Design, Overproduction and Purification of the Chimeric Phage Lysin MLTphg Fighting against Staphylococcus aureus
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. Plasmid Construction
2.3. Recombinant Protein Expression and Purification
2.4. Determination of Protein Concentrations
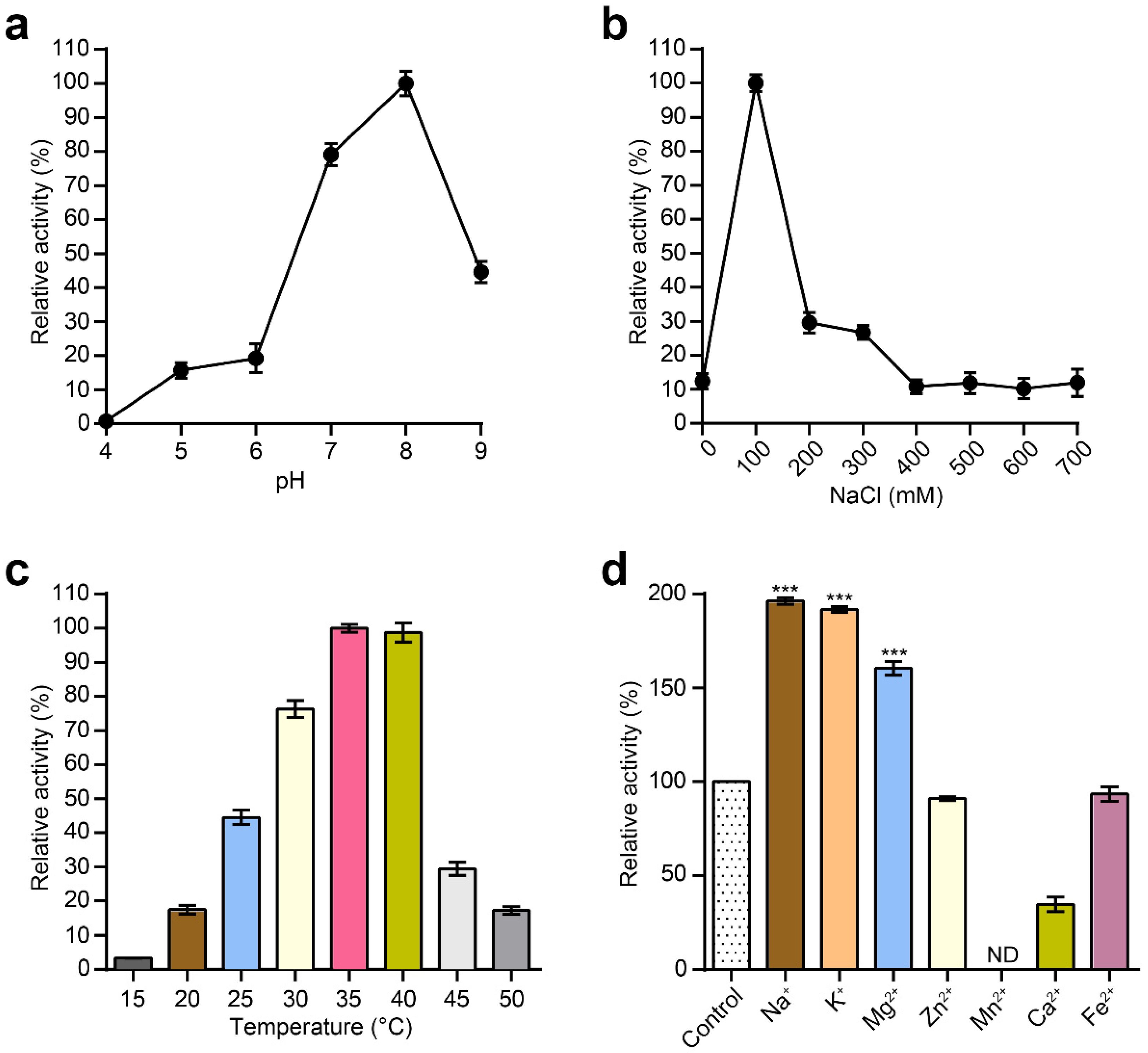
2.5. Assessing Effects of Temperature, pH, NaCl and Metal Ions on the Activity of MLTphg
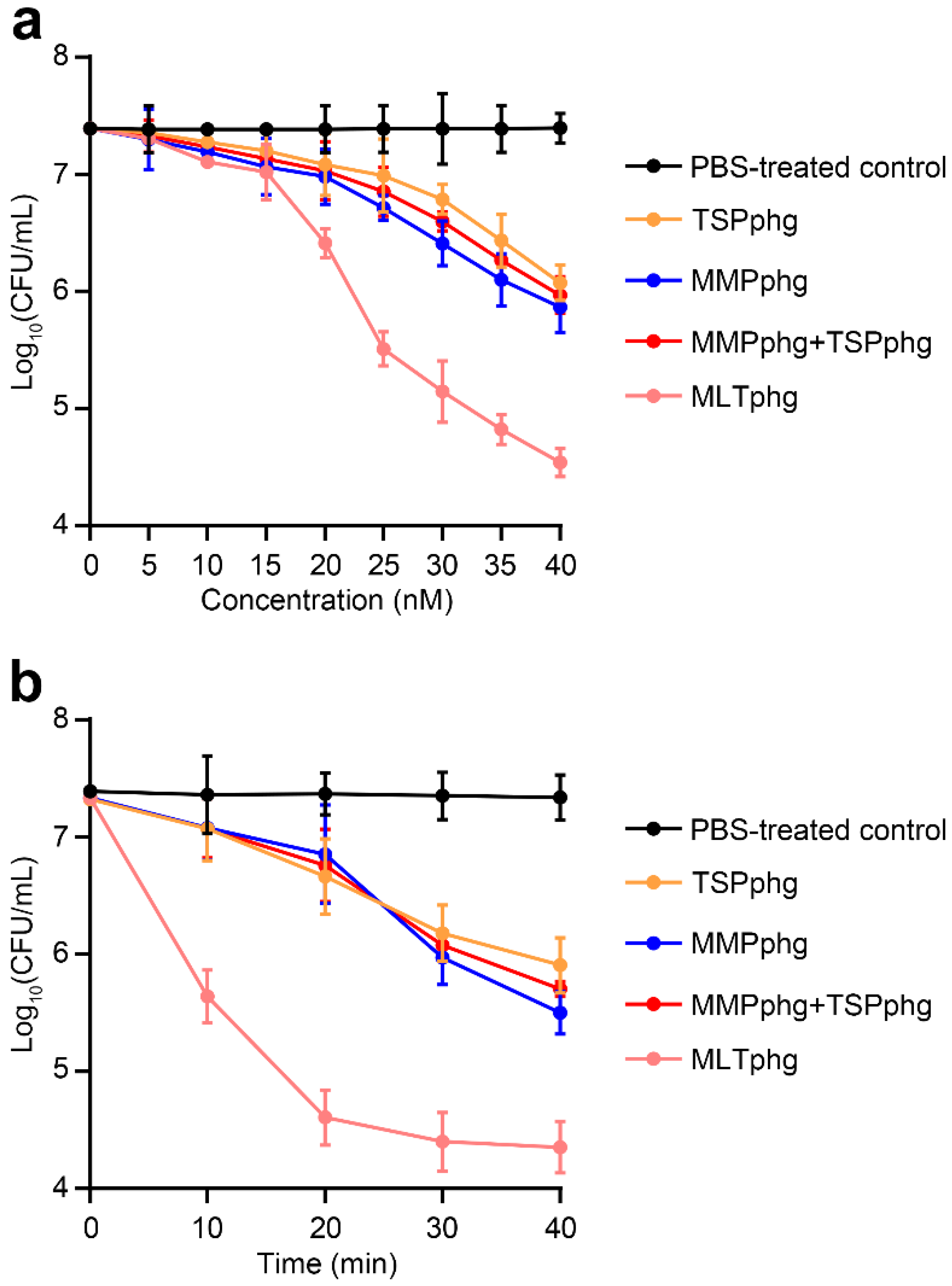
2.6. Antibacterial Assays
2.7. Statistical Analysis
3. Results
3.1. Construction of the Expression Vector for Chimeric Lysin MLTphg via Chimeolysin Bioengineering
3.2. Overproduction and Purification of the Chimeric MLTphg Protein
3.3. Characterization of Purified MLTphg Activity
3.4. Chimeric MLTphg Kills Staphylococcus aureus More Effectively than Its Parental Lysins
3.5. Comparison of Bactericidal Activity between MLTphg and Its Parental Lysins against Various Gram-Negative or Gram-Positive Bacteria
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Fischetti, V.A. Development of phage lysins as novel therapeutics: A historical perspective. Viruses 2018, 10, 310. [Google Scholar] [CrossRef] [PubMed]
- Nakonieczna, A.; Cooper, C.J.; Gryko, R. Bacteriophages and bacteriophage-derived endolysins as potential therapeutics to combat Gram-positive spore forming bacteria. J. Appl. Microbiol. 2015, 119, 620–631. [Google Scholar] [CrossRef] [PubMed]
- Sao-Jose, C. Engineering of phage-derived lytic enzymes: Improving their potential as antimicrobials. Antibiotics 2018, 7, 29. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yu, J.; Wei, H. Engineered bacteriophage lysins as novel anti-infectives. Front. Microbiol. 2014, 5, 542. [Google Scholar] [CrossRef]
- Gerstmans, H.; Criel, B.; Briers, Y. Synthetic biology of modular endolysins. Biotechnol. Adv. 2018, 36, 624–640. [Google Scholar] [CrossRef]
- Roach, D.R.; Donovan, D.M. Antimicrobial bacteriophage-derived proteins and therapeutic applications. Bacteriophage 2015, 5, 1062590. [Google Scholar] [CrossRef]
- Blázquez, B.; Fresco-Taboada, A.; Iglesias-Bexiga, M.; Menéndez, M.; García, P. Pl3 amidase, a tailor-made lysin constructed by domain shuffling with potent killing activity against Pneumococci and related species. Front. Microbiol. 2016, 7, 1156. [Google Scholar] [CrossRef]
