Hydrogen Production by Partial Oxidation Reforming of Methane over Ni Catalysts Supported on High and Low Surface Area Alumina and Zirconia
Abstract
1. Introduction
2. Experiment
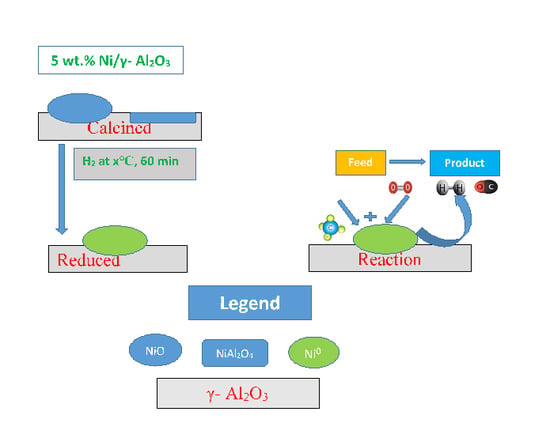
2.1. Catalyst Preparation
2.2. Catalytic Reaction
2.3. Catalyst Characterization
2.3.1. N2 Physisorption
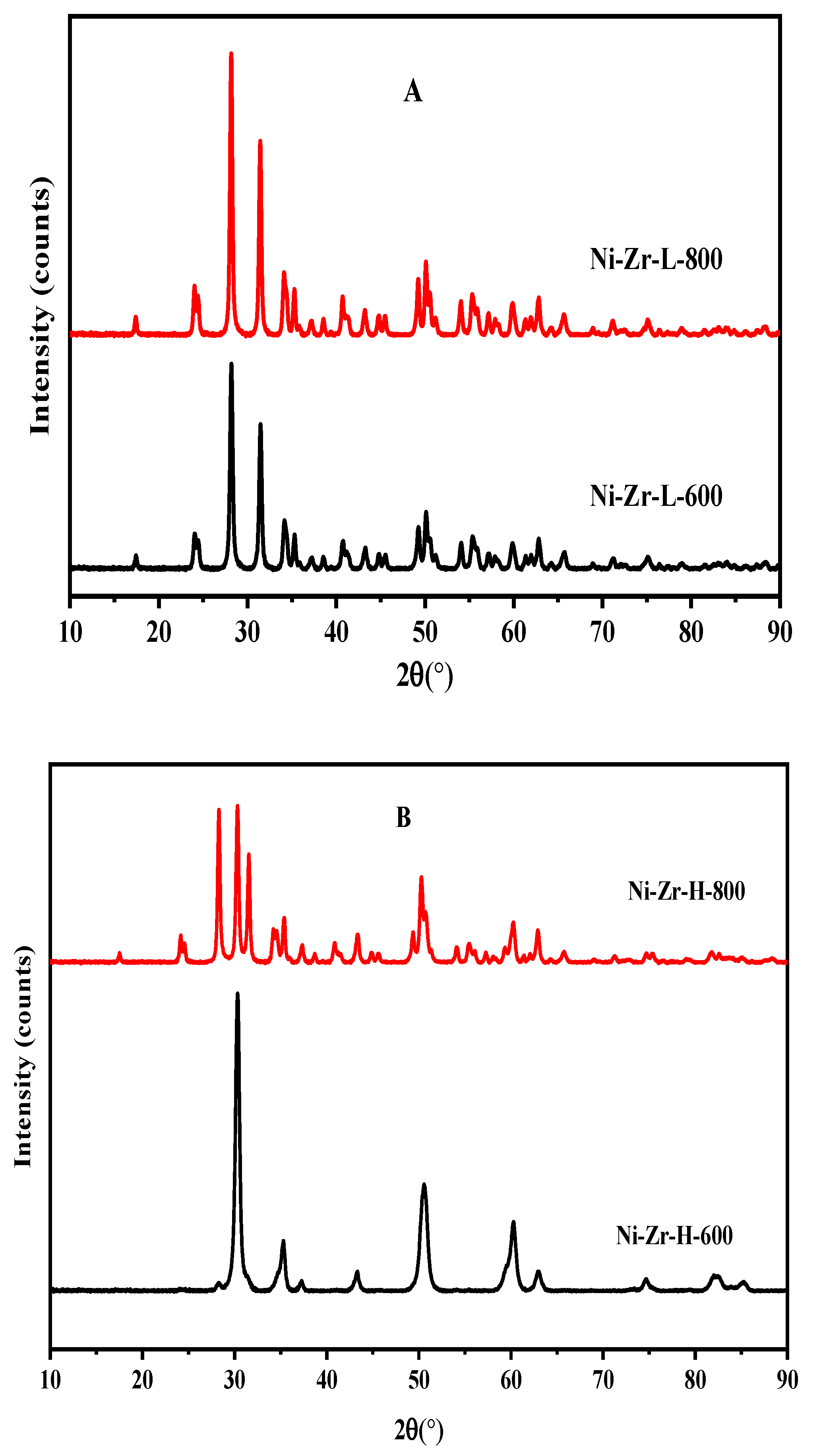
2.3.2. XRD
2.3.3. TPR
2.3.4. TGA
2.3.5. CO2-TPD
2.3.6. TPO
2.3.7. Raman Spectroscopy
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- He, S.; Castello, D.; Krishnamurthy, K.R.; Al-Fatesh, A.S.; Winkelman, J.G.M.; Seshan, K.; Fakeeha, A.H.; Kersten, S.R.A.; Heeres, H.J. Kinetics of long chain n-paraffin dehydrogenation over a commercial Pt-Sn-K-Mg/γ-Al2O3 catalyst: Model studies using n-dodecane. Appl. Catal. A Gen. 2019, 579, 130–140. [Google Scholar] [CrossRef]
- Vermeiren, W.J.M.; Blomsma, E.; Jacobs, P.A. Catalytic and thermodynamic approach of the oxyreforming reaction of methane. Catal. Today 1992, 13, 427–436. [Google Scholar] [CrossRef]
- Gillessen, B.; Heinrichs, H.; Hake, J.F.; Allelein, H.J. Natural gas as a bridge to sustainability: Infrastructure expansion regarding energy security and system transition. Appl. Energy 2019, 251, 113377. [Google Scholar] [CrossRef]
- Kaddeche, D.; Djaidja, A.; Barama, A. Partial oxidation of methane on co-precipitated Ni–Mg/Al catalysts modified with copper or iron. Int. J. Hydrog. Energy 2017, 42, 15002–15009. [Google Scholar] [CrossRef]
- Pruksawan, S.; Kitiyanan, B.; Ziff, R.M. Partial oxidation of methane on a nickel catalyst: Kinetic Monte-Carlo simulation study. Chem. Eng. Sci. 2016, 147, 128–136. [Google Scholar] [CrossRef]
