Review on Carbon Nanotube Varieties for Healthcare Application: Effect of Preparation Methods and Mechanism Insight
Abstract
1. Introduction
2. Summary of the Review Article
3. Results and Discussion
3.1. Effect of Preparation Method and Functionalization on CNT for Biosensor and Antibacterial Activity
3.2. Antimicrobial Activity and Mechanistic Insights of CNT (SWCMT and MWCNT)
3.3. Funtionalization of Carbon Nanotube for Antimicrobial Action to Control Infections
3.4. Role of CNT in Oxidative Stress Property in Cell Membrane
3.5. Role of Particle Size and Morphology in Antimicrobial Action
3.6. Adsorption Activity CNT and Its Antimicrobial Application
3.7. Role of Electronic Structure of CNT in Antimicrobial Application
3.8. Antimicrobial Mechanisim Aspect of CNT Modified Materials for Water Treatment and Electrode Application
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Han, Z.J.; Rider, A.E.; Fisher, C.; Van Der Laan, T.; Kumar, S.; Levchenko, I.; Ostrikov, K. Biological Application of Carbon Nanotubes and Graphene. In Carbon Nanotubes and Graphene, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 279–312. [Google Scholar]
- Aqel, A.; El-Nour, K.M.A.; Ammar, R.A.; Al-Warthan, A. Carbon nanotubes, science and technology part (I) structure, synthesis and characterisation. Arab. J. Chem. 2012, 5, 1–23. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nat. Cell Biol. 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Gao, J.; Yu, A.; Itkis, M.E.; Bekyarova, E.; Zhao, B.; Niyogi, S.; Haddon, R.C. Large-Scale Fabrication of Aligned Single-Walled Carbon Nanotube Array and Hierarchical Single-Walled Carbon Nanotube Assembly. J. Am. Chem. Soc. 2004, 126, 16698–16699. [Google Scholar] [CrossRef]
- Chung, C.; Kim, Y.-K.; Shin, D.; Ryoo, S.-R.; Hong, B.H.; Min, D.-H. Biomedical Applications of Graphene and Graphene Oxide. Acc. Chem. Res. 2013, 46, 2211–2224. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Jiang, Z.; Zhang, Y.; Morozov, S.V.; Stormer, H.L.; Zeitler, U.; Maan, J.C.; Boebinger, G.S.; Kim, P.; Geim, A.K. Room-Temperature Quantum Hall Effect in Graphene. Science 2007, 315, 1379. [Google Scholar] [CrossRef]
- Park, S.; Ruoff, R.S. Chemical methods for the production of graphenes. Nat. Nanotechnol. 2009, 4, 217–224. [Google Scholar] [CrossRef]
- Slattery, A.D.; Blanch, A.J.; Quinton, J.S.; Gibson, C.T. Efficient attachment of carbon nanotubes to conventional and high-frequency AFM probes enhanced by electron beam processes. Nanotechnology 2013, 24, 235705. [Google Scholar] [CrossRef]
- Slattery, A.D.; Shearer, C.J.; Shapter, J.G.; Gibson, C.T.; Gibson, C.T. Solution Based Methods for the Fabrication of Carbon Nanotube Modified Atomic Force Microscopy Probes. Nanomaterials 2017, 7, 346. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Si, C.; Sun, Z.; Liu, F. Strain engineering of graphene: A review. Nanoscale 2016, 8, 3207–3217. [Google Scholar] [CrossRef]
