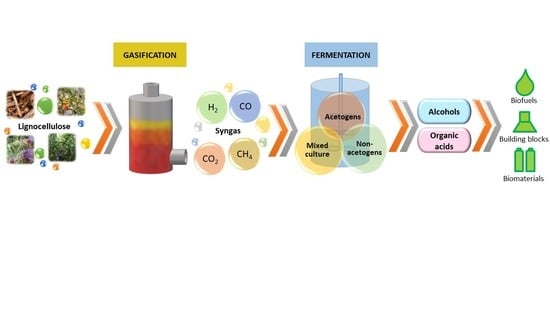
Syngas Derived from Lignocellulosic Biomass Gasification as an Alternative Resource for Innovative Bioprocesses
Abstract
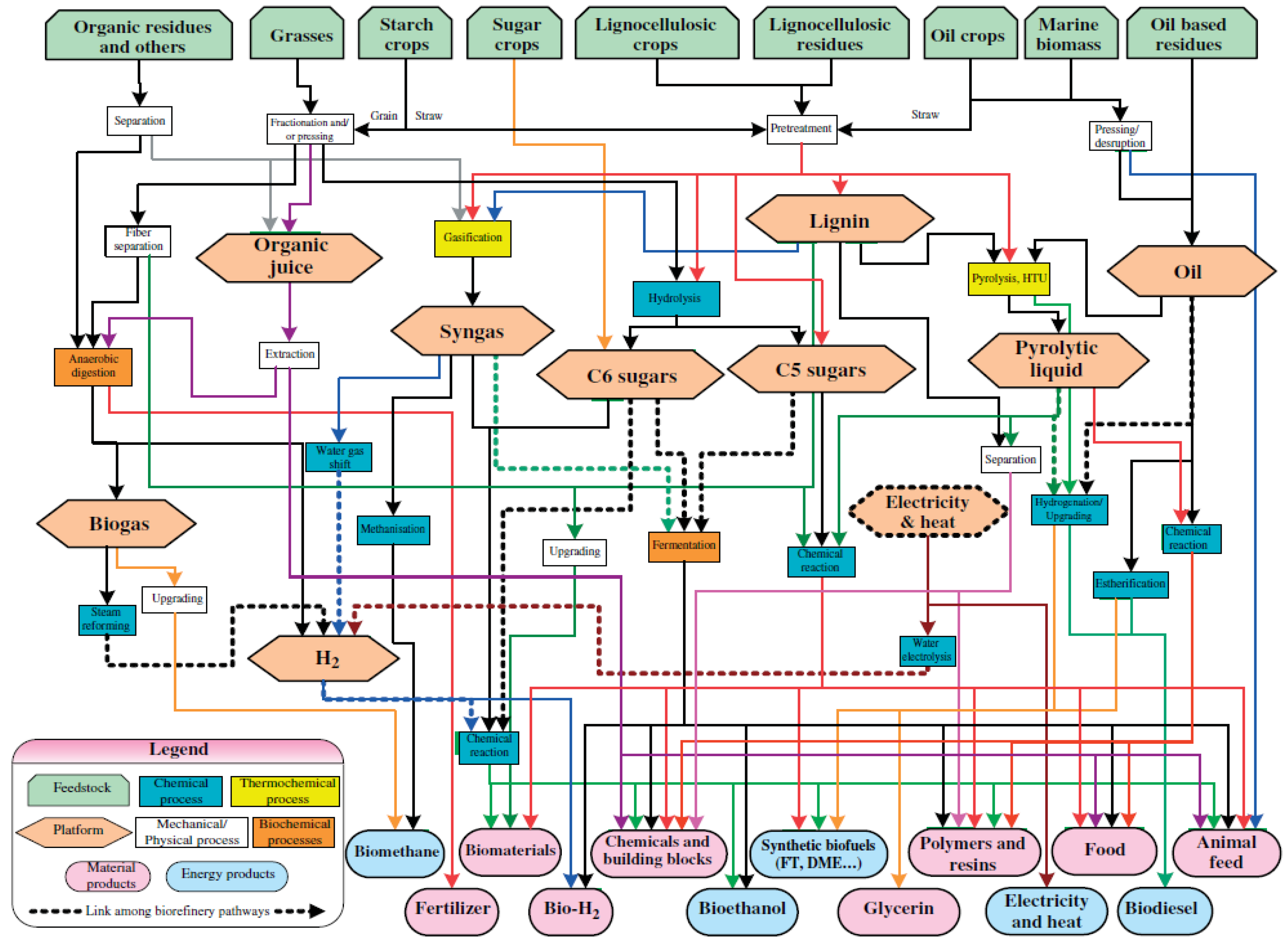
1. Introduction
2. Biomass Gasification
3. Syngas Fermentation
3.1. Metabolism Insight of Syngas Fermentation
3.1.1. Acetogens and the Wood–Ljungdahl Pathway
3.1.2. Energy Conservation Model in Acetogens
3.2. Syngas Fermenting Microorganisms
3.2.1. Pure Culture Syngas Fermentation
3.2.2. Other Strains
3.2.3. Mixed-Cultures Syngas Fermentation
| Strain | Gram | pHopt | Topt | Autotrophic Growth | Heterotrophic Growth | Products | Reference |
|---|---|---|---|---|---|---|---|
| A. woodii | + | 6.8 | 30 °C | CO, CO2, H2 | fructose, glucose, lactate, glycerate, formic acid, and o-methylated organic compounds | Acetate, EtOH | [78,93] |
| C. aceticum | − | 8.3 | 30 °C | CO, CO2, H2 | Fructose, ribose, glutamate, fumarate, malate, serine, pyruvate, formic acid, ethylene glycol, and ethanol | Acetate, EtOH | [79,94] |
| C. autoethanogenum | + | 5.8–6.0 | 37 °C | CO, CO2, H2 | Fructose, xylose, arabinose, rhamnose, glutamate, and pyruvate | Acetate, EtOH, 2,3-butanediol, lactate | [75,80] |
| C. carboxidivorans | + | 6.2 | 38 °C | CO, CO2, H2 | Ribose, xylose, fructose, glucose, galactose, arabinose, mannose, rhamnose, sucrose, cellobiose, trehalose, melezitose, pectin, starch, cellulose, inositol, mannitol, glycerol, ethanol, propanol, 2-propanol, butanol, citrate, serine, alanine, histidine, glutamate, aspartate, asparagine, casamino acids, betaine, choline, and syringate | Acetate, EtOH, butyrate, butanol, caproate, hexanol | [81,95,96] |
| C. ljungdahlii | − | 6.0 | 37 °C | CO, CO2, H2 | Fructose, glucose, xylose, arabinose, triose, erythrose, fumarate, formic acid, ethanol, and pyruvate | Acetate, EtOH, 2,3-butanediol, lactate | [82,97] |
| C. ragsdalei | + | 6.3 | 37 °C | CO, CO2, H2 | Pyruvate, threose, xylose, mannose, fructose, glucose, sucrose, ethanol, 1-propanol, casamino acids, glutamate, serine, choline, and alanine | Acetate, EtOH, butanol, 2,3-butanediol, and lactate | [75,83] |