- Pantaleo, G.; Parola, V.L.; Deganello, F.; Singha, R.K.; Bal, R.; Venezia, A.M. Ni/CeO2 catalysts for methane partial oxidation: Synthesis driven structural and catalytic effects. Appl. Catal. B Environ. 2016, 189, 233–241. [Google Scholar] [CrossRef]
- Gao, H.; Zhou, L.; Luo, X.; Liang, Z. Optimized process configuration for CO2 recovery from crude synthesis gas via a rectisol wash process. Int. J. Greenh. Gas Control 2018, 79, 83–90. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Q.; Liu, Y.; Zhang, Y. Dry reforming of methane over Ni/MgO-Al2O3 catalysts prepared by two-step hydrothermal method. Appl. Surf. Sci. 2016, 389, 25–33. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Abu-Dahrieh, J.K.; Atia, H.; Armbruster, U.; Ibrahim, A.A.; Khan, W.U.; Abasaeed, A.E.; Fakeeha, A.H. Effect of pre-treatment and calcination temperature on Al2O3-ZrO2 supported Ni-Co catalysts for dry reforming of methane. Int. J. Hydrog. Energy 2019, 44, 21546–21558. [Google Scholar] [CrossRef]
- Yang, W.; Fan, A.; Yao, H.; Liu, W. Effect of reduced pressures on the combustion efficiency of lean H2/air flames in a micro cavity-combustor. Int. J. Hydrog. Energy 2016, 41, 15354–15361. [Google Scholar] [CrossRef]
- Gan, Y.; Luo, Y.; Wang, M.; Shi, Y.; Yan, Y. Effect of alternating electric fields on the behaviour of small-scale laminar diffusion flames. Appl. Therm. Eng. 2015, 89, 306–315. [Google Scholar] [CrossRef]
- Radfarnia, H.R.; Iliuta, M.C. Hydrogen production by sorption-enhanced steam methane reforming process using CaO-Zr/Ni bifunctional sorbent–catalyst. Chem. Eng. Process. Process Intensif. 2014, 86, 96–103. [Google Scholar] [CrossRef]