- Jain, K.K. Advances in use of functionalized carbon nanotubes for drug design and discovery. Expert Opin. Drug Discov. 2012, 7, 1029–1037. [Google Scholar] [CrossRef]
- He, H.; Pham-Huy, L.A.; Dramou, P.; Xiao, D.; Zuo, P.; Pham-Huy, C. Carbon Nanotubes: Applications in Pharmacy and Medicine. BioMed Res. Int. 2013, 2013, 1–12. [Google Scholar] [CrossRef]
- Amenta, V.; Aschberger, K. Carbon nanotubes: Potential medical applications and safety concerns. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2014, 7, 371–386. [Google Scholar] [CrossRef]
- Kang, S.; Pinault, M.; Pfefferle, L.D.; Elimelech, M. Single-Walled Carbon Nanotubes Exhibit Strong Antimicrobial Activity. Langmuir 2007, 23, 8670–8673. [Google Scholar] [CrossRef]
- Kang, S.; Herzberg, M.; Rodrigues, D.F.; Elimelech, M. Antibacterial Effects of Carbon Nanotubes: Size Does Matter! Langmuir 2008, 24, 6409–6413. [Google Scholar] [CrossRef]
- Kang, S.; Behrer, A.P.; Elimelech, M. Physicochemical Determinants of Multiwalled Carbon Nanotube Bacterial Cytotoxicity. Environ. Sci. Technol. 2008, 42, 7528–7534. [Google Scholar] [CrossRef]
- Liu, S.; Wei, L.; Hao, L.; Fang, N.; Chang, M.W.; Xu, R.; Yang, Y.; Chen, Y. Sharper and Faster “Nano Darts” Kill More Bacteria: A Study of Antibacterial Activity of Individually Dispersed Pristine Single-Walled Carbon Nanotube. ACS Nano 2009, 3, 3891–3902. [Google Scholar] [CrossRef] [PubMed]
- Pasquini, L.M.; Hashmi, S.M.; Sommer, T.J.; Elimelech, M.; Zimmerman, J.B. Impact of Surface Functionalization on Bacterial Cytotoxicity of Single-Walled Carbon Nanotubes. Environ. Sci. Technol. 2012, 46, 6297–6305. [Google Scholar] [CrossRef]
- Wang, K.; Shi, L.; Linthicum, W.; Man, K.; He, X.; Wen, Q.; Rojanasakul, L.W.; Rojanasakul, Y.; Yang, Y.; Wang, L. Substrate Stiffness-Dependent Carbon Nanotube-Induced Lung Fibrogenesis. Nano Lett. 2019, 19, 5443–5451. [Google Scholar] [CrossRef]
- Smith, S.C.; Rodrigues, D.F. Carbon-based nanomaterials for removal of chemical and biological contaminants from water: A review of mechanisms and applications. Carbon 2015, 91, 122–143. [Google Scholar] [CrossRef]
- Deokar, A.R.; Lin, L.-Y.; Chang, C.-C.; Ling, Y.-C. Single-walled carbon nanotube coated antibacterial paper: Preparation and mechanistic study. J. Mater. Chem. B 2013, 1, 2639–2646. [Google Scholar] [CrossRef] [PubMed]
- Mocan, T.; Matea, C.T.; Pop, T.; Mosteanu, O.; Buzoianu, A.D.; Suciu, Ș.; Puia, C.; Zdrehus, C.; Iancu, C.; Mocan, L. Carbon nanotubes as anti-bacterial agents. Cell. Mol. Life Sci. 2017, 74, 3467–3479. [Google Scholar] [CrossRef] [PubMed]
- Rajavel, K.; Gomathi, R.; Manian, S.; Kumar, R.T.R. In vitro bacterial cytotoxicity of CNT: Reactive oxygen species mediate cell damage edges over direct physical puncturing. Langmuir 2014, 30, 592–601. [Google Scholar] [CrossRef] [PubMed]
- Maas, M. Carbon Nanomaterials as Antibacterial Colloids. Materials 2016, 9, 617. [Google Scholar] [CrossRef] [PubMed]
