A Review of Control-Oriented Bioelectrochemical Mathematical Models of Microbial Fuel Cells
Abstract
1. Introduction
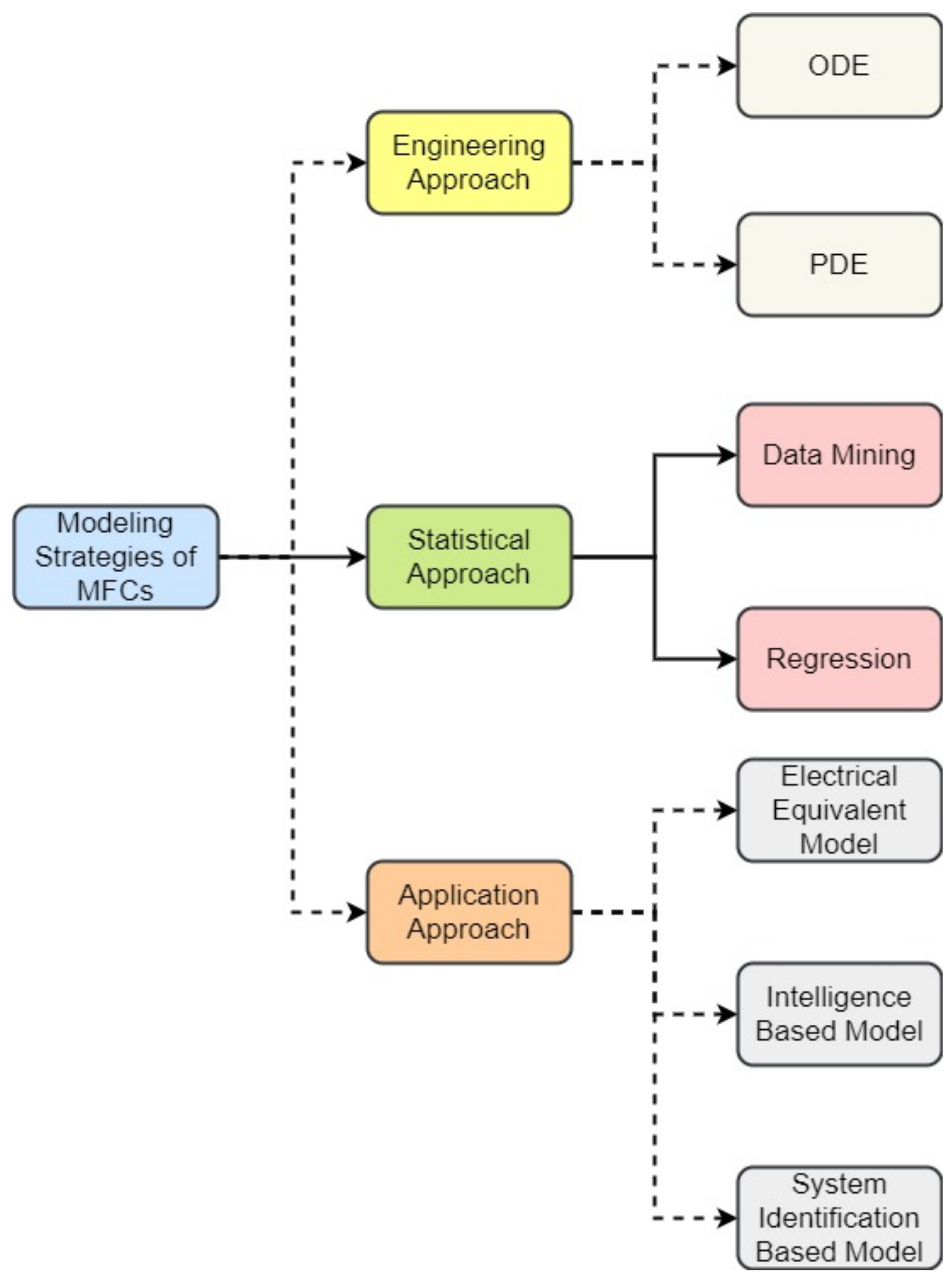
2. Modeling Strategies and Description of Mathematical Models
3. Control-Oriented Mathematical Models
3.1. Single Chamber MFC Model with a Single Microorganism
3.2. Single Chamber MFC Model with Two Microorganisms
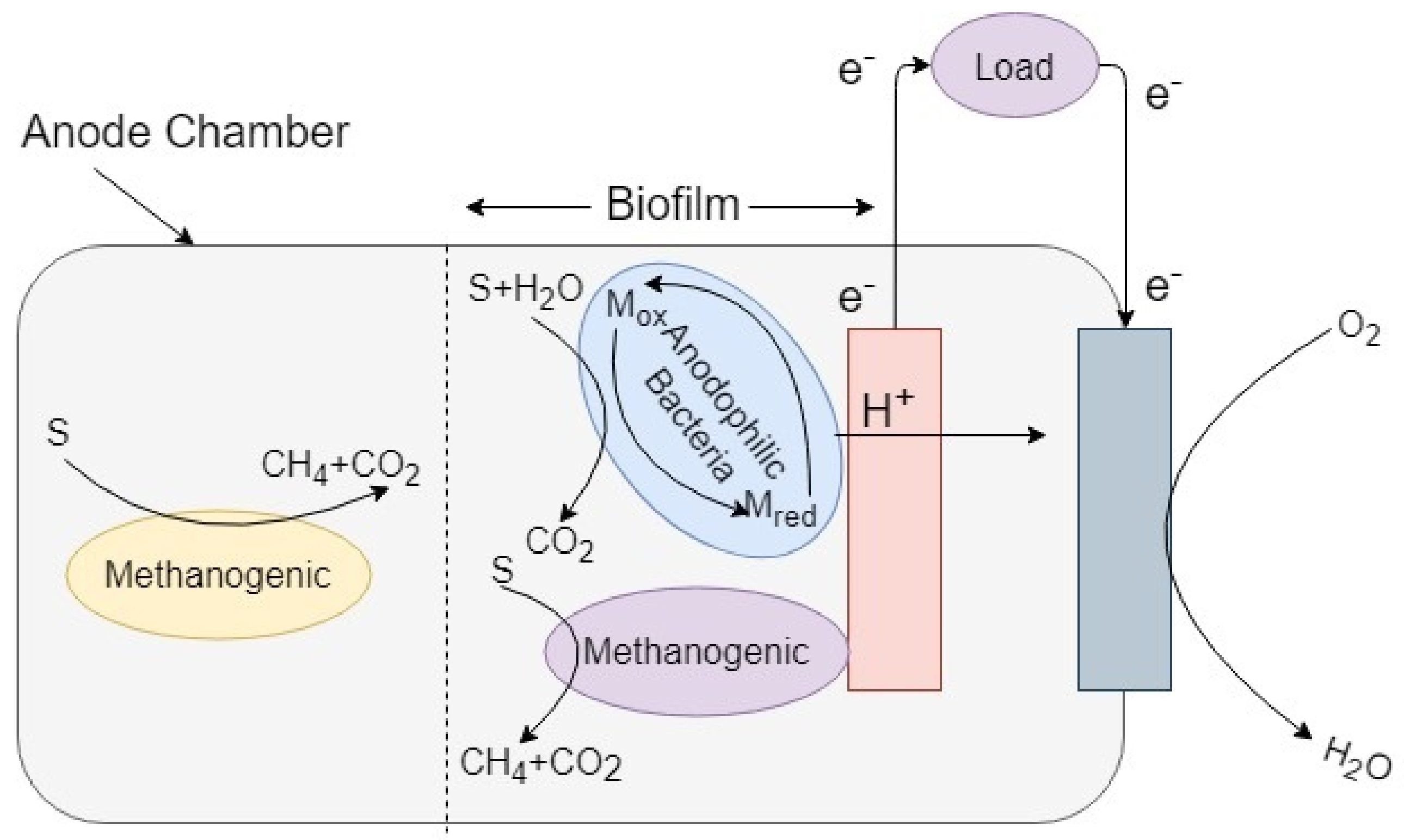
3.3. Dual Chamber MFC Model with Single Microorganism
4. Modeling of MFCs Based on Applications
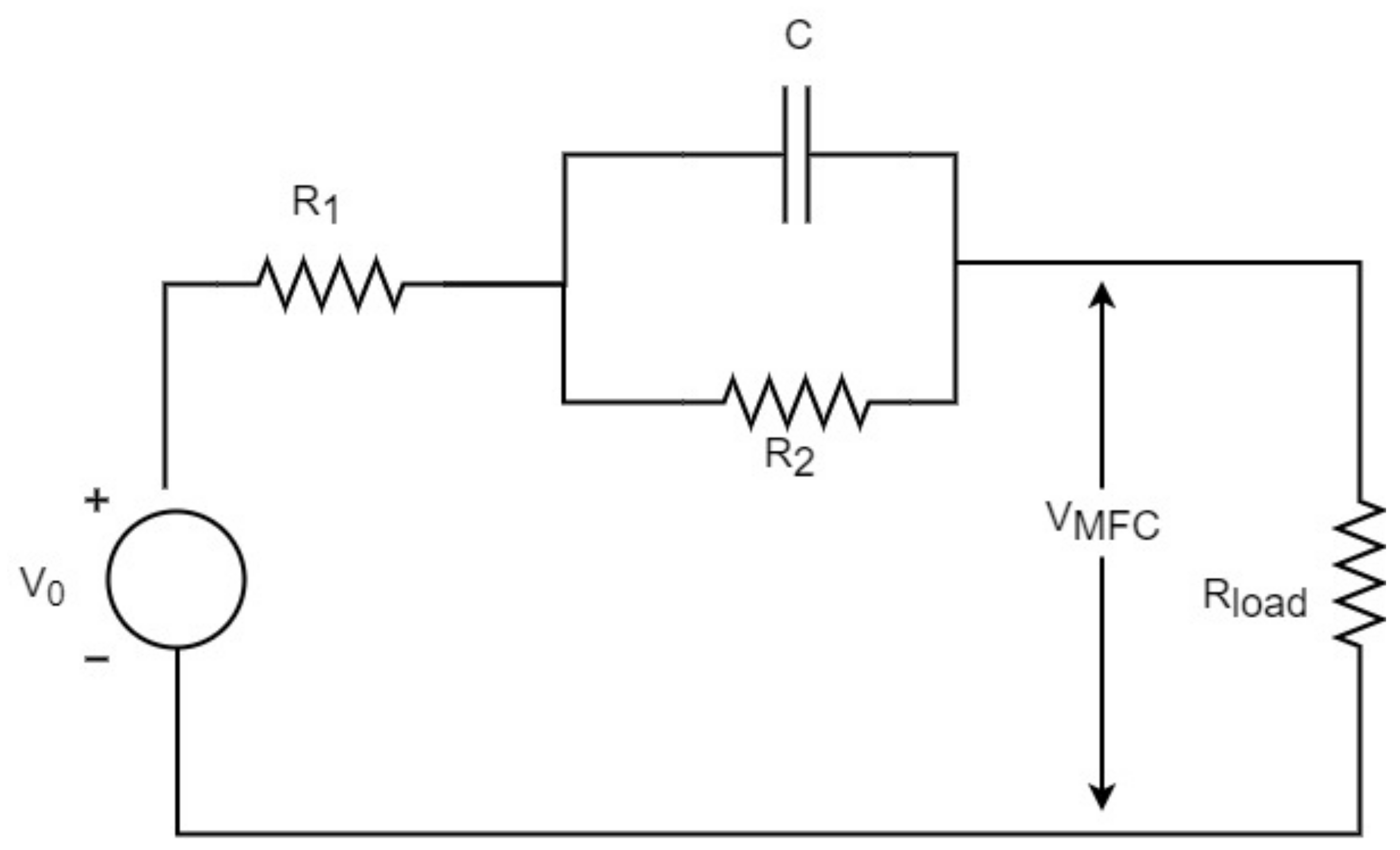
4.1. Equivalent Electrical Circuit Based MFC Models
4.2. Intelligence Based MFC Models
4.3. System Identification Based MFC Models
5. Developments in Control Strategies of MFCs
6. Perspectives and Challenges
7. Conclusions
- Mathematical model of MFCs, and its control and optimization strategies are chosen based on the specific applications and operational requirements.
- Bounds of uncertain parameters should be practical with a higher confidence level. It is advisable to do more experiments to get accurate practical bounds.
- Several reasonable assumptions may be required in mathematical modeling while developing advanced control and optimization strategies to boost the overall performance of MFCs.
- Selection of appropriate manipulated input variables for successful development of efficient control actions is required.
- Development of control and optimization strategies for MFCs should be economical, environment friendly, and reliable.
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| MFC | Microbial fuel cell |
| ODE | Ordinary differential equation |
| PDE | Partial differential equation |
| PID | Proportional integral derivative |
| LMI | Linear matrix inequality |
| MRAC | Model reference adaptive control |
| MIT | Massachusetts Institute of Technology |
| PEM | Proton exchange membrane |
| CEM | Cation exchange membrane |
| ARX | Auto regressive with external input |
| MPPT | Maximum power point tracking |
| RVM | Relevance vector machine |
| ANN | Artificial neural network |
| ANFIS | Adaptive neuro-fuzzy inference system |
| MGGP | Multi-gene genetic programming |
| SVR | Support vector regression |
| SVM | Support vector machine |
References
- Logan, B.; Hamelers, B.; Rozendal, R.; Schröder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.; Rabaey, K. Microbial Fuel Cells: Methodology and Technology†. Environ. Sci. Technol. 2006, 40, 5181–5192. [Google Scholar] [CrossRef]
- Akdeniz, F.; Çağlar, A.; Güllü, D. Recent energy investigations on fossil and alternative nonfossil resources in Turkey. Energy Convers. Manag. 2002, 43, 575–589. [Google Scholar] [CrossRef]
- Solé, J.; García-Olivares, A.; Turiel, A.; Ballabrera-Poy, J. Renewable transitions and the net energy from oil liquids: A scenarios study. Renew. Energy 2018, 116, 258–271. [Google Scholar] [CrossRef]
