Abstract
Inflammatory bowel disease (IBD) is one of the predominant intestinal diseases associated with chronic inflammation and ulceration of the colon. This study explored the ameliorative effect of Aloe vera extract (Aloe) and/or heat-killed Lactobacillus plantarum L.137 (HK L.137) on dextran sodium sulfate (DSS)-induced colitis in mice. Aloe and/or HK L.137 were supplied for 9 days and the mice were challenged with DSS for 7 days. The DSS group demonstrated bloody diarrhea, colitis of high histologic grade, increased nuclear factor-kappa B (NF-κB) p65, inducible nitric oxide synthase (iNOS), myeloperoxidase (MPO), interleukin (IL)-6, and tumor necrosis factor (TNF)-α, and decreased IL-10 expression. These alterations were dwindled in DSS-induced mice treated with Aloe and HK L.137 separately. Aloe and HK L.137 together have augmented the therapeutic effect of each other. In conclusion, our findings demonstrated that Aloe and/or HK L.137 ameliorated DSS-induced colitis by promoting the secretion of anti-inflammatory cytokines and suppressing pro-inflammatory mediators. This study indicated that A. vera may function synergistically with HK L.137 to confer an effective strategy to prevent colitis.
1. Introduction
Inflammatory bowel disease (IBD) is a chronic immune-mediated disease characterized by inflammation and ulceration of mucosal and submucosal layers of the colon. So far, the etiology of the disease is not precisely known; however, several different genetic and environmental factors might be involved [1]. IBDs include two basic types, namely Crohn’s disease (CD) and ulcerative colitis (UC). The disease is manifested by chronic diarrhea, rectal bleeding, and abdominal cramps [2]. A vast number of inflammatory mediators were implicated in the pathogenesis of the disease. Therefore, most medications formulated for the treatment and/or management of the IBD depend mainly on the anti-inflammatory/immunosuppressive actions [3]. Unfortunately, the treatment is mostly not effective in some instances and accompanied mainly by frequent relapsing and progressive nature. Therefore, surgical interference and resection of parts of the digestive system are necessary for 20%–50% of IBD patients [4]. The mechanism of the inflammatory response of IBD is mostly associated with activation of inflammatory cells, such as macrophages, dendritic cells, neutrophils, and different subsets of T lymphocytes [5]. These cells were accompanied with massive production of reactive nitrogen species (RNS) and reactive oxygen species (ROS) and several inflammatory mediators, such as tumor necrosis factor-alpha (TNF-α), interleukin (IL)-6, IL-17, IL-21, and cyclooxygenases (COX-1 and COX-2). These cytokines were mostly associated with increased epithelial permeability, mucosal injury, and subsequent invasion of commensal bacteria into the sub-epithelial space [6].
Dextran sodium sulfate (DSS) is a sulfated polysaccharide that has been reported to induce colitis for the first time in hamsters [7]. Although not fully understood, the mechanism of DSS-induced colitis is related to direct injury of the crypt epithelium and crossing of intestinal contents, particularly the commensal bacteria and/or their products, leading to inflammation [8,9]. Besides, the histopathological findings have closely resembled that of human UC [10]. Therefore, the DSS-induced colitis has been permitted as a valuable model for evaluating the potential of agents of interest as therapeutics for IBD [11].
Probiotics are non-pathogenic microorganisms supplemented to promote the intestinal microbial balance [12]. They include Saccharomyces boulardii and lactic acid-producing bacteria, such as Bifidobacterium and Lactobacillus spp. Lactobacillus species are Gram-positive bacteria playing a central role in food fermentation and offer a plausible model to explore the molecular effects of food-associated bacteria in human mucosa. The health-promoting effects of probiotics in IBD have been acknowledged and Escherichia coli Nissle 1917 has been approved for the treatment of UC [13]. Lactobacillus paracasei reduced circulating pro-inflammatory cytokines in UC [14]. Lactobacillus rhamnosus has a prophylactic activity on colorectal carcinogenesis by inducing apoptosis and ameliorating inflammation, thereby represent promising candidates as a bio-therapeutic dietary agent [15]. Interestingly, altered gastrointestinal microbiota may participate completely or in part in the mucosal immune response [16]. Therefore, the intestinal mucosal barrier in IBD may allow bacterial access to the submucosal tissues, thereby enhancing the inflammatory response. Thus, probiotic therapy depends upon altering the bacterial composition that strengthens the immune status and competes for the inflammation. The premise of dysbiosis is therefore considered the rationale for administering live beneficial bacterial strains for IBD [17].
Aloe vera (Aloe barbadensis Mill) possesses many beneficial biological effects, including anti-inflammatory, antioxidant, immunostimulant, antidiabetic, and antitumor activities [18,19]. It originates mostly within the dry areas of Southern Europe, Africa, Asia, and the Mediterranean region [20]. Dietary supplementation of Aloe components such as aloesin has shown a beneficial role in the treatment of colitis in rats [21]. A. vera gel has been shown to prevent azoxymethane/DSS-induced colon carcinogenesis by inhibiting chronic inflammation as well as cell cycle progression [22]. In a rodent model of colitis, dietary supplementation of A. vera gel ameliorated intestinal inflammation [21]. Given its anti-inflammatory potential, the current study scrutinized the preventive effect of A. vera and/or HK L.137 on DSS-induced acute colitis in mice. Although A. vera and Lactobacillus plantarum have shown multiple pharmacological effects, this is the first study assessing their ameliorative effect on clinical signs, histopathological changes, and tissue cytokines in DSS-induced colitis in mice.
2. Materials and Methods
2.1. Chemicals
DSS (M.W. 36–50 KD) was supplied by MP Biomedicals (Solon, OH, USA), and HK L.137 was supplied by the House Wellness Foods Corporation (Hyogo, Japan). Lyophilized A. vera powder was obtained from Coral Vegetable (Miyakojima, Japan). All other chemicals and kits were purchased from standard commercial sources.
2.2. Experimental Animals
The present study was carried out using 7-week old male Balb/c mice (25.32 ± 3.25 g) obtained from the Laboratory Animal Center of Theodor Bilharz Research Institute (Cairo, Egypt). The animals were divided randomly into eight groups of six mice each and housed in polypropylene cages. All mice were maintained at 25 °C under 12 h light-dark cycle with free access to water and food (Teklad global 18% protein rodent diet, Harlan Laboratories, Livermore, CA). Mice were acclimatized for 1 week before the experiment. All procedures were approved by the Institutional Animal Care and Use Committee at the Faculty of Science, Kafrelsheikh University (Ethical approval number, KFS-2016/10).
