Improved Catalytic Properties of Thermomyces lanuginosus Lipase Immobilized onto Newly Fabricated Polydopamine-Functionalized Magnetic Fe3O4 Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Catalysts and Chemicals
2.2. Preparation of Fe3O4@PDA
2.3. Preparation of Immobilized Lipase
2.4. Assay of Enzyme Activity
2.5. Surface Characterization of the Immobilized Lipase
2.6. Effect of pH and Temperature on the Free and Immobilized Lipase
2.7. pH Stability Studies of Free and Immobilized Lipase
2.8. Thermal Stability Studies of Free and Immobilized Lipase
2.9. Solvent Tolerance Stability Studies of Free and Immobilized Lipase
2.10. Operational Stability Studies of Free and Immobilized Lipase
3. Results and Discussion
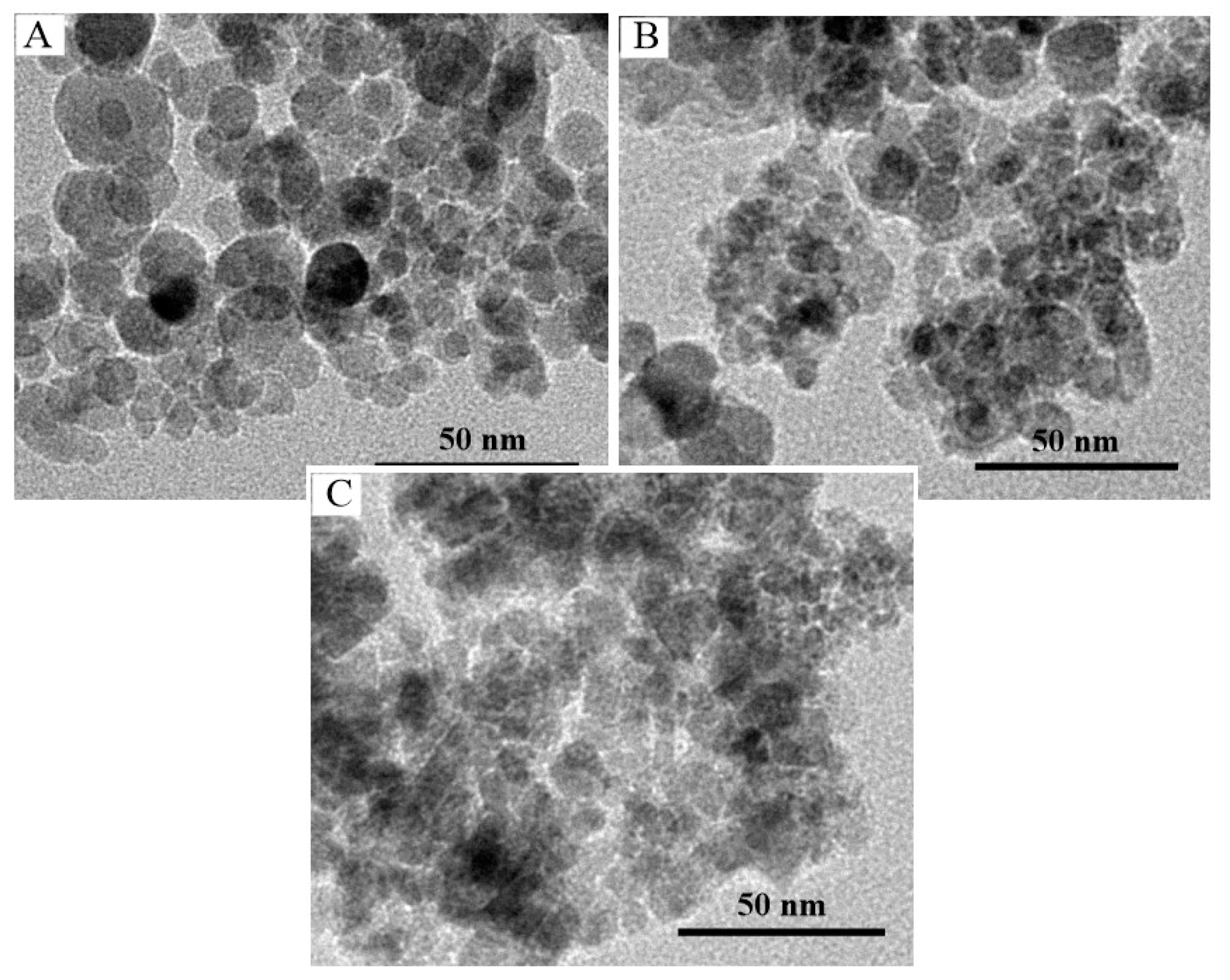
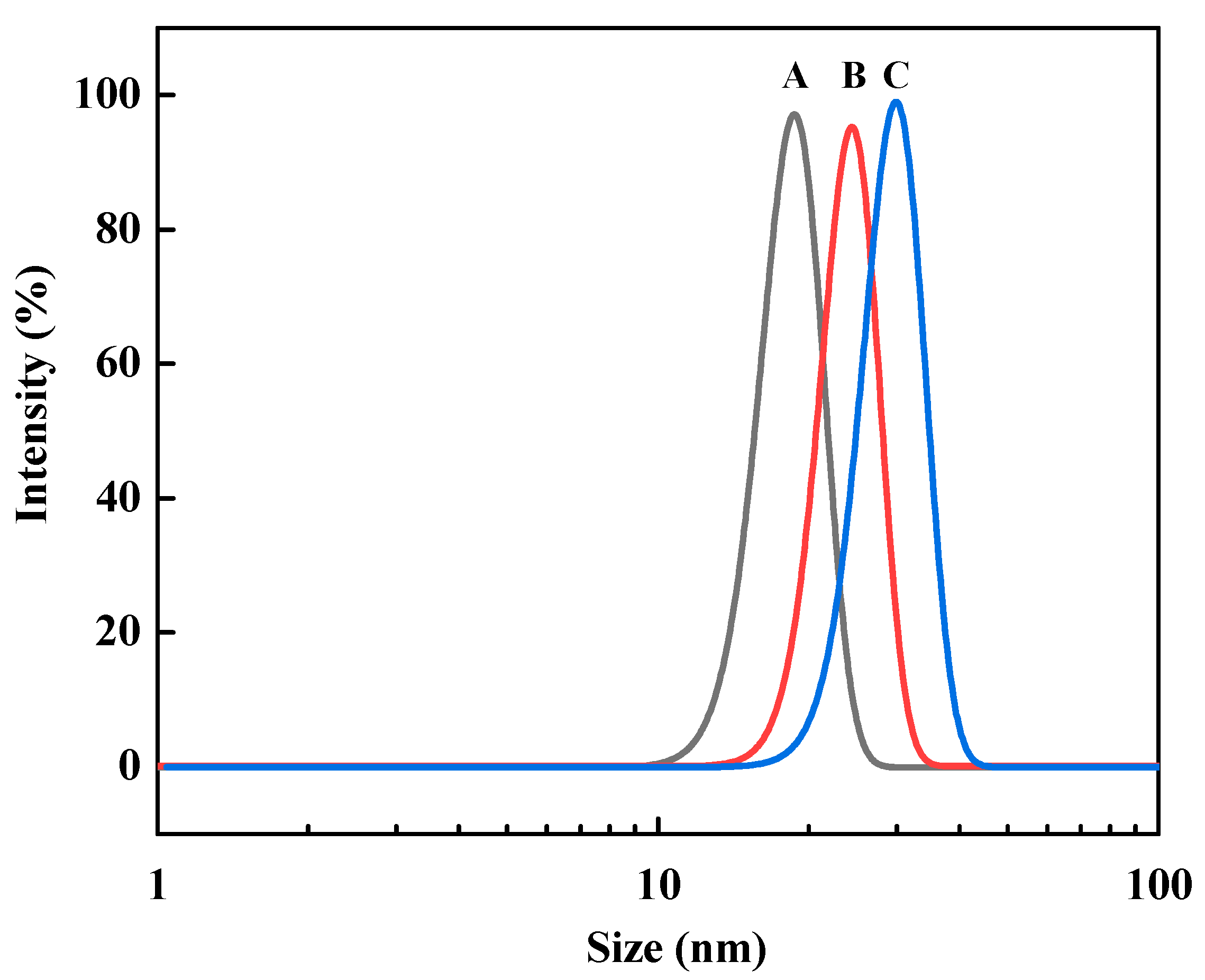
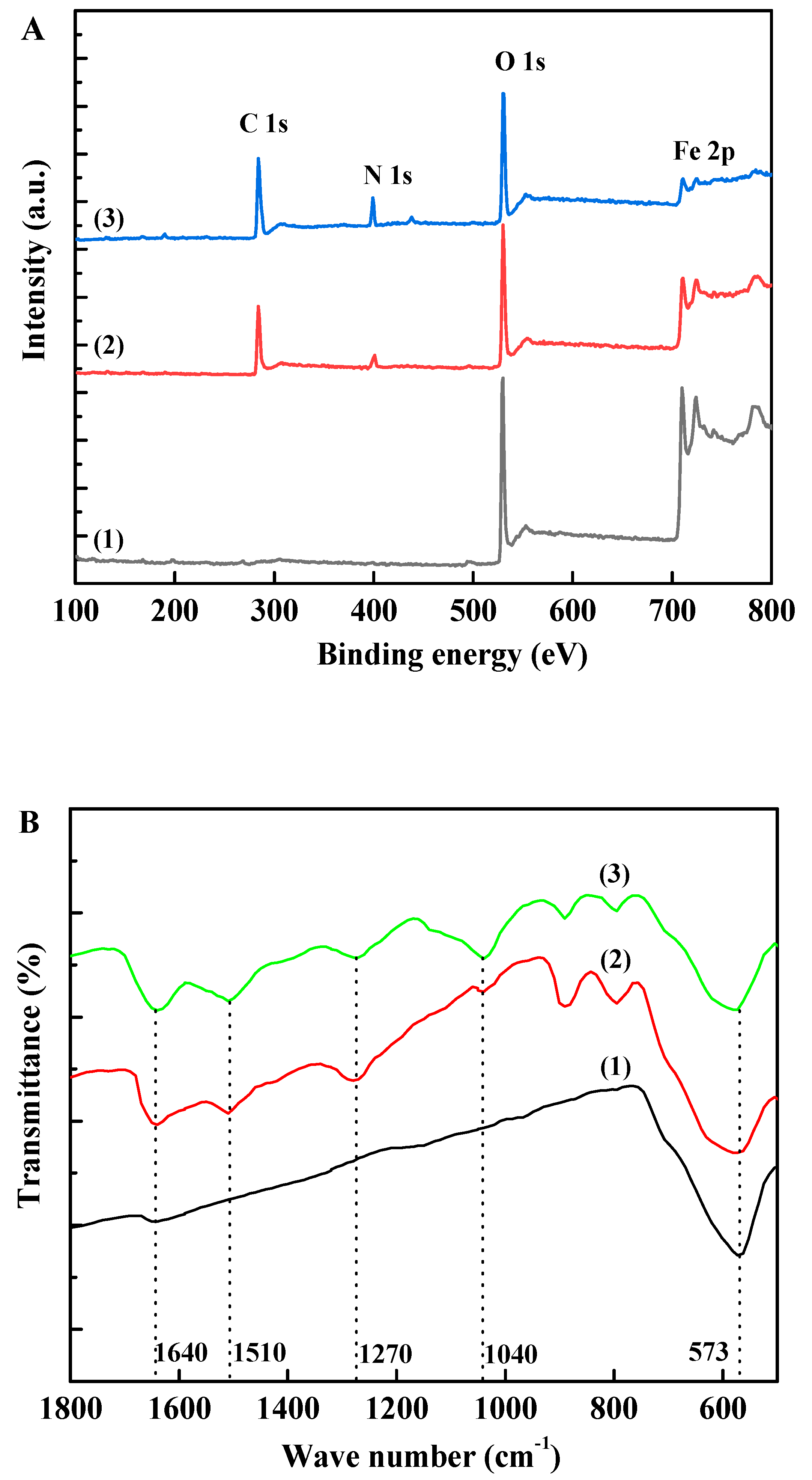
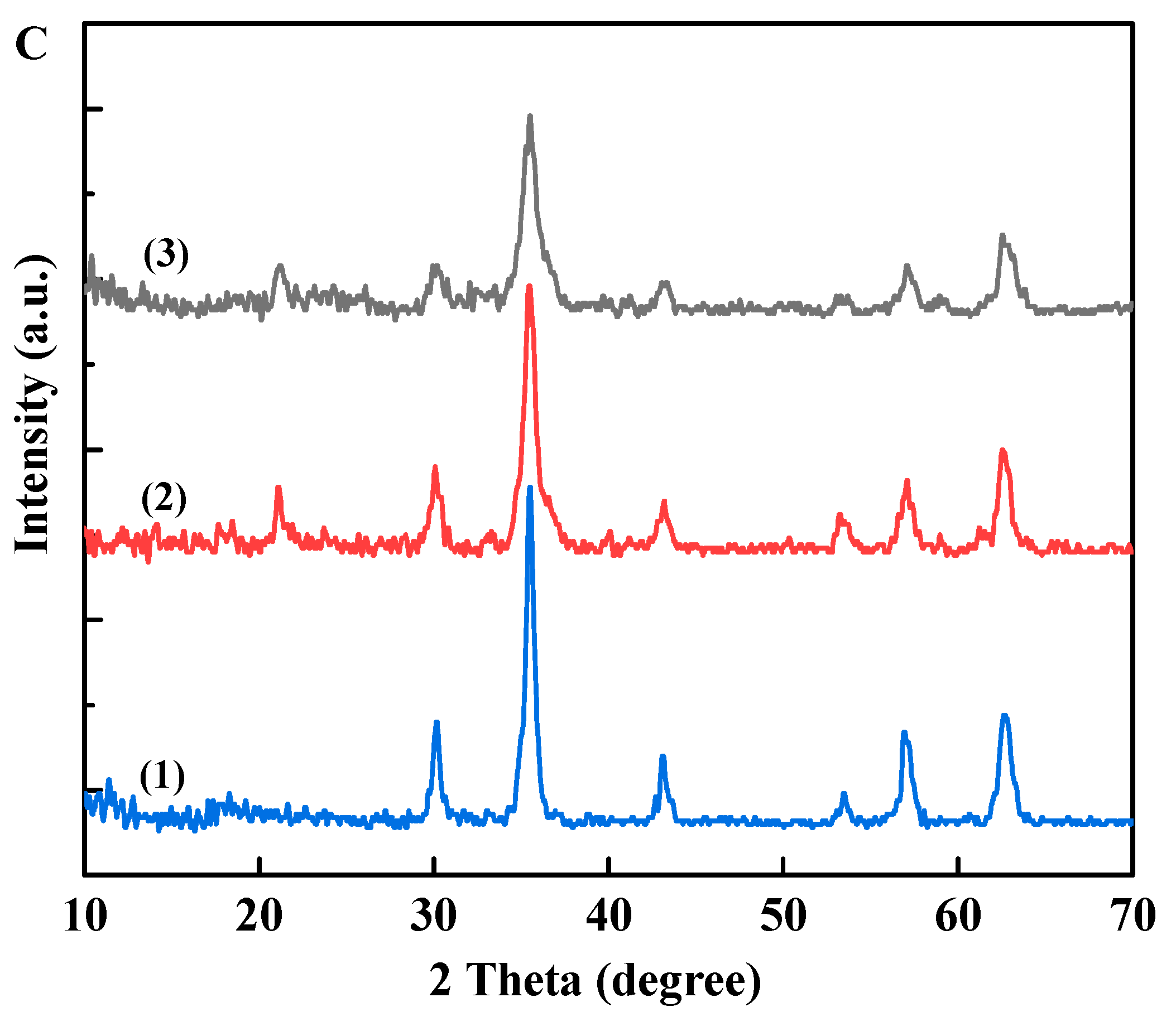
3.1. Preparation and Characterization of Fe3O4@PDA@TLL
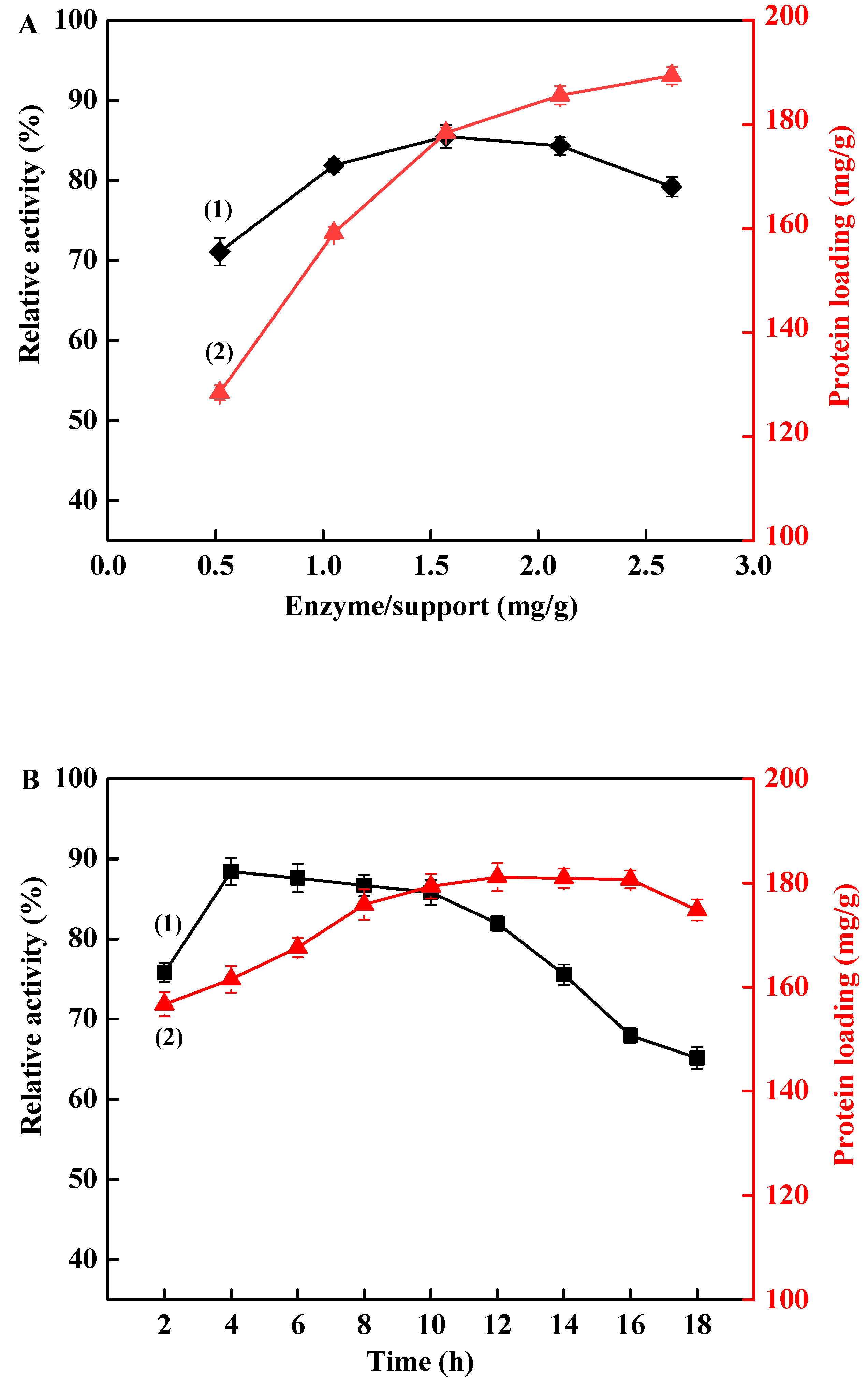
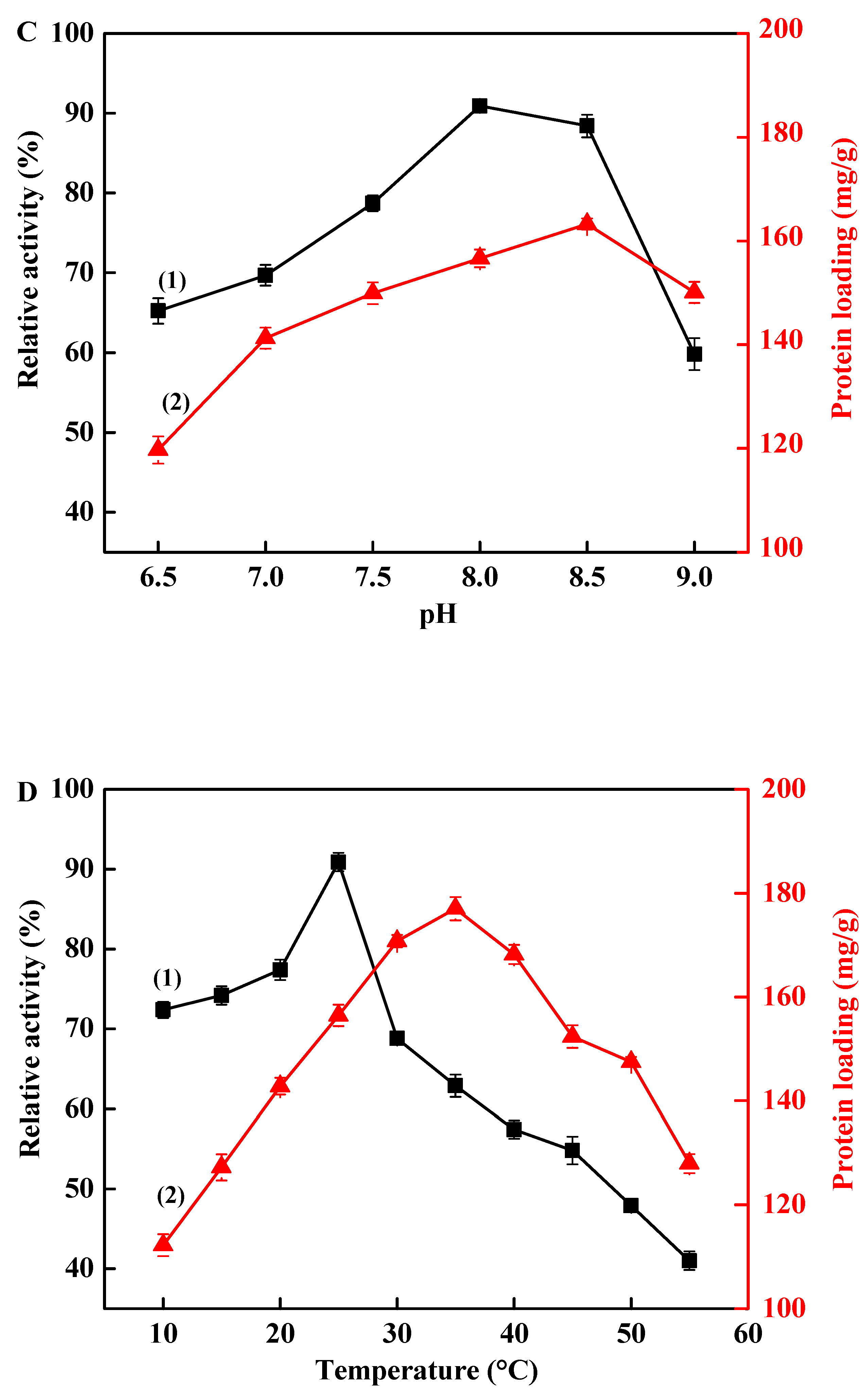
3.2. Immobilization of TLL onto Fe3O4@PDA
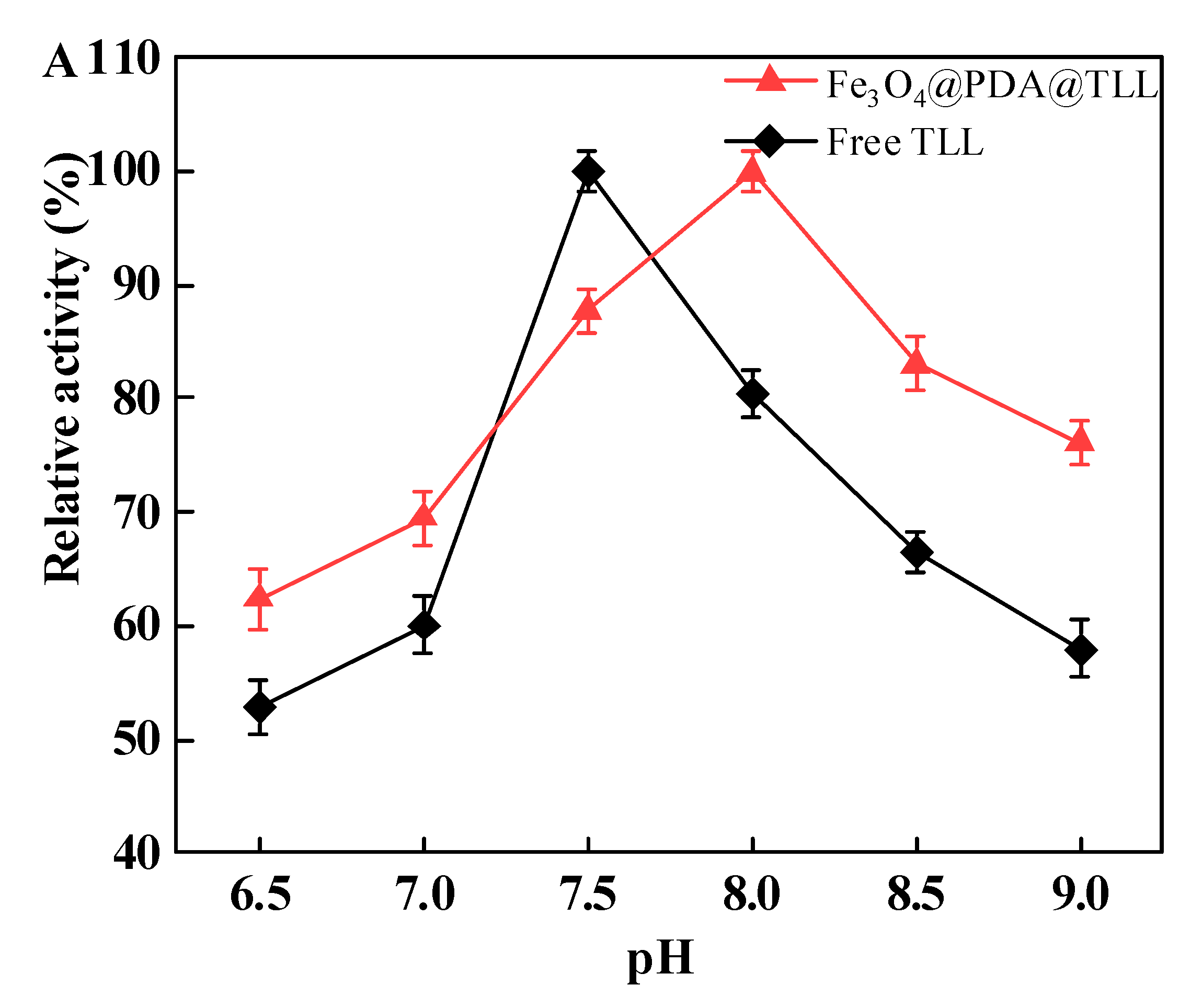
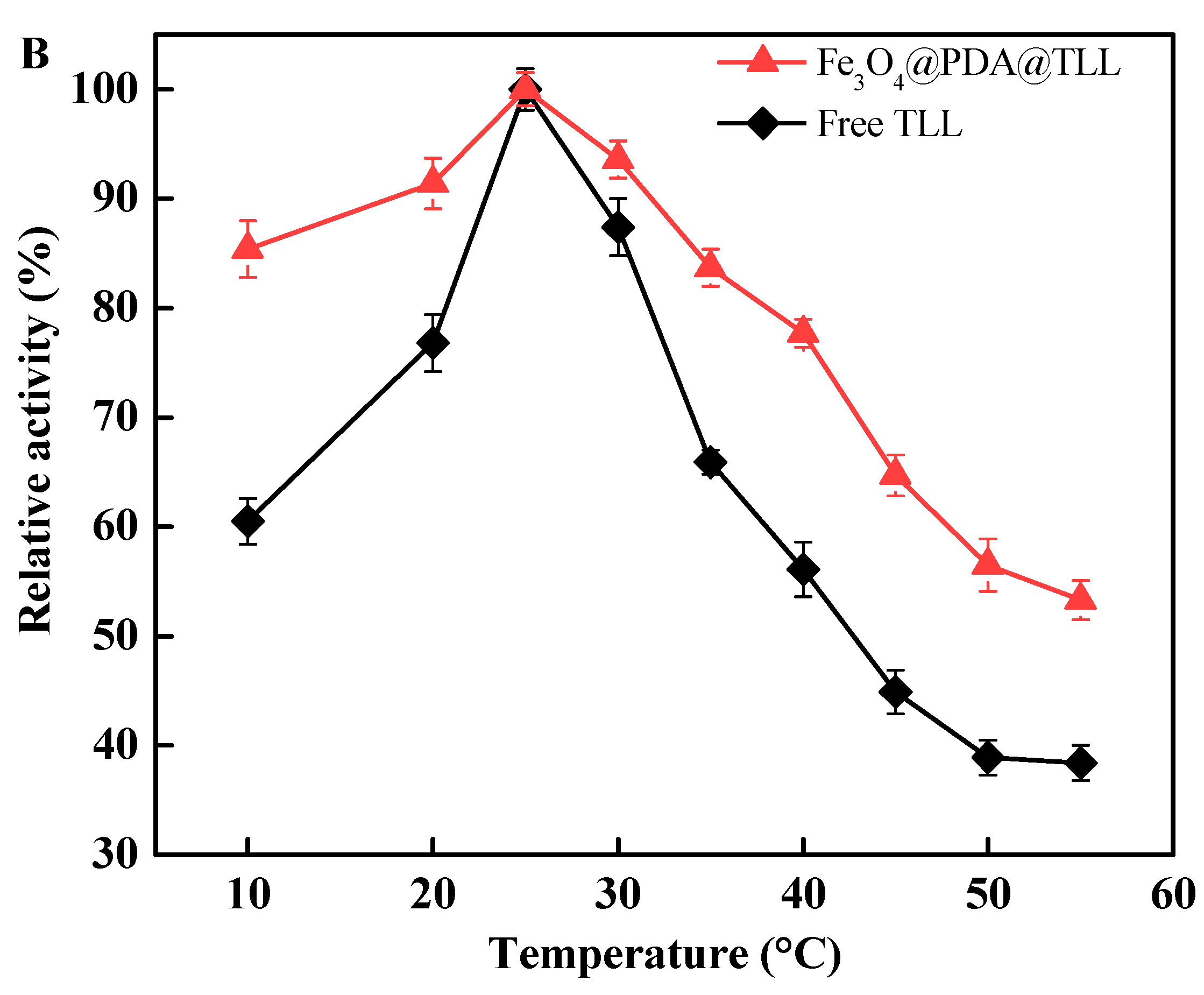