- Kaczmarek, D.; Atakan, B.; Kasper, T. Investigation of the partial oxidation of methane/n-heptane-mixtures and the interaction of methane and n-heptane under ultra-rich conditions. Combust. Flame 2019, 205, 345–357. [Google Scholar] [CrossRef]
- Melchiori, T.; Di Felice, L.; Mota, N.; Navarro, R.M.; Fierro, J.L.G.; Annaland, M.V.S.; Gallucci, F. Methane partial oxidation over a LaCr0.85Ru0.15O3 catalyst: Characterization, activity tests and kinetic modeling. Appl. Catal. A Gen. 2014, 486, 239–249. [Google Scholar] [CrossRef]
- Costa, D.S.; Gomes, R.S.; Rodella, C.B.; da Silva, R.B.; Fréty, R.; Teixeira Neto, É.; Brandão, S.T. Study of nickel, lanthanum and niobium-based catalysts applied in the partial oxidation of methane. Catal. Today 2018. [Google Scholar] [CrossRef]
- Wang, F.; Li, W.-Z.; Lin, J.-D.; Chen, Z.-Q.; Wang, Y. Crucial support effect on the durability of Pt/MgAl2O4 for partial oxidation of methane to syngas. Appl. Catal. B Environ. 2018, 231, 292–298. [Google Scholar] [CrossRef]
- Hou, Z.; Chen, P.; Fang, H.; Zheng, X.; Yashima, T. Production of synthesis gas via methane reforming with CO2 on noble metals and small amount of noble-(Rh-) promoted Ni catalysts. Int. J. Hydrog. Energy 2006, 31, 555–561. [Google Scholar] [CrossRef]
- Fasolini, A.; Abate, S.; Barbera, D.; Centi, G.; Basile, F. Pure H2 production by methane oxy-reforming over Rh-Mg-Al hydrotalcite-derived catalysts coupled with a Pd membrane. Appl. Catal. A Gen. 2019, 581, 91–102. [Google Scholar] [CrossRef]