2.3. Experimental Design
Treatment with Aloe and/or HK L.137 was started 2 days before DSS administration and continued for 7 days with DSS. 5% (w/v) DSS solution in drinking water was administered to the animals for 3 days, followed by a 3% (w/v) DSS for 4 days [23].
Forty-eight mice were randomized into eight groups as follows (Figure 1):
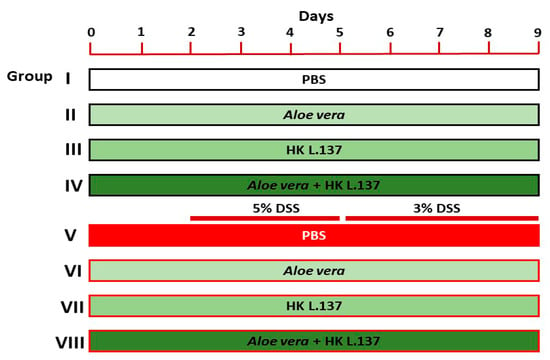
Figure 1.
A schematic diagram showing the experimental groups and treatments.
- (1)
- Group I (Control): mice received 0.5 mL of phosphate buffer saline (PBS) orally for 9 days.
- (2)
- Group II (Aloe): mice received 200 mg/kg Aloe [24] dissolved in PBS orally for 9 days.
- (3)
- Group III (HK L.137): mice received 100 mg/kg HK L.137 [25] dissolved in PBS orally for 9 days.
- (4)
- Group IV (Aloe/HK L.137): mice received 200 mg/kg Aloe and 100 mg/kg HK L.137 in PBS orally for 9 days.
- (5)
- Group V (DSS): mice received 5% DSS for 3 days and 3% DSS for 4 days.
- (6)
- Group VI (Aloe + DSS): mice received Aloe for 9 days and DSS as in group V.
- (7)
- Group VII (HK L.137 + DSS): mice received HK L.137 for 9 days and DSS as in group V.
- (8)
- Group VIII (Aloe/HK L.137 + DSS): mice received Aloe and HK L.137 for 9 days and DSS as in group V.
The mice were euthanized 24 h after the last administration of Aloe and/or HK L137 and samples were collected for analyses.
2.4. Assessment of Disease Activity Index (DAI)
Food intake and water consumption were recorded throughout the experiment (Supplementary Figure S1). Disease activity index (DAI) was determined according to stool consistency, rectal bleeding, diarrhea, and body weight loss. Each parameter was given a score according to the previously proposed criteria [26] and used in calculating an average daily DAI.
2.5. Histology
Following sacrifice, the colon was excised up to cecum, the length was determined and then emptied with PBS. Samples were divided into two portions; one preserved in formalin and the other was kept at −80 °C. For histopathology, samples from the colon were fixed in 10% neutral buffered formalin. The samples were processed for paraffin embedding, and then 5-µm sections were cut and stained with hematoxylin and eosin (H&E).
2.6. Histological Score Assessment of Colitis
The histological alterations were assessed blindly following a previously validated intestinal histologic inflammatory score [27] where the infiltration of neutrophils and eosinophils in the lamina propria and crypt degeneration were monitored (Table 1).

Table 1.
Histopathological criteria of colitis grades.
2.7. Immunohistochemical Expression of NF-κB p65 and iNOS
Paraffin sections were cleared in xylene, rehydrated in descending grade of ethanol, immersed in distal water, and then an antigen retrieval (EDTA solution, pH 8) was carried out. The slides were incubated in 0.3% hydrogen peroxide and blocked for 2 h in 5% bovine serum albumin in Tris-buffered saline (TBS). The sections were immunostained with rabbit anti-NF-κB p65 (RB1638P0, Thermo Fisher Scientific, Waltham, MA, USA; 1:100 dilution) anti-iNOS polyclonal primary antibodies (PA1-036, Invitrogen, USA; 1:20 dilution) overnight at 4 °C. After washing with PBS, the sections were incubated with secondary antibody (anti-rabbit IgG, EnVision + System HRP; Dako) for 30 min at room temperature, washed and incubated for 2 min in diaminobenzidine (DAB; Dako). Counterstaining was performed using hematoxylin stain, and the slides were visualized under a light microscope (Leica, supplied with DFC Leica digital camera). Quantitative analysis of NF-κB p65 and iNOS immunostaining was carried out using ImageJ (version 1.32j, NIH, USA) and expressed as percent of control.
2.8. Determination of Myeloperoxidase (MPO) and Cytokines
A total of 100 mg of the whole colon tissue was weighed, homogenized in 1000 µL EDTA-PBS, and centrifuged for 5 min at 4 °C (1000× g). The protein content in the supernatant was measured using a NanoDrop. MPO activity and the cytokines (TNF-α, IL-6, and IL-10) were assayed in the homogenate using ELISA kits supplied by Kamiya Biomedical Company (Tukwila, WA, USA) and RayBiotech (Peachtree Corners, GA, USA), respectively, following the provided instructions.
2.9. Statistical Analysis
The results are presented as mean ± standard deviation (SD). All statistical comparisons were performed by one-way ANOVA followed by Tukey′s Kramer test using GraphPad Prism version 7 (GraphPad Software Inc., La Jolla, CA, USA). A p value less than 0.05% was considered significant.
3. Results
3.1. Effect of Aloe and/or HK L.137 on DAI and Colon Length in DSS-Induced Mice
Mice received DSS showed an increase in DAI (diarrhea, stool consistency, bleeding, and body weight loss) when compared with the control group (p < 0.001). DSS-induced mice treated either with Aloe or HK L.137 showed significantly reduced colitis degree (p < 0.01), and the combined therapy effectively decreased the severity of colitis when compared with Aloe or HK L.137 (p < 0.001), as represented in Figure 2A.
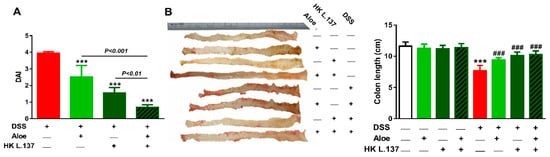
Figure 2.
Effect of Aloe vera extract (Aloe) and/or heat-killed Lactobacillus plantarum L.137 (HK L.137) on disease activity index (DAI) (A) and colon length (B) in control and dextran sodium sulfate (DSS)-induced mice. Data are mean ± SD, n = 6. *** p < 0.001 versus DSS. *** p < 0.001 versus Control and ### p < 0.001 versus DSS.
