Green Pathway in Utilizing CO2 via Cycloaddition Reaction with Epoxide—A Mini Review
Abstract
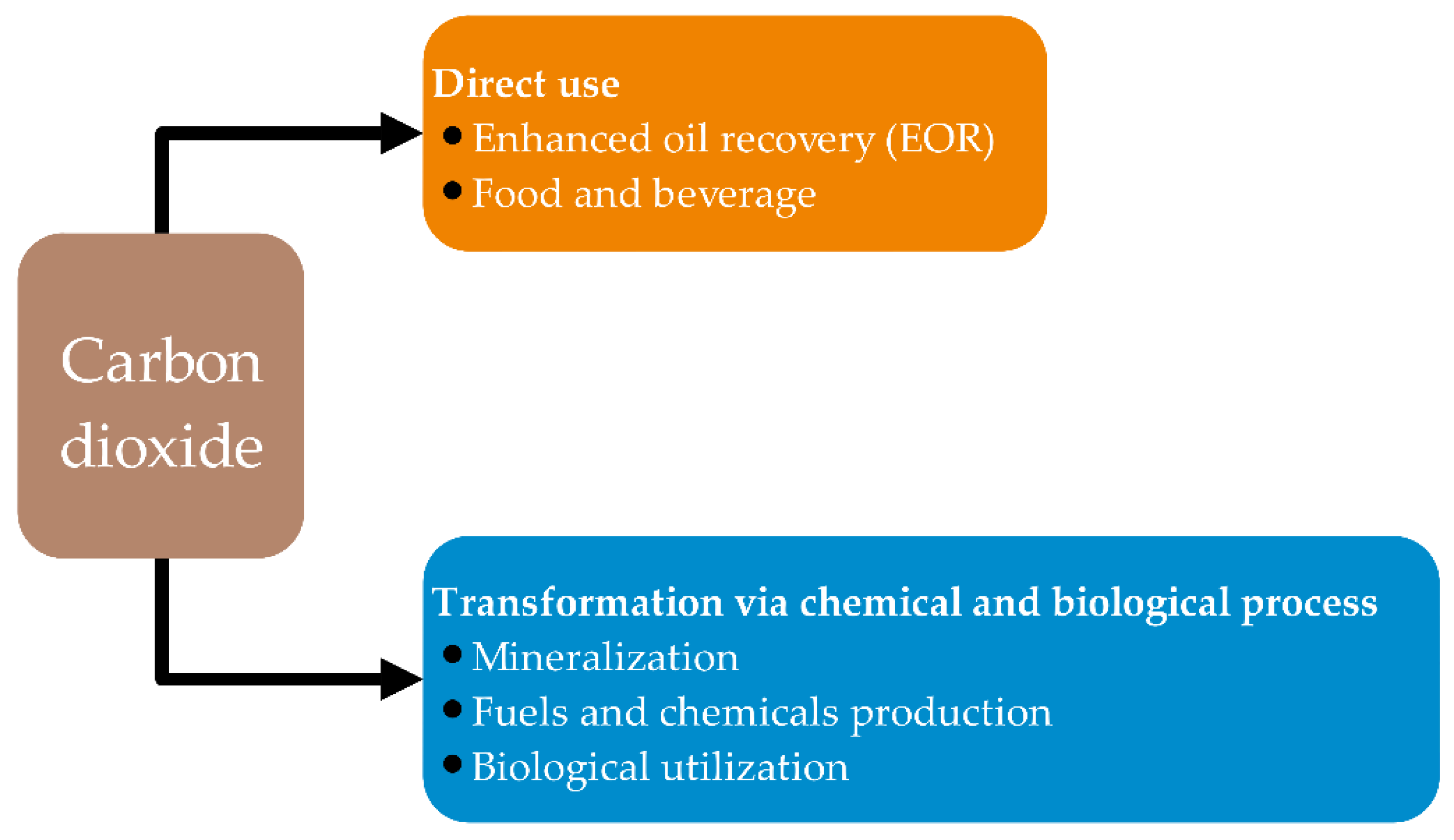
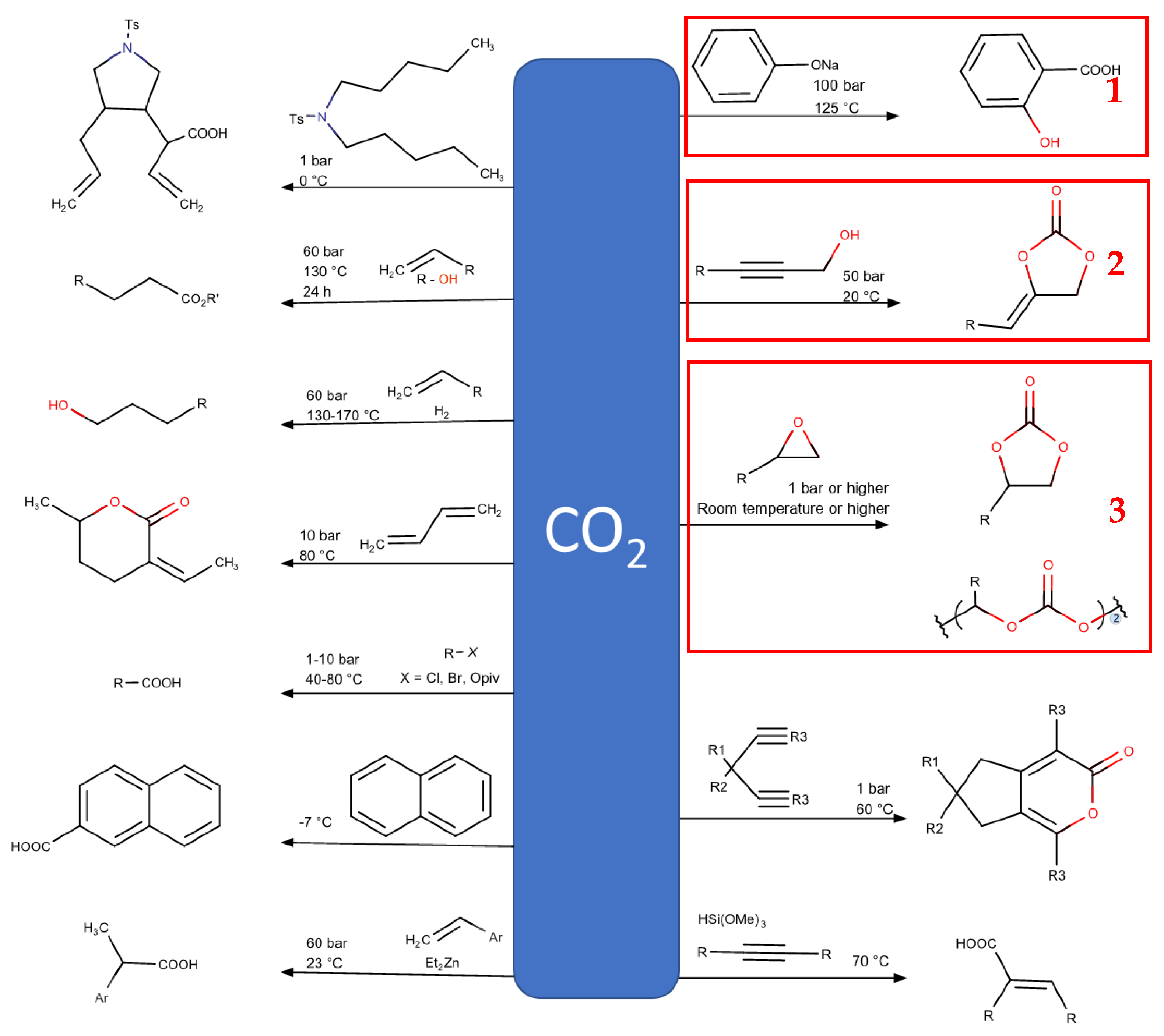
1. Introduction
2. Cycloaddition Reaction Mechanism of Epoxide with CO2
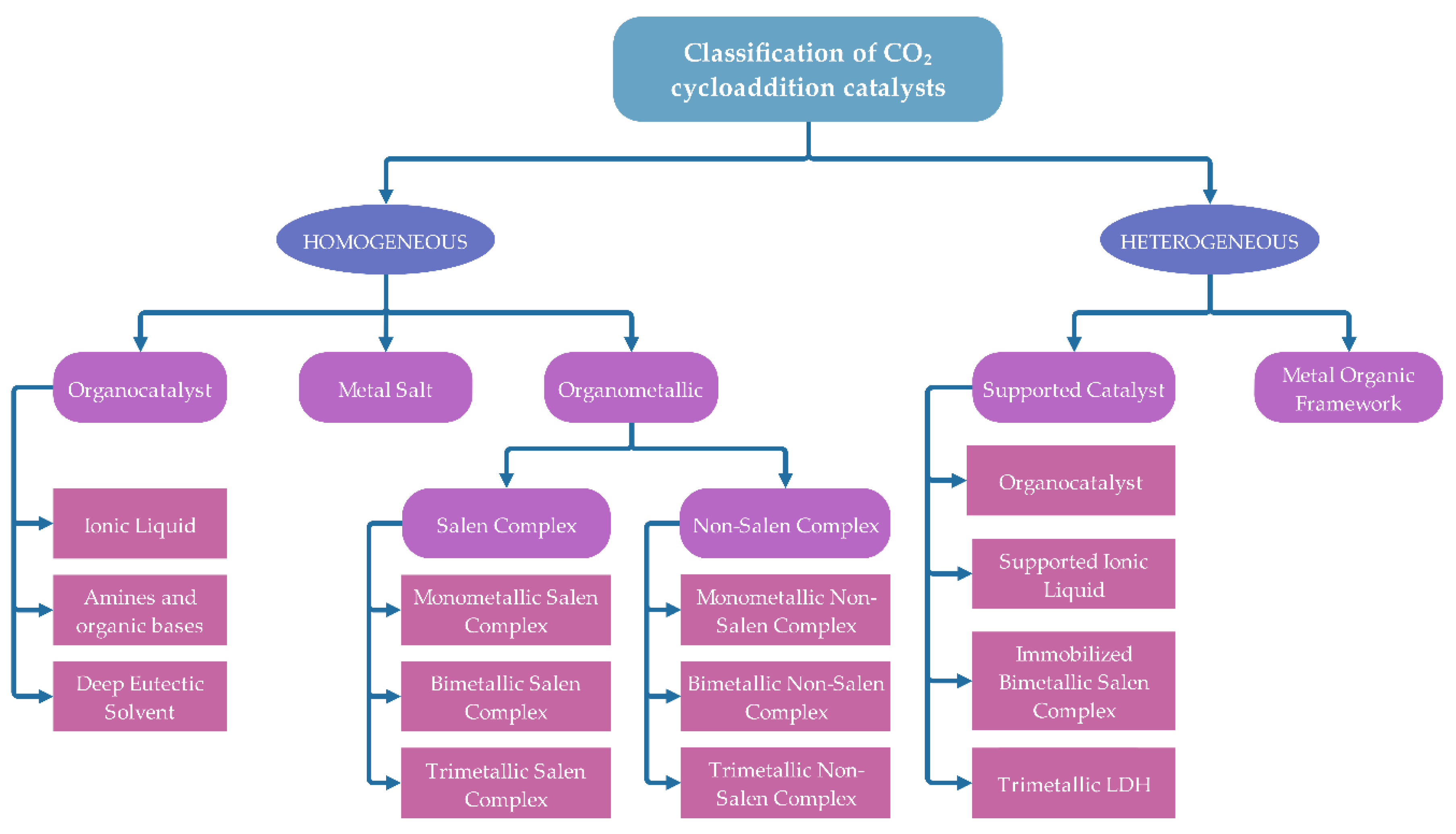
3. Catalysts for Cycloaddition of Epoxide with CO2
3.1. Homogeneous Catalyst
3.1.1. Organocatalysts
3.1.2. Metal Salt
3.1.3. Metallic Salen Complex
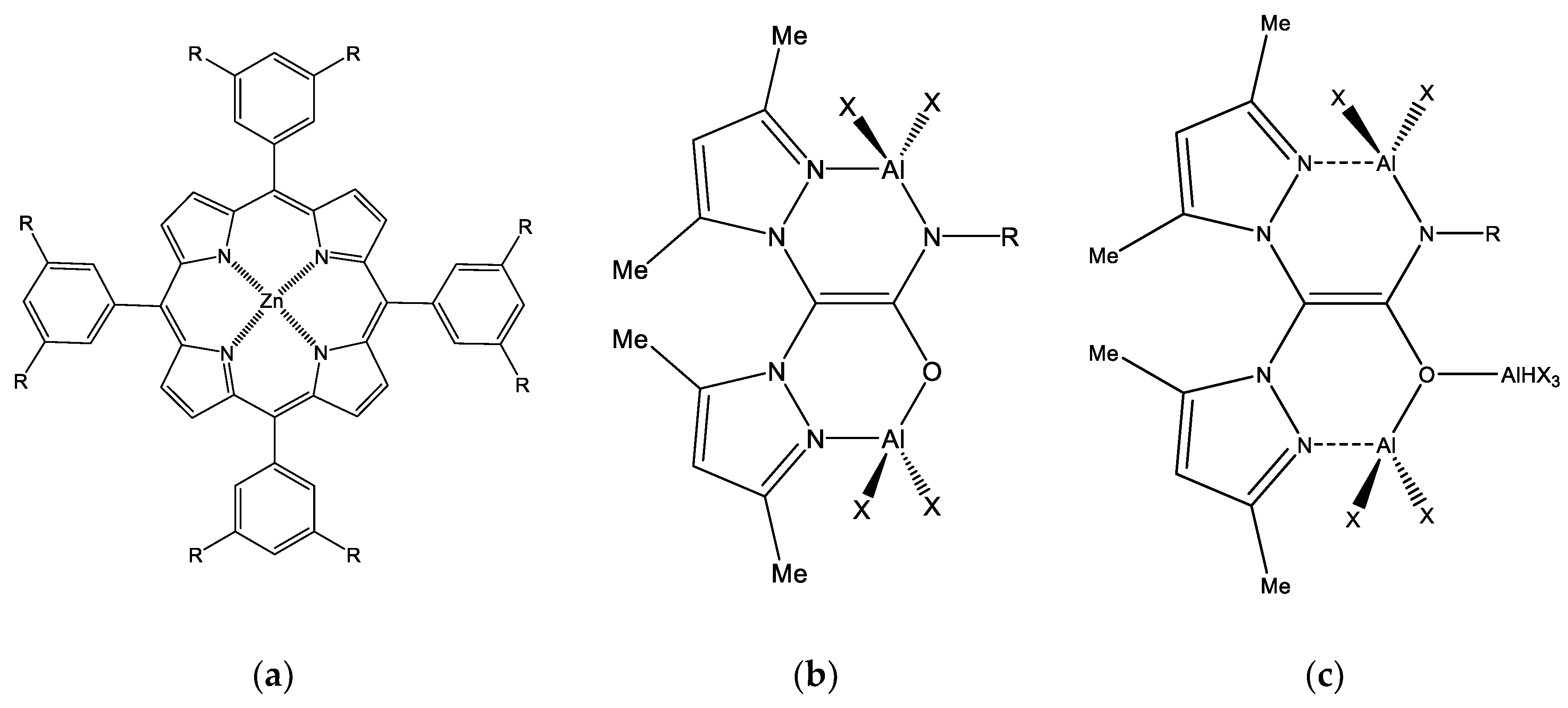
Monometallic Salen Complex
Bimetallic Salen Complexes
3.1.4. Metallic Non-Salen-Based Complexes
Monometallic Non-Salen-Based Complexes
Bimetallic Non-Salen Complexes
Trimetallic Non-Salen Complexes
3.2. Heterogeneous Catalyst
3.2.1. Supported Catalyst
Supported Ionic Liquid
Supported Bimetal–Organic Salen Complexes
Trimetallic Layered Double Hydroxide (LDH)
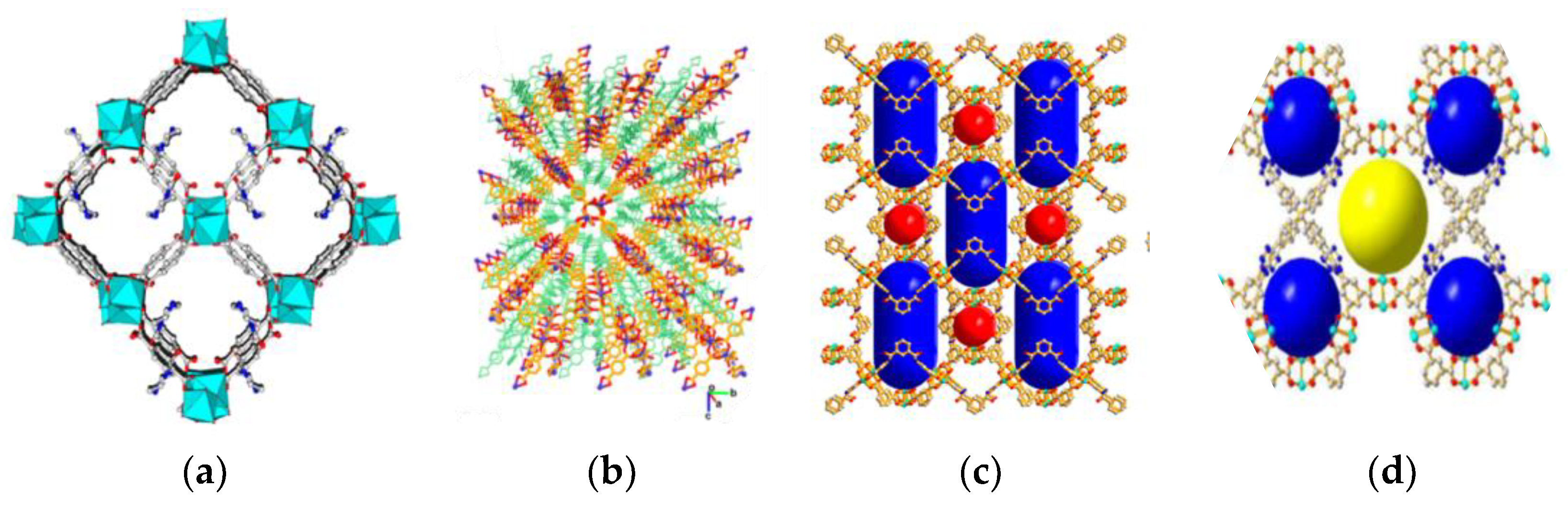
3.2.2. Metal–Organic Framework
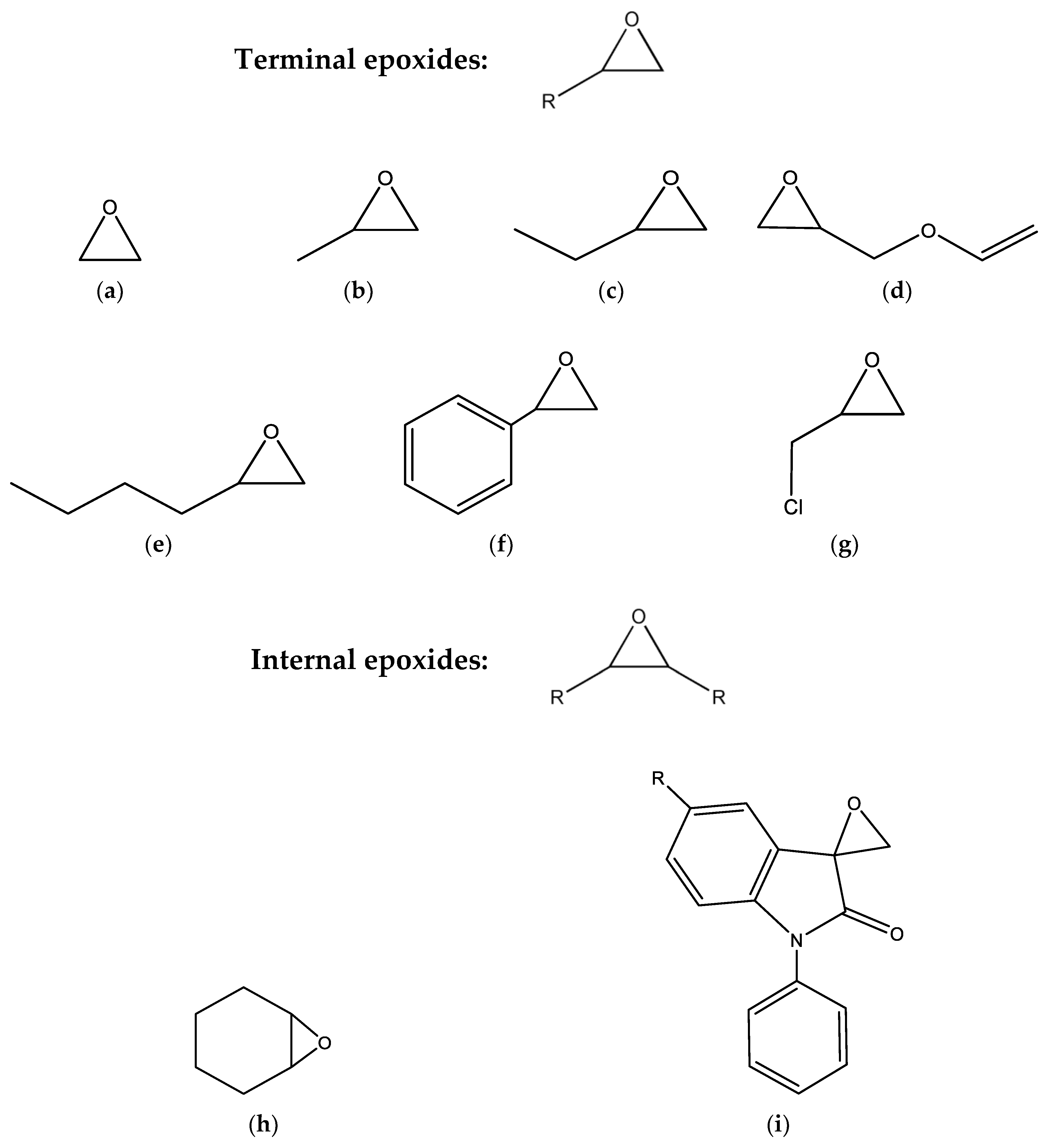
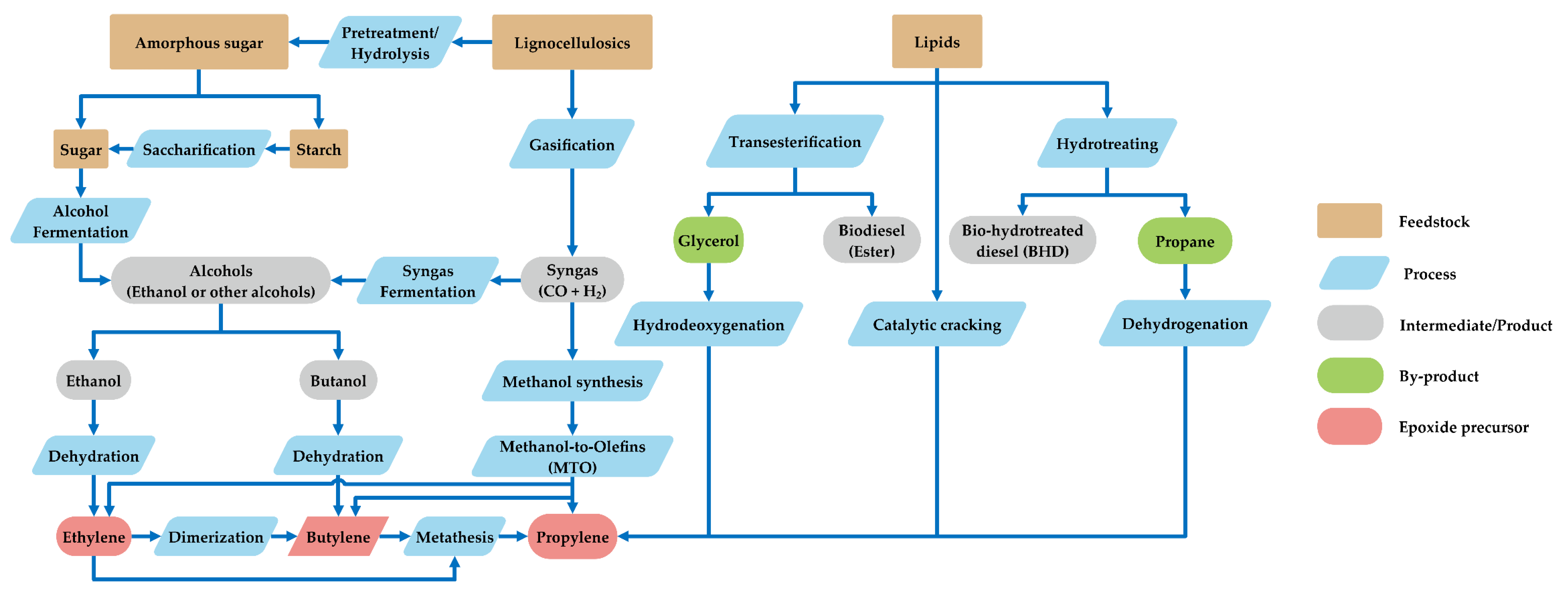
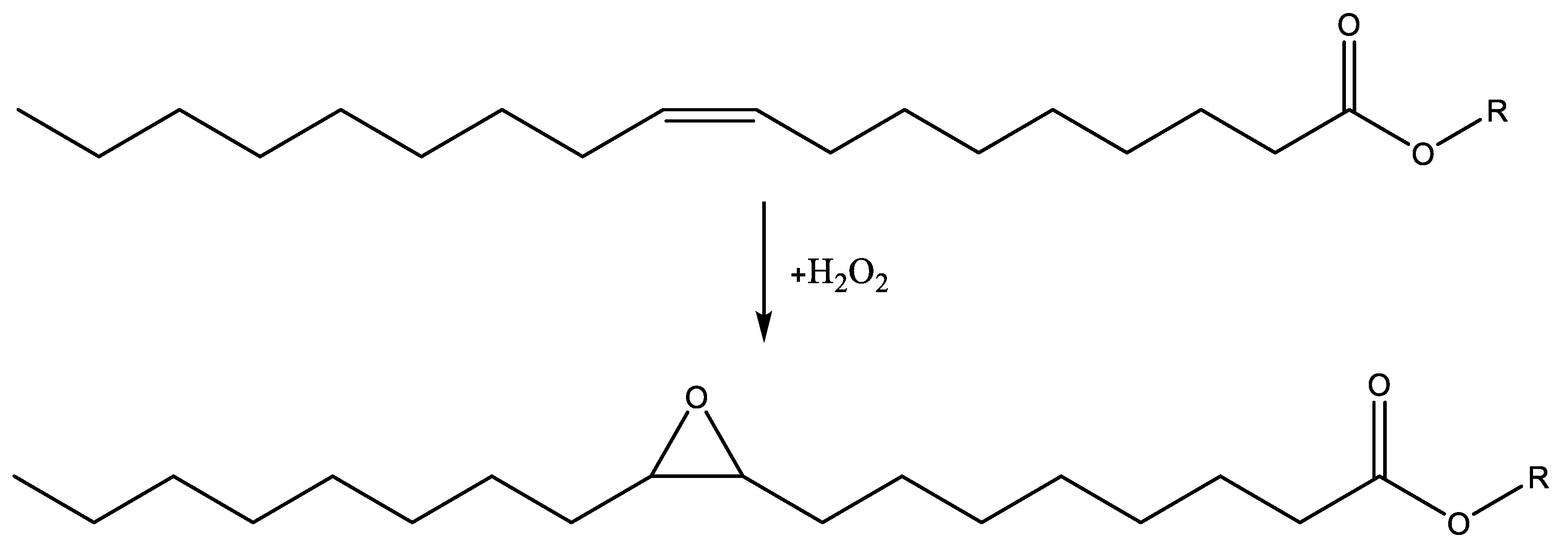
4. Sustainable Epoxide Sources for Cycloaddition Reaction
5. Potential Enhancement of Cycloaddition Reaction
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, L.; Wang, S.M.; Han, Z.B.; Ding, M.; Yuan, D.Q.; Jiang, H.L. Exceptionally Robust In-Based Metal-Organic Framework for Highly Efficient Carbon Dioxide Capture and Conversion. Inorg. Chem. 2016, 55, 3558–3565. [Google Scholar] [CrossRef] [PubMed]
- Bals, C.; Bellmann, E.; Bode, A.; Edenhofer, O.; Fischedick, M.; Gaertner, L.-E.; Gerling, P.; Helseth, J.M.; Kühn, M.; Liebscher, A. CCU and CCS–Building Blocks for Climate Protection in Industry. Analysis, Options and Recommendations; Utzverlag GmbH: München, Germany, 2019. [Google Scholar]
- Zhang, Z.; Pan, S.Y.; Li, H.; Cai, J.; Olabi, A.G.; Anthony, E.J.; Manovic, V. Recent advances in carbon dioxide utilization. Renew. Sustain. Energy Rev. 2020, 125, 109799. [Google Scholar] [CrossRef]
- Liang, S.; Liu, H.; Jiang, T.; Song, J.; Yang, G.; Han, B. Highly efficient synthesis of cyclic carbonates from CO2 and epoxides over cellulose/KI. Chem. Commun. 2011, 47, 2131–2133. [Google Scholar] [CrossRef]
- Ng, C.K.; Toh, R.W.; Lin, T.T.; Luo, H.K.; Hor, T.S.A.; Wu, J. Metal-salen molecular cages as efficient and recyclable heterogeneous catalysts for cycloaddition of CO2 with epoxides under ambient conditions. Chem. Sci. 2019, 10, 1549–1554. [Google Scholar] [CrossRef]
- Lu, B.B.; Yang, J.; Liu, Y.Y.; Ma, J.F. A Polyoxovanadate-Resorcin[4]arene-Based Porous Metal-Organic Framework as an Efficient Multifunctional Catalyst for the Cycloaddition of CO2 with Epoxides and the Selective Oxidation of Sulfides. Inorg. Chem. 2017, 56, 11710–11720. [Google Scholar] [CrossRef] [PubMed]