- Vázquez, R.; Domenech, M.; Iglesias-Bexiga, M.; Menéndez, M.; García, P. Csl2, a novel chimeric bacteriophage lysin to fight infections caused by Streptococcus suis, an emerging zoonotic pathogen. Sci. Rep. 2017, 7, 16506. [Google Scholar] [CrossRef]
- Díez-Martínez, R.; De Paz, H.D.; García-Fernández, E.; Bustamante, N.; Euler, C.W.; Fischetti, V.A.; Menendez, M.; García, P. A novel chimeric phage lysin with high in vitro and in vivo bactericidal activity against Streptococcus pneumoniae. J. Antimicrob. Chemother. 2015, 70, 1763–1773. [Google Scholar] [CrossRef]
- Crasto, C.J.; Feng, J.A. Linker: A program to generate linker sequences for fusion proteins. Protein Eng. 2000, 13, 309–312. [Google Scholar] [CrossRef]
- Lu, P.; Feng, M.G. Bifunctional enhancement of a beta-glucanase-xylanase fusion enzyme by optimization of peptide linkers. Appl. Microbiol. Biotechnol. 2008, 79, 579–587. [Google Scholar] [CrossRef]
- Trinh, R.; Gurbaxani, B.; Morrison, S.L.; Seyfzadeh, M. Optimization of codon pair use within the (GGGGS)3 linker sequence results in enhanced protein expression. Mol. Immunol. 2004, 40, 717–722. [Google Scholar] [CrossRef]
- Wang, F.; Xiao, Y.; Xiong, Y.; Jiao, Y.; Zhang, Q.; Lin, L. Complete genome sequence of MMP7, a novel Meiothermus bacteriophage of the family Myoviridae isolated from a hot spring. Arch. Virol. 2020, 165, 753–756. [Google Scholar] [CrossRef]
- Wang, F.; Ji, X.; Li, Q.; Zhang, G.; Peng, J.; Hai, J.; Zhang, Y.; Ci, B.; Li, H.; Xiong, Y.; et al. TSPphg lysin from the extremophilic Thermus bacteriophage TSP4 as a potential antimicrobial agent against both gram-negative and gram-positive pathogenic bacteria. Viruses 2020, 12, 192. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Hong, W.; Ji, X.; Han, J.; Huang, L.; Wei, Y. Isolation and characterization of an extremely long tail Thermus bacteriophage from tengchong hot springs in China. J. Basic. Microbiol. 2010, 50, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Han, J.; Ji, X.; Hong, W.; Huang, L.; Wei, Y. Isolation and characterization of a new bacteriophage MMP17 from Meiothermus. Extremophiles 2011, 15, 253–258. [Google Scholar] [CrossRef]