- Gojiya, A.; Deb, D.; Iyer, K. Feasibility Study Of Power Generation From Agricultural Residue In Comparison With Soil Incorporation Of Residue. Renew. Energy 2018, 134, 416–425. [Google Scholar] [CrossRef]
- Deb, D. Intelligent decision-making device for residue incorporation in soil or biomass power plants. J. Intell. Fuzzy Syst. 2020, 1–10. [Google Scholar] [CrossRef]
- Choudhury, P.; Uday, U.S.P.; Mahata, N.; Nath Tiwari, O.; Narayan, R.; Kanti Bandyopadhyay, T.; Bhunia, B. Performance improvement of microbial fuel cells for waste water treatment along with value addition: A review on past achievements and recent perspectives. Renew. Sustain. Energy Rev. 2017, 9, 372–389. [Google Scholar] [CrossRef]
- Peighambardoust, S.J.; Rowshanzamir, S.; Amjadi, M. Review of the Proton Exchange Membranes for Fuel Cell Applications; Elsevier Ltd.: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Hu, P.; Ouyang, Y.; Wu, L.; Shen, L.; Luo, Y.; Christie, P. Effects of water management on arsenic and cadmium speciation and accumulation in an upland rice cultivar. J. Environ. Sci. 2015, 27, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Mathuriya, A.S.; Sharma, V.N. Bioelectricity production from paper industry waste using a microbial fuel cell by Clostridium species. J. Biochem. Technol. 2009, 1, 49–52. [Google Scholar]
- Qin, M.; Hynes, E.A.; Abu-Reesh, I.M.; He, Z. Ammonium removal from synthetic wastewater promoted by current generation and water flux in an osmotic microbial fuel cell. J. Clean. Prod. 2017, 149, 856–862. [Google Scholar] [CrossRef]
- HaoYu, E.; Cheng, S.; Scott, K.; Logan, B. Microbial fuel cell performance with non-Pt cathode catalysts. J. Power Sources 2007, 171, 275–281. [Google Scholar] [CrossRef]
- Pant, D.; Van Bogaert, G.; Diels, L.; Vanbroekhoven, K. A review of the substrates used in microbial fuel cells (MFCs) for sustainable energy production. Bioresour. Technol. 2010, 101, 1533–1543. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R. The microbe electric: Conversion of organic matter to electricity. Curr. Opin Biotechnol. 2008, 19, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R. Bug juice: Harvesting electricity with microorganisms. Nat. Rev. Microbiol. 2006, 4, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Venkata Mohan, S.; Velvizhi, G.; Annie Modestra, J.; Srikanth, S. Microbial fuel cell: Critical factors regulating bio-catalyzed electrochemical process and recent advancements. Renew. Sustain. Energy Rev. 2014, 40, 779–797. [Google Scholar] [CrossRef]
- ElMekawy, A.; Hegab, H.M.; Losic, D.; Saint, C.P.; Pant, D. Applications of graphene in microbial fuel cells: The gap between promise and reality. Renew. Sustain. Energy Rev. 2017, 72, 1389–1403. [Google Scholar] [CrossRef]
- Hindatu, Y.; Annuar, M.S.M.; Gumel, A.M. Mini-review: Anode modification for improved performance of microbial fuel cell. Renew. Sustain. Energy Rev. 2017, 73, 236–248. [Google Scholar] [CrossRef]