- Styring, P.; Jansen, D.; de Coninck, H.; Reith, H.; Armstrong, K. Carbon Capture and Utilisation in the Green Economy; Centre for Low Carbon Futures: New York, NY, USA, 2011. [Google Scholar]
- Aresta, M. Carbon dioxide utilization: Chemical, biological and technological applications. In Greenhouse Gases: Mitigation and Utilization, Proceedings of the CHEMRAWN-XVII and ICCDU-IX Conference, Kingston, ON, Canada, 8–12 July 2007; Queen’s University: Kingston, ON, Canada, 2009; pp. 123–149. [Google Scholar]
- Liu, Q.; Wu, L.; Jackstell, R.; Beller, M. Using carbon dioxide as a building block in organic synthesis. Nat. Commun. 2015, 6, 1–15. [Google Scholar] [CrossRef]
- Luo, J.; Preciado, S.; Xie, P.; Larrosa, I. Carboxylation of Phenols with CO2 at Atmospheric Pressure. Chem. Eur. J. 2016, 22, 6798–6802. [Google Scholar] [CrossRef]
- Wendling, T.; Risto, E.; Krause, T.; Gooßen, L.J. Salt-Free Strategy for the Insertion of CO2 into C−H Bonds: Catalytic Hydroxymethylation of Alkynes. Chem. Eur. J. 2018, 24, 6019–6024. [Google Scholar] [CrossRef]
- Zou, B.; Hu, C. Synthesis of Cyclic Carbonates from Alkenyl and Alkynyl Substrates. Chin. J. Chem. 2017, 35, 541–550. [Google Scholar] [CrossRef]
- Dabral, S.; Schaub, T. The Use of Carbon Dioxide (CO2) as a Building Block in Organic Synthesis from an Industrial Perspective. Adv. Synth. Catal. 2019, 361, 223–246. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, G.; Kodama, K.; Hirose, T. An efficient metal- and solvent-free organocatalytic system for chemical fixation of CO2 into cyclic carbonates under mild conditions. Green Chem. 2016, 18, 1229–1233. [Google Scholar] [CrossRef]
- Jadhav, A.H.; Thorat, G.M.; Lee, K.; Lim, A.C.; Kang, H.; Seo, J.G. Effect of anion type of imidazolium based polymer supported ionic liquids on the solvent free synthesis of cycloaddition of CO2 into epoxide. Catal. Today 2016, 265, 56–67. [Google Scholar] [CrossRef]
- North, M. Synthesis of Cyclic Carbonates from Carbon Dioxide and Epoxides; Elsevier: Amsterdam, The Netherlands, 2013; ISBN 9780444538826. [Google Scholar]
- Bobadilla, L.F.; Lima, S.; Urakawa, A. Cycloaddition of CO2 and Epoxides over Reusable Solid Catalysts. Adv. Catal. Mater. 2015, 271–312. [Google Scholar] [CrossRef]
- Shaikh, R.R.; Pornpraprom, S.; D’Elia, V. Catalytic Strategies for the Cycloaddition of Pure, Diluted, and Waste CO2 to Epoxides under Ambient Conditions. ACS Catal. 2018, 8, 419–450. [Google Scholar] [CrossRef]
- Gruttadauria, M.; Giacalone, F. Catalytic Methods in Asymmetric Synthesis: Advanced Materials, Techniques, and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2011; ISBN 9780470641361. [Google Scholar]
- Tak, R.K.; Patel, P.; Subramanian, S.; Kureshy, R.I.; Khan, N.U.H. Cycloaddition Reaction of Spiro-Epoxy Oxindole with CO2 at Atmospheric Pressure Using Deep Eutectic Solvent. ACS Sustain. Chem. Eng. 2018, 6, 11200–11205. [Google Scholar] [CrossRef]
