Selective Photocatalytic Oxidation of 5-HMF in Water over Electrochemically Synthesized TiO2 Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
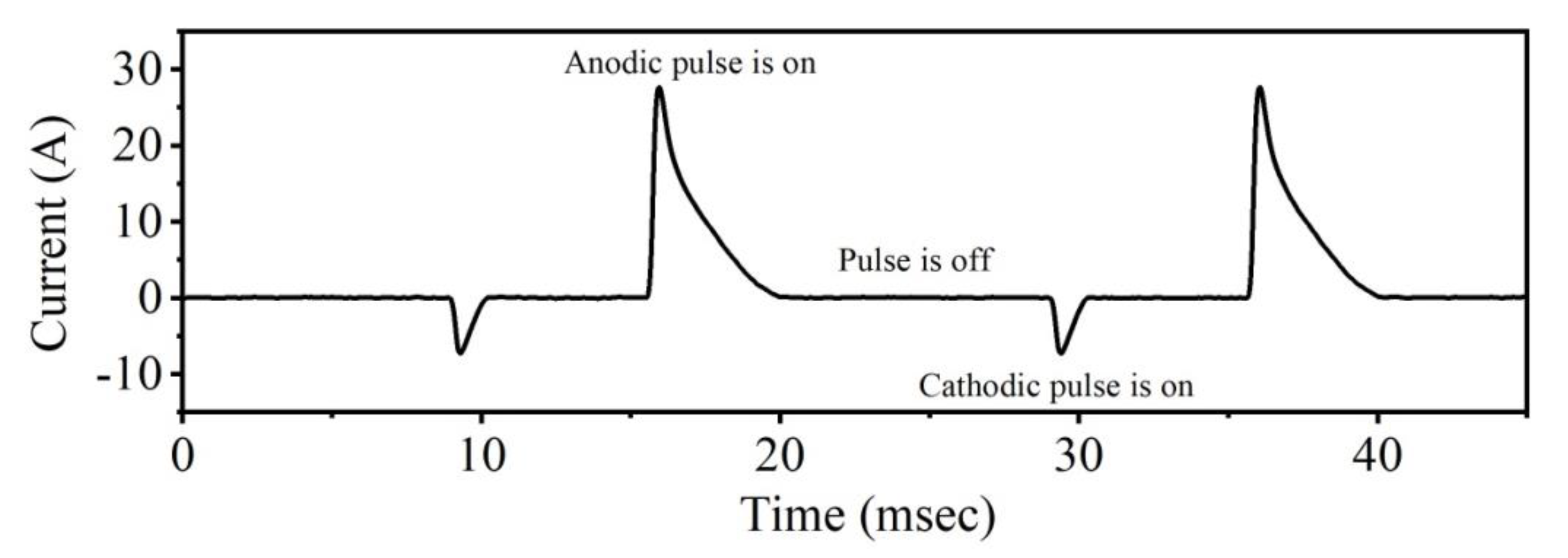
2.1. Photocatalyst Preparation
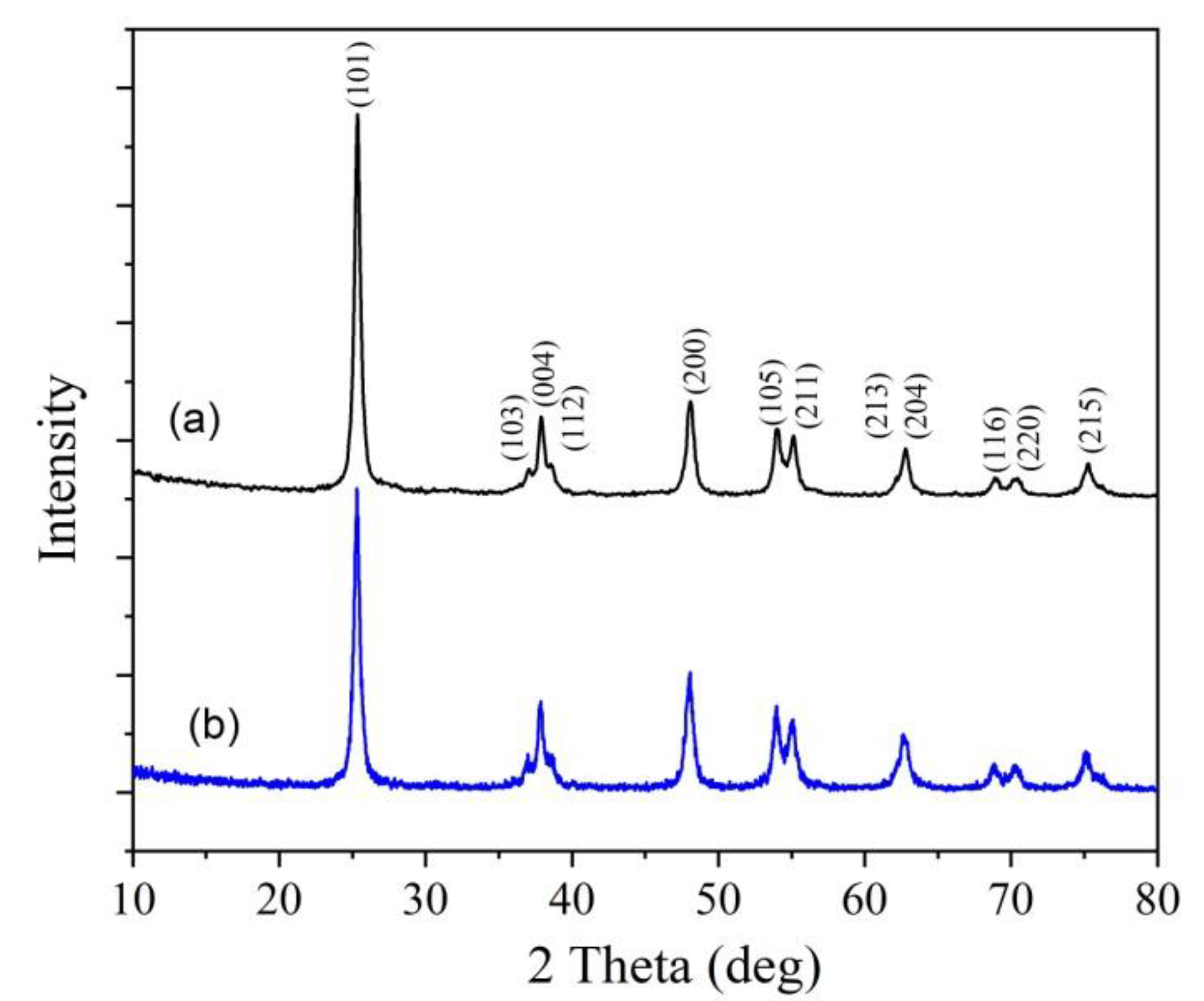
2.2. Characterization
2.3. Photocatalytic Procedure
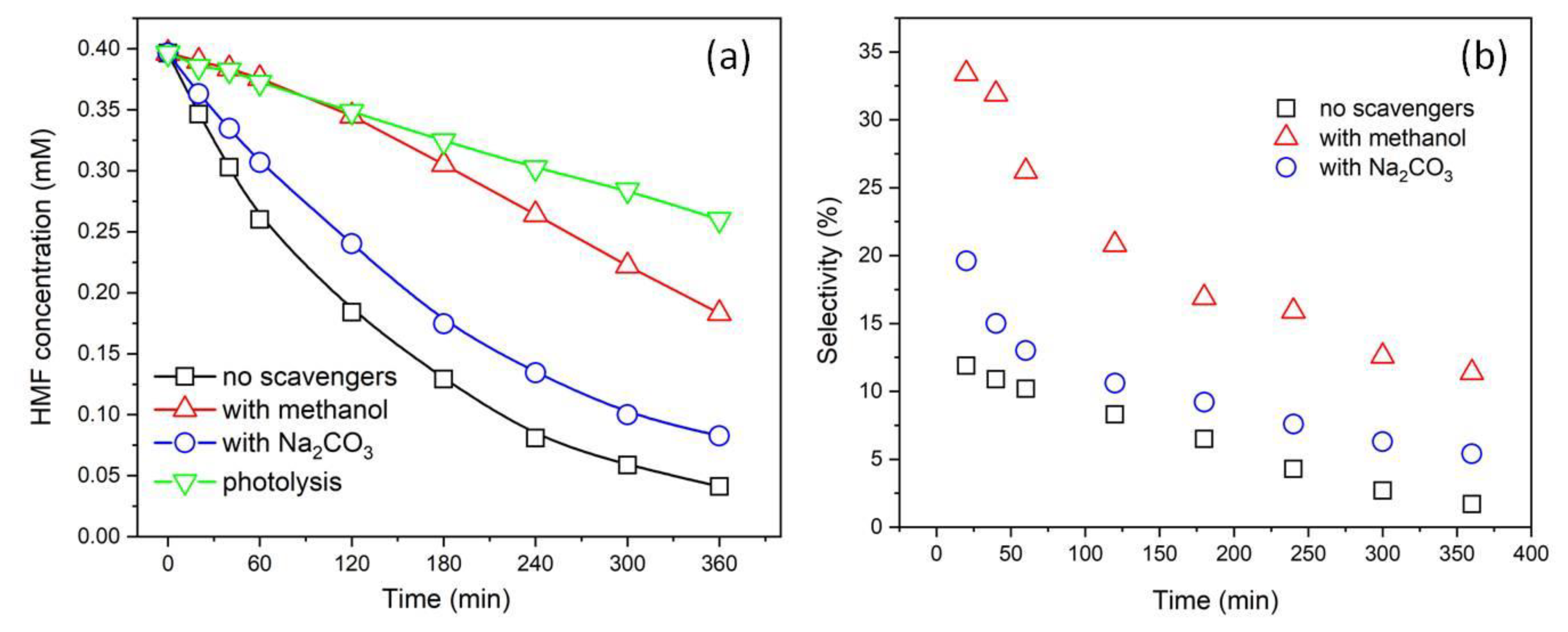
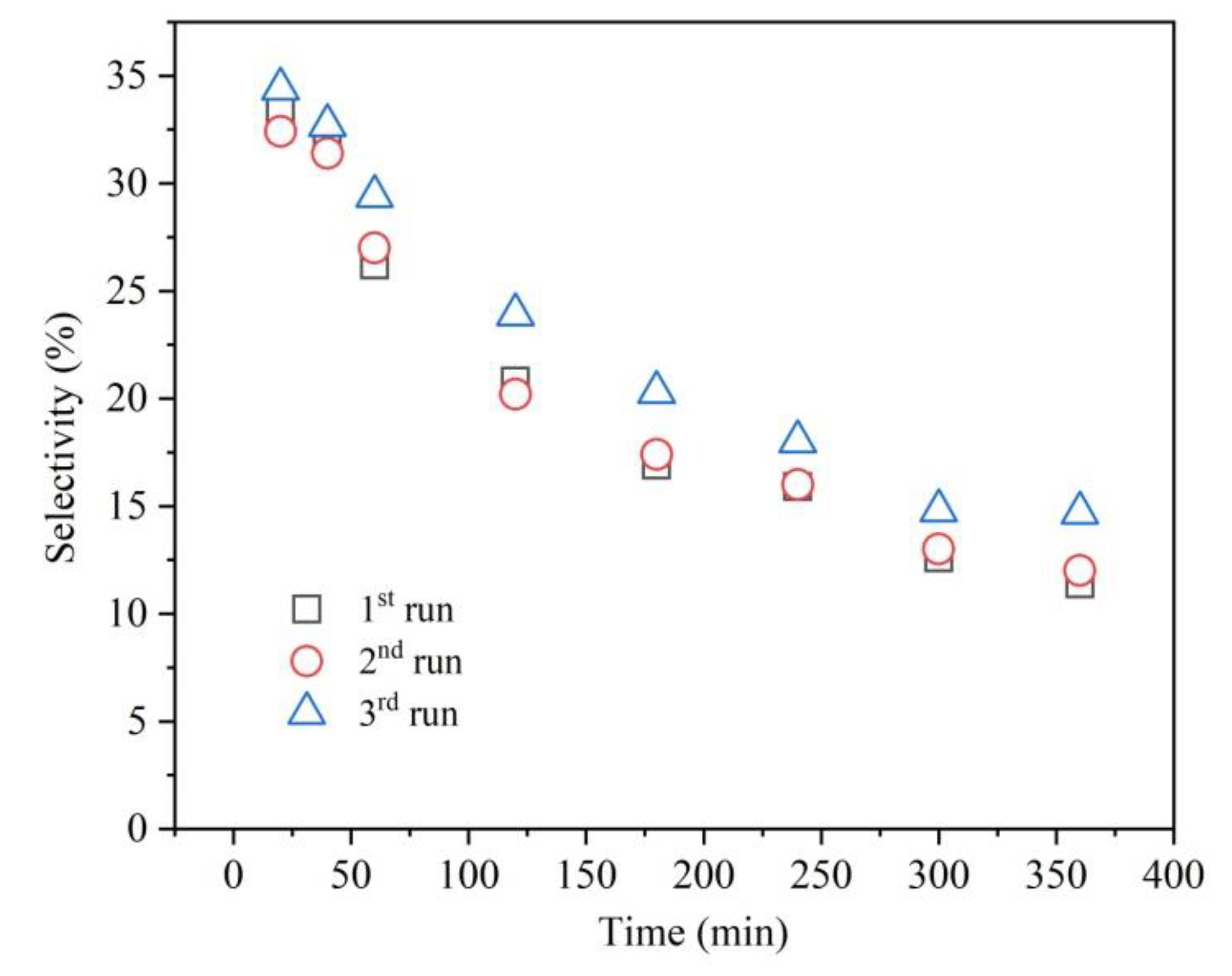
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Klushin, V.A.; Galkin, K.I.; Kashparova, V.P.; Krivodaeva, E.A.; Kravchenko, O.A.; Smirnova, N.V.; Chernyshev, V.M.; Ananikov, V.P. Technological aspects of fructose conversion to high-purity 5-hydroxymethylfurfural, a versatile platform chemical. Russ. J. Org. Chem. 2016, 52, 767–771. [Google Scholar] [CrossRef]
- Ghosh, K.; Molla, R.A.; Iqubal, M.A.; Islam, S.S.; Islam, S.M. Ruthenium nanoparticles supported on N-containing mesoporous polymer catalyzed aerobic oxidation of biomass-derived 5-hydroxymethylfurfural (HMF) to 2,5-diformylfuran (DFF). Appl. Catal. A Gen. 2016, 520, 44–52. [Google Scholar] [CrossRef]
- Teng, N.; Li, J.-L.; Lu, B.-Q.; Wang, Y.-Q.; Jia, S.-Y.; Wang, Y.-X.; Hou, X.-L. The selective aerobic oxidation of 5-hydroxymethylfurfural to produce 2,5-diformylfuran using Nitrogen-doped porous carbons as catalysts. New Carbon Mater. 2019, 34, 593–599. [Google Scholar] [CrossRef]