- Nitisoravut, R.; Regmi, R. Plant microbial fuel cells: A promising biosystems engineering. Renew. Sustain. Energy Rev. 2017, 76, 81–89. [Google Scholar] [CrossRef]
- Oh, S.T.; Kim, J.R.; Premier, G.C.; Lee, T.H.; Kim, C.; Sloan, W.T. Sustainable wastewater treatment: How might microbial fuel cells contribute. Biotechnol. Adv. 2010, 28, 871–881. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, L.; Zularisam, A.W.; Hai, F.I. Microbial fuel cell is emerging as a versatile technology: A review on its possible applications, challenges and strategies to improve the performances. Int. J. Energy Res. 2017, 42, 369–394. [Google Scholar] [CrossRef]
- Xiao, L.; He, Z. Applications and perspectives of phototrophic microorganisms for electricity generation from organic compounds in microbial fuel cells. Renew. Sustain. Energy Rev. 2014, 37, 550–559. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, M.; Guo, J.; Sun, G. Bacterial extracellular electron transfer in bioelectrochemical systems. Process Biochem. 2012, 47, 1707–1714. [Google Scholar] [CrossRef]
- Schröder, U. Anodic electron transfer mechanisms in microbial fuel cells and their energy efficiency. Phys. Chem. Chem. Phys. 2007, 9, 2619–2629. [Google Scholar] [CrossRef] [PubMed]
- Kundu, A.; Sahu, J.N.; Redzwan, G.; Hashim, M.A. An overview of cathode material and catalysts suitable for generating hydrogen in microbial electrolysis cell. Int. J. Hydrogen Energy 2013, 38, 1745–1757. [Google Scholar] [CrossRef]
- Santoro, C.; Arbizzani, C.; Erable, B.; Ieropoulos, I. Microbial fuel cells: From fundamentals to applications. A review. J. Power Sources 2017, 356, 225–244. [Google Scholar] [CrossRef]
- Saba, B.; Christy, A.D.; Yu, A.; Co, A.C. Sustainable power generation from bacterio-algal microbial fuel cells (MFCs): An overview. Renew. Sustain. Energy Rev. 2017, 73, 75–84. [Google Scholar] [CrossRef]
- He, L.; Du, P.; Chen, Y.; Lu, H.; Cheng, X.; Chang, B.; Wang, Z. Advances in microbial fuel cells for wastewater treatment. Renew. Sustain. Energy Rev. 2017, 71, 388–403. [Google Scholar] [CrossRef]
- Ortiz-Martínez, V.M.; Salar-García, M.J.; De los Ríos, A.P.; Hernández-Fernández, F.J.; Egea, J.A.; Lozano, L.J. Developments in microbial fuel cell modeling. Chem. Eng. J. 2015, 271, 50–60. [Google Scholar] [CrossRef]
- Recio-Garrido, D.; Perrier, M.; Tartakovsky, B. Modeling, optimization and control of bioelectrochemical systems. Chem. Eng. J. 2016, 289, 180–190. [Google Scholar] [CrossRef]
- Janicek, A.; Fan, Y.; Liu, H. Design of microbial fuel cells for practical application: A review and analysis of scale-up studies. Biofuels 2014, 5, 79–92. [Google Scholar] [CrossRef]
- Luo, S.; Sun, H.; Ping, Q.; Jin, R.; He, Z. A review of modeling bioelectrochemical systems: Engineering and statistical aspects. Energies 2016, 9, 111. [Google Scholar] [CrossRef]