3.2. Aloe and/or HK L.137 Suppress Histopathological Alterations in DSS-Induced Mice
H&E-stained sections from the colon of control (Figure 3A) and Aloe and HK L.137-treated mice (Figure 3B) showed normal mucosa consisted of perpendicular crypts. In contrast, the DSS-induced group revealed severe colitis associated with extensive necrosis of the crypts extended along the whole mucosa to the lamina propria and the muscle layer. The other inflammatory lesions, such as hemorrhage, edema, and neutrophilic infiltration were obviously seen in this group (Figure 3C). DSS-induced animals treated with Aloe (Figure 3D) or HK L.137 (Figure 3E) revealed a decrease in the colitis degree that appeared within the last third of the mucosal surface, whereas the combined treatment improved the intestinal mucosa with apparent mucosal covering (Figure 3F).
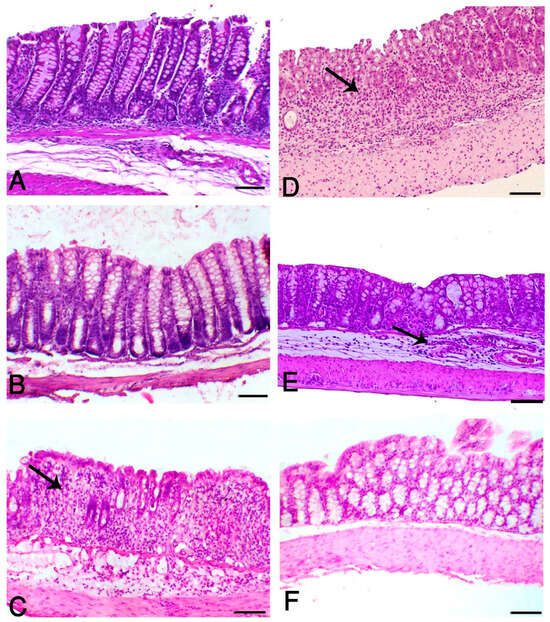
Figure 3.
Aloe and/or HK L.137 suppress histopathological alterations in DSS-induced mice. Hematoxylin and eosin (H&E)-stained colon sections from (A) Control and (B) Aloe/HK L.137-treated mice showing normal mucosal lining without any inflammatory or degenerative lesions; (C) DSS-induced mice showing G3 degree of colitis manifested by loss of intestinal crypts and replaced with marked inflammatory cells infiltration (arrow); (D) DSS-induced mice received Aloe revealing moderate inflammatory cells infiltration (arrow) towards the submucosa and representing G1; (E) DSS-induced mice treated with HK L.137 revealing edema and moderate submucosal infiltration of inflammatory cells (arrow) mostly of G2; and (F) DSS-induced mice treated with Aloe and HK L.137 showing an increase in normal mucosal lining of G0. (Scale bar = 50 μm).
The quantitative histological grades of each segment indicated a decrease in G0 percentage (normal tissue grade). The DSS group showed low levels of G0 with markedly increased G3 and G4 inflammation score. DSS-induced animals treated with Aloe and/or HK L.137 demonstrated marked increase in the G0 and G1 score and decreased percentage of G3 and G4 (Table 2).

Table 2.
Histological score of colitis grades within different animal groups.
3.3. Aloe and/or HK L.137 Suppress NF-κB and iNOS Expression in the Colon of DSS-Induced Mice
Colon sections of control (Figure 4A) and Aloe and HK L.137-treated mice (Figure 4B) showed mild interstitial expression of NF-κB p65. DSS significantly increased NF-κB p65 mostly within the infiltrated inflammatory cells (p < 0.001; Figure 4C,G). Treatment of the DSS-induced mice with Aloe (Figure 4D), HK L1.37 (Figure 4E), or their combination (Figure 4F) demonstrated marked decrease the expression of NF-κB p65 (p < 0.001; Figure 4G).
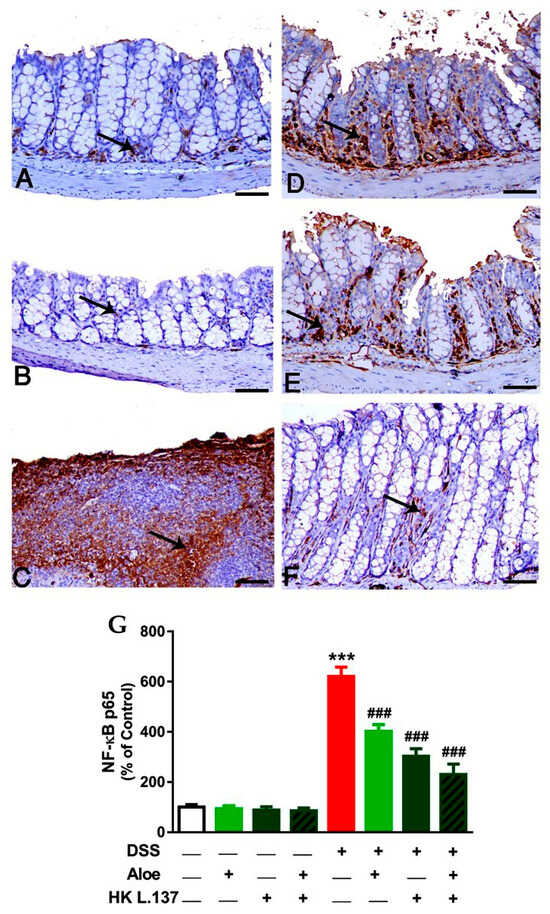
Figure 4.
Aloe and/or HK L.137 attenuate NF-κB p65 expression in the colon of DSS-induced mice. NF-κB p65 immunostained colon sections from (A) Control and (B) Aloe/HK L.137-treated mice showing mild interstitial expression, (C) DSS-induced mice revealing upregulation of NF-κB p65 mostly within the infiltrated inflammatory cells, and (D–F) DSS-induced mice treated with Aloe (D), HK L.137 (E), and their combination (F) showing markedly reduced expression of NF-κB p65. Arrows indicate positive immunostaining. (G) Mean ± SD of the NF-κB p65 expression in colon of different groups. *** p < 0.001 versus Control and ### p < 0.001 versus DSS. (Scale bar = 50 μm).