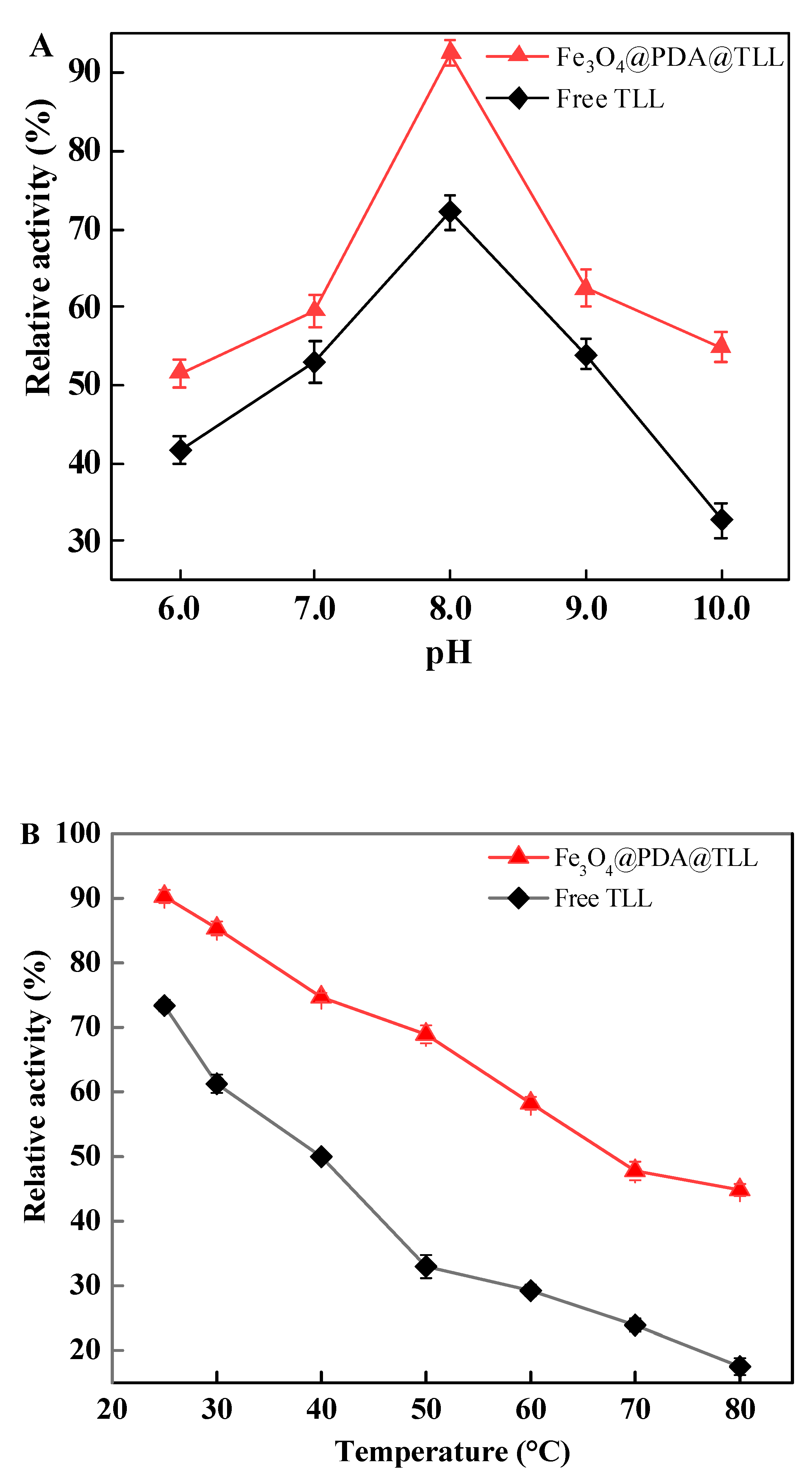
3.3. Effect of the pH and Temperature on Free and Immobilized TLL
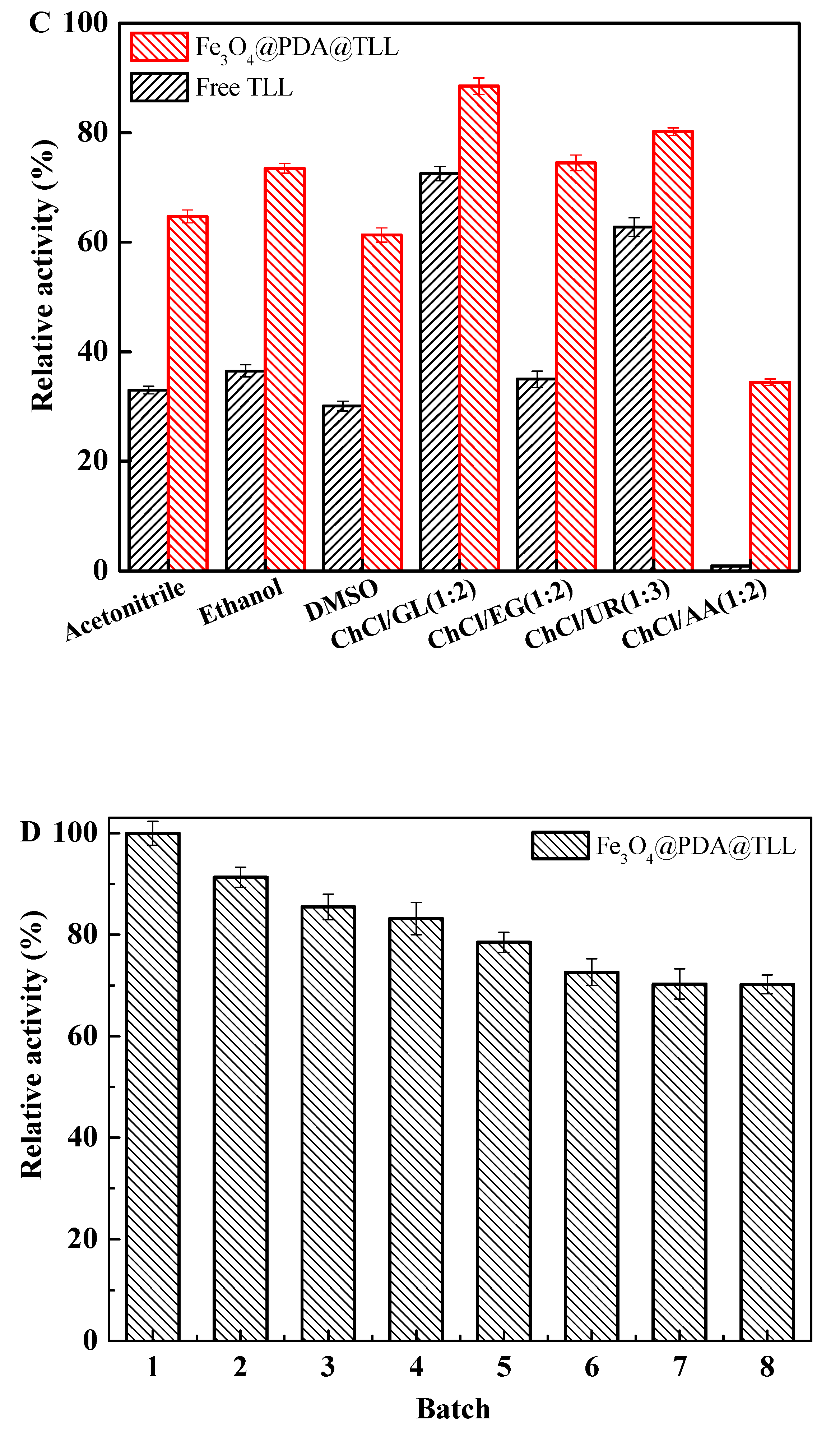
3.4. Stability of Free and Immobilized TLL
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sheldon, R.A.; Van Pelt, S. Enzyme immobilisation in biocatalysis: Why, what and how. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guisan, J.M.; Bolivar, J.M.; López-Gallego, F.; Rocha-Martín, J. Immobilization of Enzymes and Cells: Methods and Protocols; Methods in Molecular Biology; Humana: New York, NY, USA, 2020; Available online: https://link.springer.com/book/10.1007%2F978-1-0716-0215-7 (accessed on 5 May 2020).
- Liang, S.; Wu, X.L.; Xiong, J.; Zong, M.H.; Lou, W.Y. Metal-organic frameworks as novel matrices for efficient enzyme immobilization: An update review. Coordin. Chem. Rev. 2020, 406, 213149. [Google Scholar] [CrossRef]
- Adlercreutz, P. Immobilisation and application of lipases in organic media. Chem. Soc. Rev. 2013, 42, 6406–6436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, S.L.; Xu, P.; Ma, Y.Z.; Yao, X.X.; Yao, Y.; Zong, M.H.; Li, X.H.; Lou, W.Y. Recent advances in immobilized enzymes on nanocarriers. Chinese J. Catal. 2016, 37, 1814–1823. [Google Scholar] [CrossRef]