- Xia, C.; Zhang, D.; Pedrycz, W.; Zhu, Y.; Guo, Y. Models for Microbial Fuel Cells: A critical review. J. Power Sources 2018, 373, 119–131. [Google Scholar] [CrossRef]
- Jadhav, G.S.; Ghangrekar, M.M. Bioresource Technology Performance of microbial fuel cell subjected to variation in pH, temperature, external load and substrate concentration. Bioresour. Technol. 2009, 100, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Merkey, B.V.; Chopp, D.L. The Performance of a Microbial Fuel Cell Depends Strongly on Anode Geometry: A Multidimensional Modeling Study. Bull. Math. Biol. 2012, 74, 834–857. [Google Scholar] [CrossRef]
- Picioreanu, C.; Katuri, K.p.; Head, I.M.; Van Loosdrecht, M.C.M.; Scott, K. Mathematical model for microbial fuel cells with anodic biofilms and anaerobic digestion. Water Sci. Technol. 2008, 57, 965–971. [Google Scholar] [CrossRef] [PubMed]
- Maier, R.; Pepper, I. Environmental Microbiology, 3rd ed.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Zhang, X.C.; Halme, A. Modelling of a microbial fuel cell process. Biotechnol. Lett. 1995, 17, 809–814. [Google Scholar] [CrossRef]
- Abul, A.; Zhang, J.; Steidl, R.; Reguera, G.; Tan, X. Microbial fuel cells: Control-oriented modeling and experimental validation. Am. Control Conf. 2016, 412–417. [Google Scholar] [CrossRef]
- Pinto, R.P.; Srinivasan, B.; Manuel, M.F.; Tartakovsky, B. A two-population bio-electrochemical model of a microbial fuel cell. Bioresour. Technol. 2010, 101, 5256–5265. [Google Scholar] [CrossRef]
- Recio-Garrido, D.; Perrier, m.; Tartakovsky, B. Combined bioelectrochemical-electrical model of a microbial fuel cell. Bioprocess Biosyst. Eng. 2016, 39, 267–276. [Google Scholar] [CrossRef]
- Zeng, Y.; Choo, Y.F.; Kim, B.H.; Wu, P. Modelling and simulation of two-chamber microbial fuel cell. J. Power Sources 2010, 195, 79–89. [Google Scholar] [CrossRef]
- Oliveira, V.B.; Simões, M.; Melo, L.F.; Pinto, A.M.F.R. A 1D mathematical model for a microbial fuel cell. Energy 2013, 61, 463–471. [Google Scholar] [CrossRef]
- Esfandyari, M.; Fanaei, M.A.; Gheshlaghi, R.; Mahdavi, M.A. Mathematical modeling of two-chamber batch microbial fuel cell with pure culture of Shewanella. Chem. Eng. Res. Des. 2016, 117, 34–42. [Google Scholar] [CrossRef]
- Shankar, R.; Mondal, P.; Chand, S. Modelling and simulation of double chamber microbial fuel cell: Cell voltage, power density and temperature variation with process parameters. Green 2013, 3, 181–194. [Google Scholar] [CrossRef]
- Torres, I.; Rittmann, B.E.; Marcus, A.K. Conduction-Based Modeling of the Biofilm Anode of a Microbial Fuel Cell. Biotechnol. Bioeng. 2007, 98, 1171–1182. [Google Scholar]
- Jayasinghea, N.; Franksb, A.; Nevinb, K.P.; Mahadevan, R. Metabolic Modelling of Spatial Heterogeneity of Biofilms in Microbial Fuel Cells Reveals Substrate Limitations in Electrical Current Generation. Biotechnol. J. 2014, 9, 1350–1361. [Google Scholar] [CrossRef] [PubMed]