- Yeh, Y.-T.; Gulino, K.; Zhang, Y.; Sabestien, A.; Chou, T.-W.; Zhou, B.; Lin, Z.; Albert, I.; Lu, H.; Swaminathan, V.; et al. A rapid and label-free platform for virus capture and identification from clinical samples. Proc. Natl. Acad. Sci. USA 2020, 117, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.L.; Nguyen, T.T.; Chu, V.T.; Tran, Q.T.; Mai, A.T. Detection of influenza A virus using carbon nanotubes field effect transistor based DNA sensor. Phys. E Low Dimens. Syst. Nanostruct. 2017, 93, 83–86. [Google Scholar] [CrossRef]
- Govindasamy, M.; Chen, S.-M.; Mani, V.; Devasenathipathy, R.; Umamaheswari, R.; Santhanaraj, K.J.; Sathiyan, A. Molybdenum disulfide nanosheets coated multiwalled carbon nanotubes composite for highly sensitive determination of chloramphenicol in food samples milk, honey and powdered milk. J. Colloid Interface Sci. 2017, 485, 129–136. [Google Scholar] [CrossRef]
- Mani, V.V.; Mani, G.; Chen, S.M.; Karthik, R.; Huang, S.T. Determination of dopamine using a glassy carbon electrode modified with a graphene and carbon nanotube hybrid decorated with molybdenum disulfide flowers. Microchim Acta 2016, 183, 2267–2275. [Google Scholar] [CrossRef]
- Svadlakova, T.; Hubatka, F.; Knotigova, P.T.; Kulich, P.; Masek, J.; Kotoucek, J.; Macak, J.; Motola, M.; Kalbac, M.; Kolackova, M.; et al. Proinflammatory Effect of Carbon-Based Nanomaterials: In Vitro Study on Stimulation of Inflammasome NLRP3 via Destabilisation of Lysosomes. Nanomaterials 2020, 10, 418. [Google Scholar] [CrossRef]
- Moor, K.J.; Osuji, C.O.; Kim, J.-H. Dual-Functionality Fullerene and Silver Nanoparticle Antimicrobial Composites via Block Copolymer Templates. ACS Appl. Mater. Interfaces 2016, 8, 33583–33591. [Google Scholar] [CrossRef]
- De Volder, M.; Tawfick, S.H.; Baughman, R.H.; Hart, A.J. Carbon Nanotubes: Present and Future Commercial Applications. Science 2013, 339, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Cha, C.; Shin, S.R.; Annabi, N.; Dokmeci, M.R.; Khademhosseini, A. Carbon-Based Nanomaterials: Multifunctional Materials for Biomedical Engineering. ACS Nano 2013, 7, 2891–2897. [Google Scholar] [CrossRef] [PubMed]
- Ren, P.; Zheng, A.; Xiao, J.; Pan, X.; Bao, X. Exploring the ring current of carbon nanotubes by first-principles calculations. Chem. Sci. 2015, 6, 902–908. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kaicheng, Z.; Dougherty, C.A.; Zhu, K.; Hong, H. Theranostic applications of carbon nanomaterials in cancer: Focus on imaging and cargo delivery. J. Control. Release 2015, 210, 230–245. [Google Scholar]
- Kharaziha, M.; Shin, S.R.; Nikkhah, M.; Topkaya, S.N.; Masoumi, N.; Annabi, N.; Dokmeci, M.R.; Khademhosseini, A. Tough and flexible CNT–polymeric hybrid scaffolds for engineering cardiac constructs. Biomaterials 2014, 35, 7346–7354. [Google Scholar] [CrossRef]
- Costa, P.M.; Bourgognon, M.; Wang, J.T.-W.; Al-Jamal, K.T. Functionalised carbon nanotubes: From intracellular uptake and cell-related toxicity to systemic brain delivery. J. Control. Release 2016, 241, 200–219. [Google Scholar] [CrossRef]