- Ding, L.; Yang, W.; Chen, L.; Cheng, H.; Qi, Z. Fabrication of spinel CoMn2O4 hollow spheres for highly selective aerobic oxidation of 5-hydroxymethylfurfural to 2,5-diformylfuran. Catal. Today 2018. [Google Scholar] [CrossRef]
- Mamaghani, A.H.; Haghighat, F.; Lee, C.-S. Hydrothermal/solvothermal synthesis and treatment of TiO2 for photocatalytic degradation of air pollutants: Preparation, characterization, properties, and performance. Chemosphere 2019, 219, 804–825. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Ma, X.; Liu, T.; Li, Y.; Song, L.; Tjong, S.C.; Cao, L.; Wang, W.; Yu, Q.; Wang, Z. Degradation aspects of endocrine disrupting chemicals: A review on photocatalytic processes and photocatalysts. Appl. Catal. A Gen. 2020, 597, 117547. [Google Scholar] [CrossRef]
- Akbari, A.; Amini, M.; Tarassoli, A.; Eftekhari-Sis, B.; Ghasemian, N.; Jabbari, E. Transition metal oxide nanoparticles as efficient catalysts in oxidation reactions. Nano Struct. Nano Objects 2018, 14, 19–48. [Google Scholar] [CrossRef]