- Girija, A.S.; Priyadharsini, J.V. CLSI based antibiogram profile and the detection of MDR and XDR strains of Acinetobacter baumannii isolated from urine samples. Med. J. Islam Repub. Iran 2019, 33, 3. [Google Scholar] [CrossRef]
- Crump, J.A.; Sjölund-Karlsson, M.; Gordon, M.A.; Parry, C.M. Epidemiology, clinical presentation, laboratory diagnosis, antimicrobial resistance, and antimicrobial management of invasive Salmonella infections. Clin. Microbiol. Rev. 2015, 28, 901–937. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Ji, X.; Chen, M.; Guo, J.; Deng, X.; Lin, L. Rapid purification of bacteriophage endolysin TSPphg and its exogenous treatment could act as an alternative bacterial cell disruption method. Protein Expr. Purif. 2018, 148, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Li, Z.; Li, T.; Wang, S.; Zhang, L. Negative-pressure wound therapy in a Pseudomonas aeruginosa infection model. Biomed. Res. Int. 2018, 2018, 9496183. [Google Scholar] [CrossRef] [PubMed]
- Plotka, M.; Kaczorowska, A.K.; Morzywolek, A.; Makowska, J.; Kozlowski, L.P.; Thorisdottir, A.; Skirnisdottir, S.; Hjorleifsdottir, S.; Fridjonsson, O.H.; Hreggvidsson, G.O.; et al. Biochemical characterization and validation of a catalytic site of a highly thermostable Ts2631 endolysin from the Thermus scotoductus phage vB_Tsc2631. PLoS ONE 2015, 10, 0137374. [Google Scholar] [CrossRef] [PubMed]
- Plotka, M.; Kapusta, M.; Dorawa, S.; Kaczorowska, A.K.; Kaczorowski, T. Ts2631 endolysin from the extremophilic Thermus scotoductus bacteriophage vB_Tsc2631 as an antimicrobial agent against Gram-negative multidrug-resistant bacteria. Viruses 2019, 11, 657. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, M.; Yu, J.; Wei, H. Antibacterial activity of a novel peptide-modified lysin against Acinetobacter baumannii and Pseudomonas aeruginosa. Front. Microbiol. 2015, 6, 1471. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Xiong, Y.; Xiao, Y.; Han, J.; Deng, X.; Lin, L. MMPphg from the thermophilic Meiothermus bacteriophage MMP17 as a potential antimicrobial agent against both gram-negative and gram-positive bacteria. Virol. J. 2020, 17, 130. [Google Scholar] [CrossRef]