- Sirinutsomboon, B. Modeling of a membraneless single-chamber microbial fuel cell with molasses as an energy source. Int. J. Energy Environ. Eng. 2014, 5, 1–9. [Google Scholar] [CrossRef]
- Picioreanu, C.; Katuri, A.P.; Van Loosdrecht, M.C.M.; Head, I.M.; Scott, K. Modelling microbial fuel cells with suspended cells and added electron transfer mediator. J. Appl. Electrochem. 2010, 40, 151–162. [Google Scholar] [CrossRef]
- Picioreanu, C.; Head, I.M.; Katuri, K.P.; Van Loosdrecht, M.C.M.; Scott, K. A computational model for biofilm-based microbial fuel cells. Water Res. 2007, 41, 2921–2940. [Google Scholar] [CrossRef]
- Esfandyari, M.; Fanaei, M.A.; Gheshlaghi, R.; Mahdavi, M.A. Dynamic modeling of a continuous two-chamber microbial fuel cell with pure culture of Shewanella. Int. J. Hydrogen Energy 2017, 42, 21198–21202. [Google Scholar] [CrossRef]
- Pinto, R.P.; Tartakovsky, B.; Perrier, M.; Srinivasan, B. Optimizing Treatment Performance of Microbial Fuel Cells by Reactor Staging. Ind. Eng. Chem. Res. 2010, 49, 9222–9229. [Google Scholar] [CrossRef]
- Naureen, Z.; Ali, Z.; Al, R.; Nasser, M.; Jabri, A.; Khalfan, S.; Housni, A.; Gilani, S.A.; Mabood, F.; Farooq, S.; et al. Generation of Electricity by Electrogenic Bacteria in a Microbial Fuel Cell Powered by Waste Water. Adv. Biosci. Biotechnol. 2016, 7, 329–335. [Google Scholar] [CrossRef][Green Version]
- Patel, R.; Deb, D.; Dey, R.; Balas, V.E. Adaptive Control of Single Population Single Chamber MFC. In Adaptive and Intelligent Control of Microbial Fuel Cells; Intelligent Systems Reference Library; Springer Nature Switzerland AG: Basel, Switzerland, 2020; Volume 161. [Google Scholar]
- Patel, R.; Deb, D. Parametrized Control-Oriented Mathematical Model and Adaptive Backstepping Control of a Single Chamber Single Population Microbial Fuel Cell. J. Power Sources 2018, 396, 599–604. [Google Scholar] [CrossRef]
- Babanova, S.; Hubenova, Y.; Mitov, M.; Mandjukov, P. Uncertainties of Yeast-Based Biofuel Cell Operational Characteristics. Fuel Cells 2011, 11, 824–837. [Google Scholar] [CrossRef]
- Coronado, J.; Tartakovsky, B.; Perrier, M. On-line monitoring of microbial fuel cells operated with pulse-width modulated electrical load. J. Process Control 2015, 35, 59–64. [Google Scholar] [CrossRef]
- Park, J.; Roane, T.; Ren, Z.; Alaraj, M. Dynamic modeling of a microbial fuel cell considering anodic electron flow and electrical charge storage. Appl. Energy 2017, 193, 507–514. [Google Scholar] [CrossRef]
- Rismani-Yazdi, H.; Carver, S.M.; Christy, A.D.; Tuovinen, O.H. Cathodic limitations in microbial fuel cells: An overview. J. Power Sources 2008, 180, 683–694. [Google Scholar] [CrossRef]
- Manohar, A.K.; Mansfeld, F. The internal resistance of a microbial fuel cell and its dependence on cell design and operating conditions. Electrochim. Acta 2009, 54, 1664–1670. [Google Scholar] [CrossRef]