3.3. Strategies for Improving the Syngas Fermentation Process
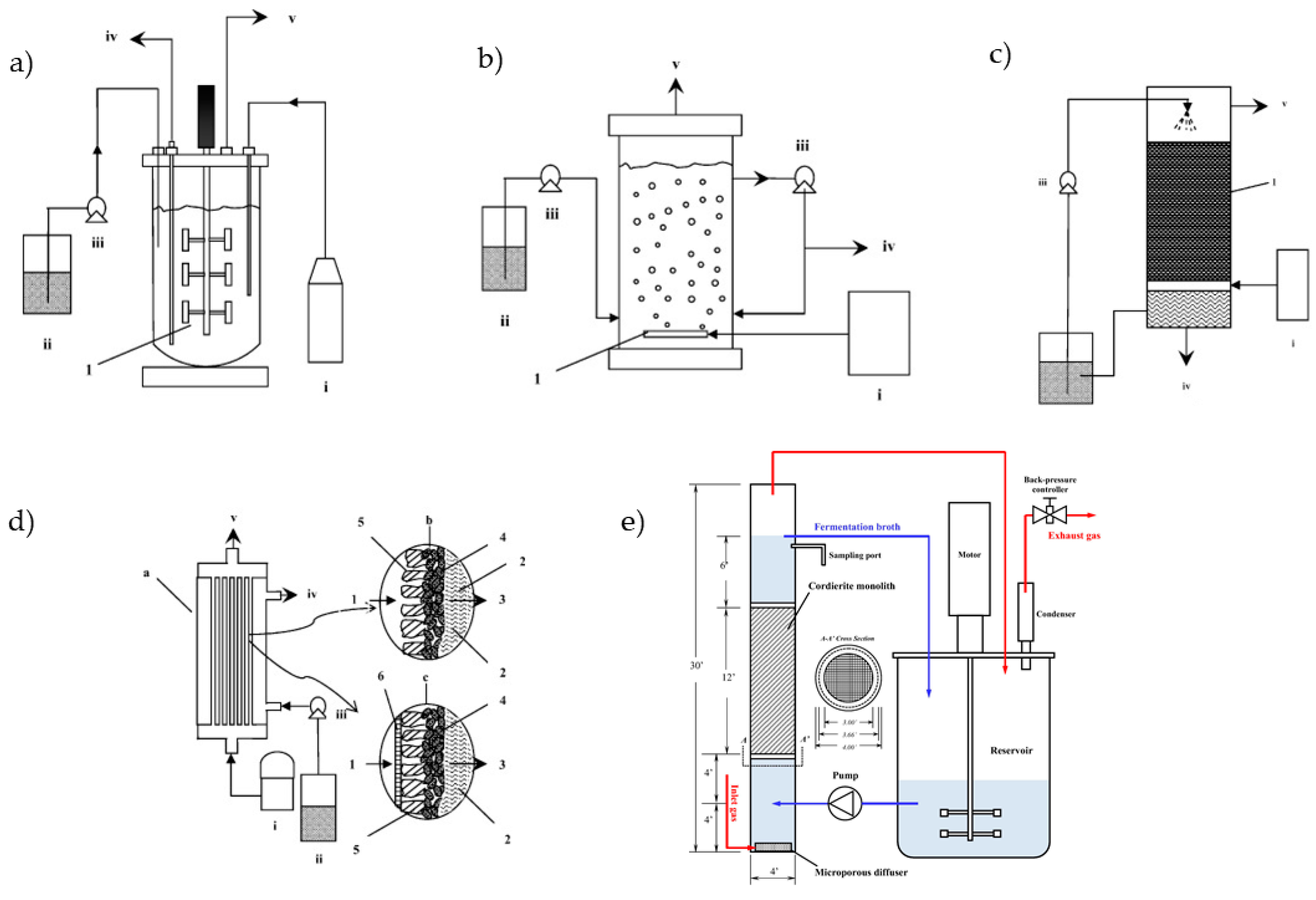
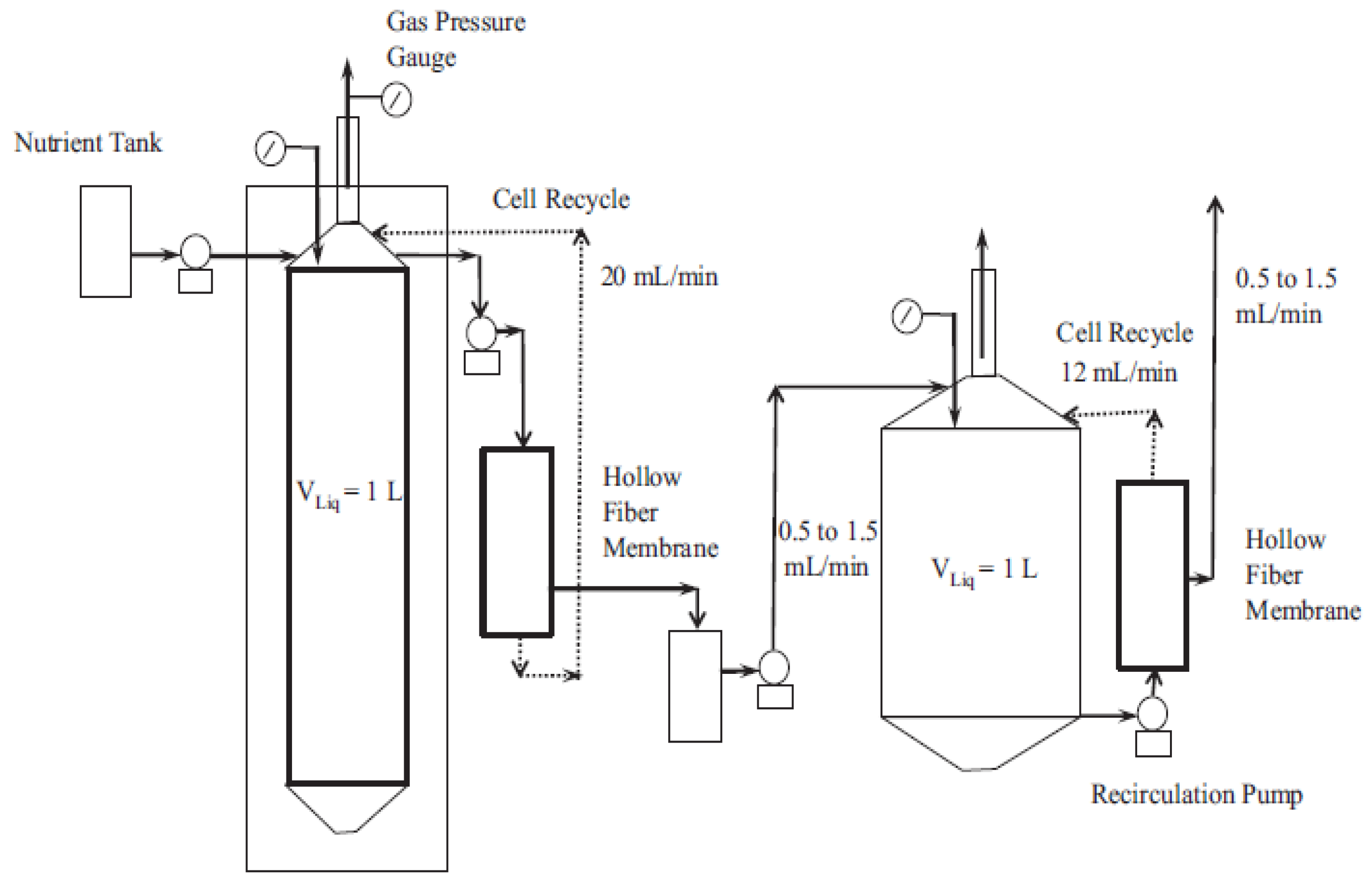
3.4. Mass Transfer Limitations and Bioreactor Optimization
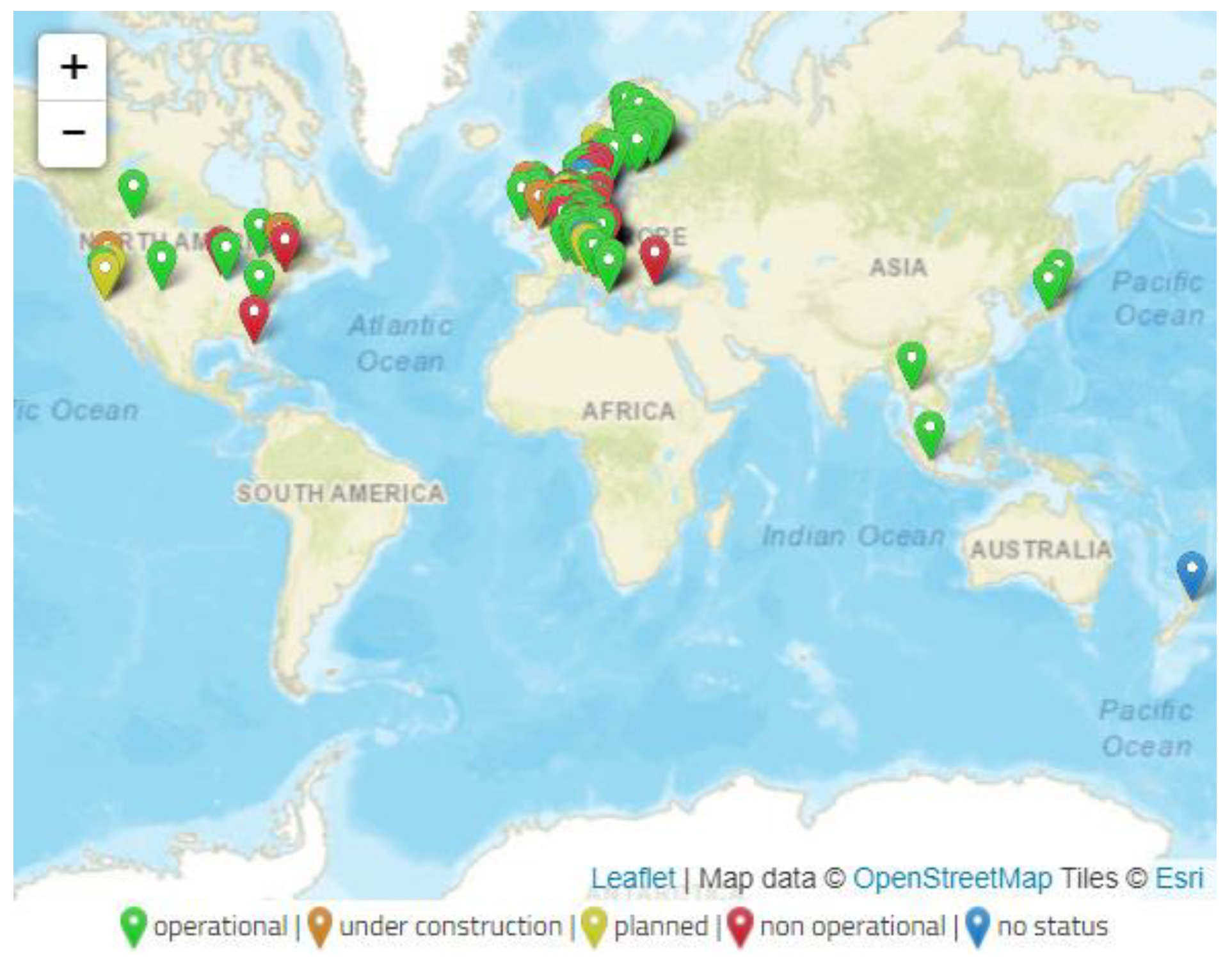
4. Biomass Gasification and Syngas Fermentation at a Large Scale
5. Future Perspectives on Strategies for a Sustainable Biomass-Derived Syngas Valorization
5.1. Effects of Syngas Impurities and Syngas Clean-Up
5.2. Cascade Approaches
5.2.1. Syngas into Elongated Carboxylic Acids
5.2.2. Syngas into Dicarboxylic Acids
5.2.3. Syngas into Lipids
5.2.4. Syngas into Polyhydroxyalkanoates
| ||||||
| Stage 1: Syngas fermentation | Microorganism | Substrate | Operative Conditions | Reactor | Products | Ref. |
| C. ljungdahlii | 65% CO, 30% H2, 5% CO2 | N.A. | N.A. | Ethanol (11.4 g/L) Acetate (2.3 g/L) | [171] | |
| Stage 2: Chain elongation process | Mixed culture | Ethanol | T: 30 °C pHi: 6.5 to pHf: 5.5 | Anaerobic filter reactor (700 mL) | Acetic acid (7.9 g/L) Butyric acid (19.4 g/L) Caproic acid (1.0 g/L) | |
| ||||||
| Stage 1: Syngas fermentation | C. ljungdahlii | 32.5% H2, 32.5% CO, 16% CO2, 19% N2 | T: 37 °C pH: 5.9 GFR: 0.02 L/min Agitation speed: 800 rpm | CSTR (2.5 L) | Ethanol (2.0 g/L) Acetate (15.9 g/L) | [172] |
| Stage 2: Malic acid production | Aspergillus oryzae | Acetic acid | T: 35 °C pH: 6.5 Aeration rate: 0.6 L/min Agitation speed: 300 rpm | CSTR (2.5 L) | Malic acid (1.8 g/L) | |
| ||||||
| Stage 1: Syngas fermentation | M. thermoacetica | CO/CO2 (4/1) H2/CO2 (4/1) | T: 60 °C pH: 6.0 GFL: 1 L/min LFR: 0.5 mL/min | BCR (1 L) | Acetate (25.0 g/L) | [51] |
| Stage 2: Lipids production | Y. lipolytica | Acetic acid | T: 35 °C pH: 7.3 DO: 20% | CSTR (2 L) | Lipids (18.0 g/L) | |
| ||||||
| Stage 1: Syngas fermentation | C. autoethanogenum | 30% CO, 10% CO2, 20% H2, 40% N2 | T: 30 °C pH: 5.75 GFR: 0.01 L/min Agitation speed: 250 rpm | CSTR (2 L) | Acetate (2.7 g/L) Ethanol (3.8 g/L) 2,3-Butanediol (1.6 g/L) | [173] |
| Stage 2: PHA production | Mixed microbial culture (MMC) | Acetic acid | T: 30 °C | Glass bioreactor (1 L) | PHA (24% of cell dry weight) | |
