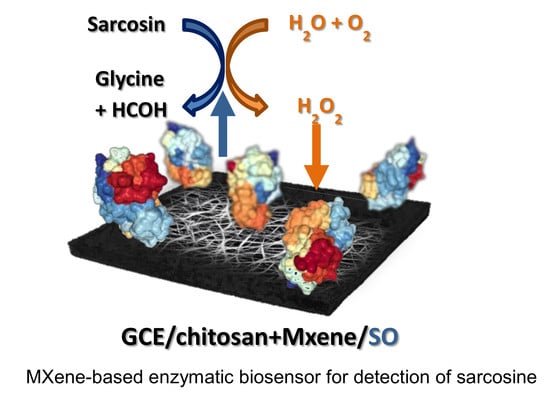
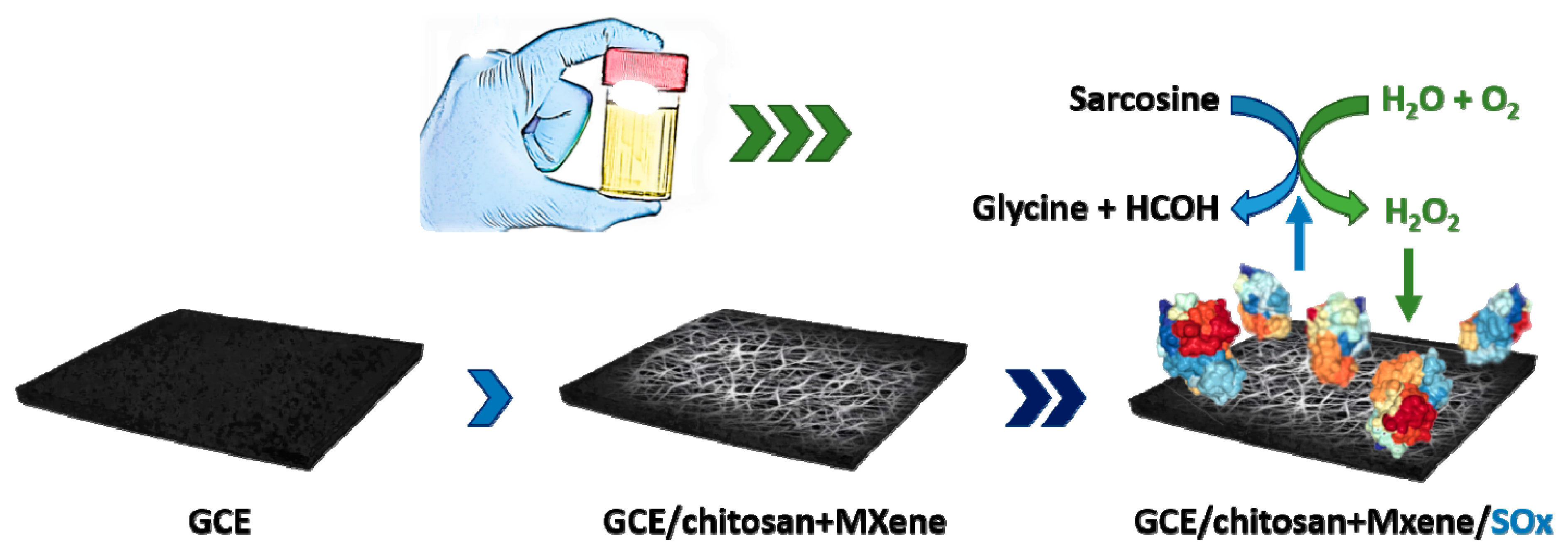
Ultrasensitive Ti3C2TX MXene/Chitosan Nanocomposite-Based Amperometric Biosensor for Detection of Potential Prostate Cancer Marker in Urine Samples
Abstract
1. Introduction
2. Material and Methods
2.1. Materials
2.2. Preparation of MXene
2.3. Electrochemical Procedures
2.4. Construction of MXene-Based Sarcosine Biosensor
2.5. Contact Angle Measurements
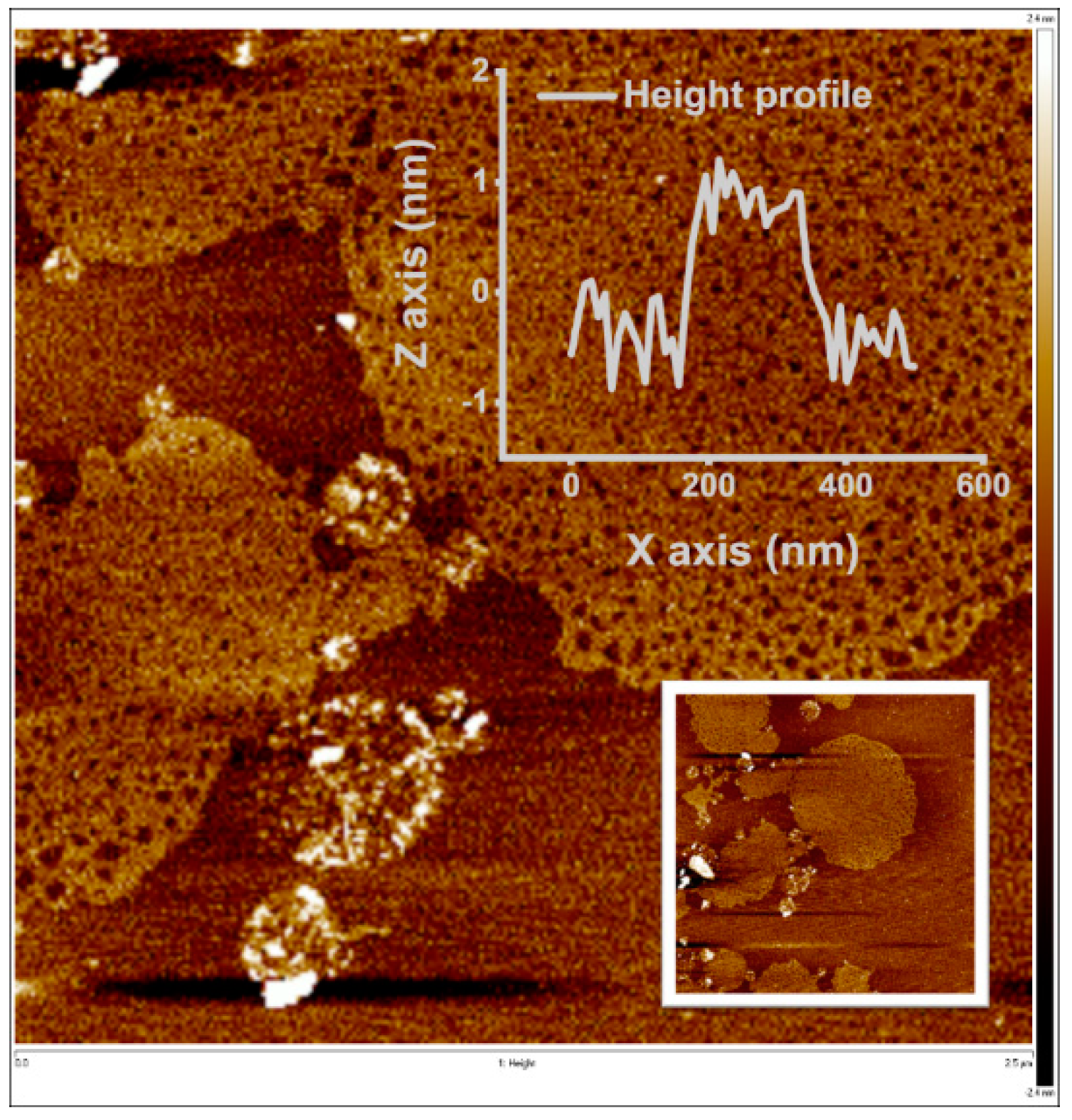
2.6. Characterisation of MXene and MXene-Chitosan Composite
3. Results and Discussion
3.1. Characterisation of MXene
3.2. Electrochemical Measurements
3.3. Clinical Application of SOx/MXene-chi/GCE Biosensor
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Tkac, J.; Gajdosova, V.; Hroncekova, S.; Bertok, T.; Hires, M.; Jane, E.; Lorencova, L.; Kasak, P. Prostate-specific antigen glycoprofiling as diagnostic and prognostic biomarker of prostate cancer. Interface Focus. 2019, 9, 20180077. [Google Scholar] [CrossRef] [PubMed]
- Bertok, T.; Lorencova, L.; Hroncekova, S.; Gajdosova, V.; Jane, E.; Hires, M.; Kasak, P.; Kaman, O.; Sokol, R.; Bella, V.; et al. Advanced impedimetric biosensor configuration and assay protocol for glycoprofiling of a prostate oncomarker using Au nanoshells with a magnetic core. Biosens. Bioelectron. 2019, 131, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Damborský, P.; Damborská, D.; Belický, Š.; Tkáč, J.; Katrlík, J. Sweet Strategies in Prostate Cancer Biomarker Research: Focus on a Prostate Specific Antigen. BioNanoScience 2017, 8, 690–700. [Google Scholar] [CrossRef]
- Markin, P.A.; Brito, A.; Moskaleva, N.; Fodor, M.; Lartsova, E.V.; Shpot, Y.V.; Lerner, Y.V.; Mikhajlov, V.Y.; Potoldykova, N.V.; Enikeev, D.V. Plasma Sarcosine Measured by Gas Chromatography-Mass Spectrometry Distinguishes Prostatic Intraepithelial Neoplasia and Prostate Cancer from Benign Prostate Hyperplasia. Lab. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Mazzu-Nascimento, T.; Gomes Carneiro Leão, P.A.; Catai, J.R.; Morbioli, G.G.; Carrilho, E. Towards low-cost bioanalytical tools for sarcosine assays for cancer diagnostics. Anal. Methods 2016, 8, 7312–7318. [Google Scholar] [CrossRef]
- Burton, C.; Gamagedara, S.; Ma, Y. A novel enzymatic technique for determination of sarcosine in urine samples. Anal. Methods 2012, 4, 141–146. [Google Scholar] [CrossRef]
