Efficacies of Carbon-Based Adsorbents for Carbon Dioxide Capture
Abstract
1. Introduction
2. Efficacy of CBMs for CO2 Capture
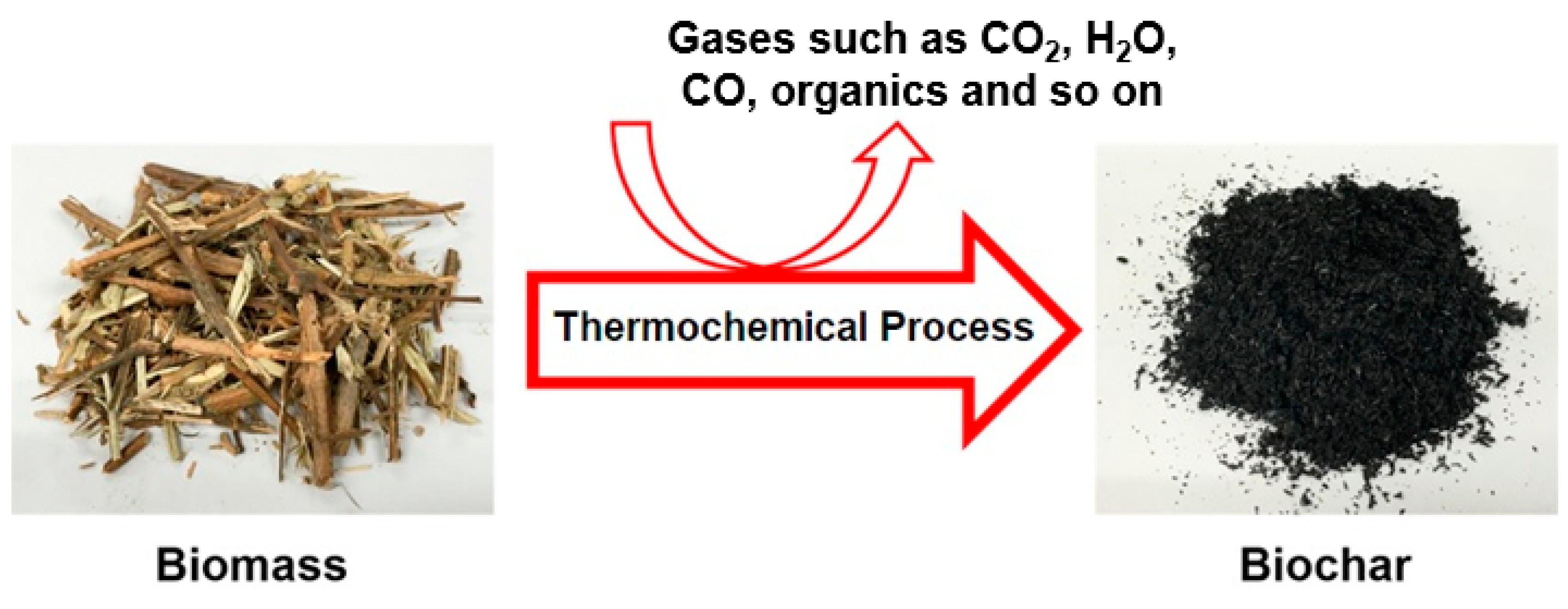
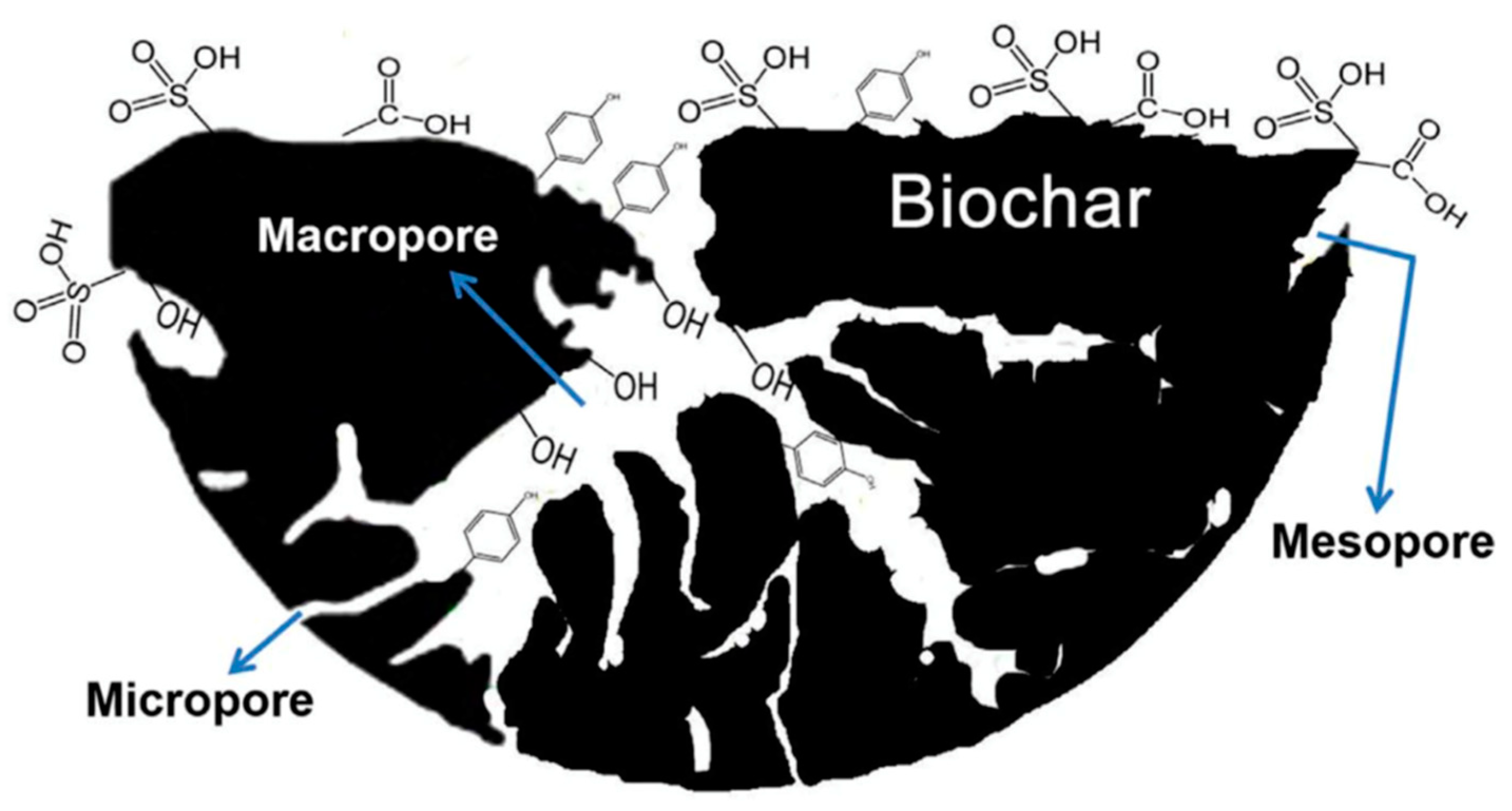
2.1. Biochar for CO2 Capture
2.2. Graphene, Graphene Oxide, and Carbon Nanotubes (CNTs) for CO2 Capture
2.3. Activated Carbons (ACs) for CO2 Capture