- Zou, X.; Zhang, L.; Wang, Z.; Luo, Y. Mechanisms of the Antimicrobial Activities of Graphene Materials. J. Am. Chem. Soc. 2016, 138, 2064–2077. [Google Scholar] [CrossRef]
- Kronenberger, T.; Asse, L.R.; Wrenger, C.; Trossini, G.H.G.; Honorio, K.M.; Maltarollo, V.G. Studies of Staphylococcus aureus FabI inhibitors: Fragment-based approach based on holographic structure-activity relationship analyses. Futur. Med. Chem. 2017, 9, 135–151. [Google Scholar] [CrossRef]
- Hu, W.; Peng, C.; Luo, W.; Lv, M.; Li, X.; Li, D.; Huang, Q.; Fan, C. Graphene-Based Antibacterial Paper. ACS Nano 2010, 4, 4317–4323. [Google Scholar] [CrossRef]
- Liu, C.; Shi, H.; Yang, H.; Yan, S.; Luan, S.; Li, Y.; Teng, M.; Khan, A.F.; Yin, J. Fabrication of antibacterial electrospun nanofibers with vancomycin-carbon nanotube via ultrasonication assistance. Mater. Des. 2017, 120, 128–134. [Google Scholar] [CrossRef]
- Nepal, D.; Balasubramanian, S.; Simonian, A.L.; Davis, V.A. Strong Antimicrobial Coatings: Single-Walled Carbon Nanotubes Armored with Biopolymers. Nano Lett. 2008, 8, 1896–1901. [Google Scholar] [CrossRef] [PubMed]
- Tiraferri, A.; Vecitis, C.D.; Elimelech, M. Covalent Binding of Single-Walled Carbon Nanotubes to Polyamide Membranes for Antimicrobial Surface Properties. ACS Appl. Mater. Interfaces 2011, 3, 2869–2877. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Mansukhani, N.D.; Guiney, L.M.; Ji, Z.; Zhao, Y.; Chang, C.H.; French, C.T.; Miller, J.F.; Hersam, M.C.; Nel, A.E.; et al. Identification and Optimization of Carbon Radicals on Hydrated Graphene Oxide for Ubiquitous Antibacterial Coatings. ACS Nano 2016, 10, 10966–10980. [Google Scholar] [CrossRef] [PubMed]
- Simmons, T.J.; Lee, S.H.; Park, T.J.; Hashim, D.P.; Ajayan, P.M.; Linhardt, R.J. Antiseptic single wall carbon nanotube bandages. Carbon 2009, 47, 1561–1564. [Google Scholar] [CrossRef]
- Simmons, T.J.; Rivet, C.J.; Singh, G.; Beaudet, J.; Sterner, E.; Guzman, D.; Hashim, D.P.; Lee, S.-H.; Qian, G.; Lewis, K.M. Application of carbon nanotubes to wound healing biotechnology. In Nanomaterials for Biomedicine; ACS: Washington, DC, USA, 2012; pp. 155–174. [Google Scholar]
- Tong, Z.; Bischoff, M.; Nies, L.F.; Myer, P.; Applegate, B.; Turco, R.F. Response of soil microorganisms to As-produced and functionalized single-wall carbon nanotubes (SWNTs). Environ. Sci. Technol. 2012, 46, 13471–13479. [Google Scholar] [CrossRef]
- Vecitis, C.D.; Zodrow, K.R.; Kang, S.; Elimelech, M. Electronic-Structure-Dependent Bacterial Cytotoxicity of Single-Walled Carbon Nanotubes. ACS Nano 2010, 4, 5471–5479. [Google Scholar] [CrossRef]
- Chen, H.; Wang, B.; Gao, D.; Guan, M.; Zheng, L.; Ouyang, H.; Chai, Z.; Zhao, Y.; Feng, W. Broad-Spectrum Antibacterial Activity of Carbon Nanotubes to Human Gut Bacteria. Small 2013, 9, 2735–2746. [Google Scholar] [CrossRef]
- Zhao, C.; Deng, B.; Chen, G.; Lei, B.; Hua, H.; Peng, H.-L.; Yan, Z. Large-area chemical vapor deposition-grown monolayer graphene-wrapped silver nanowires for broad-spectrum and robust antimicrobial coating. Nano Res. 2016, 9, 963–973. [Google Scholar] [CrossRef]