- Cernei, N.; Zitka, O.; Ryvolova, M.; Adam, V.; Masarik, M.; Hubalek, J.; Kizek, R. Spectrometric and Electrochemical Analysis of Sarcosine as a Potential Prostate Carcinoma Marker. Int. J. Electrochem. Sci. 2012, 7, 4286–4301. [Google Scholar]
- Jiang, Y.; Cheng, X.; Wang, C.; Ma, Y. Quantitative Determination of Sarcosine and Related Compounds in Urinary Samples by Liquid Chromatography with Tandem Mass Spectrometry. Anal. Chem. 2010, 82, 9022–9027. [Google Scholar] [CrossRef]
- Narwal, V.; Kumar, P.; Joon, P.; Pundir, C.S. Fabrication of an amperometric sarcosine biosensor based on sarcosine oxidase/chitosan/CuNPs/c-MWCNT/Au electrode for detection of prostate cancer. Enzyme Microb. Technol. 2018, 113, 44–51. [Google Scholar] [CrossRef]
- Naguib, M.; Mashtalir, O.; Carle, J.; Presser, V.; Lu, J.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Transition Metal Carbides. ACS Nano 2012, 6, 1322–1331. [Google Scholar] [CrossRef]
- Naguib, M.; Gogotsi, Y. Synthesis of two-dimensional materials by selective extraction. Acc. Chem. Res. 2015, 48, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional nanocrystals produced by exfoliation of Ti3 AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef] [PubMed]
- Barsoum, M.W. The MN+1AXN Phases: A New Class of Solids; Thermodynamically Stable Nanolaminates. Prog. Solid State Chem. 2000, 28, 201–281. [Google Scholar] [CrossRef]
- Naguib, M.; Mochalin, V.N.; Barsoum, M.W.; Gogotsi, Y. 25th anniversary article: MXenes: A new family of two-dimensional materials. Adv. Mater. 2014, 26, 9921005. [Google Scholar] [CrossRef]
- Lorencova, L.; Bertok, T.; Dosekova, E.; Holazova, A.; Paprckova, D.; Vikartovska, A.; Sasinkova, V.; Filip, J.; Kasak, P.; Jerigova, M.; et al. Electrochemical performance of Ti3C2Tx MXene in aqueous media: Towards ultrasensitive H2O2 sensing. Electrochim. Acta 2017, 235, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Lorencova, L.; Bertok, T.; Filip, J.; Jerigova, M.; Velic, D.; Kasak, P.; Mahmoud, K.A.; Tkac, J. Highly stable Ti3C2Tx (MXene)/Pt nanoparticles-modified glassy carbon electrode for H2O2 and small molecules sensing applications. Sens. Actuat. B. Chem. 2018, 263, 360–368. [Google Scholar] [CrossRef]