- He, Z.; Mansfeld, F. Exploring the use of electrochemical impedance spectroscopy (EIS) in microbial fuel cell studies. Energy Environ. Sci. 2009, 2, 215–219. [Google Scholar] [CrossRef]
- Cooper, K.R.; Smith, M. Electrical test methods for online fuel cell ohmic resistance measurement. J. Power Sources 2006, 160, 1088–1095. [Google Scholar] [CrossRef]
- Ren, Z.; Yan, H.; Wang, W.; Mench, M.M.; Regan, J.M. Characterization of Microbial Fuel Cells at Microbially and Electrochemically Meaningful Time scales. Environ. Sci. Technol. 2011, 45, 2435–2441. [Google Scholar] [CrossRef]
- Mingant, R.; Bernard, J.; Sauvant-Moynot, V. Novel state-of-health diagnostic method for Li-ion battery in service. Applied Energy 2016, 183, 390–398. [Google Scholar] [CrossRef]
- Hernández-Flores, G.; Poggi-Varaldo, H.M.; Solorza-Feria, O.; Ponce Noyola, M.T.; Romero-Castañón, T.; Rinderknecht-Seijas, N. Tafel equation based model for the performance of a microbial fuel cell. Int. J. Hydrogen Energy 2015, 40, 17421–17432. [Google Scholar] [CrossRef]
- Sindhuja, M.; Kumar, N.S.; Sudha, V.; Harinipriya, S. Equivalent circuit modeling of microbial fuel cells using impedance spectroscopy. J. Energy Storage 2016, 7, 136–146. [Google Scholar] [CrossRef]
- Zhihao, L.; Peter, G.; Peng, L.; Haifeng, S.; Guangtuan, H.; Lankun, C.; Lehua, Z. Biological capacitance studies of anodes in microbial fuel cells using electrochemical impedance spectroscopy. Bioprocess Biosyst. Eng. 2015, 38, 1325–1333. [Google Scholar]
- Dhiman, H.S.; Deb, D.; Guerrero, J.M. Hybrid Machine Intelligent SVR Variants For Wind Forecasting In addition, Ramp Events. Renew. Sustain. Energy Rev. 2018, 108, 369–379. [Google Scholar] [CrossRef]
- Dhiman, H.S.; Deb, D.; Balas, V.E. Supervised Machine Learning in Wind Forecasting and Ramp Event Prediction; Apple Academic Press, Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Fang, F.; Zang, G.; Sun, M.; Yu, H. Optimizing multi-variables of microbial fuel cell for electricity generation with an integrated modeling and experimental approach. Appl. Energy 2013, 110, 98–103. [Google Scholar] [CrossRef]
- Esfandyari, M.; Fanaei, M.; Gheshlaghi, R.; Mahdavi, M. Neural network and neuro-fuzzy modeling to investigate the power density and Columbic efficiency of microbial fuel cell. J. Taiwan Inst. Chem. Eng. 2015, 58, 84–91. [Google Scholar] [CrossRef]
- Garg, A.; Vijayaraghavan, V.; Mahapatra, S.; Tai, K.; Wong, C. Performance evaluation of microbial fuel cell by artificial intelligence methods. Expert Syst. Appl. 2014, 41, 1389–1399. [Google Scholar] [CrossRef]
- Boghani, H.; Kim, J.; Dinsdale, R.; Guwy, A.; Premier, G. Analysis of the dynamic performance of a microbial fuel cellusing a system identification approach. J. Power Sources 2013, 238, 218–226. [Google Scholar] [CrossRef]
- Boghani, H.; Michie, I.I.; Dinsdale, R.; Guwy, A.; Premier, G. Control of microbial fuel cell voltage using a gain scheduling control strategy. J. Power Sources 2016, 322, 106–115. [Google Scholar] [CrossRef]