- Hossain, F.; Perales-Perez, O.J.; Hwang, S.; Román, F. Antimicrobial nanomaterials as water disinfectant: Applications, limitations and future perspectives. Sci. Total Environ. 2014, 466, 1047–1059. [Google Scholar] [CrossRef]
- Arias, L.R.; Yang, L. Inactivation of Bacterial Pathogens by Carbon Nanotubes in Suspensions. Langmuir 2009, 25, 3003–3012. [Google Scholar] [CrossRef]
- Deng, S.; Upadhyayula, V.K.; Smith, G.B.; Mitchell, M.C. Adsorption Equilibrium and Kinetics of Microorganisms on Single-Wall Carbon Nanotubes. IEEE Sens. J. 2008, 8, 954–962. [Google Scholar] [CrossRef]
- Goy, R.C.; Morais, S.T.; Assis, O.B. Evaluation of the antimicrobial activity of chitosan and its quaternized derivative on E. coli and S. aureus growth. Rev. Bras. Farm. 2016, 26, 122–127. [Google Scholar] [CrossRef]
- Kell, A.J.; Stewart, G.; Ryan, S.; Peytavi, R.; Boissinot, M.; Huletsky, A.; Bergeron, M.G.; Simard, B. Vancomycin-Modified Nanoparticles for Efficient Targeting and Preconcentration of Gram-Positive and Gram-Negative Bacteria. ACS Nano 2008, 2, 1777–1788. [Google Scholar] [CrossRef] [PubMed]
- Imlay, J.A. The molecular mechanisms and physiological consequences of oxidative stress: Lessons from a model bacterium. Nat. Rev. Genet. 2013, 11, 443–454. [Google Scholar] [CrossRef]
- Brinkman, C.L.; Schmidt-Malan, S.M.; Karau, M.J.; Greenwood-Quaintance, K.; Hassett, D.J.; Mandrekar, J.N.; Patel, R. Exposure of Bacterial Biofilms to Electrical Current Leads to Cell Death Mediated in Part by Reactive Oxygen Species. PLoS ONE 2016, 11, e0168595. [Google Scholar] [CrossRef] [PubMed]
- Jeyaraj, M.; Rajesh, M.; Arun, R.; Mubarakali, D.; Sathishkumar, G.; Sivanandhan, G.; Kapil, G.; Manickavasagam, M.; Premkumar, K.; Thajuddin, N.; et al. Colloids and Surfaces B: Biointerfaces An investigation on the cytotoxicity and caspase-mediated apoptotic effect of biologically synthesized silver nanoparticles using Podophyllum hexandrum on human cervical carcinoma cells. Colloids Surf. B Biointerfaces 2013, 102, 708–717. [Google Scholar] [CrossRef]
- Vatansever, F.; De Melo, W.C.; Avci, P.; Vecchio, D.; Sadasivam, M.; Gupta, A.; Chandran, R.; Karimi, M.; Parizotto, N.A.; Yin, R.; et al. Antimicrobial strategies centered around reactive oxygen species–bactericidal antibiotics, photodynamic therapy, and beyond. FEMS Microbiol. Rev. 2013, 37, 955–989. [Google Scholar] [CrossRef]
- Upadhyayula, V.K.; Deng, S.; Mitchell, M.C.; Smith, G.B. Application of carbon nanotube technology for removal of contaminants in drinking water: A review. Sci. Total Environ. 2009, 408, 1–13. [Google Scholar] [CrossRef]
- Kang, S.; Mauter, M.S.; Elimelech, M. Microbial Cytotoxicity of Carbon-Based Nanomaterials: Implications for River Water and Wastewater Effluent. Environ. Sci. Technol. 2009, 43, 2648–2653. [Google Scholar] [CrossRef]
- Yang, C.; Mamouni, J.; Tang, Y.; Yang, L. Antimicrobial Activity of Single-Walled Carbon Nanotubes: Length Effect. Langmuir 2010, 26, 16013–16019. [Google Scholar] [CrossRef]