- Castro-Osma, J.A.; Lamb, K.J.; North, M. Cr(salophen) Complex Catalyzed Cyclic Carbonate Synthesis at Ambient Temperature and Pressure. ACS Catal. 2016, 6, 5012–5025. [Google Scholar] [CrossRef]
- Wang, T.T.; Xie, Y.; Deng, W.Q. Reaction mechanism of epoxide cycloaddition to CO2 catalyzed by salen-M (M = Co, Al, Zn). J. Phys. Chem. A 2014, 118, 9239–9243. [Google Scholar] [CrossRef]
- Maeda, C.; Shimonishi, J.; Miyazaki, R.; Hasegawa, J.Y.; Ema, T. Highly Active and Robust Metalloporphyrin Catalysts for the Synthesis of Cyclic Carbonates from a Broad Range of Epoxides and Carbon Dioxide. Chem. Eur. J. 2016, 22, 6556–6563. [Google Scholar] [CrossRef]
- De, D.; Bhattacharyya, A.; Bharadwaj, P.K. Enantioselective Aldol Reactions in Water by a Proline-Derived Cryptand and Fixation of CO2 by Its Exocyclic Co(II) Complex. Inorg. Chem. 2017, 56, 11443–11449. [Google Scholar] [CrossRef]
- Castro-Osma, J.A.; Alonso-Moreno, C.; Lara-Sánchez, A.; Martínez, J.; North, M.; Otero, A. Synthesis of cyclic carbonates catalysed by aluminium heteroscorpionate complexes. Catal. Sci. Technol. 2014, 4, 1674–1684. [Google Scholar] [CrossRef]
- Li, C.Y.; Su, Y.C.; Lin, C.H.; Huang, H.Y.; Tsai, C.Y.; Lee, T.Y.; Ko, B.T. Synthesis and characterization of trimetallic cobalt, zinc and nickel complexes containing amine-bis(benzotriazole phenolate) ligands: Efficient catalysts for coupling of carbon dioxide with epoxides. Dalt. Trans. 2017, 46, 15399–15406. [Google Scholar] [CrossRef] [PubMed]
- Lichenwalter, M.; Cooper, J. Catalytic Process for Producing Alkylene Carbonates. U.S. Patent Application No. 2,773,070, 4 December 1956. [Google Scholar]
- Büttner, H.; Longwitz, L.; Steinbauer, J.; Wulf, C.; Werner, T. Recent Developments in the Synthesis of Cyclic Carbonates from Epoxides and CO2. In Topics in Current Chemistry; Springer: Berlin/Heidelberg, Germany, 2017; Volume 375, pp. 89–144. [Google Scholar]
- Peng, J.; Deng, Y. Cycloaddition of carbon dioxide to propylene oxide catalyzed by ionic liquids. New J. Chem. 2001, 25, 639–641. [Google Scholar] [CrossRef]
- Alves, M.; Grignard, B.; Mereau, R.; Jerome, C.; Tassaing, T.; Detrembleur, C. Organocatalyzed coupling of carbon dioxide with epoxides for the synthesis of cyclic carbonates: Catalyst design and mechanistic studies. Catal. Sci. Technol. 2017, 7, 2651–2684. [Google Scholar] [CrossRef]
- Zhou, X.; Yang, X.; Yao, J.; Wang, G. Synthesis of propylene carbonate from CO2 and propylene oxide with KI and inorganic ammonium salts as catalyst. Acta Chim. Sin. 2010, 68, 870–874. [Google Scholar]
- Kihara, N.; Hara, N.; Endo, T. Catalytic Activity of Various Salts in the Reaction of 2,3-Epoxypropyl Phenyl Ether and Carbon Dioxide under Atmospheric Pressure. J. Org. Chem. 1993, 58, 6198–6202. [Google Scholar] [CrossRef]