- Patel, R.; Deb, D.; Dey, R.; Balas, E.V. Model Reference Adaptive Control of Microbial Fuel Cells. In Adaptive and Intelligent Control of Microbial Fuel Cells; Intelligent Systems Reference Library; Springer Nature Switzerland AG: Basel, Switzerland, 2020; Volume 161. [Google Scholar]
- Yewale, A.; Methekar, R.; Agrawal, S. Dynamic analysis and multiple model control of continuous microbial fuel cell (CMFC). Chem. Eng. Res. Des. 2019, 48, 403–416. [Google Scholar] [CrossRef]
- Yan, M.; Fan, L. Constant Voltage Output in Two-Chamber Microbial Fuel Cell Under Fuzzy PID Control. Int. J. Electrochem. Sci. 2013, 8, 3321–3332. [Google Scholar]
- Fan, L.; Li, C.; Boshnakov, K. Performance improvement of a microbial fuel cell based on adaptive fuzzy control. Pak J. Pharm. Sci. 2014, 27, 685–690. [Google Scholar] [PubMed]
- Fan, L.; Zhang, J.; Shi, X. Performance Improvement of a Microbial Fuel Cell based on Model Predictive Control. Int. J. Electrochem. Sci. 2015, 10, 737–748. [Google Scholar]
- Patel, R.; Deb, D. Nonlinear adaptive control of microbial fuel cell with two species in a single chamber. J. Power Sources 2019, 434, 226739. [Google Scholar] [CrossRef]
- Patel, R.; Deb, D.; Dey, R.; Balas, V.E. Robust Control Design of SPSC Microbial Fuel Cell with Norm Bounded Uncertainty. In Adaptive and Intelligent Control of Microbial Fuel Cells; Intelligent Systems Reference Library; Springer Nature Switzerland AG: Basel, Switzerland, 2020; Volume 161. [Google Scholar]
- Patel, R.; Deb, D.; Dey, R.; Balas, V.E. Exact Linearization of Two Chamber Microbial Fuel Cell. In Adaptive and Intelligent Control of Microbial Fuel Cells; Intelligent Systems Reference Library; Springer Nature Switzerland AG: Basel, Switzerland, 2020; Volume 161. [Google Scholar]
- Luo, Q.; An, A.; Wang, M. Model Reference Adaptive Control for Microbial Fuel Cell (MFC). In Proceedings of the 2019 4th International Conference on Robotics, Control and Automation—ICRCA, Guangzhou, China, 26–28 July 2019. [Google Scholar]
- Zheng, G.; Zhen, H. Long-term performance of a 200 liter modularized microbial fuel cell system treating municipal wastewater: Treatment, energy, and cost. Environ. Sci. Water Res. Technol. 2016, 2, 235–406. [Google Scholar]
- Mojtaba, M.; Ewa, Z.; Jacek, M. Achieving energy neutrality in wastewater treatment plants through energy savings and enhancing renewable energy production. Rev. Env. Sci. Biotechnol. 2018, 17, 655–689. [Google Scholar]
- Vilajeliu-Pons, A.; Puig, S.; Salcedo-Dávila, I.; Balaguer, M.D.; Colprim, J. Long-term assessment of six-stacked scaled-up MFCs treating swine manure with different electrode materials. Environ. Sci. Water Res. Technol. 2017, 3, 947–959. [Google Scholar] [CrossRef]




| Model Approach | Compartment Modeled | Mediator | No. of Bacteria | Reaction Equations | Time and Space Resolution | Ref. |