- Chen, L.; Tang, J.; Song, L.-N.; Chen, P.; He, J.; Au, C.-T.; Yin, S.-F. Heterogeneous photocatalysis for selective oxidation of alcohols and hydrocarbons. Appl. Catal. B Environ. 2019, 242, 379–388. [Google Scholar] [CrossRef]
- Yurdakal, S.; Tek, B.S.; Alagöz, O.; Augugliaro, V.; Loddo, V.; Palmisano, G.; Palmisano, L. Photocatalytic Selective Oxidation of 5-(Hydroxymethyl)-2-furaldehyde to 2,5-Furandicarbaldehyde in Water by Using Anatase, Rutile, and Brookite TiO2 Nanoparticles. ACS Sustain. Chem. Eng. 2013, 1, 456–461. [Google Scholar] [CrossRef]
- Wu, Q.; He, Y.; Zhang, H.; Feng, Z.; Wu, Y.; Wu, T. Photocatalytic selective oxidation of biomass-derived 5-hydroxymethylfurfural to 2,5-diformylfuran on metal-free g-C3N4 under visible light irradiation. Mol. Catal. 2017, 436, 10–18. [Google Scholar] [CrossRef]
- Zhang, H.; Feng, Z.; Zhu, Y.; Wu, Y.; Wu, T. Photocatalytic selective oxidation of biomass-derived 5-hydroxymethylfurfural to 2,5-diformylfuran on WO3/g-C3N4 composite under irradiation of visible light. J. Photochem. Photobiol. A Chem. 2019, 371, 1–9. [Google Scholar] [CrossRef]