- Huang, J.W.; Shi, M. Chemical fixation of carbon dioxide by NaI/PPh3/PhOH. J. Org. Chem. 2003, 68, 6705–6709. [Google Scholar] [CrossRef]
- North, M.; Quek, S.C.Z.; Pridmore, N.E.; Whitwood, A.C.; Wu, X. Aluminum(salen) complexes as catalysts for the kinetic resolution of terminal epoxides via CO2 coupling. ACS Catal. 2015, 5, 3398–3402. [Google Scholar] [CrossRef]
- Ghazali-Esfahani, S.; Song, H.; Pǎunescu, E.; Bobbink, F.D.; Liu, H.; Fei, Z.; Laurenczy, G.; Bagherzadeh, M.; Yan, N.; Dyson, P.J. Cycloaddition of CO2 to epoxides catalyzed by imidazolium-based polymeric ionic liquids. Green Chem. 2013, 15, 1584–1589. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, Z.; Jiang, T.; He, J.; Han, B.; Wu, T.; Ding, K. CO2 cycloaddition reactions catalyzed by an ionic liquid grafted onto a highly cross-linked polymer matrix. Angew. Chem. Int. Ed. 2007, 46, 7255–7258. [Google Scholar] [CrossRef]
- Udayakumar, S.; Lee, M.K.; Shim, H.L.; Park, S.W.; Park, D.W. Imidazolium derivatives functionalized MCM-41 for catalytic conversion of carbon dioxide to cyclic carbonate. Catal. Commun. 2009, 10, 659–664. [Google Scholar] [CrossRef]
- Cheng, W.; Chen, X.; Sun, J.; Wang, J.; Zhang, S. SBA-15 supported triazolium-based ionic liquids as highly efficient and recyclable catalysts for fixation of CO2 with epoxides. Catal. Today 2013, 200, 117–124. [Google Scholar] [CrossRef]
- Han, L.; Choi, H.J.; Kim, D.K.; Park, S.W.; Liu, B.; Park, D.W. Porous polymer bead-supported ionic liquids for the synthesis of cyclic carbonate from CO2 and epoxide. J. Mol. Catal. A Chem. 2011, 338, 58–64. [Google Scholar] [CrossRef]
- Meléndez, J.; North, M.; Villuendas, P. One-component catalysts for cyclic carbonate synthesis. Chem. Commun. 2009, 2577–2579. [Google Scholar] [CrossRef] [PubMed]
- Meléndez, J.; North, M.; Villuendas, P.; Young, C. One-component bimetallic aluminium(salen)-based catalysts for cyclic carbonate synthesis and their immobilization. Dalt. Trans. 2011, 40, 3885–3902. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, Q.; Puthiaraj, P.; Ahn, W.S. MgFeAl layered double hydroxide prepared from recycled industrial solid wastes for CO2 fixation by cycloaddition to epoxides. J. CO2 Util. 2019, 34, 395–403. [Google Scholar] [CrossRef]
- Li, P.Z.; Wang, X.J.; Liu, J.; Phang, H.S.; Li, Y.; Zhao, Y. Highly Effective Carbon Fixation via Catalytic Conversion of CO2 by an Acylamide-Containing Metal-Organic Framework. Chem. Mater. 2017, 29, 9256–9261. [Google Scholar] [CrossRef]
- Parmar, B.; Patel, P.; Pillai, R.S.; Tak, R.K.; Kureshy, R.I.; Khan, N.U.H.; Suresh, E. Cycloaddition of CO2 with an Epoxide-Bearing Oxindole Scaffold by a Metal-Organic Framework-Based Heterogeneous Catalyst under Ambient Conditions. Inorg. Chem. 2019, 58, 10084–10096. [Google Scholar] [CrossRef]