- Bilal, M.; Zhao, Y.P.; Rasheed, T.; Iqbal, H.M. Magnetic nanoparticles as versatile carriers for enzymes immobilization: A review. Int. J. Biol. Macromol. 2018, 120, 2530–2544. [Google Scholar] [CrossRef]
- Xiao, D.L.; Lu, T.; Zeng, R.; Bi, Y.P. Preparation and highlighted applications of magnetic microparticles and nanoparticles: A review on recent advances. Microchim. Acta 2016, 183, 2655–2675. [Google Scholar] [CrossRef]
- Oroujeni, M.; Kaboudin, B.; Xia, W.; Jönsson, P.; Ossipov, D.A. Conjugation of cyclodextrin to magnetic Fe3O4 nanoparticles via polydopamine coating for drug delivery. Prog. Org. Coat. 2018, 114, 154–161. [Google Scholar] [CrossRef]
- Cipolatti, E.P.; Valerio, A.; Henriques, R.O.; Moritz, D.E.; Ninow, J.L.; Freire, D.M.; Manoel, E.A.; Fernandez-Lafuente, R.; de Oliveira, D. Nanomaterials for biocatalyst immobilization–state of the art and future trends. RSC Adv. 2016, 6, 104675–104692. [Google Scholar] [CrossRef]