- Lolli, A.; Maslova, V.; Bonincontro, D.; Basile, F.; Ortelli, S.; Albonetti, S. Selective Oxidation of HMF via Catalytic and Photocatalytic Processes Using Metal-Supported Catalysts. Molecules 2018, 23, 2792. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Morales, S.A.; Morales, G.; López Zavala, M.Á.; Arce-Sarria, A.; Machuca-Martínez, F. Photocatalytic Treatment of Paracetamol Using TiO2 Nanotubes: Effect of pH. Processes 2019, 7, 319. [Google Scholar] [CrossRef]
- Ulyankina, A.A.; Kuriganova, A.B.; Smirnova, N.V. Photocatalytic properties of SnO2–SnO nanocomposite prepared via pulse alternating current synthesis. Mendeleev Commun. 2019, 29, 215–217. [Google Scholar] [CrossRef]
- Ulyankina, A.; Leontyev, I.; Avramenko, M.; Zhigunov, D.; Smirnova, N. Large-scale synthesis of ZnO nanostructures by pulse electrochemical method and their photocatalytic properties. Mater. Sci. Semicond. Process. 2018, 76, 7–13. [Google Scholar] [CrossRef]
- Chernysheva, D.; Vlaic, C.; Leontyev, I.; Pudova, L.; Ivanov, S.; Avramenko, M.; Allix, M.; Rakhmatullin, A.; Maslova, O.; Bund, A.; et al. Synthesis of Co3O4/CoOOH via electrochemical dispersion using a pulse alternating current method for lithium-ion batteries and supercapacitors. Solid State Sci. 2018, 86, 53–59. [Google Scholar] [CrossRef]