- Lorencova, L.; Gajdosova, V.; Hroncekova, S.; Bertok, T.; Blahutova, J.; Vikartovska, A.; Parrakova, L.; Gemeiner, P.; Kasak, P.; Tkac, J. 2D MXenes as Perspective Immobilization Platforms for Design of Electrochemical Nanobiosensors. Electroanalysis 2019, 31, 1833–1844. [Google Scholar] [CrossRef]
- Dong, Y.; Wu, Z.S.; Zheng, S.; Wang, X.; Qin, J.; Wang, S.; Shi, X.; Bao, X. Ti3C2 MXene-Derived Sodium/Potassium Titanate Nanoribbons for High-Performance Sodium/Potassium Ion Batteries with Enhanced Capacities. ACS Nano 2017, 11, 4792–4800. [Google Scholar] [CrossRef]
- Lukatskaya, M.R.; Mashtalir, O.; Ren, C.E.; Dall’Agnese, Y.; Rozier, P.; Taberna, P.L.; Naguib, M.; Simon, P.; Barsoum, M.W.; Gogotsi, Y. Cation intercalation and high volumetric capacitance of two-dimensional titanium carbide. Science 2013, 341, 1502–1505. [Google Scholar] [CrossRef]
- Xie, X.; Chen, S.; Ding, W.; Nie, Y.; Wei, Z. An extraordinarily stable catalyst: Pt NPs supported on two-dimensional Ti3C2X2 (X = OH, F) nanosheets for oxygen reduction reaction. Chem. Commun. 2013, 49, 10112–10114. [Google Scholar] [CrossRef]
- Mashtalir, O.; Naguib, M.; Mochalin, V.N.; Dall’Agnese, Y.; Heon, M.; Barsoum, M.W.; Gogotsi, Y. Intercalation and delamination of layered carbides and carbonitrides. Nat. Commun. 2013, 4, 1716. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yang, C.; Duan, M.; Tang, Y.; Zhu, J. TiO2 nanoparticle modified organ-like Ti3C2 MXene nanocomposite encapsulating hemoglobin for a mediator-free biosensor with excellent performances. Biosens. Bioelectron. 2015, 74, 1022–1028. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yang, C.; Duan, C.; Xiao, D.; Tang, Y.; Zhu, J. An Organ-Like Titanium Carbide Material (MXene) with Multilayer Structure Encapsulating Hemoglobin for a Mediator-Free Biosensor. J. Electrochem. Soc. 2015, 162, B16–B21. [Google Scholar] [CrossRef]
- Liu, H.; Duan, C.; Yang, C.; Shen, W.; Wang, F.; Zhu, Z. A novel nitrite biosensor based on the direct electrochemistry of hemoglobin immobilized on MXene-Ti3C2. Sens. Actuat. B. Chem. 2015, 218, 60–66. [Google Scholar] [CrossRef]
- Rakhi, R.B.; Nayak, P.; Xia, C.; Alshareef, H.N. Novel amperometric glucose biosensor based on MXene nanocomposite. Sci. Rep. 2016, 6, 36422. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhang, X.; Ma, L.; Gao, J.; Jiang, Y. Acetylcholinesterase/chitosan-transition metal carbides nanocomposites-based biosensor for the organophosphate pesticides detection. Biochem. Eng. J. 2017, 128, 243249. [Google Scholar] [CrossRef]