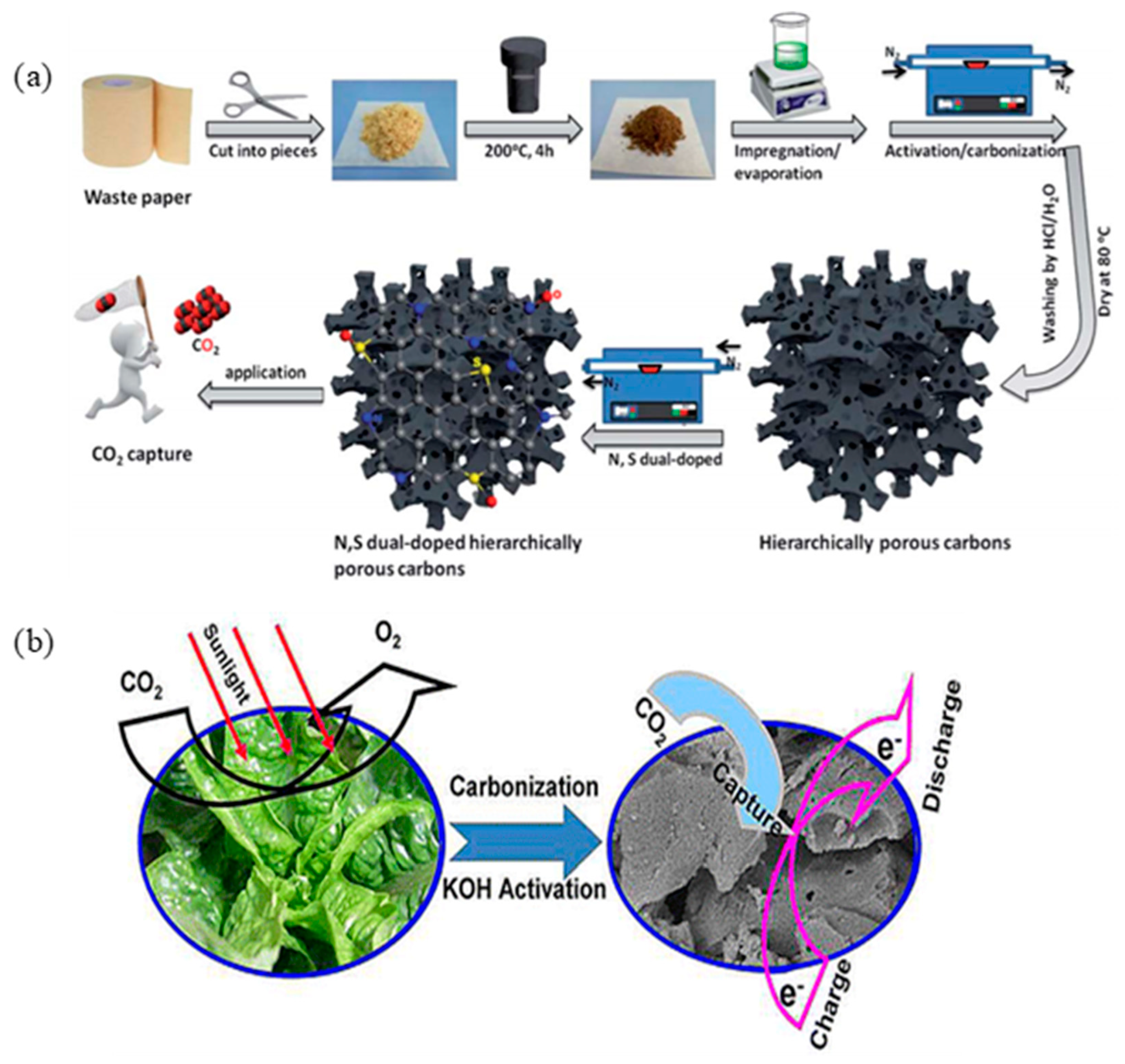
2.4. Microporous, Mesoporous, and Hierarchical Porous Carbons for CO2 Capture
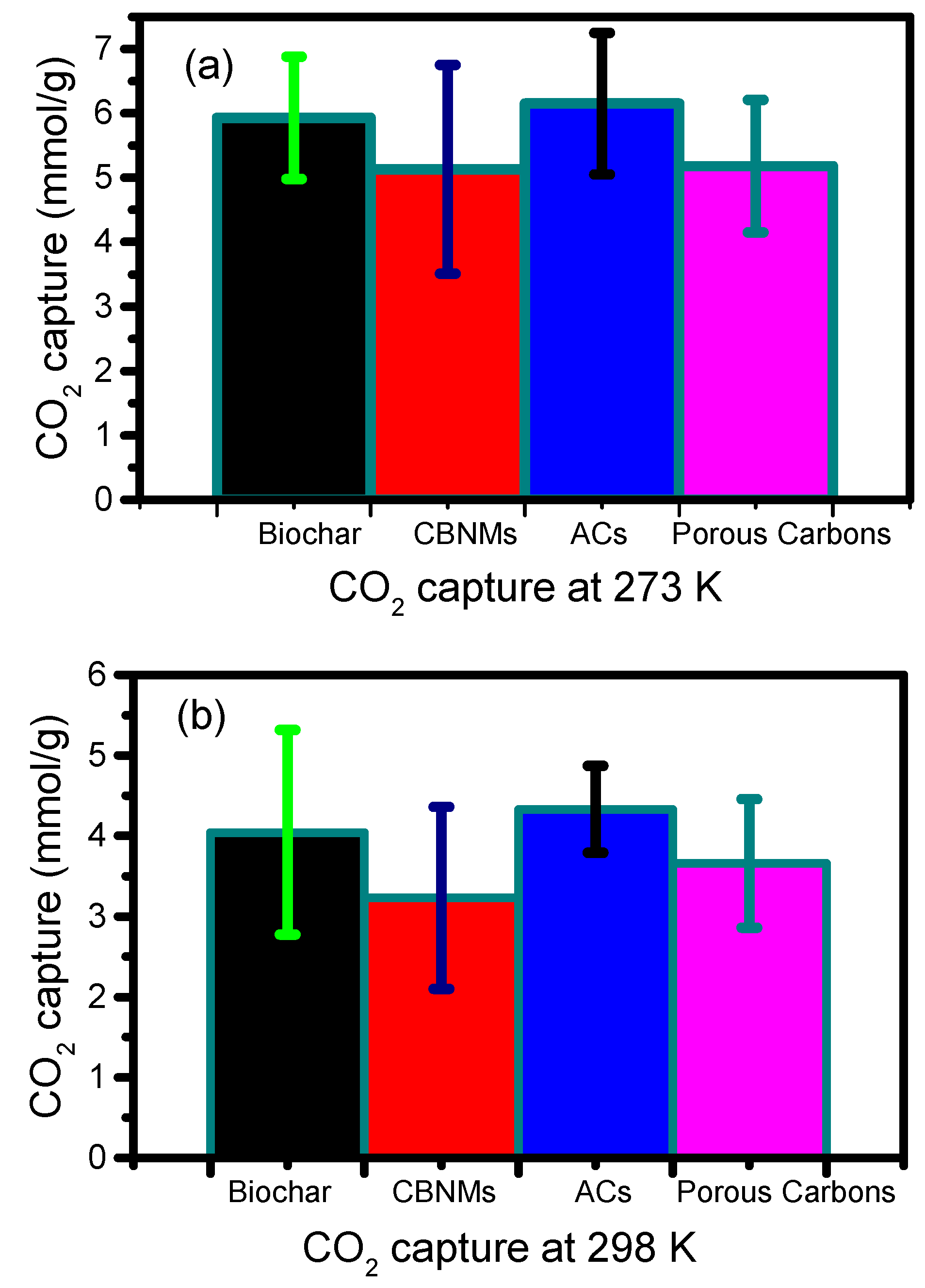
3. Comparative Analysis of CBMs Performances
4. Future Challenges and Opportunities
- Developments of the novel composite to improve the capture performance of CO2 of CBMs.