- Cao, S.L.; Xu, H.; Lai, L.H.; Gu, W.M.; Xu, P.; Xiong, J.; Yin, H.; Li, X.H.; Ma, Y.Z.; Zhou, J. Magnetic ZIF-8/cellulose/Fe3O4 nanocomposite: Preparation, characterization, and enzyme immobilization. Bioresour. Bioprocess. 2017, 4, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.F.; Liu, Z.M.; Zhou, Z.M. Improving pullulanase catalysis via reversible immobilization on modified Fe3O4@ polydopamine nanoparticles. Appl. Biochem. Biotech. 2017, 182, 1467–1477. [Google Scholar] [CrossRef]
- Fernandez-Lafuente, R. Lipase from Thermomyces lanuginosus: Uses and prospects as an industrial biocatalyst. J. Mol. Catal. B-Enzym. 2010, 62, 197–212. [Google Scholar] [CrossRef]
- Gonçalves Filho, D.; Silva, A.G.; Guidini, C.Z. Lipases: Sources, immobilization methods, and industrial applications. Appl. Microbiol. Biot. 2019, 103, 7399–7423. [Google Scholar] [CrossRef] [PubMed]
- Matuoog, N.; Li, K.; Yan, Y.J. Thermomyces lanuginosus lipase immobilized on magnetic nanoparticles and its application in the hydrolysis of fish oil. J. Food Biochem. 2018, 42, e12549. [Google Scholar] [CrossRef]