- Wu, L.; Lu, X.; Lu, X.; Wu, Z.S.; Dong, Y.; Wang, X.; Zheng, S.; Chen, J. 2D transition metal carbide MXene as a robust biosensing platform for enzyme immobilization and ultrasensitive detection of phenol. Biosens. Bioelectron. 2018, 107, 69–75. [Google Scholar] [CrossRef]
- Jornet-Martínez, N.; Henderson, C.J.; Campíns-Falcó, P.; Daly, R.; Hall, E.A.H. Towards sarcosine determination in urine for prostatic carcinoma detection. Sens. Actuat. B: Chem. 2019, 287, 380–389. [Google Scholar]
- Cernei, N.; Heger, Z.; Gumulec, J.; Zitka, O.; Masarik, M.; Babula, P.; Eckschlager, T.; Stiborova, M.; Kizek, R.; Adam, V. Sarcosine as a potential prostate cancer biomarker—A review. Int. J. Mol. Sci. 2013, 14, 13893–13908. [Google Scholar] [CrossRef]
- Hu, J.; Wei, W.; Ke, S.; Zeng, X.; Lin, P. A novel and sensitive sarcosine biosensor based on organic electrochemical transistor. Electrochim. Acta 2019, 307, 100–106. [Google Scholar] [CrossRef]
- Wagner, M.A.; Trickey, P.; Chen, Z.-w.; Mathews, F.S.; Jorns, M.S. Monomeric Sarcosine Oxidase: 1. Flavin Reactivity and Active Site Binding Determinants. Biochemistry 2000, 39, 8813–8824. [Google Scholar] [CrossRef] [PubMed]
- Alhabeb, M.; Maleski, K.; Anasori, B.; Lelyukh, P.; Clark, L.; Sin, S.; Gogotsi, Y. Guidelines for Synthesis and Processing of Two-Dimensional Titanium Carbide (Ti3C2Tx MXene). Chem. Mater. 2017, 29, 7633–7644. [Google Scholar] [CrossRef]
- Matsuda, Y.; Hoshika, H.; Inouye, Y.; Ikuta, S.; Matsuura, K.; Nakamura, S. Purificationand Characterization of Sarcosine oxidase of Bacillus Origin. Chem. Pharm. Bull. 1987, 35, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, Q.; Dang, Y.; Shu, G. The Effect of Glutaraldehyde Cross-Linking on the Enzyme Activity of Immobilized &beta-Galactosidase on Chitosan Bead. Advance J. Food Sci. Technol. 2013, 5, 932–935. [Google Scholar]
- Ang, L.F.; Por, L.Y.; Yam, M.F. Development of an amperometric-based glucose biosensor to measure the glucose content of fruit. PLoS ONE 2015, 10, e0111859. [Google Scholar] [CrossRef]
- Wang, H.-S.; Pan, Q.-X.; Wang, G.-X. A Biosensor Based on Immobilization of Horseradish Peroxidase in Chitosan Matrix Cross-linked with Glyoxal for Amperometric Determination of Hydrogen Peroxide. Sensors 2005, 5, 266–276. [Google Scholar] [CrossRef]