| ||||||
| Stage 1: Syngas fermentation | A. woodii | 20–65% CO, 2–14% H2, 16–42% CO2, 18–56% N2 | N.A. | CSTR | Formate (1.89–2.79 g/L) | [176] |
| Stage 2: PHA production | M. extorquens | Formate | N.A. | CSTR (1 L) | Polyhydroxybutyrate (PHB) (0.097 g/L) | |
5.3. Non-acetogenic Microorganisms as a Valid Alternative to Acetogens
6. Summary and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cherubini, F. The biorefinery concept: Using biomass instead of oil for producing energy and chemicals. Energy Convers. Manag. 2010, 51, 1412–1421. [Google Scholar] [CrossRef]
- Halkos, G.E.; Gkampoura, E.C. Reviewing usage, potentials, and limitations of renewable energy sources. Energies 2020, 13, 2906. [Google Scholar] [CrossRef]
- Michaelides, E.E. Alternative Energy Sources; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- The European Commission. The European Green Deal; The European Commission: Brussels, Belgium, 2019. [Google Scholar]
- Org, S.U. Transforming Our World: The 2030 Agenda for Sustainable Development; United Nations: New York, NY, USA, 2015. [Google Scholar]
- Clark, J.H.; Deswarte, F.E.I. Introduction to Chemicals from Biomass; John Wiley & Sons: Hoboken, NJ, USA, 2014; ISBN 9781118714461. [Google Scholar]
- Panwar, N.L.; Kaushik, S.C.; Kothari, S. Role of renewable energy sources in environmental protection: A review. Renew. Sustain. Energy Rev. 2011, 15, 1513–1524. [Google Scholar] [CrossRef]
- European Commission. Directive 2009/28/EC of the European Parliament and of the Council of 23 April 2009 on the Promotion of the Use of Energy from Renewable Sources and Amending and Subsequently Repealing Directives 2001/77/EC and 2003/30/EC (Text with EEA Relevance); Council of the European Community: Brussels, Belgium, 2009. [Google Scholar]
- Dubois, O.; Gomez San Juan, M. How sustainability is addressed in official bioeconomy strategies at international, national, and regional leveles-An overview. Environ. Nat. Resour. Manag. Work. Pap. (FAO) 2016, 63, 1–33. [Google Scholar]