- A need to properly understate the CO2 interactions with CBMs. For this reason, new analytical tools are needed to develop.
- Ensuring the regeneration efficiency for repeatable applications. Regeneration mechanisms also need to study in detail.
- Development of new technologies for the efficient capture of CO2.
- A highly efficient carbon-based catalyst needs to develop for the conversion of CO2 into valuable fuels, such as methane.
- Low-cost materials with high adsorption capacity need to develop.
- Most of the CBMs have been used for CO2 capture on a lab-scale basis, i.e., from ambient air. However, studies are not enough. Therefore, more studies are required.
- Other types of materials, such as metal-organic frameworks, porous silica, resin, amine derivatives sorbents, and new types of materials, need to produce with lower cost for the scale-up process.
- These coatings of sorbents can help for faster heat and mass transfer, as well as can reduce energy losses. Therefore, these kinds of sorbents need to develop.
- Detailed kinetics of sorption and mechanisms need to be focused on more clearly.
- Combining together and application of the different existing technologies can reduce the cost of the capture of CO2.
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siriwardane, R.V.; Shen, M.; Fisher, E.P.; Poston, J.A. Adsorption of CO2 on molecular sieves and activated carbon. Energy Fuels 2001, 15, 279–284. [Google Scholar] [CrossRef]
- Lopes, F.V.S.; Grande, C.A.; Ribeiro, A.M.; Loureiro, J.M.; Evaggelos, O.; Nikolakis, V.; Rodrigues, A.E. Adsorption of H2, CO2, CH4, CO, N2 and H2O in activated carbon and zeolite for hydrogen production. Sep. Sci. Technol. 2009, 44, 1045–1073. [Google Scholar] [CrossRef]
- Haque, E.; Islam, M.M.; Pourazadi, E.; Sarkar, S.; Harris, A.T.; Minett, A.I.; Yanmaz, E.; Alshehri, S.M.; Ide, Y.; Wu, K.C.W. Boron-functionalized graphene oxide-organic frameworks for highly efficient CO2 capture. Chem. Asian. J. 2017, 12, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Creamer, A.E.; Gao, B. Carbon-based adsorbents for postcombustion CO2 capture: A critical review. Environ. Sci. Technol. 2016, 50, 7276–7289. [Google Scholar] [CrossRef]
- Alam, M.M.; Hossain, M.A.; Hossain, M.D.; Johir, M.; Hossen, J.; Rahman, M.S.; Zhou, J.L.; Hasan, A.; Karmakar, A.K.; Ahmed, M.B. The potentiality of rice husk-derived activated carbon: From synthesis to application. Processes 2020, 8, 203. [Google Scholar] [CrossRef]
- Rubin, E.; De Coninck, H. IPCC special report on carbon dioxide capture and storage. In TNO (2004): Cost Curves for CO2 Storage, Part 2; Cambridge University Press: Cambridge, UK, 2005; Volume 2, p. 14. [Google Scholar]
- Li, J.R.; Ma, Y.; McCarthy, M.C.; Sculley, J.; Yu, J.; Jeong, H.K.; Balbuena, P.B.; Zhou, H.C. Carbon dioxide capture-related gas adsorption and separation in metal-organic frameworks. Coord. Chem. Rev. 2011, 255, 1791–1823. [Google Scholar] [CrossRef]
- D’Alessandro, D.M.; Smit, B.; Long, J.R. Carbon dioxide capture: Prospects for new materials. Angew. Chem. Int. Ed. 2010, 49, 6058–6082. [Google Scholar] [CrossRef]
- Shi, X.; Xiao, H.; Lackner, K.S.; Chen, X. Capture CO2 from ambient air using nanoconfined ion hydration. Angew. Chem. 2016, 128, 4094–4097. [Google Scholar] [CrossRef]
- Shi, X.; Xiao, H.; Azarabadi, H.; Song, J.; Wu, X.; Chen, X.; Lackner, K.S. Sorbents for direct capture of CO2 from ambient air. Angew. Chem. Int. Ed. 2020, 99, 6984–7006. [Google Scholar] [CrossRef]
- Ren, X.; Li, H.; Chen, J.; Wei, L.; Modak, A.; Yang, H.; Yang, Q. N-doped porous carbons with exceptionally high CO2 selectivity for CO2 capture. Carbon 2017, 114, 473–481. [Google Scholar] [CrossRef]
- Hao, G.P.; Jin, Z.Y.; Sun, Q.; Zhang, X.Q.; Zhang, J.T.; Lu, A.H. Porous carbon nanosheets with precisely tunable thickness and selective CO2 adsorption properties. Energy Environ. Sci. 2013, 6, 3740–3747. [Google Scholar] [CrossRef]
- Zhang, L.H.; Li, W.C.; Tang, L.; Wang, Q.G.; Hu, Q.T.; Zhang, Y.; Lu, A.H. Primary amine modulated synthesis of two-dimensional porous nanocarbons with tunable ultramicropores. J. Mater. Chem. A 2018, 6, 24285–24290. [Google Scholar] [CrossRef]
- Qian, D.; Lei, C.; Wang, E.M.; Li, W.C.; Lu, A.H. A method for creating microporous carbon materials with excellent CO2-adsorption capacity and selectivity. ChemSusChem 2014, 7, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Drage, T.C.; Kozynchenko, O.; Pevida, C.; Plaza, M.G.; Rubiera, F.; Pis, J.; Snape, C.E.; Tennison, S. Developing activated carbon adsorbents for pre-combustion CO2 capture. Energy Proced. 2009, 1, 599–605. [Google Scholar] [CrossRef]
- Lu, C.; Bai, H.; Wu, B.; Su, F.; Hwang, J.F. Comparative study of CO2 capture by carbon nanotubes, activated carbons, and zeolites. Energy Fuels 2008, 22, 3050–3056. [Google Scholar] [CrossRef]
- Yu, C.H.; Huang, C.H.; Tan, C.S. A review of CO2 capture by absorption and adsorption. Aerosol Air Qual. Res. 2012, 12, 745–769. [Google Scholar] [CrossRef]
- Kim, I.; Svendsen, H.F. Heat of absorption of carbon dioxide (CO2) in monoethanolamine (MEA) and 2-(aminoethyl) ethanolamine (AEEA) solutions. Ind. Eng. Chem. 2007, 46, 5803–5809. [Google Scholar] [CrossRef]
- Choi, S.; Drese, J.H.; Jones, C.W. Adsorbent materials for carbon dioxide capture from large anthropogenic point sources. ChemSusChem: Chem. Sust. Energy Mater. 2009, 2, 796–854. [Google Scholar] [CrossRef]
- Qian, K.; Kumar, A.; Zhang, H.; Bellmer, D.; Huhnke, R. Recent advances in utilization of biochar. Renew. Sust. Energy Rev. 2015, 42, 1055–1064. [Google Scholar] [CrossRef]