- Yang, Q.G.; Li, N.; Li, Q.; Chen, S.Q.; Wang, H.L.; Yang, H.P. Amperometric sarcosine biosensor based on hollow magnetic Pt-Fe3O4@C nanospheres. Anal. Chim. Acta 2019, 1078, 161–167. [Google Scholar] [CrossRef]
- Kumar, P.; Narwal, V.; Jaiwal, R.; Pundir, C.S. Construction and application of amperometric sarcosine biosensor based on SOxNPs/AuE for determination of prostate cancer. Biosens Bioelectron. 2018, 122, 140–146. [Google Scholar] [CrossRef]
- Henderson, C.J.; Pumford, E.; Seevaratnam, D.J.; Daly, R.; Hall, E.A.H. Gene to diagnostic: Self immobilizing protein for silica microparticle biosensor, modelled with sarcosine oxidase. Biomaterials 2019, 193, 58–70. [Google Scholar] [CrossRef]
- Zhao, S.; Volkner, J.; Riedel, M.; Witte, G.; Yue, Z.; Lisdat, F.; Parak, W.J. Multiplexed Readout of Enzymatic Reactions by Means of Laterally Resolved Illumination of Quantum Dot Electrodes. ACS Appl. Mater. Interf. 2019, 11, 21830–21839. [Google Scholar] [CrossRef]
- Pundir, C.S.; Deswal, R.; Kumar, P. Quantitative analysis of sarcosine with special emphasis on biosensors: A review. Biomarkers 2019, 24, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Rebelo, T.S.; Pereira, C.M.; Sales, M.G.; Noronha, J.P.; Costa-Rodrigues, J.; Silva, F.; Fernandes, M.H. Sarcosine oxidase composite screen-printed electrode for sarcosine determination in biological samples. Anal Chim Acta 2014, 850, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhao, Y.; Yang, Q.; Du, D.; Yang, H.; Lin, Y. Amperometric sarcosine biosensor with strong anti-interference capabilities based on mesoporous organic-inorganic hybrid materials. Biosens. Bioelectron. 2019, 141, 111431. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Fu, B.; Chen, J.; Li, K. An electrochemical sarcosine sensor based on biomimetic recognition. Microchim. Acta 2019, 186, 136. [Google Scholar] [CrossRef]
- Yang, H.; Wang, J.; Yang, C.; Zhao, X.; Xie, S.; Ge, Z. Nano Pt@ZIF8 Modified Electrode and Its Application to Detect Sarcosine. J. Electrochem. Soc. 2018, 165, H247–H250. [Google Scholar] [CrossRef]

| Enzyme/Protein | MXene Patterning | Immobilization | Analyte | LOD (nM) | Reference |
|---|---|---|---|---|---|
| haemoglobin (Hb) | MXene | Hb glued via Nf | H2O2 | 20 | [23] |
| haemoglobin (Hb) | MXene | Hb glued via Nf | NO2− | 120 | [24] |