- Li, P.Z.; Wang, X.J.; Liu, J.; Lim, J.S.; Zou, R.; Zhao, Y. A Triazole-Containing Metal-Organic Framework as a Highly Effective and Substrate Size-Dependent Catalyst for CO2 Conversion. J. Am. Chem. Soc. 2016, 138, 2142–2145. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Qu, Y.K.; Li, G.R.; Zhuang, Z.Z.; Chang, Z.; Hu, T.L.; Xu, J.; Bu, X.H. Zn(II)-benzotriazolate clusters based amide functionalized porous coordination polymers with high CO2 adsorption selectivity. Inorg. Chem. 2014, 53, 8842–8844. [Google Scholar] [CrossRef]
- Zheng, B.; Bai, J.; Duan, J.; Wojtas, L.; Zaworotko, M.J. Enhanced CO2 binding affinity of a high-uptake rht-type metal-organic framework decorated with acylamide groups. J. Am. Chem. Soc. 2011, 133, 748–751. [Google Scholar] [CrossRef]
- Li, P.Z.; Wang, X.J.; Zhang, K.; Nalaparaju, A.; Zou, R.; Zou, R.; Jiang, J.; Zhao, Y. “Click”-extended nitrogen-rich metal-organic frameworks and their high performance in CO2-selective capture. Chem. Commun. 2014, 50, 4683–4685. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Yang, Z.; Bai, J.; Li, Y.; Li, S. High and selective CO2 capture by two mesoporous acylamide-functionalized rht-type metal-organic frameworks. Chem. Commun. 2012, 48, 7025–7027. [Google Scholar] [CrossRef] [PubMed]
- Diao, K.S.; Wang, F.; Wang, H. jun Ab initio theoretical study of the interactions between CFCs and CO2. J. Mol. Struct. 2009, 913, 195–199. [Google Scholar] [CrossRef]
- Zhang, M.; Yu, Y. Dehydration of ethanol to ethylene. Ind. Eng. Chem. Res. 2013, 52, 9505–9514. [Google Scholar] [CrossRef]
- Mendieta, C.M.; Vallejos, M.E.; Felissia, F.E.; Chinga-Carrasco, G.; Area, M.C. Review: Bio-polyethylene from Wood Wastes. J. Polym. Environ. 2020, 28, 1–16. [Google Scholar] [CrossRef]
- Broeren, M. Production of Bio-Ethylene; IEA-ETSAP: Paris, France; IRENA: Abu Dhabi, UAE, 2013; pp. 1–20. [Google Scholar]
- Zacharopoulou, V.; Vasiliadou, E.S.; Lemonidou, A.A. One-step propylene formation from bio-glycerol over molybdena-based catalysts. Green Chem. 2015, 17, 903–912. [Google Scholar] [CrossRef]
- Jong, E.D.; Higson, A.; Walsh, P.; Wellisch, M. Task 42 Biobased Chemicals—Value Added Products from Biorefineries. A Rep. Prep. IEA Bioenergy-Task; IEA: Paris, France, 2011; Volume 42. [Google Scholar]
- Resul, M.F.M.G.; López Fernández, A.M.; Rehman, A.; Harvey, A.P. Development of a selective, solvent-free epoxidation of limonene using hydrogen peroxide and a tungsten-based catalyst. React. Chem. Eng. 2018, 3, 747–756. [Google Scholar] [CrossRef]
- Shaarani, F.W.; Bou, J.J. Synthesis of vegetable-oil based polymer by terpolymerization of epoxidized soybean oil, propylene oxide and carbon dioxide. Sci. Total Environ. 2017, 598, 931–936. [Google Scholar] [CrossRef]