Both the control (Figure 5A) and Aloe and HK L.137-treated mice (Figure 5B) exhibited a scanty iNOS immunostaining, whereas the DSS-induced animals showed marked iNOS expression mostly from the inflammatory cells and necrotic crypts (p < 0.001; Figure 5C,G). Treatment with Aloe (Figure 5D), HK L1.37 (Figure 5E), or their combination (Figure 5F) suppressed iNOS expression significantly in DSS-induced mice (p < 0.001; Figure 5G).
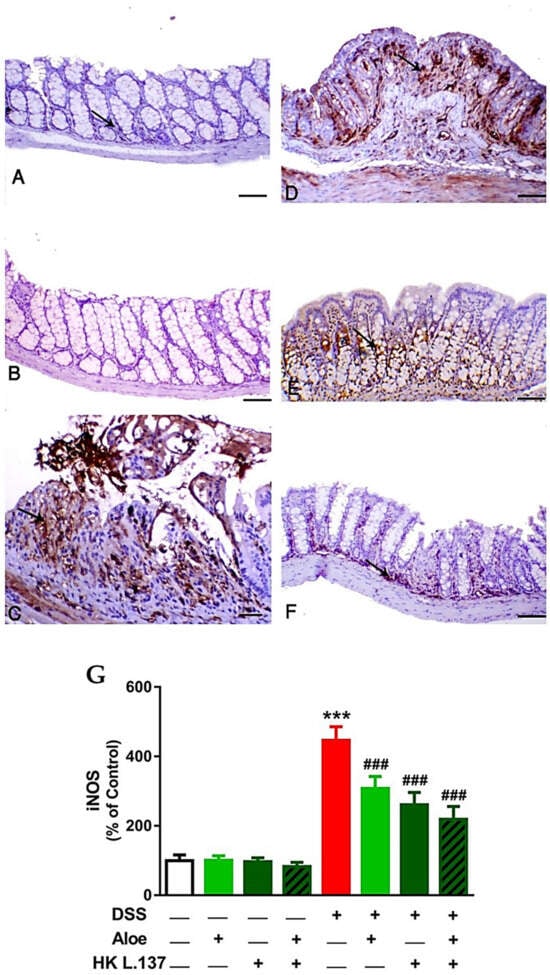
Figure 5.
Aloe and/or HK L.137 suppress iNOS expression in the colon of DSS-challenged mice. iNOS-immunostained colon sections from (A) Control and (B) Aloe/HK L.137-treated mice showing mild expression, (C) DSS-induced mice showing marked expression, and (D–F) DSS-induced mice treated with Aloe (D), HK L.137 (E), and their combination (F) showed decreased expression. Arrows indicate positive immunostaining which is expressed mostly from the inflammatory cells. (G) Mean ± SD of the iNOS expression in colon of different groups. *** p < 0.001 versus Control and ### p < 0.001 versus DSS. (Scale bar = 50 μm).
3.4. Aloe and/or HK L.137 Reduce MPO Activity and Attenuate Inflammation in DSS-Induced Mice
DSS induced a significant activation of MPO in mice colon (p < 0.001), as depicted in Figure 6A. While Aloe and/or HK L.137 exerted no effect in normal mice (p > 0.05), MPO was significantly reduced in DSS-induced animals (p < 0.001).
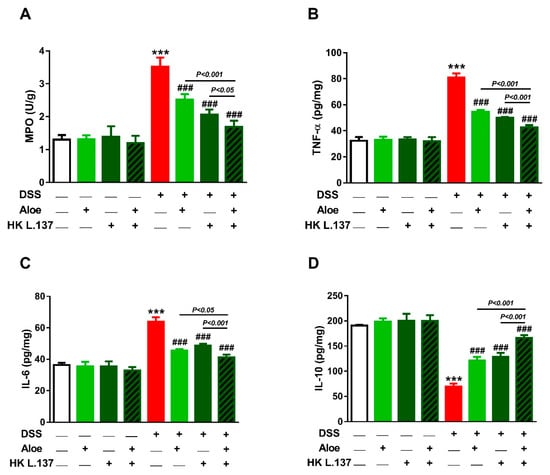
Figure 6.
Aloe and/or HK L.137 reduce myeloperoxidase (MPO) activity (A), tumor necrosis factor (TNF)-α (B), and interleukin (IL)-6 (C), and increased IL-10 (D) in the colon of DSS-induced mice. Data are mean ± SD, n = 6. *** p < 0.001 versus Control and ### p < 0.001 versus DSS.
To evaluate the anti-inflammatory effect of Aloe and or HK L.137, pro- and anti-inflammatory cytokines were determined in the colon of mice. DSS increased colon TNF-α and IL-6 and decreased IL-10 as compared to the control values (p < 0.001; Figure 6B–D). Supplementation of Aloe and/or HK L.137 significantly reduced TNF-α and IL-6, and increased IL-10 (p < 0.001). Oral supplementation of Aloe and/or HK L.137 did not affect these cytokines in normal mice.
4. Discussion
Several experimental models have been validated for the assessment of preclinical use of new treatment trials in IBD prior to provisional clinical phase. DSS is frequently used in the induction of IBD and investigation of different treatment trials [23,28]. This model mimics human UC and is of great value for understanding different morphological and pathophysiological features associated with IBD, including superficial ulceration and mucosal damage. Additionally, this model could be employed to show the efficacy of new treatment trials against inflammatory mediators and leukocyte infiltration associated with IBD [29,30]. The beneficial effects of probiotics and medicinal herbs in IBD have been reviewed by different authors [31,32]. The efficacy and safety of A. vera gel for the treatment of mildly to moderately active UC has been reported in a randomized, double-blind, placebo-controlled trial [33]. Patients that received 100 mL A. vera gel twice daily for 4 weeks showed decreased simple clinical colitis activity index and histological scores, whereas sigmoidoscopic scores and laboratory variables were not significantly changed [33]. Clinical trials on the symptomatic efficacy of L. plantarum 299v in patients with irritable bowel syndrome (IBS) showed contradictory results. While a 4-week treatment with L. plantarum 299v provided effective symptom relief [34], an 8-week treatment did not provide symptomatic relief in patients with IBS [35]. However, the efficacy of HK L.137 and its combination with A. vera to ameliorate UC has not been previously studied. Herein, we investigated for the first time the ameliorative effects of A. vera and/or HK L.137 on UC induced by DSS in mice. The results revealed that DSS colitis was associated with crypt degeneration, edema, and inflammatory cells infiltration. The severity of the lesions greatly differed from local, superficial that limited to the mucosal lining to diffuse and pancolitis extending to all layers, including the musculosa and even the serosa.