- Araújo, J.C.S.; Oton, L.F.; Bessa, B.; Neto, A.B.S.; Oliveira, A.C.; Lang, R.; Otubo, L.; Bueno, J.M.C. The role of Pt loading on La2O3-Al2O3 support for methane conversion reactions via partial oxidation and steam reforming. Fuel 2019, 254, 115681. [Google Scholar] [CrossRef]
- Zhao, A.; Ying, W.; Zhang, H.; Ma, H.; Fang, D. Ni–Al2O3 catalysts prepared by solution combustion method for syngas methanation. Catal. Commun. 2012, 17, 34–38. [Google Scholar] [CrossRef]
- Song, Y.; Liu, H.; Liu, S.; He, D. Partial Oxidation of Methane to Syngas over Ni/Al2O3 Catalysts Prepared by a Modified Sol−Gel Method. Energy Fuels 2009, 23, 1925–1930. [Google Scholar] [CrossRef]
- Beretta, A.; Bruno, T.; Groppi, G.; Tavazzi, I.; Forzatti, P. Conditioning of Rh/α-Al2O3 catalysts for H2 production via CH4 partial oxidation at high space velocity. Appl. Catal. B Environ. 2007, 70, 515–524. [Google Scholar] [CrossRef]
- Jing, Q.; Zheng, X.J.E. Combined catalytic partial oxidation and CO2 reforming of methane over ZrO2-modified Ni/SiO2 catalysts using fluidized-bed reactor. Energy 2006, 31, 2184–2192. [Google Scholar] [CrossRef]
- Dissanayake, D.; Rosynek, M.P.; Kharas, K.C.C.; Lunsford, J.H.L. Partial oxidation of methane to carbon monoxide and hydrogen over a Ni/Al2O3 catalyst. J. Catal. 1991, 132, 117–127. [Google Scholar] [CrossRef]
- Zhang, Q.; Shen, M.; Wen, J.; Wang, J.; Fei, Y. Partial oxidation of methane on Ni/CeO2-ZrO2/γ- Al2O3 prepared using different processes. J. Rare Earths 2008, 26, 347–351. [Google Scholar] [CrossRef]
- Sajjadi, S.M.; Haghighi, M. Impregnation vs. sol-gel and sol-gel-plasma dispersion of nickel nanoparticles over Al2O3 employed in combined dry reforming and partial oxidation of greenhouse gases to syngas. Int. J. Hydrog. Energy 2018, 43, 15014–15029. [Google Scholar] [CrossRef]