- Aristizábal-Marulanda, V.; Cardona Alzate, C.A. Methods for designing and assessing biorefineries: Review. Biofuels Bioprod. Biorefining 2019, 13, 789–808. [Google Scholar] [CrossRef]
- Cherubini, F.; Jungmeier, G.; Wellisch, M.; Willke, T.; Skiadas, I.; van Ree, R.; de Jong, E. Toward a common classification approach for biorefinery systems. Biofuels Bioprod. Biorefining 2009, 3, 534–546. [Google Scholar] [CrossRef]
- Nguyen, Q.; Bowyer, J.; Howe, J.; Bratkovich, S.; Groot, H.; Pepke, E.; Fernholz, K. Global Production of Second Generation Biofuels: Trends and Influences Dovetail Partners Global Production of Second Generation Biofuels: Trends and Influences; Dovetail Partners Inc.: Minneapolis, MN, USA, 2017. [Google Scholar]
- Saladini, F.; Patrizi, N.; Pulselli, F.M.; Marchettini, N.; Bastianoni, S. Guidelines for emergy evaluation of first, second and third generation biofuels. Renew. Sustain. Energy Rev. 2016, 66, 221–227. [Google Scholar] [CrossRef]
- 3. Production and Uses of Aquatic Biomass in: Biorefineries. Available online: https://www.degruyter.com/view/book/9783110331585/10.1515/9783110331585-007.xml (accessed on 30 October 2020).
- Leong, W.H.; Lim, J.W.; Lam, M.K.; Uemura, Y.; Ho, Y.C. Third generation biofuels: A nutritional perspective in enhancing microbial lipid production. Renew. Sustain. Energy Rev. 2018, 91, 950–961. [Google Scholar] [CrossRef]
- Dahmen, N.; Lewandowski, I.; Zibek, S.; Weidtmann, A. Integrated lignocellulosic value chains in a growing bioeconomy: Status quo and perspectives. GCB Bioenergy 2019, 11, 107–117. [Google Scholar] [CrossRef]
- Isikgor, F.H.; Becer, C.R. Lignocellulosic biomass: A sustainable platform for the production of bio-based chemicals and polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef]
- Sansaniwal, S.K.; Rosen, M.A.; Tyagi, S.K. Global challenges in the sustainable development of biomass gasification: An overview. Renew. Sustain. Energy Rev. 2017, 80, 23–43. [Google Scholar] [CrossRef]
- Acharya, B.; Roy, P.; Dutta, A. Review of syngas fermentation processes for bioethanol. Biofuels 2014, 5, 551–564. [Google Scholar] [CrossRef]