Oral supplementation of Aloe and/or HK L.137 prevented all histopathological alterations and decreased the score of neutrophilic infiltration in the mouse colon, demonstrating their anti-inflammatory efficacy. The hallmark of the therapeutic activity of herbs and functional foods is to prevent the disease onset through maintaining high levels of the bioactive constituents. Aloe is one of the herbal plants that has been widely applied in traditional medicine for relieving pain accompanying ulcers and burns and boosting wound healing [36]. A. vera contains a large amount of the bioactive phenolics with a potent antioxidant activity [37]. A previous study has shown that chromone-3-yl-acrylate, a bioactive constituent of A. vera, can block neutrophils adhesion to the endothelium [38]. Wan et al. have also pointed to the ability of the chromone moiety to suppress neutrophils and reduce ROS in activated neutrophils [39]. Importantly, Aloe shares a basic chromone structure that suppresses MPO activity and exerts anti-inflammatory activity. Aloe has also contracted the leukotriene production, one of the most critical chemotactic factors responsible for recruiting and activating inflammatory cells in various inflammatory diseases [40].
Moreover, HK L.137 exerted a protective effect against colitis elicited by DSS. Several lines of evidence have pointed to the existing role of microbiota that influences the mucosal immune system [41,42,43]. Previous reports demonstrated that any alteration in gut microbial diversity augments inflammation seen in both patients with UC and experimental colitis in animals. Bacteroides and Clostridium bacteria have been demonstrated to increase in colonic inflammation, whereas the beneficial bacteria as Lactobacillus and Enteobacteriaceae spp. were decreased [44,45]. It was shown that L. plantarum can ameliorate experimental colitis in murine models [46]; however, the safety of live bacteria is still debated in certain instances [47]. Therefore, there is an increased interest in the use of heat-killed probiotics. Heat killed bacteria is a type of bacteria treated with high temperatures which range between 70 and 100 °C, and/or by tyndallization [48]. Beneficial products resulted from cracking in the bacterial cell wall which lead to release of different cell components and cytoplasmic contents, including lipoteichoic acids, peptidoglycans, exopolysaccharides, and organelles as DNA and cell wall components [48], and can also inhibit pathogens [49]. The immunomodulatory activities of L. plantarum are conferred by suppressing pro-inflammatory cytokines and blocking different inflammatory signaling pathways, including toll-like receptor (TLR-4)-linked NF-κB and MAPK complexes [50,51]. Furthermore, L. plantarum possessed a powerful antioxidant function manifested by its ability to increase cellular antioxidants and suppress lipid peroxidation [52].
Besides the attenuation of histological alterations and inflammatory cells infiltration, both Aloe and HK L.137 suppressed NF-κB, iNOS, MPO, TNF-α, and IL-6 and increased IL-10 in the colon of DSS-induced mice, supporting their anti-inflammatory potential. It has been previously reported that TNF-α, IL-6, and IL-1β represent the most essential pathological mediators of IBD [53]. In the present study, TNF-α and IL-6 were dramatically increased in the colon of DSS-exposed mice, suggesting that immune cells are attracted to the site of inflammation. Therefore, the use of anti-inflammatory therapy can confer beneficial therapeutic outcomes in IBD patients. Accordingly, anti-TNF agents are effective in moderate and severe IBD in both children and adolescents, and their application resulted in limiting the use of corticosteroids [54]. A recent study has demonstrated that the therapeutic role of anti-TNF agents is critically dependent on IL-10 to resolve intestinal inflammation [55]. Hence, it is noteworthy assuming that IL-10 plays a role in the ameliorative effect of Aloe and/or HK L.137 on colitis. In addition, NF-κB p65 and iNOS were noticeably upregulated and were clearly noticed within the infiltrated inflammatory cells and damaged intestinal epithelial cells. Indeed, the UC patient is distinguished by overexpression of iNOS, and increased NO production by macrophages and colonic mucosa stimulated with interferon (IFN)-γ or IL-17A, and is correlated with the histological damage [56,57]. Furthermore, MPO was increased in the colon of mice challenged with DSS. Given its presence in neutrophils, increased activity of MPO is considered a marker of inflammation and linearly associated with neutrophilic infiltration [58]. Here, the magnitude of the inflammatory response was correlated with MPO whose activity showed several-fold increase following DSS administration. Accordingly, neutrophil accumulation is implicated in the pathogenesis of experimental colitis through the excessive release of inflammatory mediators and ROS [59]. In addition, increased activity of MPO can provoke oxidative damage and diminish antioxidant defenses culminating in cell death [58]. Although the mechanism underlying the suppression of TNF-α and IL-6 by Aloe is unclear, the role of the chromone moiety in inhibiting NF-κB activation elicited by TNF-α has been acknowledged [20,60]. In the same context, L. plantarum has been reported to attenuate inflammation by inhibiting NF-κB signaling [50,51]. Therefore, the protective activity of Aloe and HK L.137 is mediated, at least in part, via inhibiting NF-κB activation.
5. Conclusions
This investigation introduces new information on the ameliorative effect of HK L.137, alone and in combination with A. vera, on DSS-induced colitis. Aloe, HK L.137, and their combination conferred protection against experimental colitis in mice. Aloe and/or HK L.137 prevented histological alterations and neutrophils infiltration, suppressed iNOS, MPO, and pro-inflammatory cytokines, and enhanced IL-10 in the colon of mice. These findings pointed to the role of neutrophils in DSS-induced colitis and the ameliorative effect of Aloe and/or HK L.137 that was mediated via their dual antioxidant and anti-inflammatory efficacy. Therefore, the combination of Aloe vera with probiotics would represent a promising candidate for the prevention/treatment of IBDs, pending further investigations to explore their exact mechanisms of action.
Supplementary Materials
The following are available online at https://www.mdpi.com/2227-9717/8/2/225/s1, Figure S1: Effect of Aloe and/or HK L.137 on food and water intake in normal and DSS-induced mice. Data are mean ± SD, n = 6.