- Wu, P.; Li, X.; Ji, S.; Lang, B.; Habimana, F.; Li, C. Steam reforming of methane to hydrogen over Ni-based metal monolith catalysts. Catal. Today 2009, 146, 82–86. [Google Scholar] [CrossRef]
- Dong, W.-S.; Jun, K.-W.; Roh, H.-S.; Liu, Z.-W.; Park, S.-E. Comparative study on partial oxidation of methane over Ni/ZrO2, Ni/CeO2 and Ni/Ce–ZrO2, catalysts. Catal. Lett. 2002, 78, 215–222. [Google Scholar] [CrossRef]
- Hadj-Sadok Ouaguenouni, M.; Benadda, A.; Kiennemann, A.; Barama, A. Preparation and catalytic activity of nickel–manganese oxide catalysts in the reaction of partial oxidation of methane. Comptes Rendus Chim. 2009, 12, 740–747. [Google Scholar] [CrossRef]
- Moral, A.; Reyero, I.; Llorca, J.; Bimbela, F.; Gandía, L.M. Partial oxidation of methane to syngas using Co/Mg and Co/Mg-Al oxide supported catalysts. Catal. Today 2019, 333, 259–267. [Google Scholar] [CrossRef]
- Chen, L.; Lu, Y.; Hong, Q.; Lin, J.; Dautzenberg, F.M. Catalytic partial oxidation of methane to syngas over Ca-decorated-Al2O3-supported Ni and NiB catalysts. Appl. Catal. A Gen. 2005, 292, 295–304. [Google Scholar] [CrossRef]
- Wierzbicki, D.; Baran, R.; Dębek, R.; Motak, M.; Gálvez, M.E.; Grzybek, T.; Da Costa, P.; Glatzel, P. Examination of the influence of La promotion on Ni state in hydrotalcite-derived catalysts under CO2 methanation reaction conditions: Operando X-ray absorption and emission spectroscopy investigation. Appl. Catal. B Environ. 2018, 232, 409–419. [Google Scholar] [CrossRef]