- Tan, X.F.; Liu, S.B.; Liu, Y.G.; Gu, Y.L.; Zeng, G.M.; Hu, X.J.; Wang, X.; Liu, S.H.; Jiang, L.H. Biochar as potential sustainable precursors for activated carbon production: Multiple applications in environmental protection and energy storage. Bioresour. Technol. 2017, 227, 359–372. [Google Scholar] [CrossRef]
- Woolf, D.; Amonette, J.E.; Street-Perrott, F.A.; Lehmann, J.; Joseph, S. Sustainable biochar to mitigate global climate change. Nat. Commun. 2010, 1, 56. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W. Insight into biochar properties and its cost analysis. Biomass Bioenerg. 2016, 84, 76–86. [Google Scholar] [CrossRef]
- Lee, J.; Kim, K.H.; Kwon, E.E. Biochar as a catalyst. Renew. Sustain. Energy Rev. 2017, 77, 70–79. [Google Scholar] [CrossRef]
- You, S.; Ok, Y.S.; Chen, S.S.; Tsang, D.C.; Kwon, E.E.; Lee, J.; Wang, C.H. A critical review on sustainable biochar system through gasification: Energy and environmental applications. Bioresour. Technol. 2017, 246, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.; Gaunt, J.; Rondon, M. Bio-char sequestration in terrestrial ecosystems–a review. Mitig. Adapt. Strat. Glob. Change 2006, 11, 403–427. [Google Scholar] [CrossRef]
- Balahmar, N.; Mitchell, A.C.; Mokaya, R. Generalized Mechanochemical Synthesis of Biomass-derived sustainable carbons for high performance CO2 storage. Adv. Energy Mater. 2015, 5. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. Sustainable porous carbons with a superior performance for CO2 capture. Energy Environ. Sci. 2011, 4, 1765–1771. [Google Scholar] [CrossRef]
- Nanda, S.; Dalai, A.K.; Berruti, F.; Kozinski, J.A. Biochar as an exceptional bioresource for energy, agronomy, carbon sequestration, activated carbon and specialty materials. Waste Biomass Valori. 2016, 7, 201–235. [Google Scholar] [CrossRef]
- Guizani, C.; Haddad, K.; Jeguirim, M.; Colin, B.; Limousy, L. Combustion characteristics and kinetics of torrefied olive pomace. Energy 2016, 107, 453–463. [Google Scholar] [CrossRef]
- Rousset, P.; Macedo, L.; Commandre, J.M.; Moreira, A. Biomass torrefaction under different oxygen concentrations and its effect on the composition of the solid by-product. J. Anal. Appl. Pyrol. 2012, 96, 86–91. [Google Scholar] [CrossRef]
- Ullah, H.; Liu, G.J.; Yousaf, B.; Ali, M.U.; Abbas, Q.; Zhou, C.C. Combustion characteristics and retention-emission of selenium during co-firing of torrefied biomass and its blends with high ash coal. Bioresour. Technol. 2017, 245, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.J.; Jiang, H.; Yu, H.Q. Development of biochar-based functional materials: Toward a sustainable platform carbon material. Chem. Rev. 2015, 115, 12251–12285. [Google Scholar] [CrossRef] [PubMed]
- Keiluweit, M.; Nico, P.S.; Johnson, M.G.; Kleber, M. Dynamic molecular structure of plant biomass-derived black carbon (biochar). Environ. Sci. Technol. 2010, 44, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Mohd, A.; Ghani, W.A.W.A.; Resitanim, N.Z.; Sanyang, L. A Review: Carbon dioxide capture: Biomass-derived-biochar and its applications. J. Disper. Sci. Technol. 2013, 34, 974–984. [Google Scholar] [CrossRef]
- Manyà, J.J.; González, B.; Azuara, M.; Arner, G. Ultra-microporous adsorbents prepared from vine shoots-derived biochar with high CO2 uptake and CO2/N2 selectivity. Chem. Eng. 2018, 345, 631–639. [Google Scholar] [CrossRef]
- Ello, A.S.; de Souza, L.K.; Trokourey, A.; Jaroniec, M. Development of microporous carbons for CO2 capture by KOH activation of African palm shells. J. CO2 Util. 2013, 2, 35–38. [Google Scholar] [CrossRef]
- Li, D.W.; Ma, T.F.; Zhang, R.L.; Tian, Y.Y.; Qiao, Y.Y. Preparation of porous carbons with high low-pressure CO2 uptake by KOH activation of rice husk char. Fuel 2015, 139, 68–70. [Google Scholar] [CrossRef]
- Deng, S.B.; Wei, H.R.; Chen, T.; Wang, B.; Huang, J.; Yu, G. Superior CO2 adsorption on pine nut shell-derived activated carbons and the effective micropores at different temperatures. Chem. Eng. 2014, 253, 46–54. [Google Scholar] [CrossRef]
- Hong, S.M.; Jang, E.; Dysart, A.D.; Pol, V.G.; Lee, K.B. CO2 capture in the sustainable wheat-derived activated microporous carbon compartments. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Coromina, H.M.; Walsh, D.A.; Mokaya, R. Biomass-derived activated carbon with simultaneously enhanced CO2 uptake for both pre and post combustion capture applications. J. Mater. Chem. A 2016, 4, 280–289. [Google Scholar] [CrossRef]
- Serafin, J.; Narkiewicz, U.; Morawski, A.W.; Wrobel, R.J.; Michalkiewicz, B. Highly microporous activated carbons from biomass for CO2 capture and effective micropores at different conditions. J. CO2 Util. 2017, 18, 73–79. [Google Scholar] [CrossRef]
- Zhang, C.M.; Song, W.; Ma, Q.L.; Xie, L.J.; Zhang, X.C.; Guo, H. Enhancement of CO2 capture on biomass-based carbon from black locust by KOH activation and ammonia modification. Energy Fuels 2016, 30, 4181–4190. [Google Scholar] [CrossRef]
- Rouzitalab, Z.; Maklavany, D.M.; Rashidi, A.; Jafarinejad, S. Synthesis of N-doped nanoporous carbon from walnut shell for enhancing CO2 adsorption capacity and separation. J. Environ. Chem. Eng. 2018, 6, 6653–6663. [Google Scholar] [CrossRef]
- Zhu, B.J.; Shang, C.X.; Guo, Z.X. Naturally nitrogen and calcium-doped nanoporous carbon from pine cone with superior CO2 capture capacities. ACS Sustain. Chem. Eng. 2016, 4, 1050–1057. [Google Scholar] [CrossRef]
- Bamdad, H.; Hawboldt, K.; MacQuarrie, S. Nitrogen functionalized biochar as a renewable adsorbent for efficient CO2 removal. Energy Fuels 2018, 32, 11742–11748. [Google Scholar] [CrossRef]