- Mohammadi, M.; Najafpour, G.D.; Younesi, H.; Lahijani, P.; Uzir, M.H.; Mohamed, A.R. Bioconversion of synthesis gas to second generation biofuels: A review. Renew. Sustain. Energy Rev. 2011, 15, 4255–4273. [Google Scholar] [CrossRef]
- Munasinghe, P.C.; Khanal, S.K. Biomass-derived syngas fermentation into biofuels: Opportunities and challenges. Bioresour. Technol. 2010, 101, 5013–5022. [Google Scholar] [CrossRef]
- Molino, A.; Chianese, S.; Musmarra, D. Biomass gasification technology: The state of the art overview. J. Energy Chem. 2016, 25, 10–25. [Google Scholar] [CrossRef]
- Rauch, R.; Hrbek, J.; Hofbauer, H. Biomass gasification for synthesis gas production and applications of the syngas. Wiley Interdiscip. Rev. Energy Environ. 2014, 3, 343–362. [Google Scholar] [CrossRef]
- Devarapalli, M.; Atiyeh, H.K. A review of conversion processes for bioethanol production with a focus on syngas fermentation. Biofuel Res. J. 2015, 2, 268–280. [Google Scholar] [CrossRef]
- Neubauer, Y.; Liu, H. Biomass gasification. Biomass Combust. Sci. Technol. Eng. 2013, 8, 106–129. [Google Scholar]
- Sikarwar, V.S.; Zhao, M.; Clough, P.; Yao, J.; Zhong, X.; Memon, M.Z.; Shah, N.; Anthony, E.J.; Fennell, P.S. An overview of advances in biomass gasification. Energy Environ. Sci. 2016, 9, 2939–2977. [Google Scholar] [CrossRef]
- Sansaniwal, S.K.; Pal, K.; Rosen, M.A.; Tyagi, S.K. Recent advances in the development of biomass gasification technology: A comprehensive review. Renew. Sustain. Energy Rev. 2017, 72, 363–384. [Google Scholar] [CrossRef]
- Dai, J.; Saayman, J.; Grace, J.R.; Ellis, N. Gasification of Woody Biomass. Annu. Rev. Chem. Biomol. Eng. 2015, 6, 77–99. [Google Scholar] [CrossRef] [PubMed]
- Kennes, D.; Abubackar, H.N.; Diaz, M.; Veiga, M.C.; Kennes, C. Bioethanol production from biomass: Carbohydrate vs syngas fermentation. J. Chem. Technol. Biotechnol. 2016, 91, 304–317. [Google Scholar] [CrossRef]
- IEA BioEnergy Agreement Task 33: Thermal Gasification of Biomass. Available online: http://task33.ieabioenergy.com/ (accessed on 1 November 2020).