- Li, D.; Li, R.; Lu, M.; Lin, X.; Zhan, Y.; Jiang, L. Carbon dioxide reforming of methane over Ru catalysts supported on Mg-Al oxides: A highly dispersed and stable Ru/Mg(Al)O catalyst. Appl. Catal. B Environ. 2017, 200, 566–577. [Google Scholar] [CrossRef]
- Chatelier, H.L.L. Sur un énoncé général des lois des équilibres chimiques. Comptes Rendus Chim. 1884, 99, 786–789. [Google Scholar]
- Ma, Y.; Ma, Y.; Chen, Y.; Ma, S.; Li, Q.; Hu, X.; Wang, Z.; Buckley, C.E.; Dong, D. Highly stable nanofibrous La2NiZrO6 catalysts for fast methane partial oxidation. Fuel 2020, 265, 116861. [Google Scholar] [CrossRef]
- Dajiang, M.; Yaoqiang, C.; Junbo, Z.; Zhenling, W.; Di, M.; Maochu, G. Catalytic Partial Oxidation of Methane over Ni/CeO2-ZrO2-Al2O3. J. Rare Earths 2007, 25, 311–315. [Google Scholar] [CrossRef]
- Emamdoust, A.; La Parola, V.; Pantaleo, G.; Testa, M.L.; Shayesteh, S.F.; Venezia, A.M. Partial oxidation of methane over SiO2 supported Ni and NiCe catalysts. J. Energy Chem. 2020, 47, 1–9. [Google Scholar] [CrossRef]
- Padilla, R.; Benito, M.; Rodríguez, L.; Serrano, A.; Muñoz, G.; Daza, L. Nickel and cobalt as active phase on supported zirconia catalysts for bio-ethanol reforming: Influence of the reaction mechanism on catalysts performance. Int. J. Hydrog. Energy 2010, 35, 8921–8928. [Google Scholar] [CrossRef]
- Ding, M.; Tu, J.; Zhang, Q.; Wang, M.; Tsubaki, N.; Wang, T.; Ma, L. Enhancement of methanation of bio-syngas over CeO2-modified Ni/Al2O3 catalysts. Biomass Bioenergy 2016, 85, 12–17. [Google Scholar] [CrossRef]
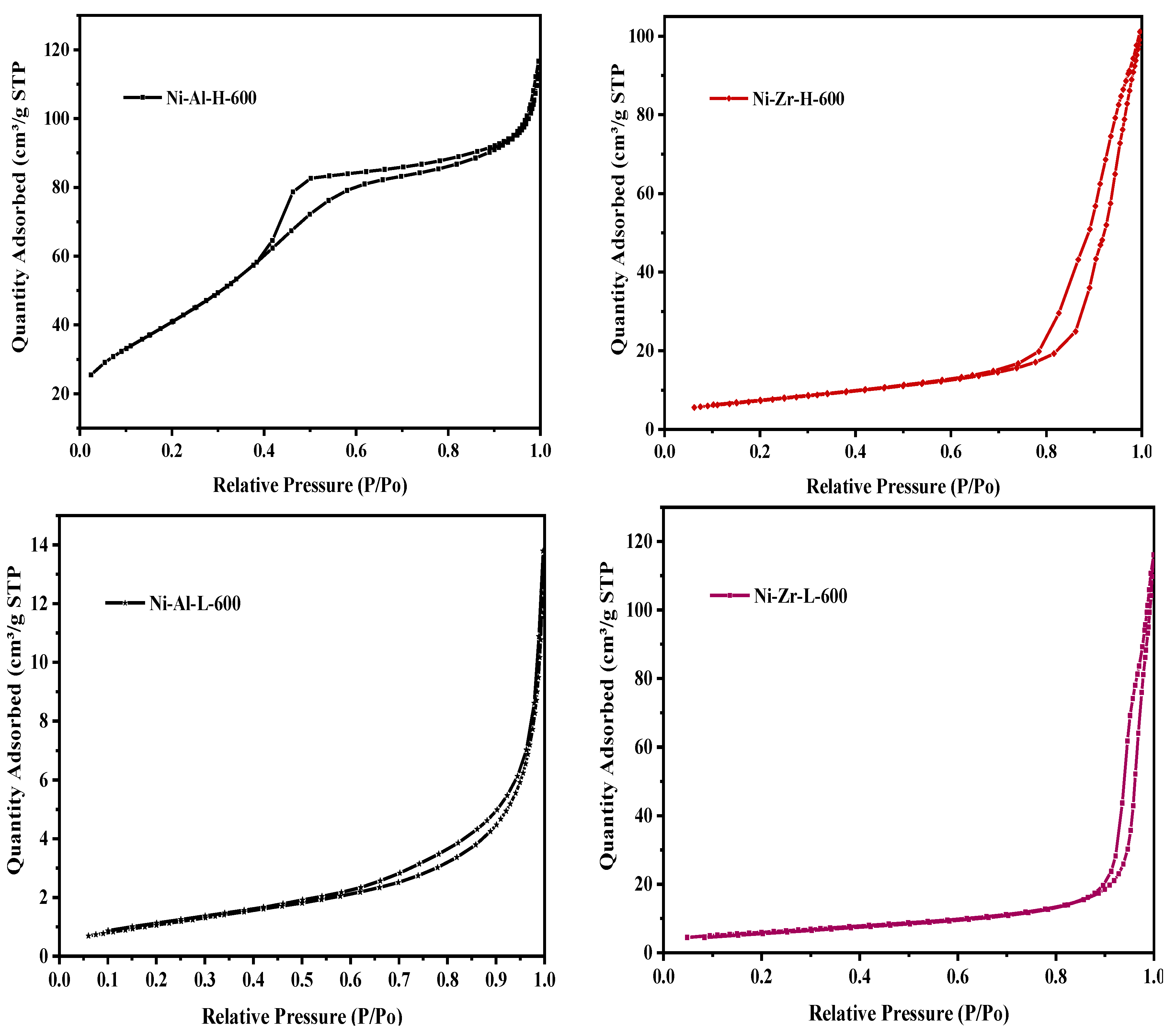
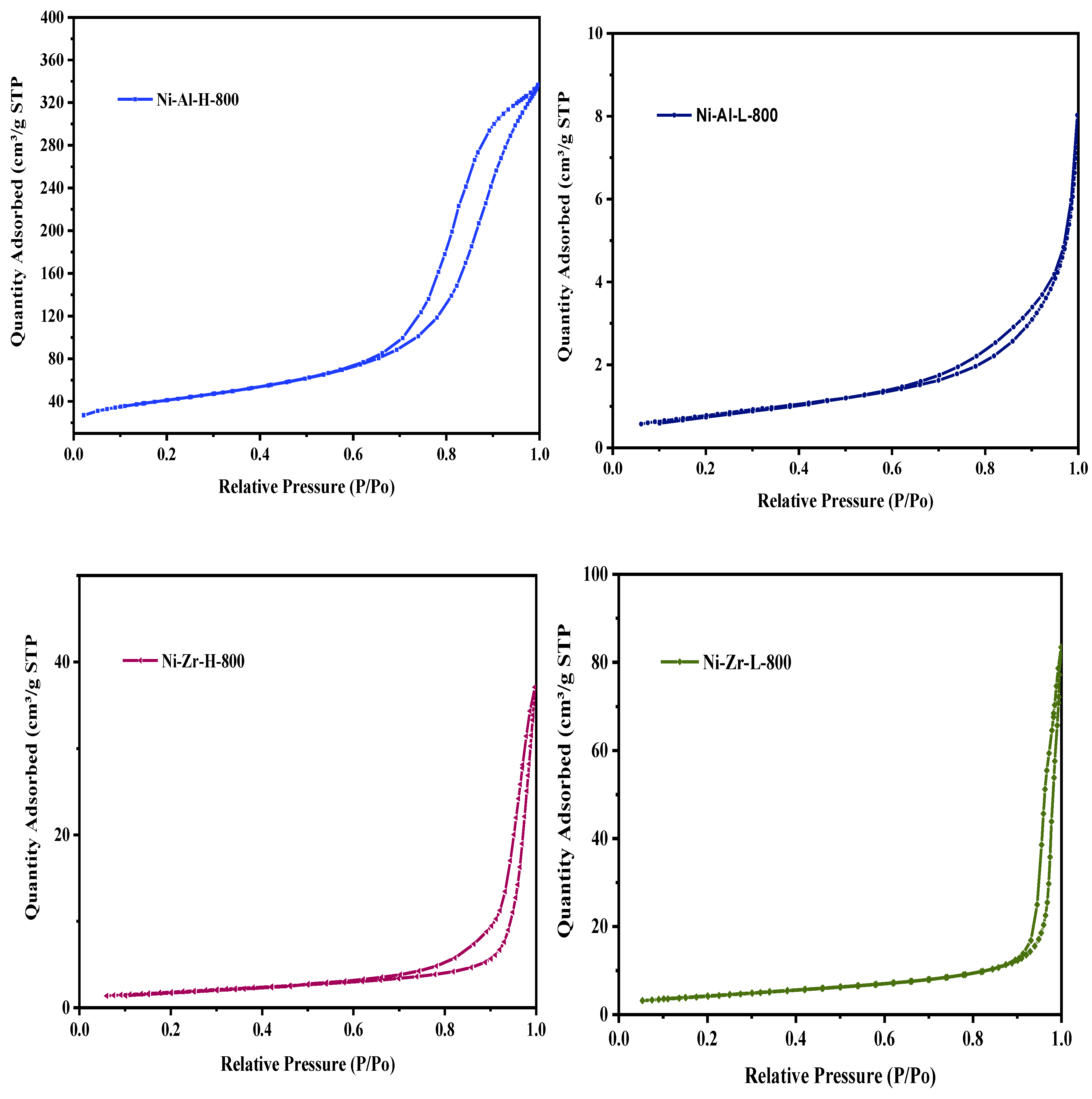