- Cui, S.; Qin, Y.; Li, Y. Sustainable Approach for the Synthesis of Biopolycarbonates from Carbon Dioxide and Soybean Oil. ACS Sustain. Chem. Eng. 2017, 5, 9014–9022. [Google Scholar] [CrossRef]
- Phimsen, S.; Yamada, H.; Tagawa, T.; Kiatkittipong, W.; Kiatkittipong, K.; Laosiripojana, N.; Assabumrungrat, S. Epoxidation of methyl oleate in a TiO2 coated-wall capillary microreactor. Chem. Eng. J. 2017, 314, 594–599. [Google Scholar] [CrossRef]
- Katryniok, B.; Kimura, H.; Skrzyńska, E.; Girardon, J.S.; Fongarland, P.; Capron, M.; Ducoulombier, R.; Mimura, N.; Paul, S.; Dumeignil, F. Selective catalytic oxidation of glycerol: Perspectives for high value chemicals. Green Chem. 2011, 13, 1960–1979. [Google Scholar] [CrossRef]
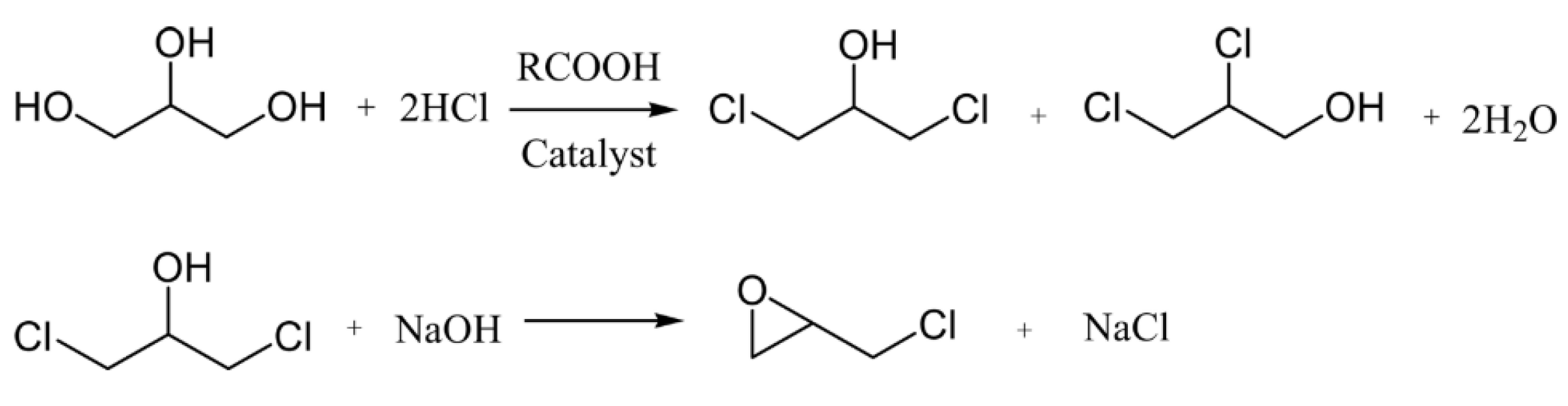
- Santacesaria, E.; Tesser, R.; Di Serio, M.; Casale, L. New process for producing epichlorohydrin via glycerol chlorination. Ind. Eng. Chem. Res. 2010, 49, 964–970. [Google Scholar] [CrossRef]
- Olson, D.H.; Camblor, M.A.; Villaescusa, L.A.; Kuehl, G.H. Light hydrocarbon sorption properties of pure silica Si-CHA and ITQ-3 and high silica ZSM-58. Microporous Mesoporous Mater. 2004, 67, 27–33. [Google Scholar] [CrossRef]
- Lari, G.M.; Pastore, G.; Mondelli, C.; Pérez-Ramírez, J. Towards sustainable manufacture of epichlorohydrin from glycerol using hydrotalcite-derived basic oxides. Green Chem. 2018, 20, 148–159. [Google Scholar] [CrossRef]
- Vitiello, R.; Tesser, R.; Santacesaria, E.; Di Serio, M. New Production Processes of Dichlorohydrins from Glycerol Using Acyl Chlorides as Catalysts or Reactants. Ind. Eng. Chem. Res. 2016, 55, 1484–1490. [Google Scholar] [CrossRef]
- Kamphuis, A.J.; Picchioni, F.; Pescarmona, P.P. CO2-fixation into cyclic and polymeric carbonates: Principles and applications. Green Chem. 2019, 21, 406–448. [Google Scholar] [CrossRef]
- Engel, R.V.; Alsaiari, R.; Nowicka, E.; Pattisson, S.; Miedziak, P.J.; Kondrat, S.A.; Morgan, D.J.; Hutchings, G.J. Oxidative Carboxylation of 1-Decene to 1,2-Decylene Carbonate. Top. Catal. 2018, 61, 509–518. [Google Scholar] [CrossRef]
- Chen, F.; Dong, T.; Xu, T.; Li, X.; Hu, C. Direct synthesis of cyclic carbonates from olefins and CO2 catalyzed by a MoO2(acac) 2-quaternary ammonium salt system. Green Chem. 2011, 13, 2518–2524. [Google Scholar] [CrossRef]


| Epoxide | Catalyst Class/Type | Cocatalyst | T (℃) | P (bar) | Time (h) | Yield (%) | Conv. (%) | Ref. | |
|---|---|---|---|---|---|---|---|---|---|
| Organocatalysts | |||||||||
| ECH | Ionic liquid/2,6-pyridinedimethanol | TBACl | 25 | 1.01 | 24 | 52 | ND | [14] | |
| TBAB | 25 | 1.01 | 24 | 67 | ND | ||||
| TBAI | 25 | 1.01 | 24 | 92 | ND | ||||