- Xu, D.; Tree, D.R.; Lewis, R.S. The effects of syngas impurities on syngas fermentation to liquid fuels. Biomass Bioenergy 2011, 35, 2690–2696. [Google Scholar] [CrossRef]
- Ciferno, J.P.; Marano, J.J. Benchmarking Biomass Gasification Technologies for Fuels, Chemicals and Hydrogen Production; US DOE National Energy Technology Laboratory: Pittsburgh, PA, USA, 2002. [Google Scholar]
- Kruse, A. Supercritical water gasification. Biofuels Bioprod. Biorefining 2008, 2, 415–437. [Google Scholar] [CrossRef]
- Yang, H.; Chen, H. Biomass Gasification for Synthetic Liquid Fuel Production; All rights reserved; Woodhead Publishing Limited: Sawston, UK, 2015; ISBN 9780857098085. [Google Scholar]
- Cheng, Y.; Thow, Z.; Wang, C.H. Biomass gasification with CO2 in a fluidized bed. Powder Technol. 2016, 296, 87–101. [Google Scholar] [CrossRef]
- Ren, J.; Cao, J.P.; Zhao, X.Y.; Yang, F.L.; Wei, X.Y. Recent advances in syngas production from biomass catalytic gasification: A critical review on reactors, catalysts, catalytic mechanisms and mathematical models. Renew. Sustain. Energy Rev. 2019, 116, 109426. [Google Scholar] [CrossRef]
- Ramachandriya, K.D.; Kundiyana, D.K.; Sharma, A.M.; Kumar, A.; Atiyeh, H.K.; Huhnke, R.L.; Wilkins, M.R. Critical factors affecting the integration of biomass gasification and syngas fermentation technology. AIMS Bioeng. 2016, 3, 188–210. [Google Scholar] [CrossRef]
- Chen, W.; Annamalai, K.; Ansley, R.J.; Mirik, M. Updraft fixed bed gasification of mesquite and juniper wood samples. Energy 2012, 41, 454–461. [Google Scholar] [CrossRef]
- Huang, F.; Jin, S. Investigation of biomass (pine wood) gasification: Experiments and Aspen Plus simulation. Energy Sci. Eng. 2019, 7, 1178–1187. [Google Scholar] [CrossRef]
- Yan, Q.; Yu, F.; Liu, J.; Street, J.; Gao, J.; Cai, Z.; Zhang, J. Catalytic conversion wood syngas to synthetic aviation turbine fuels over a multifunctional catalyst. Bioresour. Technol. 2013, 127, 281–290. [Google Scholar] [CrossRef]
- Galvagno, S.; Casciaro, G.; Casu, S.; Martino, M.; Mingazzini, C.; Russo, A.; Portofino, S. Steam gasification of tyre waste, poplar, and refuse-derived fuel: A comparative analysis. Waste Manag. 2009, 29, 678–689. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, J.L.; da Silva, J.N.; Martins, M.A.; Pereira, E.G. Gasification of waste from coffee and eucalyptus production as an alternative source of bioenergy in Brazil. Sustain. Energy Technol. Assess. 2018, 27, 159–166. [Google Scholar] [CrossRef]
- Kaewluan, S.; Pipatmanomai, S. Potential of synthesis gas production from rubber wood chip gasification in a bubbling fluidised bed gasifier. In Energy Conversion and Management; Elsevier Ltd.: Amsterdam, The Netherlands, 2011; Volume 52, pp. 75–84. [Google Scholar]
- Nipattummakul, N.; Ahmed, I.I.; Kerdsuwan, S.; Gupta, A.K. Steam gasification of oil palm trunk waste for clean syngas production. Appl. Energy 2012, 92, 778–782. [Google Scholar] [CrossRef]
- Mikeska, M.; Najser, J.; Peer, V.; Frantík, J.; Kielar, J. Quality assessment of gas produced from different types of biomass pellets in gasification process. Energy Explor. Exploit. 2020, 38, 406–416. [Google Scholar] [CrossRef]
- Peng, W.X.; Wang, L.S.; Mirzaee, M.; Ahmadi, H.; Esfahani, M.J.; Fremaux, S. Hydrogen and syngas production by catalytic biomass gasification. Energy Convers. Manag. 2017, 135, 270–273. [Google Scholar] [CrossRef]
- Carpenter, D.L.; Bain, R.L.; Davis, R.E.; Dutta, A.; Feik, C.J.; Gaston, K.R.; Jablonski, W.; Phillips, S.D.; Nimlos, M.R. Pilot-scale gasification of corn stover, switchgrass, wheat straw, and wood: 1. Parametric study and comparison with literature. Ind. Eng. Chem. Res. 2010, 49, 1859–1871. [Google Scholar] [CrossRef]
- Weiland, F.; Nordwaeger, M.; Olofsson, I.; Wiinikka, H.; Nordin, A. Entrained flow gasification of torrefied wood residues. Fuel Process. Technol. 2014, 125, 51–58. [Google Scholar] [CrossRef]
- Vonk, G.; Piriou, B.; Dos Santos, P.F.; Wolbert, D.; Vaïtilingom, G. Comparative analysis of wood and solid recovered fuels gasification in a downdraft fixed bed reactor. Waste Manag. 2019, 85, 106–120. [Google Scholar] [CrossRef]
- Arena, U.; Zaccariello, L.; Mastellone, M.L. Gasification of Natural and Waste Biomass in a Pilot Scale Fluidized Bed Reactor. Combust. Sci. Technol. 2010, 182, 625–639. [Google Scholar] [CrossRef]
- Hu, P.; Chakraborty, S.; Kumar, A.; Woolston, B.; Liu, H.; Emerson, D.; Stephanopoulos, G. Integrated bioprocess for conversion of gaseous substrates to liquids. Proc. Natl. Acad. Sci. USA 2016, 113, 3773–3778. [Google Scholar] [CrossRef]