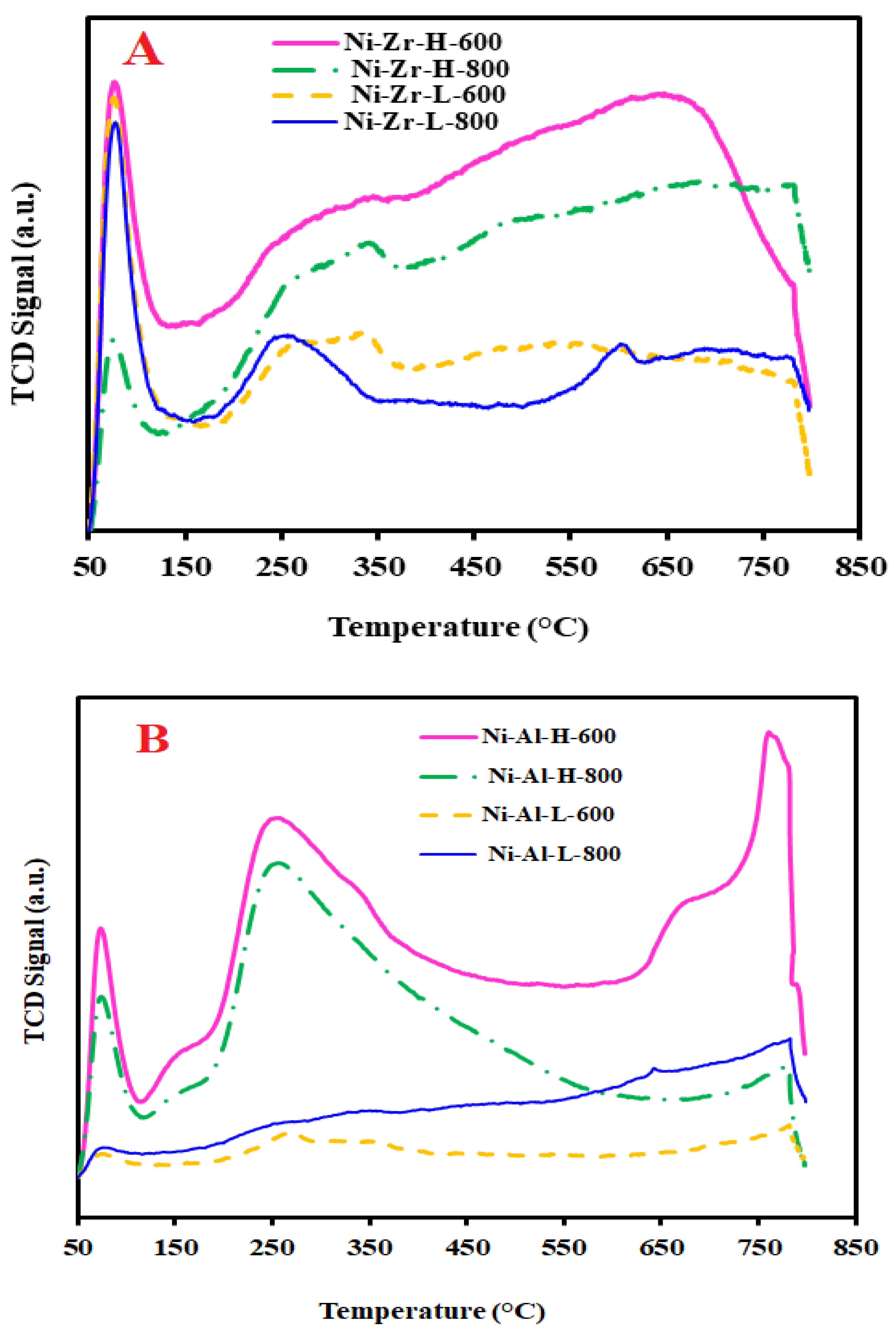
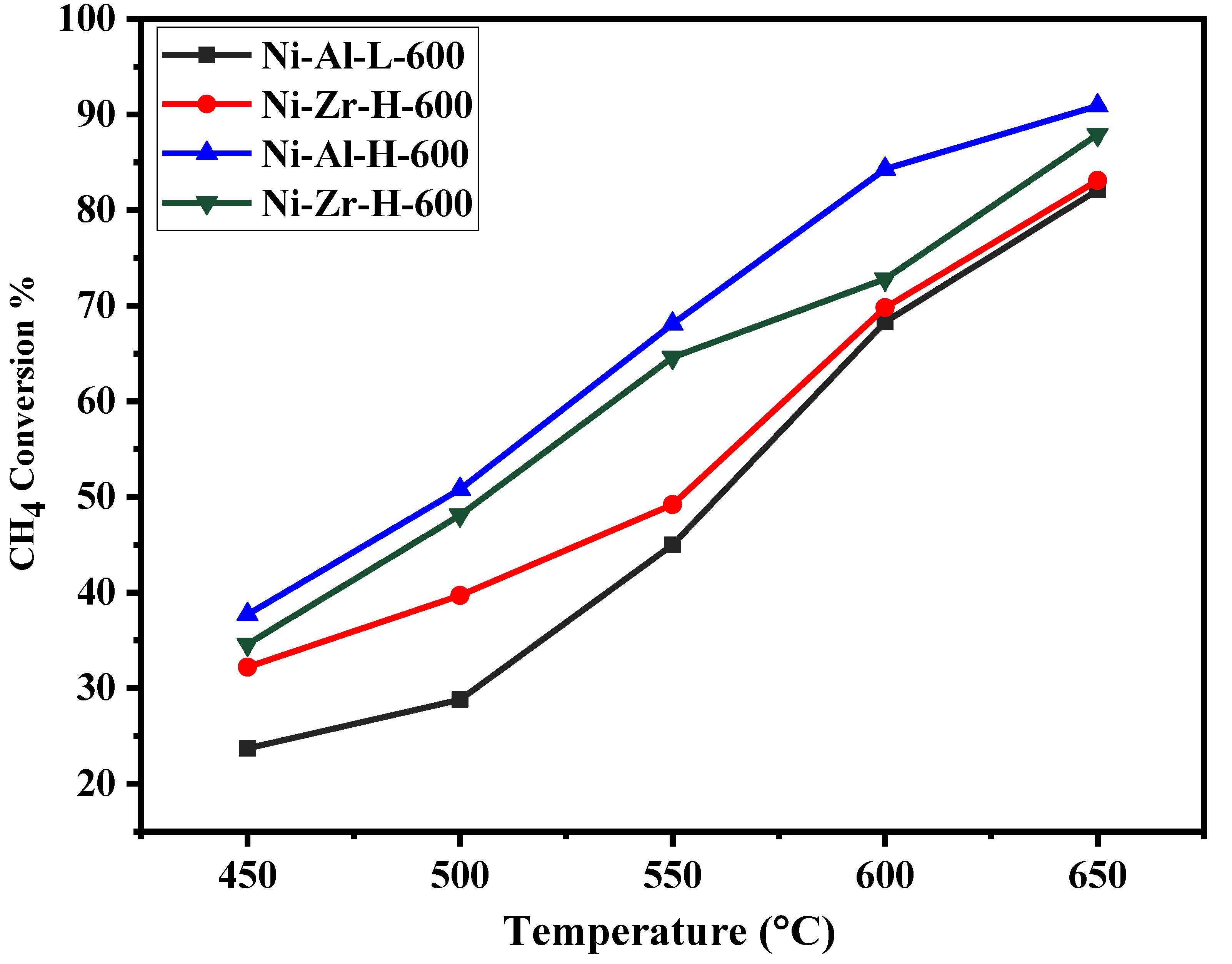
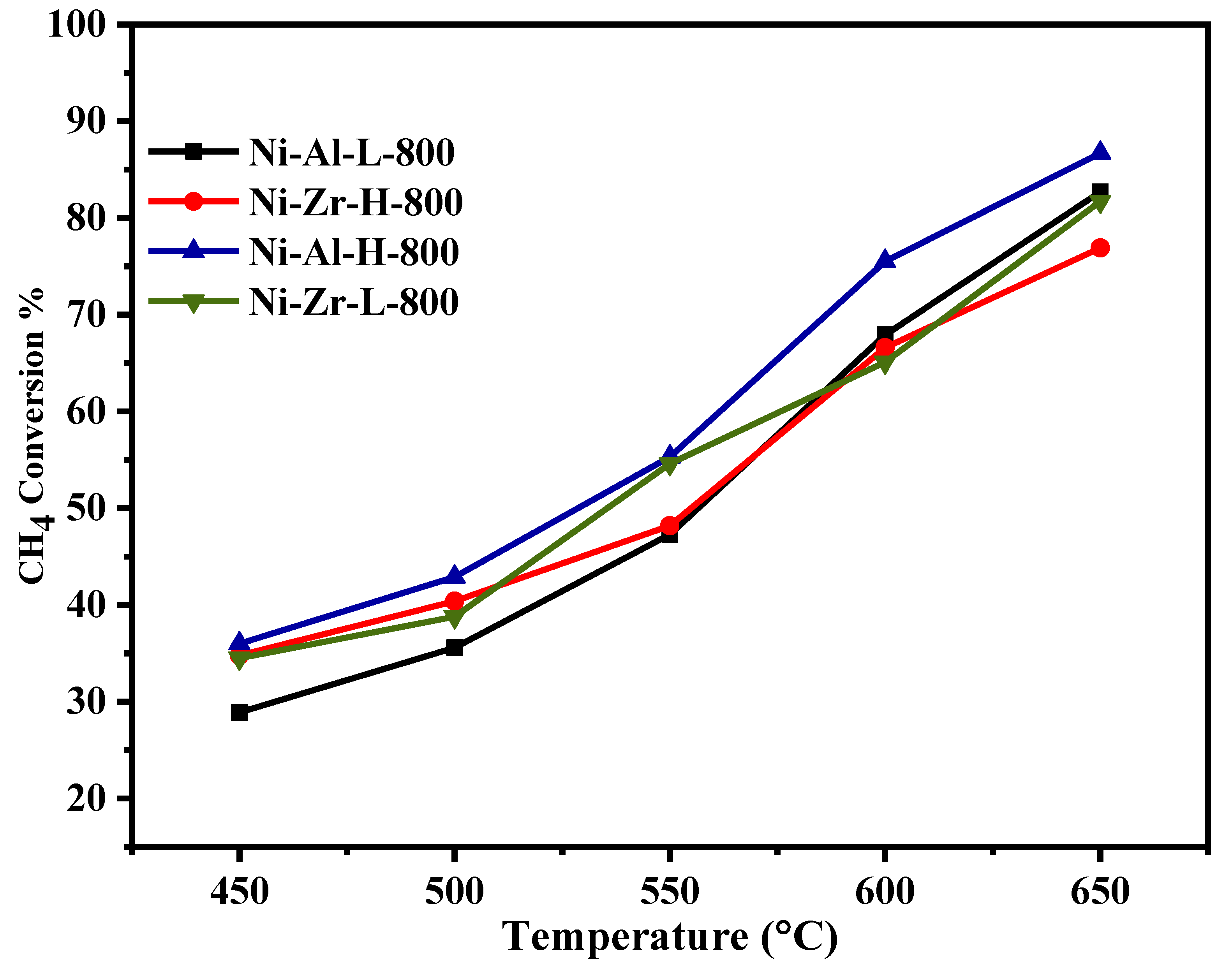
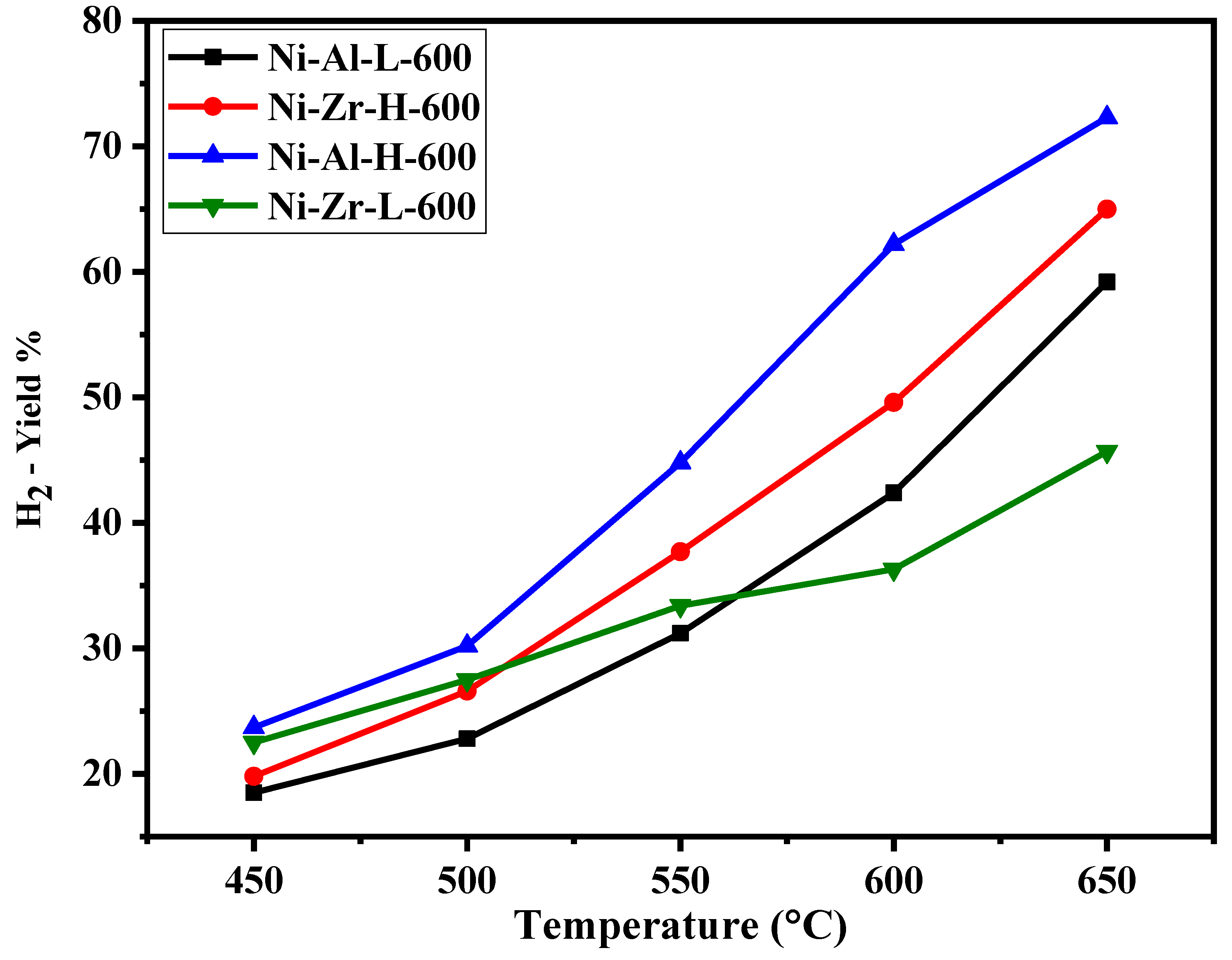

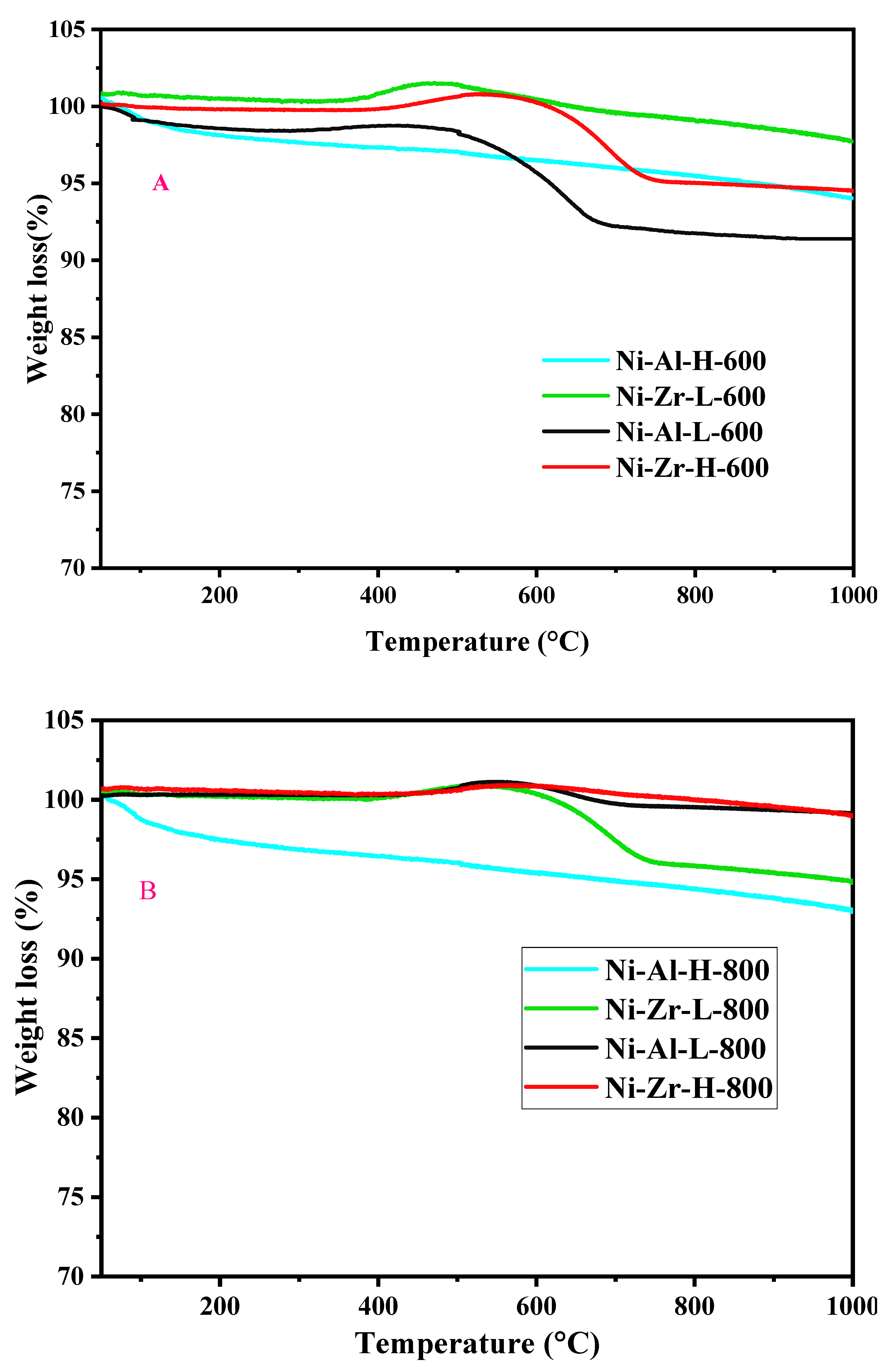
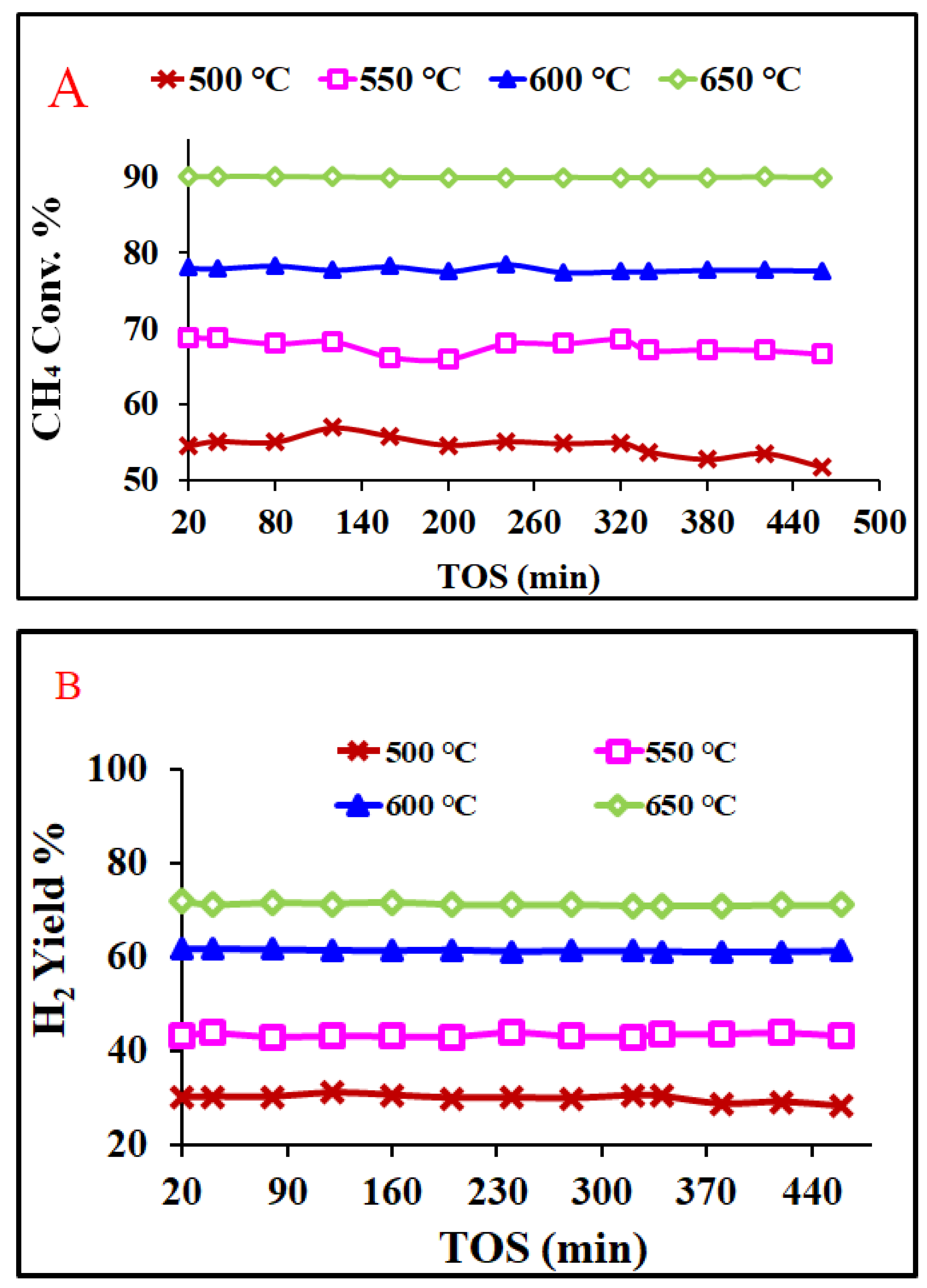
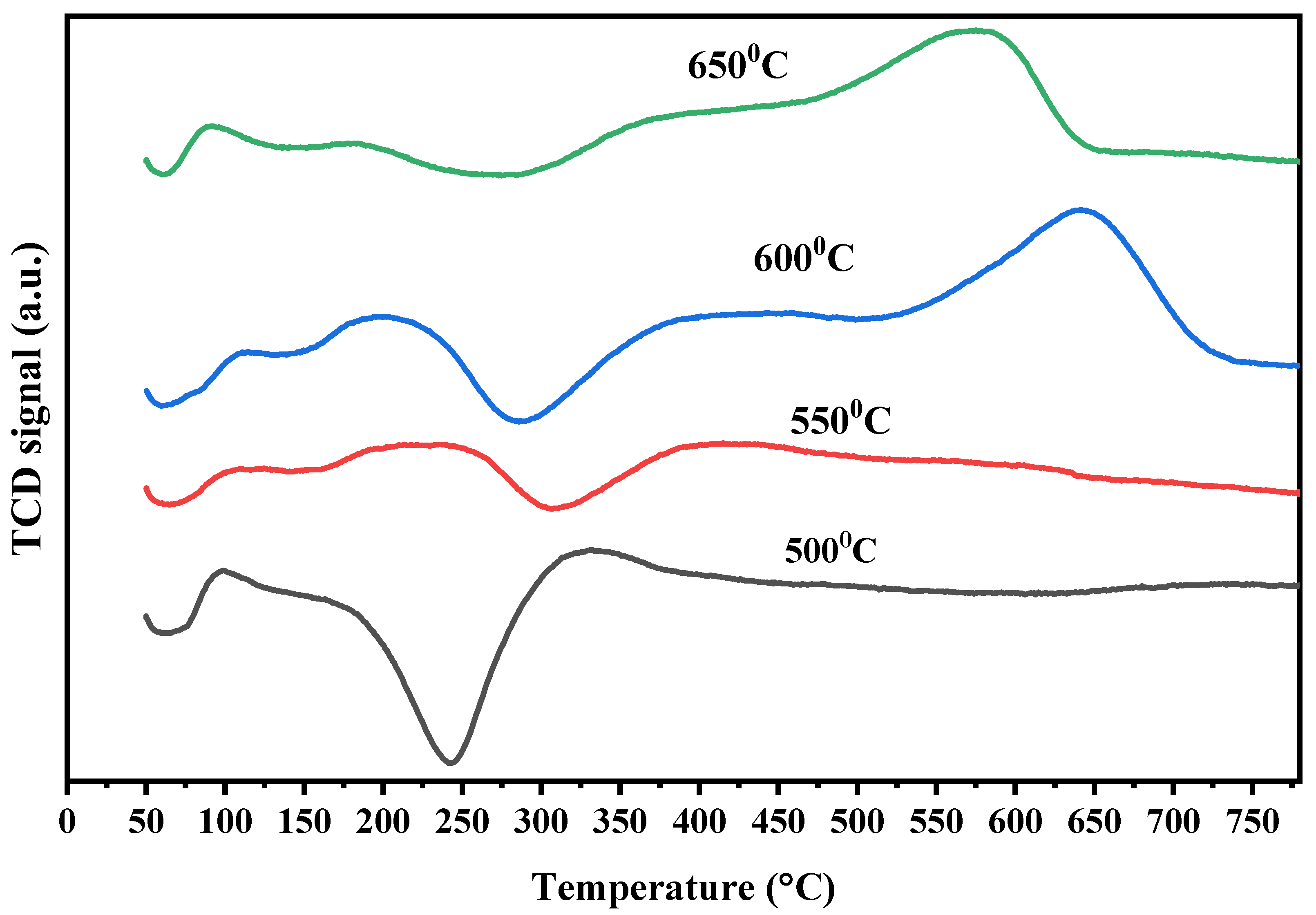
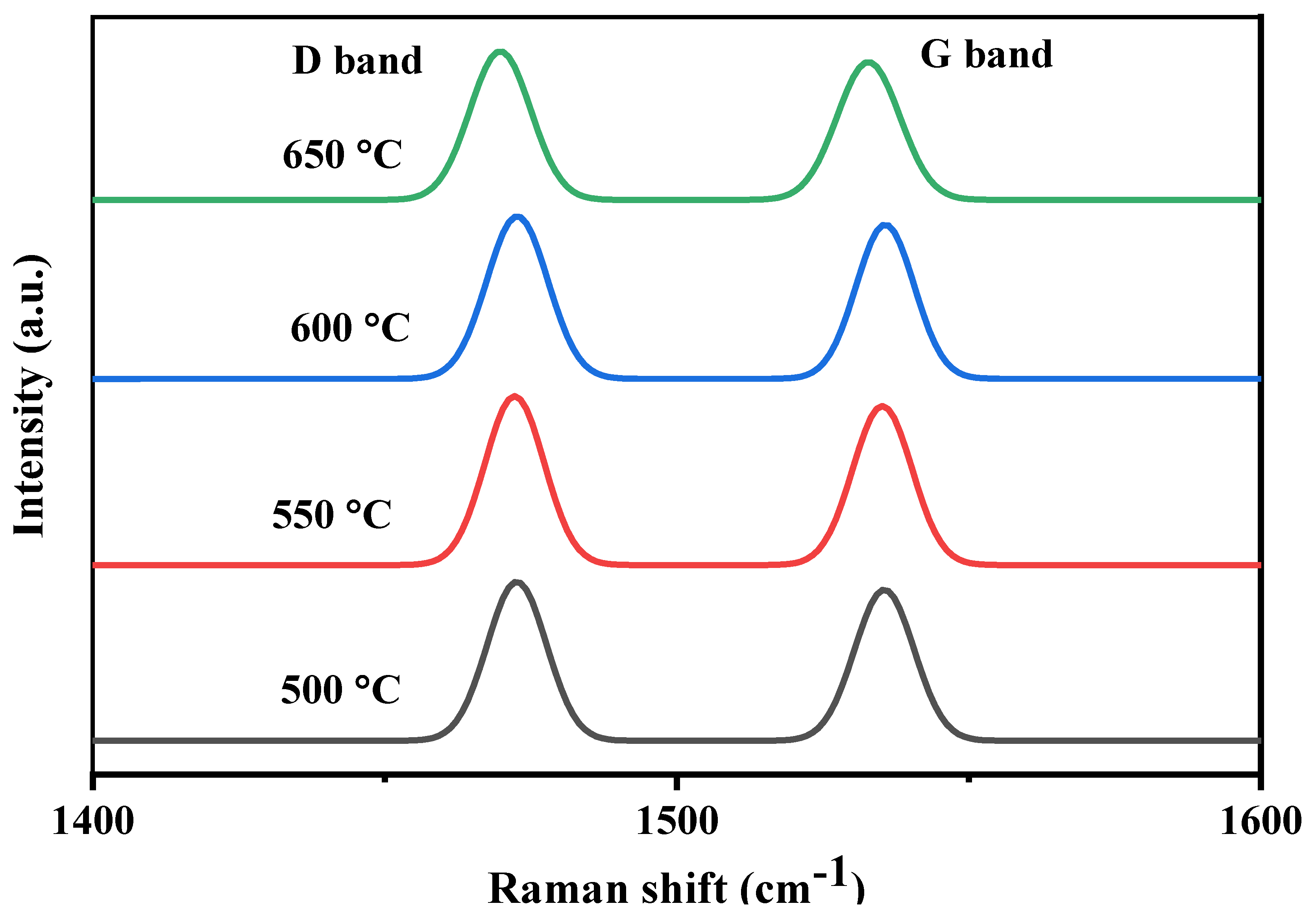
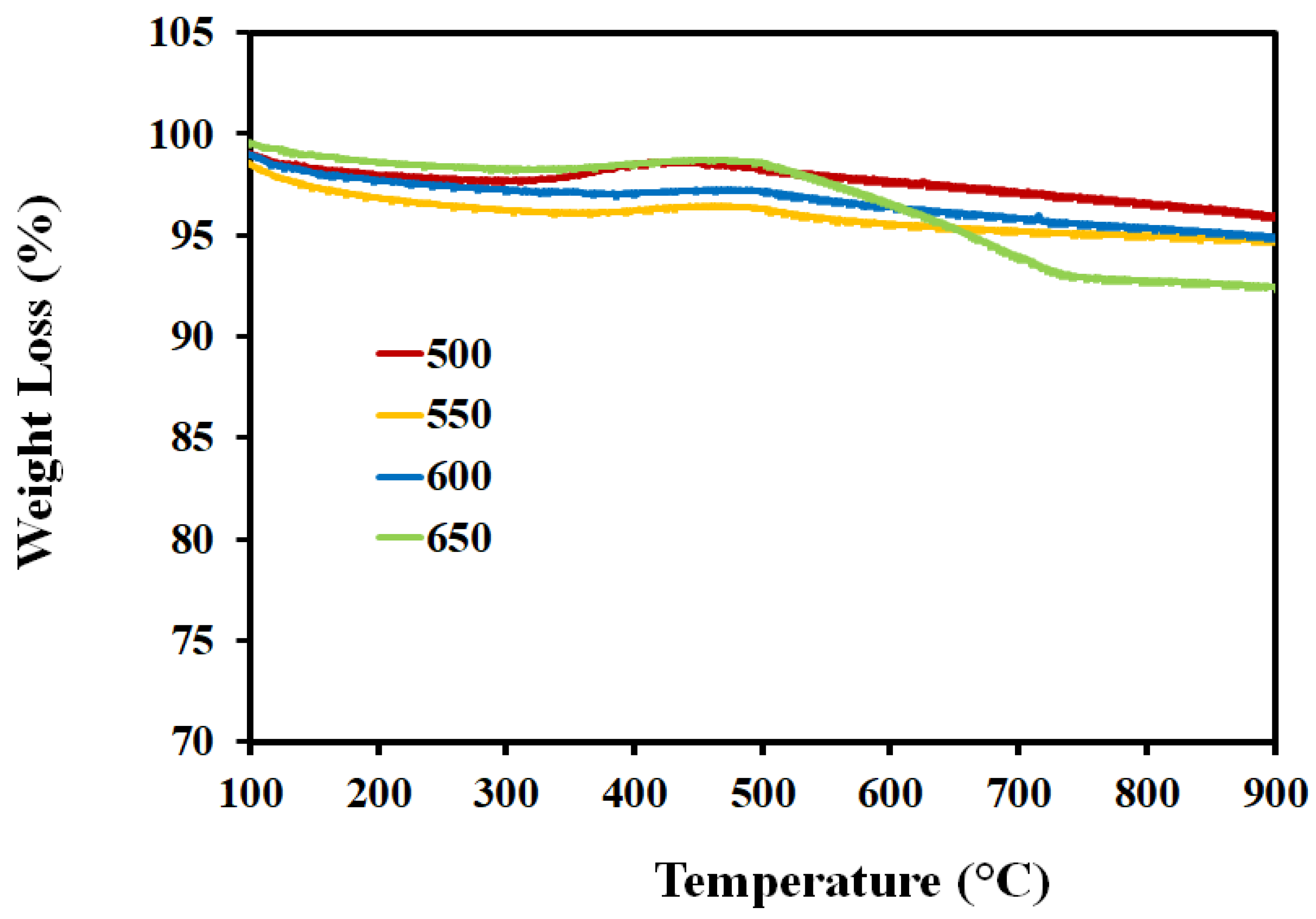
| Catalyst/Support | Designation | Calcination Temperature (°C) | Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Diameter (nm) | Total H2 Consumption (µmol/g) |
|---|---|---|---|---|---|---|
| α-Al2O3 | Al-L | 2.5 | ||||
| 10% Ni/α-Al2O3 | Ni-Al-L-600 | 600 | 4.3 | 0.02 | 14.4 | 3058.9 |
| 10% Ni/α-Al2O3 | Ni-Al-L-800 | 800 | 2.9 | 0.01 | 14.9 | 2637.3 |
| γ-Al2O3 | Al-H | 260 | ||||
| 10% Ni/γ-Al2O3 | Ni-Al-H-600 | 600 | 175.9 | 0.61 | 12.1 | 3729.8 |
| 10% Ni/γ-Al2O3 | Ni-Al-H-800 | 800 | 146.4 | 0.54 | 13.3 | 4472.9 |
| α-ZrO2 | Zr-L | 22.6 | ||||
| 10% Ni/α-ZrO2 | Ni-Zr-L-600 | 600 | 21.5 | 0.16 | 32.0 | 2349.1 |
| 10% Ni/α-ZrO2 | Ni-Zr-L-800 | 800 | 15.1 | 0.11 | 33.7 | 3116.7 |
| γ-ZrO2 | Zr-H | 325 | ||||
| 10% Ni/γ-ZrO2 | Ni-Zr-H-600 | 600 | 26.7 | 0.16 | 22.5 | 3442.9 |
| 10% Ni/γ-ZrO2 | Ni-Zr-H-800 | 800 | 6.6 | 0.05 | 34.9 | 3407.5 |
| Catalyst | Weight (g) | CH4:O2 | Space Velocity (mL/g/h) | Reaction Temperature (°C) | % CH4 Conversion | Reference |
|---|---|---|---|---|---|---|
| 25% Ni/Al2O3+TiO2+CaO | 0.05 | 1.78:1.00 | 6 × 104 | 650 | 86 | [24] |
| 10% Ni/Ce0.7Zr0.3O2-Al2O3 | 0.5 | 2.00:1.00 | 4 × 104 | 650 | 67.8 | [25] |
| La2NiZrO6 | 0.01 | 2.00:1.00 | 300 × 104 | 750 | 40 | [35] |
| 8% Ni/CeO2-ZrO2-Al2O3 | 0.15 | 2.00:1.00 | 20 × 104 | 650 | 88.5 | [36] |