- Sarno, M.; Iuliano, M.; Polichetti, M.; Ciambelli, P. High activity and selectivity immobilized lipase on Fe3O4 nanoparticles for banana flavour synthesis. Process Biochem. 2017, 56, 98–108. [Google Scholar] [CrossRef]
- Ghasemi, S.; Sadighi, A.; Heidary, M.; Bozorgi-Koushalshahi, M.; Habibi, Z.; Faramarzi, M.A. Immobilisation of lipase on the surface of magnetic nanoparticles and non-porous glass beads for regioselective acetylation of prednisolone. IET Nanobiotechnol. 2013, 7, 100–108. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Soni, S.; Dwivedee, B.P.; Banerjee, U.C. Facile fabrication of a recyclable nanobiocatalyst: Immobilization of Burkholderia cepacia lipase on carbon nanofibers for the kinetic resolution of a racemic atenolol intermediate. RSC Adv. 2018, 8, 27763–27774. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.H.; Han, H.B.; Jiang, W.; Ding, X.B.; Li, Q.S.; Wang, Y.B. Immobilization of thermostable lipase QLM on core-shell structured polydopamine-coated Fe3O4 nanoparticles. Catalysts 2017, 7, 49. [Google Scholar] [CrossRef]
- Cheng, G.; Zheng, S.Y. Construction of a high-performance magnetic enzyme nanosystem for rapid tryptic digestion. Scientific Rep. 2014, 4, 6947. [Google Scholar] [CrossRef] [Green Version]