| SEO | DES/ChCl:glycerol | - | 40 | 1.01 | 5 | 20 | ND | [20] | |
| DES/ChCl:Ethylene glycol | - | 40 | 1.01 | 5 | 10 | ND | |||
| DES/ChCl:benzoic acid | - | 40 | 1.01 | 5 | 12 | ND | |||
| DES/ChCl:Urea(100mg) | - | 40 | 1.01 | 5 | 49 | ND | |||
| - | 60 | 1.01 | 5 | 85 | ND | ||||
| - | 70 | 1.01 | 5 | 89 | ND | ||||
| DES/ChCl:Urea (200mg) | - | 70 | 1.01 | 3 | 93 | ND | |||
| DES/ChCl:Urea (300mg) | - | 70 | 1.01 | 2 | 98 | ND | |||
| Monometallic salen complexes | |||||||||
| SO | Salophen (Figure 6a; R = tert-butyl, X = Cl) | TBAB | 25 | 1 | 3 | ≈37 a | 37 | [21] | |
| 6 | ≈60 a | 60 | |||||||
| 24 | ≈100 a | 100 | |||||||
| Salophen (Figure 6a; R = MeO, X = Br) | TBAB | 25 | 1 | 3 | ≈44 a | 44 | |||
| 6 | ≈71 a | 71 | |||||||
| 24 | ≈100 a | 100 | |||||||
| Bimetallic salen complexes | |||||||||
| PO | Bimetallic Salen-Co | TBAB | 25 | 10 | 48 | 75.8 | ND | [22] | |
| PO | Bimetallic Salen-Al | TBAB | 25 | 10 | 48 | 73.2 | ND | ||
| PO | Bimetallic Salen-Zn | TBAB | 25 | 10 | 48 | 72.1 | ND | ||
| Monometallic non-salen complexes | |||||||||
| HO | Zn(II) TPP | - | 20 | 1 | 43 | 82 | ND | [23] | |
| PO | Co-cryptand | TBAB | 0 | 1.01 | 8 | 43 | ND | [24] | |
| SO | TBAB | 20 | 1.01 | 12 | 32 | ND | |||
| ECH | TBAB | 20 | 1.01 | 24 | 48 | ND | |||
| - | 20 | 1.01 | 48 | 6 | ND | ||||
| - | TBAB | 20 | 1.01 | 48 | 11 | ND | |||
| Bimetallic non-salen complexes | |||||||||
| SO | Bi-aluminium scorpionate | R = (S)-CH(PhMe; X = Et | TBAB | RT | 1 | 24 | 75.0 b | 77 | [25] |
| TBAB | RT | 10 | 24 | 97.3 b | 100 | ||||
| R = Ph; X = Me | TBAB | RT | 10 | 24 | 58.6 b | 60 | |||
| Trimetallic non-salen complexes | |||||||||
| SO | Trinuclear aluminium scorpionate | R = Bu; X = Et | TBAB | RT | 10 | 24 | 97.3 b | 100 | [25] |
| TBAB | RT | 1 | 24 | 75.0 b | 77 | ||||
| R = (S)-CH(Ph)Me; X = Me | TBAB | RT | 10 | 24 | 90.2 b | 92 | |||
| TBAB | RT | 1 | 24 | 50.4 b | 52 | ||||
| R = (S)-CH(Ph)Me; X = Et | TBAB | RT | 10 | 24 | 97.3 b | 100 | |||
| TBAB | RT | 1 | 24 | 75.0 b | 77 | ||||
| R = Ph; X = Me | TBAB | RT | 10 | 24 | 97.3 b | 100 | |||
| TBAB | RT | 1 | 24 | 97.3 b | 100 | ||||
| CHO | Trimetallic-Co | TBAB | 80 | 20.68 | 24 | 66.3 | 67 | [26] | |
| TBAI | 80 | 20.68 | 24 | 38.6 | 39 | ||||
| Trimetallic-Zn | TBAB | 80 | 20.68 | 24 | 90.0 | 91 | |||
| TBAI | 80 | 20.68 | 24 | 87.1 | 88 | ||||
| Trimetallic-Ni | TBAB | 80 | 20.68 | 24 | 42.6 | 43 | |||
| Epoxide | Catalyst | Cocatalyst | T (°C) | P (bar) | Time (h) | Yield (%) | Conv. (%) | Reusability | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| AGE | PVIm2-BuBr | - | 90 | 8.6 | 6 | 48.02 | 49 | 5 times, drop slightly after fourth run | [39] |
| - | 100 | 8.6 | 6 | 58.8 | 60 | ||||
| - | 110 | 8.6 | 6 | 64.35 | 65 | ||||
| - | 110 | 13.4 | 6 | 84.15 | 85 | ||||