| 6% Ni/SiO2 | 0.1 | 2.00:1.00 | 6 × 104 | 600 | 85 | [37] |
| Ni-Al-H-600 | 0.1 | 2.00:1.00 | 1.95× 104 | 650 | 90 | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fakeeha, A.; Ibrahim, A.A.; Aljuraywi, H.; Alqahtani, Y.; Alkhodair, A.; Alswaidan, S.; Abasaeed, A.E.; Kasim, S.O.; Mahmud, S.; Al-Fatesh, A.S. Hydrogen Production by Partial Oxidation Reforming of Methane over Ni Catalysts Supported on High and Low Surface Area Alumina and Zirconia. Processes 2020, 8, 499. https://doi.org/10.3390/pr8050499
Fakeeha A, Ibrahim AA, Aljuraywi H, Alqahtani Y, Alkhodair A, Alswaidan S, Abasaeed AE, Kasim SO, Mahmud S, Al-Fatesh AS. Hydrogen Production by Partial Oxidation Reforming of Methane over Ni Catalysts Supported on High and Low Surface Area Alumina and Zirconia. Processes. 2020; 8(5):499. https://doi.org/10.3390/pr8050499
Chicago/Turabian StyleFakeeha, Anis, Ahmed A. Ibrahim, Hesham Aljuraywi, Yazeed Alqahtani, Ahmad Alkhodair, Suliman Alswaidan, Ahmed E. Abasaeed, Samsudeen O. Kasim, Sofiu Mahmud, and Ahmed S. Al-Fatesh. 2020. "Hydrogen Production by Partial Oxidation Reforming of Methane over Ni Catalysts Supported on High and Low Surface Area Alumina and Zirconia" Processes 8, no. 5: 499. https://doi.org/10.3390/pr8050499
APA StyleFakeeha, A., Ibrahim, A. A., Aljuraywi, H., Alqahtani, Y., Alkhodair, A., Alswaidan, S., Abasaeed, A. E., Kasim, S. O., Mahmud, S., & Al-Fatesh, A. S. (2020). Hydrogen Production by Partial Oxidation Reforming of Methane over Ni Catalysts Supported on High and Low Surface Area Alumina and Zirconia. Processes, 8(5), 499. https://doi.org/10.3390/pr8050499