparticles within metal–organic frameworks for the reduction of nitro compounds. Molecules 2019, 24, 3050. [Google Scholar] [CrossRef] [PubMed]
- Serna, P.; Corma, A. Transforming nano metal nonselective particulates into chemoselective catalysts for hydrogenation of substituted nitrobenzenes. ACS Catal. 2015, 5, 7114–7121. [Google Scholar] [CrossRef]
- Primo, A.; Neatu, F.; Florea, M.; Parvulescu, V.; Garcia, H. Graphenes in the absence of metals as carbocatalysts for selective acetylene hydrogenation and alkene hydrogenation. Nat. Commun. 2014, 5, 5291. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.J.; Fuchter, M.J.; Ashley, A.E. Designing effective 'frustrated Lewis pair' hydrogenation catalysts. Chem. Soc. Rev. 2017, 46, 5689–5700. [Google Scholar] [CrossRef] [PubMed]
- Stephan, D.W.; Erker, G. Frustrated lewis pairs: Metal-free hydrogen activation and more. Angew. Chem. Int. Ed. 2010, 49, 46–76. [Google Scholar] [CrossRef]
- Ding, Y.; Huang, X.; Yi, X.; Qiao, Y.; Sun, X.; Zheng, A.; Su, D.S. A heterogeneous metal-free catalyst for hydrogenation: Lewis acid–base pairs integrated into a carbon lattice. Angew. Chem. Int. Ed. 2018, 57, 13800–13804. [Google Scholar] [CrossRef]
- Trandafir, M.-M.; Florea, M.; Neatu, F.; Primo, A.; Parvulescu, V.I.; Garcia, H. Graphene from alginate pyrolysis as a metal-free catalyst for hydrogenation of nitro compounds. ChemSusChem 2016, 9, 1565–1569. [Google Scholar] [CrossRef]
- Jurca, B.; Bucur, C.; Primo, A.; Concepción, P.; Parvulescu, V.I.; García, H. N-doped defective graphene from biomass as catalyst for CO2 hydrogenation to methane. ChemCatChem 2019, 11, 985–990. [Google Scholar] [CrossRef]
- Dou, J.; Sun, Z.C.; Opalade, A.A.; Wang, N.; Fu, W.S.; Tao, F. Operando chemistry of catalyst surfaces during catalysis. Chem. Soc. Rev. 2017, 46, 2001–2027. [Google Scholar] [CrossRef] [PubMed]
- Li, X.N.; Wang, H.Y.; Yang, H.B.; Cai, W.Z.; Liu, S.; Liu, B. In situ/operando characterization techniques to probe the electrochemical reactions for energy conversion. Small Methods 2018, 2, 1700395. [Google Scholar] [CrossRef]
- Li, X.; Wang, S.; Li, L.; Sun, Y.; Xie, Y. Progress and perspective for in situ studies of CO2 reduction. J. Am. Chem. Soc. 2020, 142, 9567–9581. [Google Scholar] [PubMed]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navalón, S.; Ong, W.-J.; Duan, X. Sustainable Catalytic Processes Driven by Graphene-Based Materials. Processes 2020, 8, 672. https://doi.org/10.3390/pr8060672
Navalón S, Ong W-J, Duan X. Sustainable Catalytic Processes Driven by Graphene-Based Materials. Processes. 2020; 8(6):672. https://doi.org/10.3390/pr8060672
Chicago/Turabian StyleNavalón, Sergio, Wee-Jun Ong, and Xiaoguang Duan. 2020. "Sustainable Catalytic Processes Driven by Graphene-Based Materials" Processes 8, no. 6: 672. https://doi.org/10.3390/pr8060672
APA StyleNavalón, S., Ong, W.-J., & Duan, X. (2020). Sustainable Catalytic Processes Driven by Graphene-Based Materials. Processes, 8(6), 672. https://doi.org/10.3390/pr8060672