- Wick, P.; Manser, P.; Limbach, L.K.; Dettlaff-Weglikowska, U.; Krumeich, F.; Roth, S.; Stark, W.J.; Bruinink, A. The degree and kind of agglomeration affect carbon nanotube cytotoxicity. Toxicol. Lett. 2007, 168, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Calvaresi, M.; Zerbetto, F. Atomistic molecular dynamics simulations reveal insights into adsorption, packing, and fluxes of molecules with carbon nanotubes. J. Mater. Chem. A 2014, 2, 12123–12135. [Google Scholar] [CrossRef]
- Prola, L.D.; Machado, F.M.; Bergmann, C.P.; De Souza, F.E.; Gally, C.R.; Lima, E.C.; Adebayo, M.A.; Dias, S.L.; Calvete, T. Adsorption of Direct Blue 53 dye from aqueous solutions by multi-walled carbon nanotubes and activated carbon. J. Environ. Manag. 2013, 130, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Upadhyayula, V.K.K.; Ruparelia, J.P.; Agrawal, A. Use of Carbon Nanotubes in Water Treatment. Nanoscale Multifunct. Mater. 2011, 321–368. [Google Scholar] [CrossRef]
- Wang, R.; Mikoryak, C.; Li, S.; Bushdiecker, D., 2nd; Musselman, I.H.; Pantano, P.; Draper, R.K. Cytotoxicity screening of single-walled carbon nanotubes: Detection and removal of cytotoxic contaminants from carboxylated carbon nanotubes. Mol. Pharm 2011, 8, 1351–1361. [Google Scholar] [CrossRef] [PubMed]
- Mauter, M.S.; Elimelech, M. Environmental Applications of Carbon-Based Nanomaterials. Environ. Sci. Technol. 2008, 42, 5843–5859. [Google Scholar] [CrossRef]
- Agnihotri, S.; Mota, J.P.; Rostam-Abadi, M.; Rood, M.J. Structural Characterization of Single-Walled Carbon Nanotube Bundles by Experiment and Molecular Simulation. Langmuir 2005, 21, 896–904. [Google Scholar] [CrossRef]
- Zhang, X.; Feng, Y.; Tang, S.; Feng, W. Preparation of a graphene oxide–phthalocyanine hybrid through strong π–π interactions. Carbon 2010, 48, 211–216. [Google Scholar] [CrossRef]
- Rodrigues, D.F.; Elimelech, M. Toxic Effects of Single-Walled Carbon Nanotubes in the Development of E. coli Biofilm. Environ. Sci. Technol. 2010, 44, 4583–4589. [Google Scholar] [CrossRef]
- Bai, Y.; Park, I.S.; Lee, S.-J.; Bae, T.-S.; Watari, F.; Uo, M.; Lee, M.-H. Aqueous dispersion of surfactant-modified multiwalled carbon nanotubes and their application as an antibacterial agent. Carbon 2011, 49, 3663–3671. [Google Scholar] [CrossRef]
- Geckeler, K.E.; Premkumar, T. Carbon nanotubes: Are they dispersed or dissolved in liquids? Nanoscale Res. Lett. 2011, 6, 136. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Li, L.; Chusuei, C.C.; Hull, R.V. Sonochemical Oxidation of Multiwalled Carbon Nanotubes. Langmuir 2005, 21, 4185–4190. [Google Scholar] [CrossRef]
- Murugan, E.; Vimala, G. Effective functionalization of multiwalled carbon nanotube with amphiphilic poly(propyleneimine) dendrimer carrying silver nanoparticles for better dispersability and antimicrobial activity. J. Colloid Interface Sci. 2011, 357, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wang, J.; Hou, J.; Zhang, Y.; Liu, J.; Van Der Bruggen, B. Graphene-based antimicrobial polymeric membranes: A review. J. Mater. Chem. A 2017, 5, 6776–6793. [Google Scholar] [CrossRef]