- Lahijani, P.; Mohammadi, M.; Mohamed, A.R. Metal incorporated biochar as a potential adsorbent for high capacity CO2 capture at ambient condition. J. CO2 Util. 2018, 26, 281–293. [Google Scholar] [CrossRef]
- Dissanayake, P.D.; You, S.; Igalavithana, A.D.; Xia, Y.; Bhatnagar, A.; Gupta, S.; Kua, H.W.; Kim, S.; Kwon, J.H.; Tsang, D.C.W.; et al. Biochar-based adsorbents for carbon dioxide capture: A critical review. Renew. Sustain. Energy Rev. 2020, 119, 109582. [Google Scholar] [CrossRef]
- Chowdhury, S.; Balasubramanian, R. Three-dimensional graphene-based porous adsorbents for postcombustion CO2 capture. Ind. Eng. Chem. 2016, 55, 7906–7916. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Atarod, M.; Jaleh, B.; Gandomirouzbahani, M. In situ green synthesis of Ag nanoparticles on graphene oxide/TiO2 nanocomposite and their catalytic activity for the reduction of 4-nitrophenol, congo red and methylene blue. Ceram. Int. 2016, 42, 8587–8596. [Google Scholar] [CrossRef]
- Liu, Y.; Xiang, M.; Hong, L. Three-dimensional nitrogen and boron codoped graphene for carbon dioxide and oils adsorption. RSC Adv. 2017, 7, 6467–6473. [Google Scholar] [CrossRef]
- Bhanja, P.; Das, S.K.; Patra, A.K.; Bhaumik, A. Functionalized graphene oxide as an efficient adsorbent for CO2 capture and support for heterogeneous catalysis. RSC Adv. 2016, 6, 72055–72068. [Google Scholar] [CrossRef]
- Politakos, N.; Barbarin, I.; Cantador, L.S.; Cecilia, J.A.; Mehravar, E.; Tomovska, R. Graphene-based Monolithic Nanostructures for CO2 Capture. Ind. Eng. Chem. Res. 2020, 59, 8612–8621. [Google Scholar] [CrossRef]
- Huang, A.; Feng, B. Facile synthesis of PEI-GO@ ZIF-8 hybrid material for CO2 capture. Int. J. Hydrog. Energy 2018, 43, 2224–2231. [Google Scholar] [CrossRef]
- Rahimi, M.; Babu, D.J.; Singh, J.K.; Yang, Y.B.; Schneider, J.J.; Müller-Plathe, F. Double-walled carbon nanotube array for CO2 and SO2 adsorption. J. Chem. Phys. 2015, 143, 124701. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Bai, J.; Francisco, J.S.; Zeng, X.C. Formation of CO2 hydrates within single-walled carbon nanotubes at ambient pressure: CO2 capture and selective separation of a CO2/H2 mixture in water. J. Phys. Chem. 2018, 122, 7951–7958. [Google Scholar] [CrossRef]
- Cortés-Súarez, J.; Celis-Arias, V.; Beltrán, H.I.; Tejeda-Cruz, A.; Ibarra, I.A.; Romero-Ibarra, J.E.; Sánchez-González, E.; Loera-Serna, S. Synthesis and characterization of an SWCNT@ HKUST-1 composite: Enhancing the CO2 adsorption properties of HKUST-1. ACS Omega 2019, 4, 5275–5282. [Google Scholar] [CrossRef]
- Kemp, K.C.; Chandra, V.; Saleh, M.; Kim, K.S. Reversible CO2 adsorption by an activated nitrogen doped graphene/polyaniline material. Nanotechnology 2013, 24, 235703. [Google Scholar] [CrossRef]
- Shin, G.J.; Rhee, K.; Park, S.J. Improvement of CO2 capture by graphite oxide in presence of polyethylenimine. Int. J. Hydrog. Energy 2016, 41, 14351–14359. [Google Scholar] [CrossRef]
- Deng, M.; Park, H.G. Spacer-assisted amine-coiled carbon nanotubes for CO2 capture. Langmuir 2019, 35, 4453–4459. [Google Scholar] [CrossRef]
- Gromov, A.; Kulur, A.; Gibson, J.; Mangano, E.; Brandani, S.; Campbell, E. Carbon nanotube/PVA aerogels impregnated with PEI: Solid adsorbents for CO2 capture. Sustain. Energy Fuels 2018, 2, 1630–1640. [Google Scholar] [CrossRef]
- Alhwaige, A.A.; Agag, T.; Ishida, H.; Qutubuddin, S. Biobased chitosan hybrid aerogels with superior adsorption: Role of graphene oxide in CO2 capture. RSC Adv. 2013, 3, 16011–16020. [Google Scholar] [CrossRef]
- Alhwaige, A.A.; Ishida, H.; Qutubuddin, S. Carbon aerogels with excellent CO2 adsorption capacity synthesized from clay-reinforced biobased chitosan-polybenzoxazine nanocomposites. ACS Sustain. Chem. Eng. 2016, 4, 1286–1295. [Google Scholar] [CrossRef]
- Sircar, S.; Golden, T.C.; Rao, M.B. Activated carbon for gas separation and storage. Carbon 1996, 3, 1–12. [Google Scholar] [CrossRef]
- Hayashi, J.; Kazehaya, A.; Muroyama, K.; Watkinson, A.P. Preparation of activated carbon from lignin by chemical activation. Carbon 2000, 38, 1873–1878. [Google Scholar] [CrossRef]
- Ge, C.; Lian, D.; Cui, S.; Gao, J.; Lu, J. Highly selective CO2 capture on waste polyurethane foam-based activated carbon. Processes 2019, 7, 592. [Google Scholar] [CrossRef]
- Borhan, A.; Yusup, S.; Lim, J.W.; Show, P.L. Characterization and modelling studies of activated carbon produced from rubber-seed shell using KOH for CO2 adsorption. Processes 2019, 7, 855. [Google Scholar] [CrossRef]
- Basheer, O.A.; Hanafiah, M.M.; Abdulhakim Alsaadi, M.; Al-Douri, Y.; Malek, M.A.; Mohammed Aljumaily, M.; Saadi Fiyadh, S. Synthesis and characterization of natural extracted precursor date palm fibre-based activated carbon for aluminum removal by RSM optimization. Processes 2019, 7, 249. [Google Scholar] [CrossRef]
- Shao, X.; Feng, Z.; Xue, R.; Ma, C.; Wang, W.; Peng, X.; Cao, D. Adsorption of CO2, CH4, CO2/N2 and CO2/CH4 in novel activated carbon beads: Preparation, measurements and simulation. AIChE J. 2011, 57, 3042–3051. [Google Scholar] [CrossRef]
- Chen, J.; Yang, J.; Hu, G.; Hu, X.; Li, Z.; Shen, S.; Radosz, M.; Fan, M. Enhanced CO2 capture capacity of nitrogen-doped biomass-derived porous carbons. ACS Sustain. Chem. Eng. 2016, 4, 1439–1445. [Google Scholar] [CrossRef]
- Li, Y.; Li, D.; Rao, Y.; Zhao, X.; Wu, M. Superior CO2, CH4, and H2 uptakes over ultrahigh-surface-area carbon spheres prepared from sustainable biomass-derived char by CO2 activation. Carbon 2016, 105, 454–462. [Google Scholar] [CrossRef]