- Ulyankina, A.; Leontyev, I.; Maslova, O.; Allix, M.; Rakhmatullin, A.; Nevzorova, N.; Valeev, R.; Yalovega, G.; Smirnova, N. Copper oxides for energy storage application: Novel pulse alternating current synthesis. Mater. Sci. Semicond. Process. 2018, 73, 111–116. [Google Scholar] [CrossRef]
- Luttrell, T.; Halpegamage, S.; Tao, J.; Kramer, A.; Sutter, E.; Batzill, M. Why is anatase a better photocatalyst than rutile?—Model studies on epitaxial TiO2 films. Sci. Rep. 2014, 4, 4043. [Google Scholar] [CrossRef] [PubMed]
- Sathiyan, K.; Bar-Ziv, R.; Mendelson, O.; Zidki, T. Controllable synthesis of TiO2 nanoparticles and their photocatalytic activity in dye degradation. Mater. Res. Bull. 2020, 126, 110842. [Google Scholar] [CrossRef]
- Ma, B.; Wang, Y.; Guo, X.; Tong, X.; Liu, C.; Wang, Y.; Guo, X. Photocatalytic synthesis of 2,5-diformylfuran from 5-hydroxymethyfurfural or fructose over bimetallic Au-Ru nanoparticles supported on reduced graphene oxides. Appl. Catal. A Gen. 2018, 552, 70–76. [Google Scholar] [CrossRef]



| Sample | HMF Concentration | Irradiation Type | HMF Conversion (%) | DFF Selectivity (%) | Reference |
|---|---|---|---|---|---|
| TiO2-m | 0.08 M | 300 W Xe-lamp (250–2500 nm) | 10 | 2 | [12] |
| 12 | 2 | ||||
| 22 | 5 | ||||