- Zardini, H.Z.; Amiri, A.; Shanbedi, M.; Maghrebi, M.; Baniadam, M. Enhanced antibacterial activity of amino acids-functionalized multi walled carbon nanotubes by a simple method. Colloids Surf. B Biointerfaces 2012, 92, 196–202. [Google Scholar] [CrossRef]
- Zhou, R.; Gao, H. Cytotoxicity of graphene: Recent advances and future perspective. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2014, 6, 452–474. [Google Scholar] [CrossRef]
- Karlsson, H.L.; Cronholm, P.; Gustafsson, J.; Gustafsson, J.; Möller, L. Copper Oxide Nanoparticles Are Highly Toxic: A Comparison between Metal Oxide Nanoparticles and Carbon Nanotubes. Chem. Res. Toxicol. 2008, 21, 1726–1732. [Google Scholar] [CrossRef]
- Doody, M.A.; Wang, D.; Bais, H.P.; Jin, Y. Differential antimicrobial activity of silver nanoparticles to bacteria Bacillus subtilis and Escherichia coli, and toxicity to crop plant Zea mays and beneficial B. subtilis-inoculated Z. mays. J. Nanoparticle Res. 2016, 18, 1–19. [Google Scholar] [CrossRef]
- Arnold, M.S.; Green, A.A.; Hulvat, J.F.; Stupp, S.I.; Hersam, M.C. Sorting carbon nanotubes by electronic structure using density differentiation. Nat. Nanotechnol. 2006, 1, 60–65. [Google Scholar] [CrossRef]
- Strano, M.S.; Dyke, C.A.; Usrey, M.L.; Barone, P.W.; Allen, M.J.; Shan, H.; Kittrell, C.; Hauge, R.H.; Tour, J.M.; Smalley, R.E. Electronic Structure Control of Single-Walled Carbon Nanotube Functionalization. Science 2003, 301, 1519–1522. [Google Scholar] [CrossRef]
- Vecitis, C.D.; Schnoor, M.H.; Rahaman, S.; Schiffman, J.D.; Elimelech, M. Electrochemical Multiwalled Carbon Nanotube Filter for Viral and Bacterial Removal and Inactivation. Environ. Sci. Technol. 2011, 45, 3672–3679. [Google Scholar] [CrossRef] [PubMed]
- Brady-Estevez, A.S.; Kang, S.; Elimelech, M. A singlewalled-carbon-nanotube filter for removal of viral and bacterial pathogens. Small 2008, 4, 481–484. [Google Scholar] [CrossRef] [PubMed]
- Brady-Estévez, A.S.; Nguyen, T.H.; Gutierrez, L.; Elimelech, M. Impact of solution chemistry on viral removal by a single walled carbon nanotube filter. Water Res. 2010, 44, 3773–3780. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.P.; Kim, J.H.; Kim, J.; Lee, S.N.; Park, H.-O. A nanofilter composed of carbon nanotube-silver composites for virus removal and antibacterial activity improvement. J. Environ. Sci. 2016, 42, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-N.; Gong, J.; Zeng, G.-M.; Ou, X.-M.; Song, B.; Guo, M.; Zhang, J.; Liu, H.-Y. Antimicrobial behavior comparison and antimicrobial mechanism of silver coated carbon nanocomposites. Process. Saf. Environ. Prot. 2016, 102, 596–605. [Google Scholar] [CrossRef]
- Ali, Q.; Ahmed, W.; Lal, S.; Sen, T. Novel Multifunctional Carbon Nanotube Containing Silver and Iron Oxide Nanoparticles for Antimicrobial Applications in Water Treatment. Mater. Today Proc. 2017, 4, 57–64. [Google Scholar] [CrossRef]