- Wu, C.Z.; Yin, X.L.; Ma, L.L.; Zhou, Z.Q.; Chen, H.P. Operational characteristics of a 1.2-MW biomass gasification and power generation plant. Biotechnol. Adv. 2009, 27, 588–592. [Google Scholar] [CrossRef] [PubMed]
- Senapati, P.K.; Behera, S. Experimental investigation on an entrained flow type biomass gasification system using coconut coir dust as powdery biomass feedstock. Bioresour. Technol. 2012, 117, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Yadav, S.; Rajesh, V.M.; Mohanty, P. Groundnut shell gasification performance in a fluidized bed gasifier with bubbling air as gasification medium. Environ. Technol. 2019, 40, 3140–3152. [Google Scholar] [CrossRef] [PubMed]
- Rapagnà, S. Steam-gasification of biomass in a fluidised-bed of olivine particles. Biomass Bioenergy 2000, 19, 187–197. [Google Scholar] [CrossRef]
- Dogru, M.; Howarth, C.R.; Akay, G.; Keskinler, B.; Malik, A.A. Gasification of hazelnut shells in a downdraft gasifier. Energy 2002, 27, 415–427. [Google Scholar] [CrossRef]
- García-Ibañez, P.; Cabanillas, A.; Sánchez, J.M. Gasification of leached orujillo (olive oil waste) in a pilot plant circulating fluidised bed reactor. Preliminary results. Biomass Bioenergy 2004, 27, 183–194. [Google Scholar] [CrossRef]
- Biagini, E.; Barontini, F.; Tognotti, L. Gasification of agricultural residues in a demonstrative plant: Corn cobs. Bioresour. Technol. 2015, 173, 110–116. [Google Scholar] [CrossRef]
- Biagini, E.; Barontini, F.; Tognotti, L. Gasification of Agricultural Residues in a Demonstrative Plant. Bioresour. Technol. 2014, 37, 110–116. [Google Scholar] [CrossRef]
- Chiodo, V.; Urbani, F.; Zafarana, G.; Prestipino, M.; Galvagno, A.; Maisano, S. Syngas production by catalytic steam gasification of citrus residues. Int. J. Hydrogen Energy 2017, 42, 28048–28055. [Google Scholar] [CrossRef]
- Maisano, S.; Urbani, F.; Cipitì, F.; Freni, F.; Chiodo, V. Syngas production by BFB gasification: Experimental comparison of different biomasses. Int. J. Hydrogen Energy 2019, 44, 4414–4422. [Google Scholar] [CrossRef]
- Erlich, C.; Fransson, T.H. Downdraft gasification of pellets made of wood, palm-oil residues respective bagasse: Experimental study. Appl. Energy 2011, 88, 899–908. [Google Scholar] [CrossRef]
- Gabra, M.; Pettersson, E.; Backman, R.; Kjellström, B. Evaluation of cyclone gasifier performance for gasification of sugar cane residue—Part 1: Gasification of bagasse. Biomass Bioenergy 2001, 21, 351–369. [Google Scholar] [CrossRef]
- Werle, S. Impact of feedstock properties and operating conditions on sewage sludge gasification in a fixed bed gasifier. Waste Manag. Res. 2014, 32, 954–960. [Google Scholar] [CrossRef]
- Xue, G.; Kwapinska, M.; Horvat, A.; Kwapinski, W.; Rabou, L.P.L.M.; Dooley, S.; Czajka, K.M.; Leahy, J.J. Gasification of torrefied Miscanthus×giganteus in an air-blown bubbling fluidized bed gasifier. Bioresour. Technol. 2014, 159, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Christodoulou, C.; Tsekos, C.; Tsalidis, G.; Fantini, M.; Panopoulos, K.D.; De Jong, W.; Kakaras, E. Attempts on cardoon gasification in two different circulating fluidized beds. Case Stud. Therm. Eng. 2014, 4, 42–52. [Google Scholar] [CrossRef]
- Torreiro, Y.; Ortiz, I.; Molina, G.; Maroño, M.; Pérez, V.; Murillo, J.M.; Ramos, R.; Fernández, M.; García, S.; Sánchez, J.M. Thermochemical assessment of Nicotiana glauca, Panicum virgatum and Elytrigia elongata as fuels for energy recovery through gasification. Fuel 2018, 225, 71–79. [Google Scholar] [CrossRef]
- Drzyzga, O.; Revelles, O.; Durante-Rodríguez, G.; Díaz, E.; García, J.L.; Prieto, A. New challenges for syngas fermentation: Towards production of biopolymers. J. Chem. Technol. Biotechnol. 2015, 90, 1735–1751. [Google Scholar] [CrossRef]
- Drake, H.L.; Gößner, A.S.; Daniel, S.L. Old acetogens, new light. Ann. N. Y. Acad. Sci. 2008, 1125, 100–128. [Google Scholar] [CrossRef]
- Min, F.; Kopke, M.; Dennis, S. Gas Fermentation for Commercial Biofuels Production. Liq. Gaseous Solid Biofuels Convers. Tech. 2013, 125–173. [Google Scholar] [CrossRef]
- Phillips, J.R.; Huhnke, R.L.; Atiyeh, H.K. Syngas fermentation: A microbial conversion process of gaseous substrates to various products. Fermentation 2017, 3, 28. [Google Scholar] [CrossRef]
- Sun, X.; Atiyeh, H.K.; Huhnke, R.L.; Tanner, R.S. Syngas fermentation process development for production of biofuels and chemicals: A review. Bioresour. Technol. Rep. 2019, 7, 100279. [Google Scholar] [CrossRef]
- Bertsch, J.; Müller, V. Bioenergetic constraints for conversion of syngas to biofuels in acetogenic bacteria. Biotechnol. Biofuels 2015, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Müller, V. Energy Conservation in Acetogenic Bacteria. Appl. Environ. Microbiol. 2003, 69, 6345–6353. [Google Scholar] [CrossRef]