|---|---|---|---|---|---|---|
| ODE | Anode | Yes | One | Monod,Tafel, | 1D, | [37] |
| (External) | Nernst. | Dynamic. | ||||
| — | One | Monod, | 1D, | [38] | ||
| Nernst. | Dynamic. | |||||
| 1D, | [39] | |||||
| Yes | Nernst, | Steady St., | ||||
| (Intracellular) | Two | Double Monod, | Dynamic | |||
| Butler–Volmer. | 1D, | [40] | ||||
| Dynamic. | ||||||
| Anode, Cathode. | No | One | Monod, | [41] | ||
| Butler–Volmer | 1D, | |||||
| No | Monod, | Steady St., | [42] | |||
| Tafel. | Dynamic. | |||||
| Yes | Monod,Tessier, | [43] | ||||
| (External) | Blackman, | |||||
| Nernst. | ||||||
| No | Two | — | 1D, | [44] | ||
| Steady St.. | ||||||
| PDE | Anode | No | One | Monod, Nernst. | 1D, | [45] |
| Steady | ||||||
| Dynamic. | ||||||
| 2D, | [46] | |||||
| Steady St.. | ||||||
| Anode, | Butler–Volmer | 1D, | [47] | |||
| Cathode. | Monod,Nernst. | Steady St.. | ||||
| PDE & ODE | Cathode, | One | Monod,Nernst. | Steady St., | [48] | |
| Anode. | Yes | 1D,2D,3D. | ||||
| (External) | Multiple | Double Monod, | 3D, | [49] | ||
| Anode | Butler–Volmer, | Steady St.. | ||||
| Nernst. |
| No. | Equation | Description |
|---|---|---|
| 1. | Monod Kinetics | It illustrates microorganisms grwoth and substrate utilization rate. |
| = specific growth rate, = maximum value of , = substrate | ||
| concentration, = Monod constant. | ||
| 2. | Tafel | It describes electrodes electrochemical current density and potentials. |
| E = electrode potential, = equilibrium potential, i = current density, | ||
| = exhcnage current density, F = Farady’s constant, R = ideal gas | ||
| constant, T = temperature, = charge transfer coefficient. | ||
| 3. | Nernst | It describes electrode potential based on an electrochemical reaction. |
| = standard electrode potential, n = number of electrons, | ||
| Q = reaction quotient. | ||
| 4. | Butler–Volmer | It illustrate electrochemical kinetics of anode and cathode reactions. |
| = charge transfer coefficients of anode and cathode. | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deb, D.; Patel, R.; Balas, V.E. A Review of Control-Oriented Bioelectrochemical Mathematical Models of Microbial Fuel Cells. Processes 2020, 8, 583. https://doi.org/10.3390/pr8050583
Deb D, Patel R, Balas VE. A Review of Control-Oriented Bioelectrochemical Mathematical Models of Microbial Fuel Cells. Processes. 2020; 8(5):583. https://doi.org/10.3390/pr8050583
Chicago/Turabian StyleDeb, Dipankar, Ravi Patel, and Valentina E. Balas. 2020. "A Review of Control-Oriented Bioelectrochemical Mathematical Models of Microbial Fuel Cells" Processes 8, no. 5: 583. https://doi.org/10.3390/pr8050583
APA StyleDeb, D., Patel, R., & Balas, V. E. (2020). A Review of Control-Oriented Bioelectrochemical Mathematical Models of Microbial Fuel Cells. Processes, 8(5), 583. https://doi.org/10.3390/pr8050583