- George, R.A.; Heringa, J. An analysis of protein domain linkers: Their classification and role in protein folding. Protein Eng. 2002, 15, 871–879. [Google Scholar] [CrossRef]
- Arai, R.; Wriggers, W.; Nishikawa, Y.; Nagamune, T.; Fujisawa, T. Conformations of variably linked chimeric proteins evaluated by synchrotron X-ray small-angle scattering. Proteins 2004, 57, 829–838. [Google Scholar] [CrossRef]
- Kabiri, M.; Tafaghodi, M.; Saberi, M.R.; Moghadam, M.; Rezaee, S.A.; Sankian, M. Separation of the epitopes in a multi-epitope chimera: Helical or flexible linkers. Protein Pept. Lett. 2020, 27, 604–613. [Google Scholar] [CrossRef]
- Yoong, P.; Schuch, R.; Nelson, D.; Fischetti, V.A. Identification of a broadly active phage lytic enzyme with lethal activity against antibiotic-resistant Enterococcus faecalis and Enterococcus faecium. J. Bacteriol. 2004, 186, 4808–4812. [Google Scholar] [CrossRef]
- Briers, Y.; Walmagh, M.; Van Puyenbroeck, V.; Cornelissen, A.; Cenens, W.; Aertsen, A.; Oliveira, H.; Azeredo, J.; Verween, G.; Pirnay, J.P.; et al. Engineered endolysin-based “Artilysins” to combat multidrug-resistant Gram-negative pathogens. mBio 2014, 5, e01379-14. [Google Scholar] [CrossRef]
- Briers, Y.; Lavigne, R. Breaking barriers: Expansion of the use of endolysins as novel antibacterials against Gram-negative bacteria. Future Microbiol. 2015, 10, 377–390. [Google Scholar] [CrossRef]
- Becker, S.C.; Roach, D.R.; Chauhan, V.S.; Shen, Y.; Foster-Frey, J.; Powell, A.M.; Bauchan, G.; Lease, R.A.; Mohammadi, H.; Harty, W.J.; et al. Triple-acting lytic enzyme treatment of drug-resistant and intracellular Staphylococcus aureus. Sci. Rep. 2016, 6, 25063. [Google Scholar] [CrossRef]
- Low, L.Y.; Yang, C.; Perego, M.; Osterman, A.; Liddington, R. Role of net charge on catalytic domain and influence of cell wall binding domain on bactericidal activity, specificity, and host range of phage lysins. J. Biol. Chem. 2011, 286, 34391–34403. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, D.; Briers, Y. Lysins breaking down the walls of gram-negative bacteria, no longer a no-go. Curr. Opin. Biotechnol. 2020, 68, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Gerstmans, H.; Grimon, D.; Gutiérrez, D.; Lood, C.; Rodríguez, A.; Van Noort, V.; Lammertyn, J.; Lavigne, R.; Briers, Y. A VersaTile-driven platform for rapid hit-to-lead development of engineered lysins. Sci. Adv. 2020, 6, eaaz1136. [Google Scholar] [CrossRef] [PubMed]