- Chen, Y.; Lv, Z.; Xu, J.; Peng, D.; Liu, Y.; Chen, J.; Sun, X.; Feng, C.; Wei, C. Stainless steel mesh coated with MnO2/carbon nanotube and polymethylphenyl siloxane as low-cost and high-performance microbial fuel cell cathode materials. J. Power Sources 2012, 201, 136–141. [Google Scholar] [CrossRef]

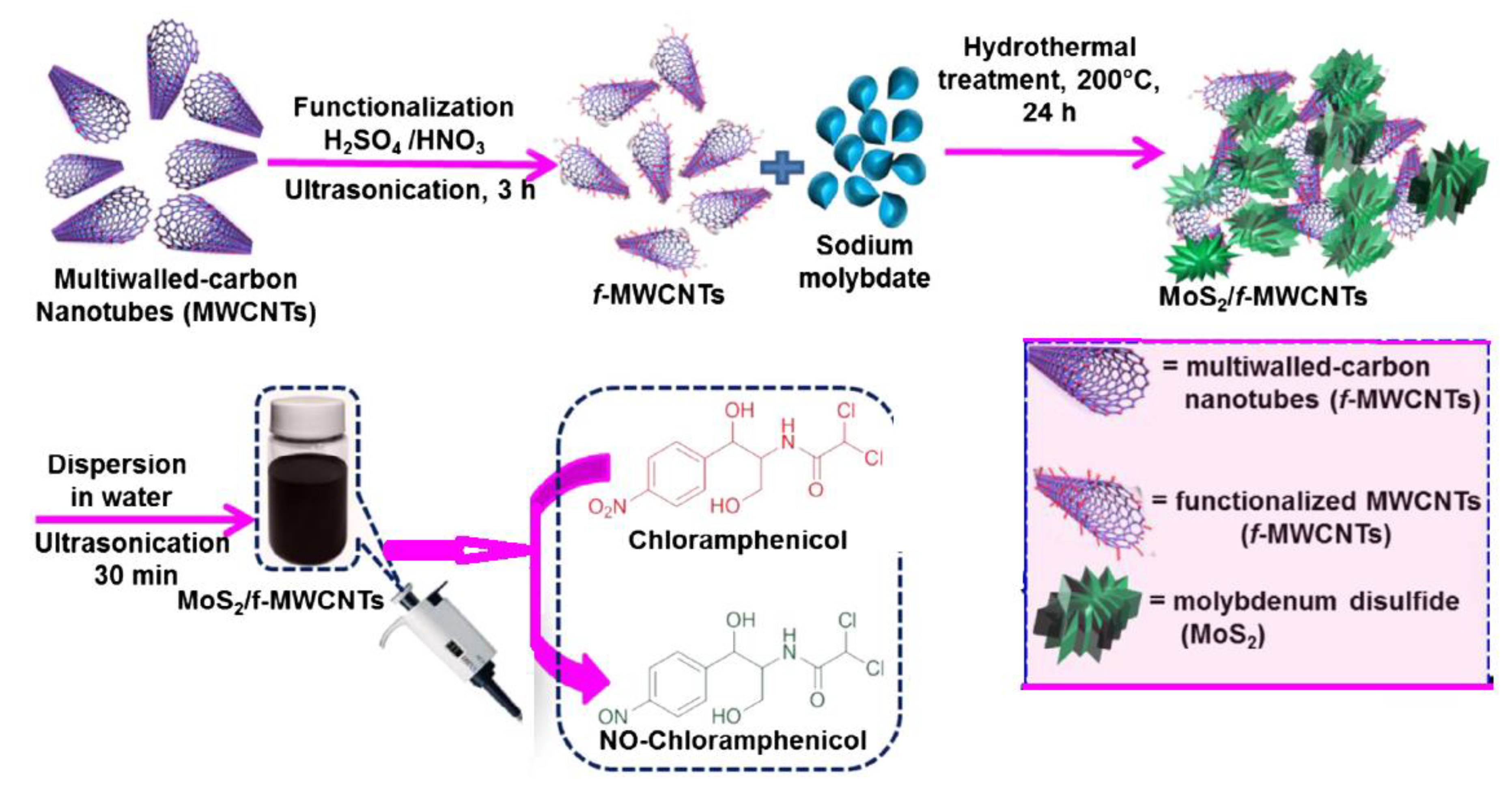
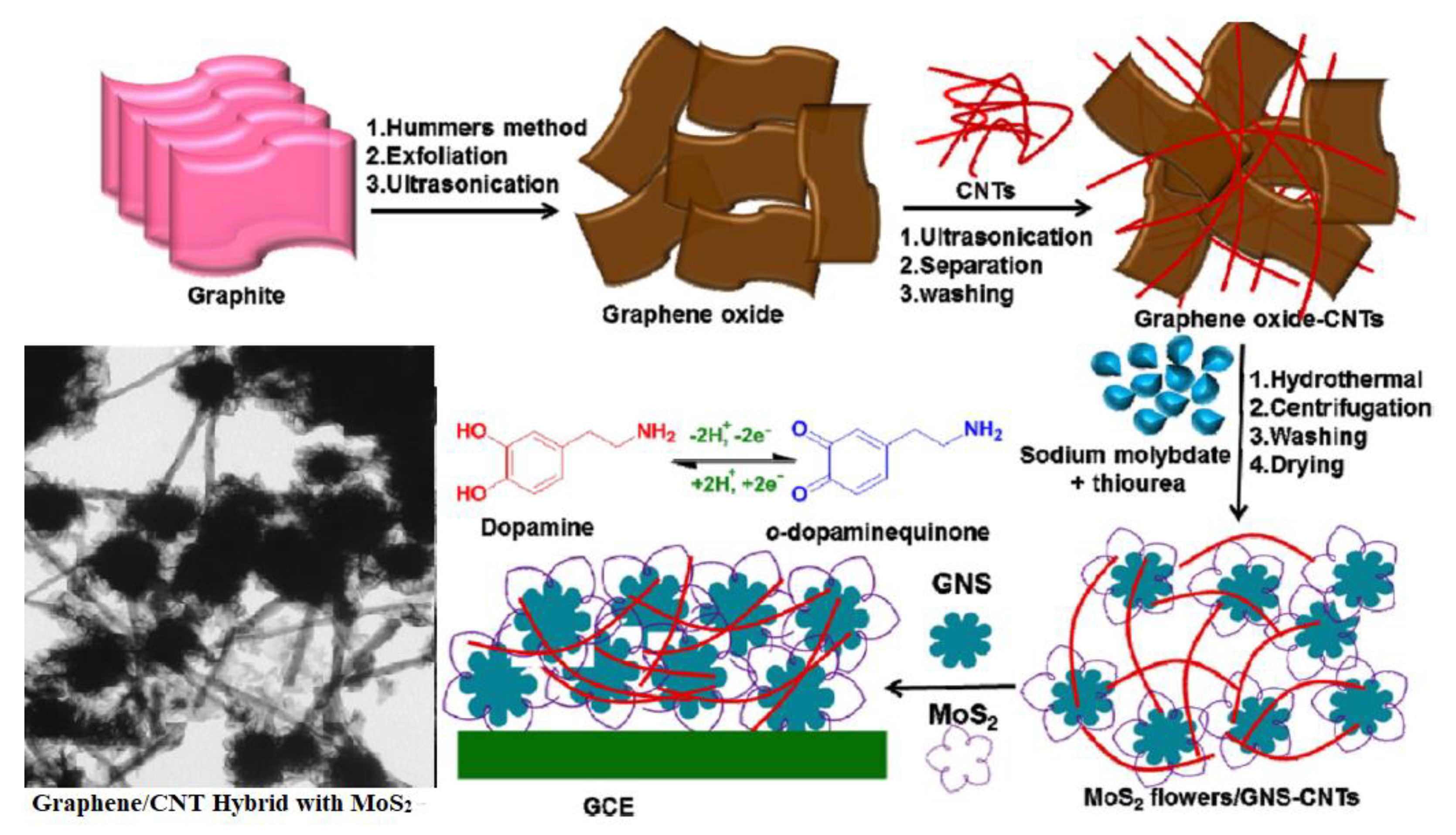
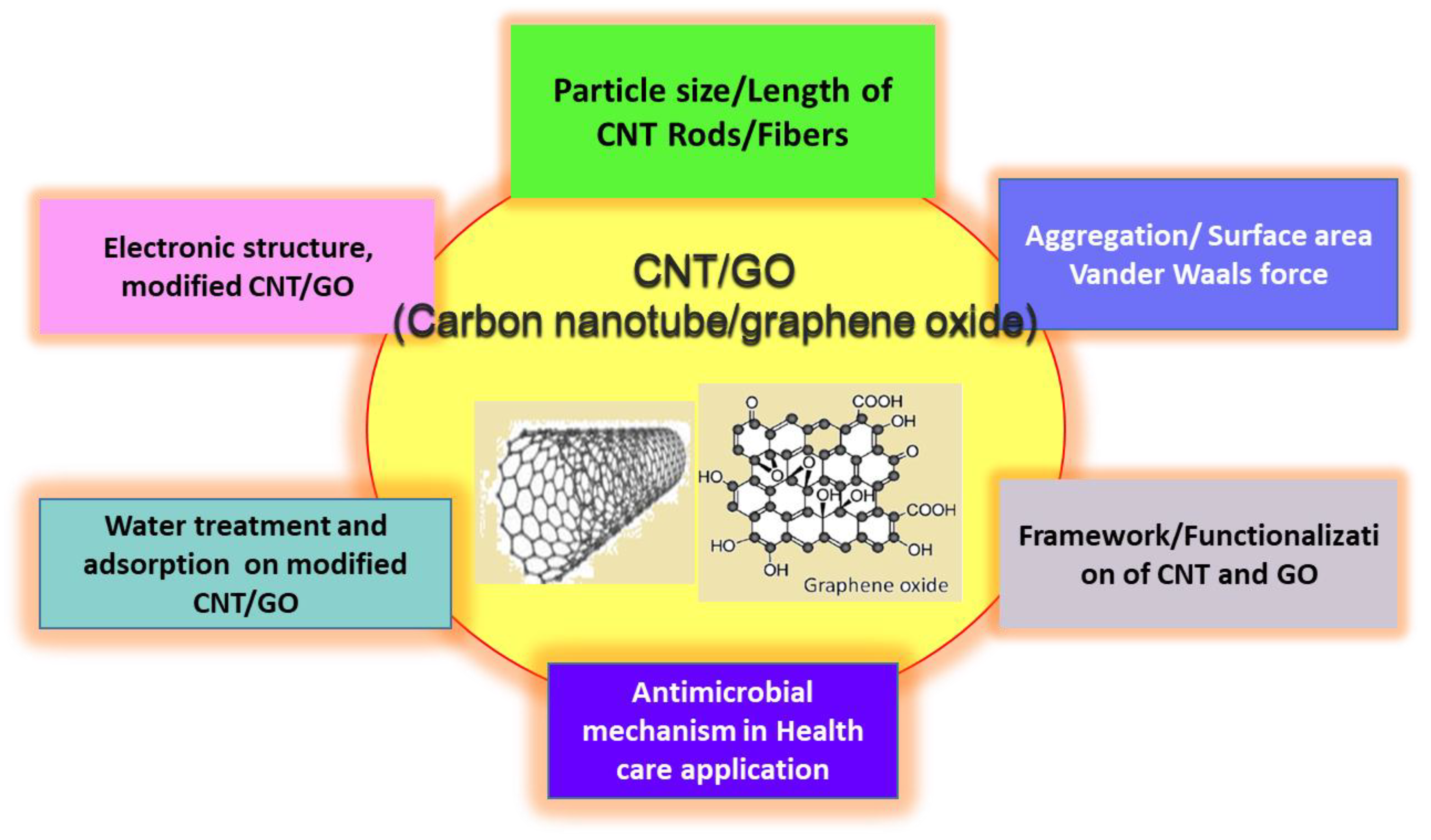
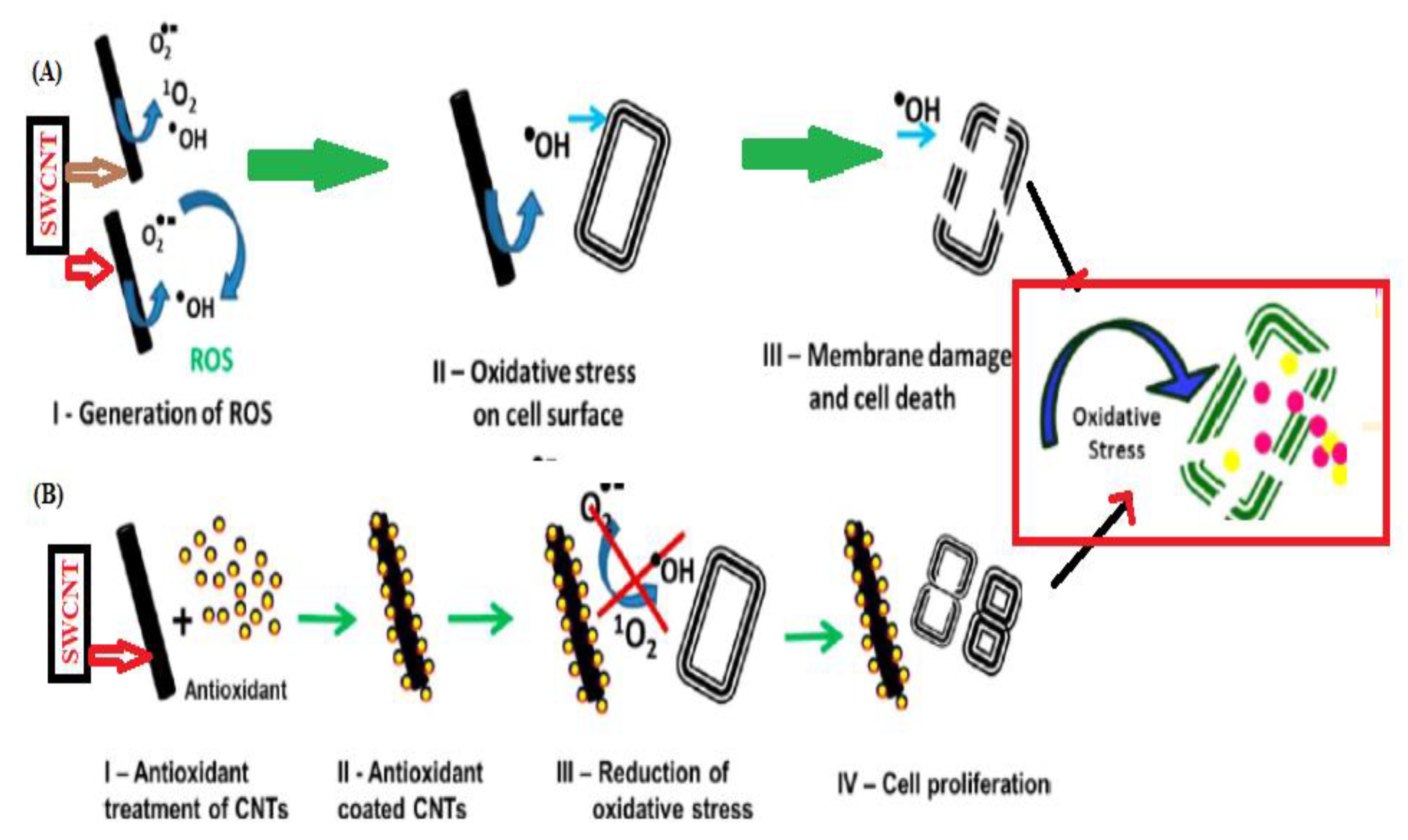


| Carbon Nanomaterials | Bacteria | Contributing Factor | Mechanism of Action | References |
|---|---|---|---|---|
| Fullerenes MWCNT | E. coli E. coli | Silver nanoparticle modification on C60 Uncapped, short, debundled and highly dispersed in solution | Synergistically target bacterial cells that increase ROS Membrane damage | [31] [17] |
| SWCNT | E. coli K12 | Direct contact between bacterial cell and SWCNT in solution | Membrane damage | [15] |
| SWCNT MWCNT | E. coli | Higher surface area of SWCNT | Membrane damage | [16] |
| SWCNT | Soil microorganisms | Raw SWCNT enhances metal toxicity in the soil | Suppressed metabolic activity | [47] |
| SWCNT | E. coli K12 | Aggregation characteristics | Bacterial inactivation | [19] |
| SWCNT | E. coli K12 | Increasing metallic fraction | Oxidative stress | [48] |
| CNT | E. coli, Shigella sonnei, Klebsiella pneumoniae, P. aeruginosa, | Presence of light | Oxidative stress | [24] |
| SWCNT MWCNT | Lactobacillus acidophilus, Bifidobacterium adolescentis, E. coli, Enterococcus faecalis, S. aureus | Wrapping mechanism influenced by length and piercing mechanism dependent on diameter | Membrane damage, release of DNA and RNA, potential reduction of bacterial membrane | [49] |
| Graphene oxide | E coli | aggregation of GO | extraction of phospholipids | [50] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajabathar, J.R.; Periyasami, G.; Alanazi, A.M.; Govindasamy, M.; Arunachalam, P. Review on Carbon Nanotube Varieties for Healthcare Application: Effect of Preparation Methods and Mechanism Insight. Processes 2020, 8, 1654. https://doi.org/10.3390/pr8121654
Rajabathar JR, Periyasami G, Alanazi AM, Govindasamy M, Arunachalam P. Review on Carbon Nanotube Varieties for Healthcare Application: Effect of Preparation Methods and Mechanism Insight. Processes. 2020; 8(12):1654. https://doi.org/10.3390/pr8121654
Chicago/Turabian StyleRajabathar, Jothi Ramalingam, Govindasami Periyasami, Amer M. Alanazi, Mani Govindasamy, and Prabhakarn Arunachalam. 2020. "Review on Carbon Nanotube Varieties for Healthcare Application: Effect of Preparation Methods and Mechanism Insight" Processes 8, no. 12: 1654. https://doi.org/10.3390/pr8121654
APA StyleRajabathar, J. R., Periyasami, G., Alanazi, A. M., Govindasamy, M., & Arunachalam, P. (2020). Review on Carbon Nanotube Varieties for Healthcare Application: Effect of Preparation Methods and Mechanism Insight. Processes, 8(12), 1654. https://doi.org/10.3390/pr8121654