Author Contributions
Conceptualization, W.A.; A.M.M., and S.A.; methodology, W.A.; A.M.M.; S.A.; H.I.; validation, W.A.; S.A.; A.M.M., and A.T.; formal analysis, W.A. and A.M.M.; investigation, H.I.; W.A.; A.M.M.; S.A.; A.T.; D.M., and E.Z.; resources, M.B.-J., and A.M.M.; data curation, W.A.; S.A.; M.B.-J., and A.M.M.; writing—original draft preparation, W.A.; H.I., A.M.M., and S.A.; writing—review and editing, A.M.M.; visualization, W.A.; S.A.; A.T.; A.M.M.; D.M., and E.Z.; supervision, W.A.; S.A., and E.Z.; project administration, W.A., and S.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Deanship of Scientific Research at Princess Nourah bint Abdulrahman University through the Fast-track Research Funding Program.
Acknowledgments
The authors would like to thank Mahmoud Dawood (Faculty of Agriculture, Kafrelsheikh University, Egypt) and Ayman Atiba (Faculty of Veterinary Medicine, Kafrelsheikh University, Egypt) for providing resources during the execution of this work. The authors acknowledge the Deanship of Scientific Research at Princess Nourah bint Abdulrahman University for funding this research through the Fast-track Research Funding Program.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Abraham, C.; Cho, J.H. Inflammatory bowel disease. N. Engl. J. Med. 2009, 361, 2066–2078. [Google Scholar] [CrossRef] [PubMed]
- Low, D.; Nguyen, D.D.; Mizoguchi, E. Animal models of ulcerative colitis and their application in drug research. Drug Des. Dev. Ther. 2013, 7, 1341–1357. [Google Scholar]
- Guan, Q.; Zhang, J. Recent advances: The imbalance of cytokines in the pathogenesis of inflammatory bowel disease. Mediat. Inflamm. 2017, 2017, 4810258. [Google Scholar] [CrossRef]
- Frolkis, A.D.; Dykeman, J.; Negron, M.E.; Debruyn, J.; Jette, N.; Fiest, K.M.; Frolkis, T.; Barkema, H.W.; Rioux, K.P.; Panaccione, R.; et al. Risk of surgery for inflammatory bowel diseases has decreased over time: A systematic review and meta-analysis of population-based studies. Gastroenterology 2013, 145, 996–1006. [Google Scholar] [CrossRef]
- Giuffrida, P.; Corazza, G.R.; Di Sabatino, A. Old and new lymphocyte players in inflammatory bowel disease. Dig. Dis. Sci. 2018, 63, 277–288. [Google Scholar] [CrossRef]
- Nagao-Kitamoto, H.; Kamada, N. Host-microbial cross-talk in inflammatory bowel disease. Immune Netw. 2017, 17, 1–12. [Google Scholar] [CrossRef]
- Ohkusa, T. Production of experimental ulcerative colitis in hamsters by dextran sulfate sodium and changes in intestinal microflora. Nihon Shokakibyo Gakkai Zasshi Jpn. J. Gastro Enterol. 1985, 82, 1327–1336. [Google Scholar]
- Bauer, C.; Duewell, P.; Mayer, C.; Lehr, H.A.; Fitzgerald, K.A.; Dauer, M.; Tschopp, J.; Endres, S.; Latz, E.; Schnurr, M. Colitis induced in mice with dextran sulfate sodium (dss) is mediated by the nlrp3 inflammasome. Gut 2010, 59, 1192–1199. [Google Scholar] [CrossRef]
- Pu, Z.; Han, C.; Zhang, W.; Xu, M.; Wu, Z.; Liu, Y.; Wu, M.; Sun, H.; Xie, H. Systematic understanding of the mechanism and effects of arctigenin attenuates inflammation in dextran sulfate sodium-induced acute colitis through suppression of nlrp3 inflammasome by sirt1. Am. J. Transl. Res. 2019, 11, 3992–4009. [Google Scholar]
- Okayasu, I.; Hatakeyama, S.; Yamada, M.; Ohkusa, T.; Inagaki, Y.; Nakaya, R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology 1990, 98, 694–702. [Google Scholar] [CrossRef]
- Melgar, S.; Karlsson, L.; Rehnstrom, E.; Karlsson, A.; Utkovic, H.; Jansson, L.; Michaelsson, E. Validation of murine dextran sulfate sodium-induced colitis using four therapeutic agents for human inflammatory bowel disease. Int. Immunopharmacol. 2008, 8, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Williams, N.T. Probiotics. Am. J. Health Syst. Pharm. AJHP Off. J. Am. Soc. Health Syst. Pharm. 2010, 67, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Kruis, W.; Fric, P.; Pokrotnieks, J.; Lukas, M.; Fixa, B.; Kascak, M.; Kamm, M.A.; Weismueller, J.; Beglinger, C.; Stolte, M.; et al. Maintaining remission of ulcerative colitis with the probiotic escherichia coli nissle 1917 is as effective as with standard mesalazine. Gut 2004, 53, 1617–1623. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.L.; Hsu, P.Y.; Pan, T.M. Therapeutic effects of lactobacillus paracasei subsp. Paracasei ntu 101 powder on dextran sulfate sodium-induced colitis in mice. J. Food Drug Anal. 2019, 27, 83–92. [Google Scholar] [CrossRef]
- Gamallat, Y.; Meyiah, A.; Kuugbee, E.D.; Hago, A.M.; Chiwala, G.; Awadasseid, A.; Bamba, D.; Zhang, X.; Shang, X.; Luo, F.; et al. Lactobacillus rhamnosus induced epithelial cell apoptosis, ameliorates inflammation and prevents colon cancer development in an animal model. Biomed. Pharmacother. Biomed. Pharmacother. 2016, 83, 536–541. [Google Scholar] [CrossRef]
- Kang, D.W.; Adams, J.B.; Gregory, A.C.; Borody, T.; Chittick, L.; Fasano, A.; Khoruts, A.; Geis, E.; Maldonado, J.; McDonough-Means, S.; et al. Microbiota transfer therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: An open-label study. Microbiome 2017, 5, 10. [Google Scholar] [CrossRef]
- Tamboli, C.P.; Neut, C.; Desreumaux, P.; Colombel, J.F. Dysbiosis in inflammatory bowel disease. Gut 2004, 53, 1–4. [Google Scholar] [CrossRef]
- Ozsoy, N.; Candoken, E.; Akev, N. Implications for degenerative disorders: Antioxidative activity, total phenols, flavonoids, ascorbic acid, beta-carotene and beta-tocopherol in aloe vera. Oxidative Med. Cell. Longev. 2009, 2, 99–106. [Google Scholar] [CrossRef]