- Usai, D.; Donadu, M.; Bua, A.; Molicotti, P.; Zanetti, S.; Piras, S.; Corona, P.; Ibba, R.; Carta, A. Enhancement of antimicrobial activity of pump inhibitors associating drugs. J. Infect. Dev. Ctries. 2019, 13, 162–164. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.W.C.; Monção, N.B.N.; Araújo, B.Q.; Arcanjo, D.D.R.; Ferreira, J.H.L.; Lima Neto, J.S.; Citó, A.M.D.G.L.; De Siqueira Júnior, J.P.; Kaatz, G.W.; Barreto, H.M.; et al. Antimicrobial activity of Mimosa caesalpiniifolia Benth and its interaction with antibiotics against Staphylococcus aureus strains overexpressing efflux pump genes. Lett. Appl. Microbiol. 2019, 69, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.M.B.; Sousa, J.N.; Costa, L.M.; Oliveira, F.A.A.; Dos Santos, R.C.; Nunes, A.S.S.; Da Silva, W.O.; Cordeiro, P.J.M.; De Sousa, J.L.N.; De Siqueira-Júnior, J.P.; et al. Antimicrobial activity of Phyllanthus amarus Schumach. & Thonn and inhibition of the NorA efflux pump of Staphylococcus aureus by Phyllanthin. Microb. Pathog. 2019, 130, 242–246. [Google Scholar] [CrossRef]
- Rineh, A.; Bremner, J.B.; Hamblin, M.R.; Ball, A.R.; Tegos, G.P.; Kelso, M.J. Attaching NorA efflux pump inhibitors to methylene blue enhances antimicrobial photodynamic inactivation of Escherichia coli and Acinetobacter baumannii in vitro and in vivo. Bioorg. Med. Chem. Lett. 2018, 28, 2736–2740. [Google Scholar] [CrossRef]


| Strain | Antibiotic Resistance a | Antimicrobial Activity (log10 Reduction) b | |||
|---|---|---|---|---|---|
| MLTphg | MMPphg | TSPphg | MMPphg + TSPphg | ||
| Salmonella ser. Paratyphi B | |||||
| CMCC(B)50094 | None | 4.72 ± 0.14 | 3.38 ± 0.10 (***) | 3.17 ± 0.09 (***) | 3.28 ± 0.12 (***) |
| Salmonella ser. Enteritidis | |||||
| CMCC(B)50335 | None | 3.06 ± 0.06 | 1.18 ± 0.10 (***) | 2.37 ± 0.32 (*) | 1.96 ± 0.16 (***) |
| Salmonella ser. Typhi | |||||
| CGMCC1.1190 | None | 3.05 ± 0.05 | 0.69 ± 0.21 (***) | 1.28 ± 0.32 (***) | 1.07 ± 0.12 (***) |