- Deng, X.; Cao, S.L.; Li, N.; Wu, H.; Smith, T.J.; Zong, M.H.; Lou, W.Y. A magnetic biocatalyst based on mussel-inspired polydopamine and its acylation of dihydromyricetin. Chin. J. Catal. 2016, 37, 584–595. [Google Scholar] [CrossRef]
- Sureshkumar, M.; Lee, C.K. Polydopamine coated magnetic-chitin (MCT) particles as a new matrix for enzyme immobilization. Carbohyd. Polym. 2011, 84, 775–780. [Google Scholar] [CrossRef]
- Wang, X.Y.; Jiang, X.P.; Li, Y.; Zeng, S.; Zhang, Y.W. Preparation Fe3O4@chitosan magnetic particles for covalent immobilization of lipase from Thermomyces lanuginosus. Int. J. Biol. Macromol. 2015, 75, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.T.; Liu, C.Z.; Hu, J.H.; Guo, C. Improved performance of immobilized laccase on amine-functioned magnetic Fe3O4 nanoparticles modified with polyethylenimine. Chem. Eng. J. 2016, 295, 201–206. [Google Scholar] [CrossRef]
- Kumar, D.; Nagar, S.; Bhushan, I.; Kumar, L.; Parshad, R.; Gupta, V.K. Covalent immobilization of organic solvent tolerant lipase on aluminum oxide pellets and its potential application in esterification reaction. J. Mol. Catal. B-Enzym. 2013, 87, 51–61. [Google Scholar] [CrossRef]