- Sumi, F.A.; Sikder, B.; Rahman, M.M.; Lubna, S.R.; Ulla, A.; Hossain, M.H.; Jahan, I.A.; Alam, M.A.; Subhan, N. Phenolic content analysis of aloe vera gel and evaluation of the effect of aloe gel supplementation on oxidative stress and fibrosis in isoprenaline-administered cardiac damage in rats. Prev. Nutr. Food Sci. 2019, 24, 254–264. [Google Scholar] [CrossRef]
- Salehi, B.; Albayrak, S.; Antolak, H.; Kregiel, D.; Pawlikowska, E.; Sharifi-Rad, M.; Uprety, Y.; Tsouh Fokou, P.V.; Yousef, Z.; Amiruddin Zakaria, Z.; et al. Aloe genus plants: From farm to food applications and phytopharmacotherapy. Int. J. Mol. Sci. 2018, 19, 2843. [Google Scholar] [CrossRef]
- Park, M.Y.; Kwon, H.J.; Sung, M.K. Dietary aloin, aloesin, or aloe-gel exerts anti-inflammatory activity in a rat colitis model. Life Sci. 2011, 88, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Im, S.A.; Kim, J.W.; Kim, H.S.; Park, C.S.; Shin, E.; Do, S.G.; Park, Y.I.; Lee, C.K. Prevention of azoxymethane/dextran sodium sulfate-induced mouse colon carcinogenesis by processed aloe vera gel. Int. Immunopharmacol. 2016, 40, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Chassaing, B.; Aitken, J.D.; Malleshappa, M.; Vijay-Kumar, M. Dextran sulfate sodium (dss)-induced colitis in mice. Curr. Protoc. Immunol. 2014, 104, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Mojahed, L.S.; Saeb, M.; Mohammadi, M.M.; Nazifi, S. Long period starvation in rat: The effect of aloe vera gel extract on oxidative stress status. Int. Arch. Med. 2016, 9, 252. [Google Scholar]
- Dawood, M.A.O.; Magouz, F.I.; Salem, M.F.I.; Abdel-Daim, H.A. Modulation of digestive enzyme activity, blood health, oxidative responses and growth-related gene expression in gift by heat-killed lactobacillus plantarum (l-137). Aquaculture 2019, 505, 127–136. [Google Scholar] [CrossRef]
- Cooper, H.S.; Murthy, S.N.; Shah, R.S.; Sedergran, D.J. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab. Investig. A J. Tech. Methods Pathol. 1993, 69, 238–249. [Google Scholar]
- Camuesco, D.; Rodriguez-Cabezas, M.E.; Garrido-Mesa, N.; Cueto-Sola, M.; Bailon, E.; Comalada, M.; Arribas, B.; Merlos, M.; Balsa, D.; Zarzuelo, A.; et al. The intestinal anti-inflammatory effect of dersalazine sodium is related to a down-regulation in il-17 production in experimental models of rodent colitis. Br. J. Pharmacol. 2012, 165, 729–740. [Google Scholar] [CrossRef]
- Valatas, V.; Vakas, M.; Kolios, G. The value of experimental models of colitis in predicting efficacy of biological therapies for inflammatory bowel diseases. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 305, G763–G785. [Google Scholar] [CrossRef]
- Hoffmann, M.; Schwertassek, U.; Seydel, A.; Weber, K.; Falk, W.; Hauschildt, S.; Lehmann, J. A refined and translationally relevant model of chronic dss colitis in balb/c mice. Lab. Anim. 2018, 52, 240–252. [Google Scholar] [CrossRef]
- Yan, Y.; Kolachala, V.; Dalmasso, G.; Nguyen, H.; Laroui, H.; Sitaraman, S.V.; Merlin, D. Temporal and spatial analysis of clinical and molecular parameters in dextran sodium sulfate induced colitis. PLoS ONE 2009, 4, e6073. [Google Scholar] [CrossRef]
- Losurdo, G.; Iannone, A.; Contaldo, A.; Ierardi, E.; Di Leo, A.; Principi, M. Escherichia coli nissle 1917 in ulcerative colitis treatment: Systematic review and meta-analysis. J. Gastrointest. Liver Dis. JGLD 2015, 24, 499–505. [Google Scholar]
- Curro, D.; Ianiro, G.; Pecere, S.; Bibbo, S.; Cammarota, G. Probiotics, fibre and herbal medicinal products for functional and inflammatory bowel disorders. Br. J. Pharmacol. 2017, 174, 1426–1449. [Google Scholar] [CrossRef]
- Langmead, L.; Feakins, R.M.; Goldthorpe, S.; Holt, H.; Tsironi, E.; De Silva, A.; Jewell, D.P.; Rampton, D.S. Randomized, double-blind, placebo-controlled trial of oral aloe vera gel for active ulcerative colitis. Aliment. Pharmacol. Ther. 2004, 19, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Ducrotte, P.; Sawant, P.; Jayanthi, V. Clinical trial: Lactobacillus plantarum 299v (dsm 9843) improves symptoms of irritable bowel syndrome. World J. Gastroenterol. 2012, 18, 4012–4018. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, C.; Blaauw, R.; Fredericks, E.; Visser, J.; Roux, S. Randomized clinical trial: Effect of lactobacillus plantarum 299 v on symptoms of irritable bowel syndrome. Nutrition 2014, 30, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Atiba, A.; Nishimura, M.; Kakinuma, S.; Hiraoka, T.; Goryo, M.; Shimada, Y.; Ueno, H.; Uzuka, Y. Aloe vera oral administration accelerates acute radiation-delayed wound healing by stimulating transforming growth factor-beta and fibroblast growth factor production. Am. J. Surg. 2011, 201, 809–818. [Google Scholar] [CrossRef]
- Kumar, S.; Yadav, A.; Yadav, M.; Yadav, J.P. Effect of climate change on phytochemical diversity, total phenolic content and in vitro antioxidant activity of aloe vera (l.) burm.F. BMC Res. Notes 2017, 10, 60. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, B.K.; Pandey, A.K.; Kumar, A.; Sharma, S.K.; Raj, H.G.; Prasad, A.K.; Van der Eycken, E.; Parmar, V.S.; Ghosh, B. A chromone analog inhibits tnf-alpha induced expression of cell adhesion molecules on human endothelial cells via blocking nf-kappab activation. Bioorganic Med. Chem. 2007, 15, 2952–2962. [Google Scholar] [CrossRef]