| Escherichia coli O157 | |||||
| KUST401 | STR, TET and AMP | 4.38 ± 0.40 | 3.33 ± 0.17 (*) | 3.61 ± 0.11 (*) | 3.50 ± 0.02 (*) |
| Klebsiella pneumoniae | |||||
| 13A14918 | CRO, AMP, CFZ, NIT, AMP, AMX and GEN | 5.52 ± 0.13 | 1.27 ± 0.18 (***) | 5.20 ± 0.35 (ns) | 3.13 ± 0.15 (***) |
| 14V0622 | CRO, AMP, CFZ, ATM, FOX and FEP | 5.40 ± 0.10 | 1.23 ± 0.10 (***) | 4.67 ± 0.29 (*) | 2.31 ± 0.02 (***) |
| 13A14165 | AMP, NIT and CFP | 3.58 ± 0.08 | 3.19 ± 0.06 (**) | 3.40 ± 0.05 (*) | 3.33 ± 0.06 (*) |
| 1501SP0351 | CRO, AMP, CFZ, ATM, AMX and FEP | 2.31 ± 0.03 | 0.55 ± 0.05 (***) | 1.39 ± 0.10 (***) | 0.92 ± 0.06 (***) |
| Meiothermus sp. TC7 | |||||
| KUST401-TC7 | None | 5.50 ± 0.20 | 5.83 ± 0.11 (ns) | 0.01 ± 0.00 (***) | 2.22 ± 0.20 (***) |
| Thermus sp. TC4 | |||||
| KUST401-TC4 | None | 6.45 ± 0.10 | 0.01 ± 0.00 (***) | 6.66 ± 0.11 (ns) | 2.94 ± 0.11 (***) |
| Lactobacillus plantarum | |||||
| CGMCC1.16089 | None | 0.01 ± 0.00 | 0.00 ± 0.01 (ns) | 0.01 ± 0.01 (ns) | 0.00 ± 0.01 (ns) |
| Staphylococcus aureus | |||||
| ATCC6538 | None | 2.85 ± 0.12 | 1.53 ± 0.22 (***) | 1.32 ± 0.15 (***) | 1.42 ± 0.16 (***) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Liu, X.; Deng, Z.; Zhang, Y.; Ji, X.; Xiong, Y.; Lin, L. Design, Overproduction and Purification of the Chimeric Phage Lysin MLTphg Fighting against Staphylococcus aureus. Processes 2020, 8, 1587. https://doi.org/10.3390/pr8121587
Wang F, Liu X, Deng Z, Zhang Y, Ji X, Xiong Y, Lin L. Design, Overproduction and Purification of the Chimeric Phage Lysin MLTphg Fighting against Staphylococcus aureus. Processes. 2020; 8(12):1587. https://doi.org/10.3390/pr8121587
Chicago/Turabian StyleWang, Feng, Xiaohang Liu, Zhengyu Deng, Yao Zhang, Xinyu Ji, Yan Xiong, and Lianbing Lin. 2020. "Design, Overproduction and Purification of the Chimeric Phage Lysin MLTphg Fighting against Staphylococcus aureus" Processes 8, no. 12: 1587. https://doi.org/10.3390/pr8121587
APA StyleWang, F., Liu, X., Deng, Z., Zhang, Y., Ji, X., Xiong, Y., & Lin, L. (2020). Design, Overproduction and Purification of the Chimeric Phage Lysin MLTphg Fighting against Staphylococcus aureus. Processes, 8(12), 1587. https://doi.org/10.3390/pr8121587