| haemoglobin (Hb) | TiO2 on MXene | Hb glued via Nf | H2O2 | 14 | [22] |
| tyrosinase (Tyr) | MXene | Tyr glued via Chi | phenol | 12 | [27] |
| glucose oxidase | AuNPs on MXene | GOx adsorbed on Nf-AuNP/MXene | glucose | 5900 | [25] |
| SOx | MXene | SOx glued via Chi | sarcosine | 18 | This work |
| Interface | LOD (nM) | Working Range (nM) | Reference |
|---|---|---|---|
| platinum-plated anodised aluminium oxide electrode | 50 | 50–100,000 | [30] |
| Hybrid: Pt nanoparticles with hollow Fe3O4 nanospheres | 430 | 500–60,000 | [37] |
| Modified SPCE | 16 | 10–100 | [42] |
| SOxNPs/AuE | 10 | 100–100,000 | [38] |
| SOx/Pt–Fe3O4@C nanocomposite/GCE | 430 | 500–60,000 | [37] |
| Pt-supported organic/inorganic hybrid mesoporous NPs | 130 | 1000–70,000 | [43] |
| Riboflavin/AuPt-PPy/graphene-chitosan-modified GCE | 680 | 2500–600,000 | [44] |
| nanoPt@porous zeolitic imidazolate framework-8 | 1060 | 5000–30,000 | [45] |
| SOx/MXene-Chi/GCE | 18 | 36–7800 | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hroncekova, S.; Bertok, T.; Hires, M.; Jane, E.; Lorencova, L.; Vikartovska, A.; Tanvir, A.; Kasak, P.; Tkac, J. Ultrasensitive Ti3C2TX MXene/Chitosan Nanocomposite-Based Amperometric Biosensor for Detection of Potential Prostate Cancer Marker in Urine Samples. Processes 2020, 8, 580. https://doi.org/10.3390/pr8050580
Hroncekova S, Bertok T, Hires M, Jane E, Lorencova L, Vikartovska A, Tanvir A, Kasak P, Tkac J. Ultrasensitive Ti3C2TX MXene/Chitosan Nanocomposite-Based Amperometric Biosensor for Detection of Potential Prostate Cancer Marker in Urine Samples. Processes. 2020; 8(5):580. https://doi.org/10.3390/pr8050580
Chicago/Turabian StyleHroncekova, Stefania, Tomas Bertok, Michal Hires, Eduard Jane, Lenka Lorencova, Alica Vikartovska, Aisha Tanvir, Peter Kasak, and Jan Tkac. 2020. "Ultrasensitive Ti3C2TX MXene/Chitosan Nanocomposite-Based Amperometric Biosensor for Detection of Potential Prostate Cancer Marker in Urine Samples" Processes 8, no. 5: 580. https://doi.org/10.3390/pr8050580
APA StyleHroncekova, S., Bertok, T., Hires, M., Jane, E., Lorencova, L., Vikartovska, A., Tanvir, A., Kasak, P., & Tkac, J. (2020). Ultrasensitive Ti3C2TX MXene/Chitosan Nanocomposite-Based Amperometric Biosensor for Detection of Potential Prostate Cancer Marker in Urine Samples. Processes, 8(5), 580. https://doi.org/10.3390/pr8050580