- Wang, J.Z.; Zhao, G.H.; Jing, L.Y.; Peng, X.M.; Li, Y.F. Facile self-assembly of magnetite nanoparticles on three-dimensional graphene oxide–chitosan composite for lipase immobilization. Biochem. Eng. J. 2015, 98, 75–83. [Google Scholar] [CrossRef]
- Marruecos, D.F.; Schwartz, D.K.; Kaar, J.L. Impact of surface interactions on protein conformation. Curr. Opin. Colloid Interface Sci. 2018, 38, 45–55. [Google Scholar] [CrossRef]
- Salihu, A.; Alam, M.Z. Solvent tolerant lipases: A review. Process Biochem. 2015, 50, 86–96. [Google Scholar] [CrossRef]
- Yang, R.L.; Zhao, X.J.; Wu, T.T.; Bilal, M.; Wang, Z.Y.; Luo, H.Z.; Yang, W.J. A novel and highly regioselective biocatalytic approach to acetylation of helicid by using whole-cell biocatalysts in organic solvents. Catal. Commun. 2019, 128, 105707. [Google Scholar] [CrossRef]
- Pätzold, M.; Siebenhaller, S.; Kara, S.; Liese, A.; Syldatk, C.; Holtmann, D. Deep eutectic solvents as efficient solvents in biocatalysis. Trends Biotechnol. 2019, 37, 943–959. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bi, Y.; Wang, Z.; Zhang, R.; Diao, Y.; Tian, Y.; Jin, Z. Improved Catalytic Properties of Thermomyces lanuginosus Lipase Immobilized onto Newly Fabricated Polydopamine-Functionalized Magnetic Fe3O4 Nanoparticles. Processes 2020, 8, 629. https://doi.org/10.3390/pr8050629
Bi Y, Wang Z, Zhang R, Diao Y, Tian Y, Jin Z. Improved Catalytic Properties of Thermomyces lanuginosus Lipase Immobilized onto Newly Fabricated Polydopamine-Functionalized Magnetic Fe3O4 Nanoparticles. Processes. 2020; 8(5):629. https://doi.org/10.3390/pr8050629
Chicago/Turabian StyleBi, Yanhong, Zhaoyu Wang, Rui Zhang, Yihan Diao, Yaoqi Tian, and Zhengyu Jin. 2020. "Improved Catalytic Properties of Thermomyces lanuginosus Lipase Immobilized onto Newly Fabricated Polydopamine-Functionalized Magnetic Fe3O4 Nanoparticles" Processes 8, no. 5: 629. https://doi.org/10.3390/pr8050629
APA StyleBi, Y., Wang, Z., Zhang, R., Diao, Y., Tian, Y., & Jin, Z. (2020). Improved Catalytic Properties of Thermomyces lanuginosus Lipase Immobilized onto Newly Fabricated Polydopamine-Functionalized Magnetic Fe3O4 Nanoparticles. Processes, 8(5), 629. https://doi.org/10.3390/pr8050629