| - | 110 | 16.2 | 6 | 93.06 | 94 | ||||
| - | 110 | 18.2 | 6 | 97.02 | 98 | ||||
| SO | Bimetallic salen-merrifield resin (single TBAB) | - | 26 | 1 | 20 | 100 | - | 100%, 94%, 74%, 70% | [40] |
| Bimetallic salen-merrifield resin (four TBAB) | - | 26 | 1 | 20 | 79 | - | 79%, 71%, 67%, 64% | ||
| Bimetallic salen-silica supported(R = t-butyl) | - | 26 | 1 | 24 | 86 | - | - | [41] | |
| Bimetallic salen-aluminium pillared clay (R = t-butyl) | - | 26 | 1 | 24 | 21 | - | |||
| ECH | MgFeAl-LDH | TBAB | 25 | 5 | 48 | 92.83 | 96.3 | - | [42] |
| TBAB | 50 | 1 | 7 | 74.28 | 75.8 | - | |||
| TBAB | 50 | 5 | 7 | 96.04 | 98.0 | - | |||
| - | 50 | 5 | 7 | 7.39 | 7.6 | - | |||
| - | - | 50 | 5 | 7 | 16.6 | 17.0 | - |
| MOF | Active Site | T (°C) | P (bar) | Time (h) | Epoxide | Yield (%) | Conv. (%) | TOF | Reusability | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| In2(OH)(btc)(Hbtc)0.4(L)0.6·3H2O | Metal | RT | 1.01 | 48 | PO | 77.9 | - | 7.1 | 5 times (almost constant) | [1] |
| BO | 60.1 | - | 5.4 | |||||||
| SO | 31.6 | - | 2.9 | |||||||
| {[Co(BDC)(L)]·2H2O·xG}n | Metal and pyridine | RT | 1.01 | 48 | SEO | 84.15 | 85 | - | 5 times (almost constant) | [44] |
| 40 | 1.01 | 24 | 98.01 | 99 | - | |||||
| {Cu2[(C20H12N2O2)(COO)4]}n | Metal and acylamide | RT | 1.01 | 48 | PO | 96 | - | - | (96%, 96%, 95%, 95%, 95%) | [43] |
| BO | 85 | - | - | |||||||
| ECH | 88 | - | - | |||||||
| Octene oxide | 10 | - | - | |||||||
| {Cu4[(C57H32N12)(COO)8]}n | Metal and triazole | RT | 1.01 | 48 | PO | 96 | - | 200 | (96%, 96%, 96%, 95%, 95%) | [45] |
| BO | 83 | - | 172.9 | |||||||
| ECH | 85 | - | 177 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiatkittipong, K.; Mohamad Shukri, M.A.A.; Kiatkittipong, W.; Lim, J.W.; Show, P.L.; Lam, M.K.; Assabumrungrat, S. Green Pathway in Utilizing CO2 via Cycloaddition Reaction with Epoxide—A Mini Review. Processes 2020, 8, 548. https://doi.org/10.3390/pr8050548
Kiatkittipong K, Mohamad Shukri MAA, Kiatkittipong W, Lim JW, Show PL, Lam MK, Assabumrungrat S. Green Pathway in Utilizing CO2 via Cycloaddition Reaction with Epoxide—A Mini Review. Processes. 2020; 8(5):548. https://doi.org/10.3390/pr8050548
Chicago/Turabian StyleKiatkittipong, Kunlanan, Muhammad Amirul Amin Mohamad Shukri, Worapon Kiatkittipong, Jun Wei Lim, Pau Loke Show, Man Kee Lam, and Suttichai Assabumrungrat. 2020. "Green Pathway in Utilizing CO2 via Cycloaddition Reaction with Epoxide—A Mini Review" Processes 8, no. 5: 548. https://doi.org/10.3390/pr8050548
APA StyleKiatkittipong, K., Mohamad Shukri, M. A. A., Kiatkittipong, W., Lim, J. W., Show, P. L., Lam, M. K., & Assabumrungrat, S. (2020). Green Pathway in Utilizing CO2 via Cycloaddition Reaction with Epoxide—A Mini Review. Processes, 8(5), 548. https://doi.org/10.3390/pr8050548