- Chiang, Y.C.; Yeh, C.Y.; Weng, C.H. Carbon Dioxide Adsorption on Porous and Functionalized Activated Carbon Fibers. Appl. Sci. 2019, 9, 1977. [Google Scholar] [CrossRef]
- Shi, W.; Wang, R.; Liu, H.; Chang, B.; Yang, B.; Zhang, Z. Biowaste-derived 3D honeycomb-like N and S dual-doped hierarchically porous carbons for high-efficient CO2 capture. RSC Adv. 2019, 9, 23241–23253. [Google Scholar] [CrossRef]
- Wang, R.; Wang, P.; Yan, X.; Lang, J.; Peng, C.; Xue, Q. Promising porous carbon derived from celtuce leaves with outstanding supercapacitance and CO2 capture performance. ACS Appl. Mater. 2012, 4, 5800–5806. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Chen, H.; Fu, N.; Chen, J.; Lan, G.; Qian, W.; Liu, Y.; Lin, H.; Han, S. Excellent electrochemical properties and large CO2 capture of nitrogen-doped activated porous carbon synthesised from waste longan shells. Electrochim. Acta 2017, 231, 403–411. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Johir, M.A.H.; Zhou, J.L.; Ngo, H.H.; Nghiem, L.D.; Richardson, C.; Moni, M.A.; Bryant, M.R. Activated carbon preparation from biomass feedstock: Clean production and carbon dioxide adsorption. J. Clean. Prod. 2019, 225, 405–413. [Google Scholar] [CrossRef]
- Ello, A.S.; de Souza, L.K.; Trokourey, A.; Jaroniec, M. Coconut shell-based microporous carbons for CO2 capture. Microporous Mesoporous Mater. 2013, 180, 280–283. [Google Scholar] [CrossRef]
- Parshetti, G.K.; Chowdhury, S.; Balasubramanian, R. Biomass derived low-cost microporous adsorbents for efficient CO2 capture. Fuel 2015, 148, 246–254. [Google Scholar] [CrossRef]
- Demir, M.; Tessema, T.D.; Farghaly, A.A.; Nyankson, E.; Saraswat, S.K.; Aksoy, B.; Islamoglu, T.; Collinson, M.M.; El-Kaderi, H.M.; Gupta, R.B. Lignin-derived heteroatom-doped porous carbons for supercapacitor and CO2 capture applications. Int. J. Energy Res. 2018, 42, 2686–2700. [Google Scholar] [CrossRef]
- Yu, D.; Hu, J.; Zhou, L.; Li, J.; Tang, J.; Peng, C.; Liu, H. Nitrogen-doped coal tar pitch based microporous carbons with superior CO2 capture performance. Energy Fuels 2018, 32, 3726–3732. [Google Scholar] [CrossRef]
- Shafeeyan, M.S.; Daud, W.M.A.W.; Houshmand, A.; Shamiri, A. A review on surface modification of activated carbon for carbon dioxide adsorption. J. Anal. Appl. Pyrolysis 2010, 89, 143–151. [Google Scholar] [CrossRef]
- Petrova, B.; Budinova, T.; Petrov, N.; Yardim, M.; Ekinci, E.; Razvigorova, M. Effect of different oxidation treatments on the chemical structure and properties of commercial coal tar pitch. Carbon 2005, 43, 261–267. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Li, W.C.; Lu, A.H. Designed porous carbon materials for efficient CO2 adsorption and separation. New Carbon Mater. 2015, 30, 481–501. [Google Scholar] [CrossRef]
- Long, L.; Jiang, X.; Liu, J.; Han, D.; Xiao, M.; Wang, S.; Meng, Y. In situ template synthesis of hierarchical porous carbon used for high performance lithium–sulfur batteries. RSC Adv. 2018, 8, 4503–4513. [Google Scholar] [CrossRef]
- Cox, M.; Mokaya, R. Ultra-high surface area mesoporous carbons for colossal pre combustion CO2 capture and storage as materials for hydrogen purification. Sustain. Energy Fuels 2017, 1, 1414–1424. [Google Scholar] [CrossRef]
- Huang, K.; Chai, S.H.; Mayes, R.T.; Tan, S.; Jones, C.W.; Dai, S. Significantly increasing porosity of mesoporous carbon by NaNH2 activation for enhanced CO2 adsorption. Microporous Mesoporous Mater. 2016, 230, 100–108. [Google Scholar] [CrossRef]
- Lu, J.; Jiao, C.; Majeed, Z.; Jiang, H. Magnesium and Nitrogen Co-Doped Mesoporous Carbon with Enhanced Microporosity for CO2 Adsorption. Nanomaterials 2018, 8, 275. [Google Scholar] [CrossRef]
- Yaumi, A.; Bakar, M.A.; Hameed, B. Reusable nitrogen-doped mesoporous carbon adsorbent for carbon dioxide adsorption in fixed-bed. Energy 2017, 138, 776–784. [Google Scholar] [CrossRef]
- Park, D.H.; Lakhi, K.S.; Ramadass, K.; Kim, M.K.; Talapaneni, S.N.; Joseph, S.; Ravon, U.; Al-Bahily, K.; Vinu, A. Energy efficient synthesis of ordered mesoporous carbon nitrides with a high nitrogen content and enhanced CO2 capture capacity. Chem. Eur. J. 2017, 23, 10753–10757. [Google Scholar] [CrossRef]
- Pei, Y.R.; Choi, G.; Asahina, S.; Yang, J.H.; Vinu, A.; Choy, J.H. A novel geopolymer route to porous carbon: High CO2 adsorption capacity. Chem. Comm. 2019, 55, 3266–3269. [Google Scholar] [CrossRef]
- Srinivas, G.; Krungleviciute, V.; Guo, Z.X.; Yildirim, T. Exceptional CO2 capture in a hierarchically porous carbon with simultaneous high surface area and pore volume. Energy Environ. Sci. 2014, 7, 335–342. [Google Scholar] [CrossRef]
- Lu, A.H.; Hao, G.P.; Zhang, X.Q. Porous carbons for carbon dioxide capture. In Porous Materials for Carbon Dioxide Capture; Springer: Berlin, Germany, 2014; pp. 15–77. [Google Scholar]
- Yuan, B.; Wu, X.; Chen, Y.; Huang, J.; Luo, H.; Deng, S. Adsorption of CO2, CH4, and N2 on ordered mesoporous carbon: Approach for greenhouse gases capture and biogas upgrading. Environ. Sci. Technol. 2013, 47, 5474–5480. [Google Scholar] [CrossRef]
- Zhang, Z.; Luo, D.; Lui, G.; Li, G.; Jiang, G.; Cano, Z.P.; Deng, Y.P.; Du, X.; Yin, S.; Chen, Y. In-situ ion-activated carbon nanospheres with tunable ultramicroporosity for superior CO2 capture. Carbon 2019, 143, 531–541. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, H.; Wang, G.; Du, J.; Zhang, Y.; Fu, X.; Chen, A. Synthesis of mesoporous carbon nanospheres via “pyrolysis-deposition” strategy for CO2 capture. J. Mater. Sci. 2017, 52, 9640–9647. [Google Scholar] [CrossRef]