- Mazzei, M.; Dondero, R.; Sottofattori, E.; Melloni, E.; Minafra, R. Inhibition of neutrophil o(2)(-) production by unsymmetrical methylene derivatives of benzopyrans: Their use as potential antiinflammatory agents. Eur. J. Med. Chem. 2001, 36, 851–861. [Google Scholar] [CrossRef]
- Liu, M.; Yokomizo, T. The role of leukotrienes in allergic diseases. Allergol. Int. Off. J. Jpn. Soc. Allergol. 2015, 64, 17–26. [Google Scholar] [CrossRef]
- Bennek, E.; Mandic, A.D.; Verdier, J.; Roubrocks, S.; Pabst, O.; Van Best, N.; Benz, I.; Kufer, T.; Trautwein, C.; Sellge, G. Subcellular antigen localization in commensal e. Coli is critical for t cell activation and induction of specific tolerance. Mucosal Immunol. 2019, 12, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Wasilewski, A.; Zielinska, M.; Storr, M.; Fichna, J. Beneficial effects of probiotics, prebiotics, synbiotics, and psychobiotics in inflammatory bowel disease. Inflamm. Bowel Dis. 2015, 21, 1674–1682. [Google Scholar] [CrossRef] [PubMed]
- Imaoka, A.; Umesaki, Y. Rationale for using of bifidobacterium probiotic strains-fermented milk against colitis based on animal experiments and clinical trials. Probiotics Antimicrob. Proteins 2009, 1, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Guarner, F. The intestinal flora in inflammatory bowel disease: Normal or abnormal? Curr. Opin. Gastroenterol. 2005, 21, 414–418. [Google Scholar] [PubMed]
- Ni, J.; Wu, G.D.; Albenberg, L.; Tomov, V.T. Gut microbiota and ibd: Causation or correlation? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Le, B.; Yang, S.H. Efficacy of lactobacillus plantarum in prevention of inflammatory bowel disease. Toxicol. Rep. 2018, 5, 314–317. [Google Scholar] [CrossRef] [PubMed]
- Adams, C.A. The probiotic paradox: Live and dead cells are biological response modifiers. Nutr. Res. Rev. 2010, 23, 37–46. [Google Scholar] [CrossRef]
- Kim, H.; Kim, H.; Bang, J.; Kim, Y.; Beuchat, L.R.; Ryu, J.H. Reduction of bacillus cereus spores in sikhye, a traditional korean rice beverage, by modified tyndallization processes with and without carbon dioxide injection. Lett. Appl. Microbiol. 2012, 55, 218–223. [Google Scholar] [CrossRef]
- Sarkar, A.; Mandal, S. Bifidobacteria-insight into clinical outcomes and mechanisms of its probiotic action. Microbiol. Res. 2016, 192, 159–171. [Google Scholar] [CrossRef]
- Anderson, R.C.; Cookson, A.L.; McNabb, W.C.; Park, Z.; McCann, M.J.; Kelly, W.J.; Roy, N.C. Lactobacillus plantarum mb452 enhances the function of the intestinal barrier by increasing the expression levels of genes involved in tight junction formation. BMC Microbiol. 2010, 10, 316. [Google Scholar] [CrossRef]
- Zhang, F.; Li, Y.; Wang, X.; Wang, S.; Bi, D. The impact of lactobacillus plantarum on the gut microbiota of mice with dss-induced colitis. BioMed Res. Int. 2019, 2019, 3921315. [Google Scholar] [PubMed]
- Tomusiak-Plebanek, A.; Heczko, P.; Skowron, B.; Baranowska, A.; Okon, K.; Thor, P.J.; Strus, M. Lactobacilli with superoxide dismutase-like or catalase activity are more effective in alleviating inflammation in an inflammatory bowel disease mouse model. Drug Des. Dev. Ther. 2018, 12, 3221–3233. [Google Scholar] [CrossRef] [PubMed]
- de Mattos, B.R.; Garcia, M.P.; Nogueira, J.B.; Paiatto, L.N.; Albuquerque, C.G.; Souza, C.L.; Fernandes, L.G.; Tamashiro, W.M.; Simioni, P.U. Inflammatory bowel disease: An overview of immune mechanisms and biological treatments. Mediat. Inflamm. 2015, 2015, 493012. [Google Scholar] [CrossRef] [PubMed]
- Aardoom, M.A.; Veereman, G.; de Ridder, L. A review on the use of anti-tnf in children and adolescents with inflammatory bowel disease. Int. J. Mol. Sci. 2019, 20, 2529. [Google Scholar] [CrossRef]
- Koelink, P.J.; Bloemendaal, F.M.; Li, B.; Westera, L.; Vogels, E.W.M.; van Roest, M.; Gloudemans, A.K.; van‘t Wout, A.B.; Korf, H.; Vermeire, S.; et al. Anti-tnf therapy in ibd exerts its therapeutic effect through macrophage il-10 signalling. Gut 2019. [Google Scholar] [CrossRef]
- Rafa, H.; Amri, M.; Saoula, H.; Belkhelfa, M.; Medjeber, O.; Boutaleb, A.; Aftis, S.; Nakmouche, M.; Touil-Boukoffa, C. Involvement of interferon-gamma in bowel disease pathogenesis by nitric oxide pathway: A study in algerian patients. J. Interferon Cytokine Res. Off. J. Int. Soc. Interferon Cytokine Res. 2010, 30, 691–697. [Google Scholar] [CrossRef]
- Rafa, H.; Saoula, H.; Belkhelfa, M.; Medjeber, O.; Soufli, I.; Toumi, R.; de Launoit, Y.; Morales, O.; Nakmouche, M.; Delhem, N.; et al. Il-23/il-17a axis correlates with the nitric oxide pathway in inflammatory bowel disease: Immunomodulatory effect of retinoic acid. J. Interferon Cytokine Res. Off. J. Int. Soc. Interferon Cytokine Res. 2013, 33, 355–368. [Google Scholar] [CrossRef]
- Khan, A.A.; Alsahli, M.A.; Rahmani, A.H. Myeloperoxidase as an active disease biomarker: Recent biochemical and pathological perspectives. Med. Sci. 2018, 6, 33. [Google Scholar] [CrossRef]
- Sung, M.K.; Park, M.Y. Nutritional modulators of ulcerative colitis: Clinical efficacies and mechanistic view. World J. Gastroenterol. 2013, 19, 994–1004. [Google Scholar] [CrossRef]
- Grace, O.M.; Simmonds, M.S.; Smith, G.F.; van Wyk, A.E. Therapeutic uses of aloe l. (asphodelaceae) in southern africa. J. Ethnopharmacol. 2008, 119, 604–614. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).