| HPA 1 | 0.5 mM | four 8 W Philips lamps (365 nm) 3.0 mW cm−2 | 20 | 26 | [9] |
| TiO2 NPs 1 | 0.4 mM | Hamamatsu, LC8 (365 nm) 3.0 mW cm−2 | 15 | 29.5 | the present study |
| comm-TiO2 | 0.4 mM | Hamamatsu, LC8 (365 nm) 3.0 mW cm−2 | 60 | 1.0 | the present study |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ulyankina, A.; Mitchenko, S.; Smirnova, N. Selective Photocatalytic Oxidation of 5-HMF in Water over Electrochemically Synthesized TiO2 Nanoparticles. Processes 2020, 8, 647. https://doi.org/10.3390/pr8060647
Ulyankina A, Mitchenko S, Smirnova N. Selective Photocatalytic Oxidation of 5-HMF in Water over Electrochemically Synthesized TiO2 Nanoparticles. Processes. 2020; 8(6):647. https://doi.org/10.3390/pr8060647
Chicago/Turabian StyleUlyankina, Anna, Sergey Mitchenko, and Nina Smirnova. 2020. "Selective Photocatalytic Oxidation of 5-HMF in Water over Electrochemically Synthesized TiO2 Nanoparticles" Processes 8, no. 6: 647. https://doi.org/10.3390/pr8060647
APA StyleUlyankina, A., Mitchenko, S., & Smirnova, N. (2020). Selective Photocatalytic Oxidation of 5-HMF in Water over Electrochemically Synthesized TiO2 Nanoparticles. Processes, 8(6), 647. https://doi.org/10.3390/pr8060647