- Zhou, J.; Li, Z.; Xing, W.; Zhu, T.; Shen, H.; Zhuo, S. N-doped microporous carbons derived from direct carbonization of K+ exchanged meta-aminophenol–formaldehyde resin for superior CO2 sorption. Chem. Comm. 2015, 51, 4591–4594. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Z.; Jia, Z.; Zhao, L.; Zhang, T.; Xing, W.; Komarneni, S.; Subhan, F.; Yan, Z. New strategy to prepare ultramicroporous carbon by ionic activation for superior CO2 capture. Chem. Eng. J. 2018, 337, 290–299. [Google Scholar] [CrossRef]
- Robertson, C.; Mokaya, R. Microporous activated carbon aerogels via a simple subcritical drying route for CO2 capture and hydrogen storage. Microporous Mesoporous Mater. 2013, 179, 151–156. [Google Scholar] [CrossRef]
- Xing, W.; Liu, C.; Zhou, Z.; Zhang, L.; Zhou, J.; Zhuo, S.; Yan, Z.; Gao, H.; Wang, G.; Qiao, S.Z. Superior CO2 uptake of N-doped activated carbon through hydrogen-bonding interaction. Energy Environ. Sci. 2012, 5, 7323–7327. [Google Scholar] [CrossRef]
- Chang, B.; Sun, L.; Shi, W.; Zhang, S.; Yang, B. Cost-Efficient Strategy for Sustainable Cross-Linked Microporous Carbon Bead with Satisfactory CO2 Capture Capacity. ACS Omega 2018, 3, 5563–5573. [Google Scholar] [CrossRef]
- Fan, X.; Zhang, L.; Zhang, G.; Shu, Z.; Shi, J. Chitosan derived nitrogen-doped microporous carbons for high performance CO2 capture. Carbon 2013, 61, 423–430. [Google Scholar] [CrossRef]
- Manmuanpom, N.; Thubsuang, U.; Dubas, S.T.; Wongkasemjit, S.; Chaisuwan, T. Enhanced CO2 capturing over ultra-microporous carbon with nitrogen-active species prepared using one-step carbonization of polybenzoxazine for a sustainable environment. J. Environ. Manag. 2018, 223, 779–786. [Google Scholar] [CrossRef]
- Seema, H.; Kemp, K.C.; Le, N.H.; Park, S.-W.; Chandra, V.; Lee, J.W.; Kim, K.S. Highly selective CO2 capture by S-doped microporous carbon materials. Carbon 2014, 66, 320–326. [Google Scholar] [CrossRef]
- Hao, G.P.; Li, W.C.; Qian, D.; Lu, A.H. Rapid synthesis of nitrogen-doped porous carbon monolith for CO2 capture. Adv. Mater. 2010, 22, 853–857. [Google Scholar] [CrossRef]
- Zhang, L.H.; Li, W.C.; Liu, H.; Wang, Q.G.; Tang, L.; Hu, Q.T.; Xu, W.J.; Qiao, W.H.; Lu, Z.Y.; Lu, A.H. Thermoregulated Phase-Transition Synthesis of Two-Dimensional Carbon Nanoplates Rich in sp2 Carbon and Unimodal Ultramicropores for Kinetic Gas Separation. Angew. Chem. Int. Ed. 2018, 57, 1632–1635. [Google Scholar] [CrossRef]
- Shen, W.; He, Y.; Zhang, S.; Li, J.; Fan, W. Yeast-based microporous carbon materials for carbon dioxide capture. ChemSusChem 2012, 5, 1274–1279. [Google Scholar] [CrossRef]
- Guo, L.P.; Hu, Q.T.; Zhang, P.; Li, W.C.; Lu, A.H. Polyacrylonitrile-Derived Sponge-Like Micro/Macroporous Carbon for Selective CO2 Separation. Chem. Eur. J. 2018, 24, 8369–8374. [Google Scholar] [CrossRef]
- Li, Q.; Yang, J.; Feng, D.; Wu, Z.; Wu, Q.; Park, S.S.; Ha, C.-S.; Zhao, D. Facile synthesis of porous carbon nitride spheres with hierarchical three-dimensional mesostructures for CO2 capture. Nano Res. 2010, 3, 632–642. [Google Scholar] [CrossRef]
- Estevez, L.; Barpaga, D.; Zheng, J.; Sabale, S.; Patel, R.L.; Zhang, J.G.; McGrail, B.P.; Motkuri, R.K. Hierarchically porous carbon materials for CO2 capture: The role of pore structure. Ind. Eng. Chem. Res. 2018, 57, 1262–1268. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Ma, C.; Qiao, W.; Ling, L. Fabrication of hierarchical carbon nanosheet-based networks for physical and chemical adsorption of CO2. J. Colloid Interf. Sci. 2019, 534, 72–80. [Google Scholar] [CrossRef]
- Chang, B.; Shi, W.; Yin, H.; Zhang, S.; Yang, B. Poplar catkin-derived self-templated synthesis of N-doped hierarchical porous carbon microtubes for effective CO2 capture. Chem. Eng. J. 2019, 358, 1507–1518. [Google Scholar] [CrossRef]
- Li, Y.; Xu, R.; Wang, X.; Wang, B.; Cao, J.; Yang, J.; Wei, J. Waste wool derived nitrogen-doped hierarchical porous carbon for selective CO2 capture. RSC Adv. 2018, 8, 19818–19826. [Google Scholar] [CrossRef]
- Gao, A.; Guo, N.; Yan, M.; Li, M.; Wang, F.; Yang, R. Hierarchical porous carbon activated by CaCO3 from pigskin collagen for CO2 and H2 adsorption. Microporous Mesoporous Mater. 2018, 260, 172–179. [Google Scholar] [CrossRef]
- Marszewska, J.; Jaroniec, M. Tailoring porosity in carbon spheres for fast carbon dioxide adsorption. J. Colloid Interf. Sci. 2017, 487, 162–174. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W. Adsorptive removal of antibiotics from water and wastewater: Progress and challenges. Sci. Total Environ. 2015, 532, 112–126. [Google Scholar] [CrossRef]
- Shi, X.; Xiao, H.; Liao, X.; Armstrong, M.; Chen, X.; Lackner, K.S. Humidity effect on ion behaviors of moisture-driven CO2 sorbents. J. Chem. Phys. 2018, 149, 164708. [Google Scholar] [CrossRef]

| Biochar Derived from | BET Surface Area (m2/g) | Pressure (Bar) | Adsorption Capacity (mmol/g) at 273 K | Adsorption Capacity (mmol/g) at 298 K | Reference |
|---|---|---|---|---|---|
| Vine shoots | 767 | 1 | 4.07 | 1.58 | [36] |
| Vine shoots | 1305 | 1 | 6.04 | 2.46 | [36] |
| Vine shoots | 1439 | 1 | 6.08 | 1.98 | [37] |
| African palm shells | 1250 | 1 | 6.3 | 4.4 | [37] |
| Rice husk | 2695 | 1 | 6.24 | 3.71 | [38] |
| Pine nut shells | 1486 | 1 | 7.7 | 5.00 | [39] |
| Wheat flour | 1438 | 1 | 5.70 | 3.48 | [40] |
| Coconut shells | 1172 | 1 | 6.04 | 4.23 | [11] |
| Jujun grass | 1512 | 1 | - | 4.9 | [41] |
| Jujun grass | 3144 | 1 | - | 4.1 | [41] |
| Camellia Japonica | 1353 | 1 | - | 5.0 | [41] |
| Camellia Japonica | 3537 | 1 | - | 2.8 | [41] |
| Pomegranate peels | 585 | 1 | 6.89 | 4.00 | [42] |
| Carrot peels | 1379 | 1 | 5.64 | 4.18 | [42] |
| Fern leaves | 1593 | 1 | 4.52 | 4.12 | [42] |
| Black locust | 2511 | 1 | - | 5.05 | [43] |
| Walnut shell | 1315 | 1 | - | 7.42 | [44] |
| Pine cone | 1680 | 1 | - | 4.7 | [45] |
| Saw dust | 394.12 | 1 | - | 3.7 | [46] |
| Mg loaded Walnut shell | 292 | 1 | - | 3.7 | [47] |
| Pristiene Walnut shell | 997 | 1 | - | 3.2 | [47] |
| Adsorbent | BET Surface Area (m2/g) | Pressure (Bar) | Adsorption Capacity (mmol/g) at 273 K | Adsorption Capacity (mmol/g) at 298 K | Reference |
|---|---|---|---|---|---|
| Reduced graphene oxide | 1300 | 1 | 3.35 | 2.45 | [49] |
| BN-graphene | 170 | 1 | 2.9 | 2.6 | [51] |
| Imine-functionalized graphene oxide | 190 | 2 | 8.1 | 2.1 | [52] |
| N-functionalized graphene | 979 | 1 | 5.8 | 2.7 | [58] |
| Polyetheleneimine (PEI)-modified graphene oxide | 29 | 1 | - | 2.0 | [59] |
| Graphene-based monolith | 328 | 1 | - | 2.1 | [53] |
| PEI-graphene oxide@ZIF-8 | 190 | 1 | 8.08 | - | [54] |
| DWCNTs | 423 | 1 | - | 3.5 (at 308 K) | [55] |
| PEI-purine-CNTs | 1 | - | 3.9 (at 323 K) | [60] | |
| PEI-CNT aerogels | 62 | 1 | - | 3.3 (at 343 K) | [61] |
| SWCNT@HKUST-1 | 1714 | 1 | - | 8.75 (at 196K) | [57] |
| Chitosan-polybenzoxazine nanocomposite aerogels | 710 | 1 | 6.70 | 5.72 | [62] |
| Adsorbent | BET Surface Area (m2/g) | Pressure (Bar) | Adsorption Capacity (mmol/g) at 273 K | Adsorption Capacity (mmol/g) at 298 K | Reference |
|---|---|---|---|---|---|
| AC beds | 3537 | 18 | - | 20.66 | [69] |
| N-doped ACs | 1535 | 1 | 7.0 | 4.80 | [70] |
| Starch-based ACs | 3350 | 1 | 4.4 | 3.4 | [71] |
| Polyurethane foam-based AC | 1360 | 1 | 5.85 | - | [67] |
| Polyacrylonitrile-based AC fibers | 1565 | 1 | - | 2.74 | [72] |
| N and S-doped ACs | 2040 | 1 | 7.76 | 5.19 | [73] |
| Celtuce leaves-derived AC | 3404 | 1 | 6.04 | 4.36 | [74] |
| Longan shells-derived AC | 3260 | 1 | 5.60 | 4.30 | [75] |
| Slash pine-derived AC | 906 | 1 | 4.93 | 3.86 (at 288K) | [76] |
| Coconut shell-derived AC | 1327 | 1 | 5.60 | 3.90 | [77] |
| Black locust-derived AC | 2511 | 1 | 5.86 | 3.75 | [44] |
| Starch and cellulose, sawdust | 1260 | 1 | 6.10 | 4.8 | [28] |
| Empty fruit bunch-derived AC | 1720 | 1 | 5.22 | 3.70 | [78] |
| Lignin-derived AC | 3500 | 1 | 8.20 | 4.8 | [79] |
| Pitch-based N-doped AC | 1505 | 1 | 7.10 | 4.58 | [80] |
| Adsorbent | BET Surface Area (m2/g) | Pressure (bar) | Adsorption Capacity (mmol/g) at 273 K | Adsorption Capacity (mmol/g) at 298 K | Reference |
|---|---|---|---|---|---|
| Mesoporous carbon | 3934 | 1 | - | 2.8 | [85] |
| NaNH2-activated mesoporous carbon | 3325 | 1 | 6.31 | 3.66 | [86] |
| Mg and N-doped mesoporous carbon | 541 | 1 | 3.68 | - | [87] |
| N-doped mesoporous carbon | 984.91 | 4.23 (at 303 K) | [88] | ||
| Ordered mesoporous carbon nitrides | 232 | 30 | 5.63 | [89] | |
| Ordered mesoporous carbon | 2255 | 1 | 3.0 | 2.1 | [93] |
| Ultramicroporous carbon | 882 | 1 | 5.91 | 4.30 | [94] |
| Mesoporous carbon nanospheres | 1240 | 1 | 4.76 | 2.36 | [95] |
| Microporous carbon | 1551 | 30 | 26.30 | - | [90] |
| N-doped microporous carbon | 664 | 1 | 5.0 | 4.0 | [96] |
| Ultramicroporous carbon | 1059 | 1 | 5.87 | 3.82 | [97] |
| Microporous carbon aerogel | 1871 | 1 | - | 3.0 | [98] |
| N-doped microporous carbon | 1060 | 1 | - | 4.24 | [99] |
| Microporous carbon beads | 1755 | 6.15 | 4.25 | [100] | |
| N-doped microporous carbon | 1381 | 1 | 5.91 | 3.86 | [101] |
| Ultra microporous carbon | 335 | 1 | - | 1.82 (at 303 K) | [102] |
| S-doped microporous carbon | 1567 | 1 | - | 4.5 | [103] |
| N-doped porous carbon | 467 | 1 | - | 3.13 | [104] |
| Ultra microporous carbon nanoplates | 800 | 1 | - | 5.2 | [105] |
| Yeast-based porous carbon | 1348 | 1 | - | 5.0 | [106] |
| Sponge-like porous carbon | 1143 | 1 | 5.6 | 4.0 | [107] |
| Hierarchical porous carbon | 2734 | 30 | - | 27.0 (at 300 K) | [91] |
| Hierarchical porous carbon nitride | 550 | 1 | - | 2.9 | [108] |
| Hierarchical porous carbon | 2698 | 1 | - | 3.7 | [109] |
| Hierarchical nanosheet | 1555.7 | 1 | 4.62 | 3.10 | [110] |
| N-doped hierarchical porous carbon | 1455.1 | 1 | 6.22 | 4.05 | [111] |
| Waste wool-derived N-doped hierarchical porous carbon | 1352 | 1 | 3.72 | 2.78 | [112] |
| N-doped hierarchical porous carbon | 2799 | 1 | 5.3 | 4.4 | [113] |
| Si-doped porous carbon | 1500 | 1 | 7.8 | 4.0 (at 296 K) | [114] |
| Adsorbents | Lower Price ($/kg) | Higher Price ($/kg) |
|---|---|---|
| Biochar/activated biochar | 0.4 | 0.90 |
| Activated carbons | 2.90 | 8.20 |
| CNTs | 1000 | 10,000 |
| Graphene | 50 | 200 |
| Graphene oxide | 200 | 400 |
| Other carbons | Depends on processing | Depends on processing |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khandaker, T.; Hossain, M.S.; Dhar, P.K.; Rahman, M.S.; Hossain, M.A.; Ahmed, M.B. Efficacies of Carbon-Based Adsorbents for Carbon Dioxide Capture. Processes 2020, 8, 654. https://doi.org/10.3390/pr8060654
Khandaker T, Hossain MS, Dhar PK, Rahman MS, Hossain MA, Ahmed MB. Efficacies of Carbon-Based Adsorbents for Carbon Dioxide Capture. Processes. 2020; 8(6):654. https://doi.org/10.3390/pr8060654
Chicago/Turabian StyleKhandaker, Tasmina, Muhammad Sarwar Hossain, Palash Kumar Dhar, Md. Saifur Rahman, Md. Ashraf Hossain, and Mohammad Boshir Ahmed. 2020. "Efficacies of Carbon-Based Adsorbents for Carbon Dioxide Capture" Processes 8, no. 6: 654. https://doi.org/10.3390/pr8060654
APA StyleKhandaker, T., Hossain, M. S., Dhar, P. K., Rahman, M. S., Hossain, M. A., & Ahmed, M. B. (2020). Efficacies of Carbon-Based Adsorbents for Carbon Dioxide Capture. Processes, 8(6), 654. https://doi.org/10.3390/pr8060654