- Koepke, M.; Liew, F. Recombinant microorganism and methods of production thereof. U.S. Patent Application No. 2011/0236941, 29 September 2011. [Google Scholar]
- Bengelsdorf, F.R.; Straub, M.; Dürre, P. Bacterial synthesis gas (syngas) fermentation. Environ. Technol. 2013, 34, 1639–1651. [Google Scholar] [CrossRef]
- Schuchmann, K.; Müller, V. Autotrophy at the thermodynamic limit of life: A model for energy conservation in acetogenic bacteria. Nat. Rev. Microbiol. 2014, 12, 809–821. [Google Scholar] [CrossRef]
- Balch, W.E.; Schoberth, S.; Tanner, R.S.; Wolfe, R.S. Acetobacterium, a new genus of hydrogen-oxidizing, carbon dioxide-reducing, anaerobic bacteria. Int. J. Syst. Bacteriol. 1977, 27, 355–361. [Google Scholar] [CrossRef]
- Wieringa, K.T. The formation of acetic acid from carbon dioxide and hydrogen by anaerobic spore-forming bacteria. Antonie Van Leeuwenhoek 1939, 6, 251–262. [Google Scholar] [CrossRef]
- Abrini, J.; Naveau, H.; Nyns, E. Clostridium autoethanogenum, sp. nov., an anaerobic bacterium that produces ethanol from carbon monoxide. Arch. Microbiol. 1994, 161, 345–351. [Google Scholar] [CrossRef]
- Liou, J.S.C.; Balkwill, D.L.; Drake, G.R.; Tanner, R.S. Clostridium carboxidivorans sp. nov., a solvent-producing clostridium isolated from an agricultural settling lagoon, and reclassification of the acetogen Clostridium scatologenes strain SL1 as Clostridium drakei sp. nov. Int. J. Syst. Evol. Microbiol. 2005, 55, 2085–2091. [Google Scholar] [CrossRef]
- Huhnke, R.L.; Lewis, R.S.; Tanner, R.S. Isolation and Characterization of Novel Clostridial Species. U.S. Patent 7,704,723, 27 April 2010. [Google Scholar]
- Tanner, R.S.; Miller, L.M.; Yang, D. Clostridial rRNA Homology Group I. Int. J. Syst. Bacteriol. 1993, 43, 232–236. [Google Scholar] [CrossRef]
- Ragsdale, S.W.; Pierce, E. Acetogenesis and the Wood-Ljungdahl pathway of CO2 fixation. Biochim. Biophys. Acta Proteins Proteom. 2008, 1784, 1873–1898. [Google Scholar] [CrossRef] [PubMed]
- Heiskanen, H.; Virkajärvi, I.; Viikari, L. The effect of syngas composition on the growth and product formation of Butyribacterium methylotrophicum. Enzyme Microb. Technol. 2007, 41, 362–367. [Google Scholar] [CrossRef]
- Gößner, A.S.; Picardal, F.; Tanner, R.S.; Drake, H.L. Carbon metabolism of the moderately acid-tolerant acetogen Clostridium drakei isolated from peat. FEMS Microbiol. Lett. 2008, 287, 236–242. [Google Scholar] [PubMed]
- Chang, I.S.; Kim, B.H.; Lovitt, R.W.; Bang, J.S. Effect of CO partial pressure on cell-recycled continuous CO fermentation by Eubacterium limosum KIST612. Process Biochem. 2001, 37, 411–421. [Google Scholar] [CrossRef]
- Xu, S.; Fu, B.; Zhang, L.; Liu, H. Bioconversion of H2/CO2 by acetogen enriched cultures for acetate and ethanol production: The impact of pH. World J. Microbiol. Biotechnol. 2015, 31, 941–950. [Google Scholar] [CrossRef]
- Chakraborty, S.; Rene, E.R.; Lens, P.N.L.; Veiga, M.C.; Kennes, C. Enrichment of a solventogenic anaerobic sludge converting carbon monoxide and syngas into acids and alcohols. Bioresour. Technol. 2019, 272, 130–136. [Google Scholar] [CrossRef]
- Alves, J.I.; Stams, A.J.M.; Plugge, C.M.; Madalena Alves, M.; Sousa, D.Z. Enrichment of anaerobic syngas-converting bacteria from thermophilic bioreactor sludge. FEMS Microbiol. Ecol. 2013, 86, 590–597. [Google Scholar] [CrossRef]
- Park, S.; Yasin, M.; Kim, D.; Park, H.D.; Kang, C.M.; Kim, D.J.; Chang, I.S. Rapid enrichment of (homo)acetogenic consortia from animal feces using a high mass-transfer gas-lift reactor fed with syngas. J. Ind. Microbiol. Biotechnol. 2013, 40, 995–1003. [Google Scholar] [CrossRef]
- Singla, A.; Verma, D.; Lal, B.; Sarma, P.M. Enrichment and optimization of anaerobic bacterial mixed culture for conversion of syngas to ethanol. Bioresour. Technol. 2014, 172, 41–49. [Google Scholar] [CrossRef]
- Buschhorn, H.; Durre, P.; Gottschalk, G. Production and utilization of ethanol by the homoacetogen Acetobacterium woodii. Appl. Environ. Microbiol. 1989, 55, 1835–1840. [Google Scholar] [CrossRef]
- Braun, M.; Mayer, F.; Gottschalk, G. Clostridium aceticum (Wieringa), a microorganism producing acetic acid from molecular hydrogen and carbon dioxide. Arch. Microbiol. 1981, 128, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.R.; Atiyeh, H.K.; Tanner, R.S.; Torres, J.R.; Saxena, J.; Wilkins, M.R.; Huhnke, R.L. Butanol and hexanol production in Clostridium carboxidivorans syngas fermentation: Medium development and culture techniques. Bioresour. Technol. 2015, 190, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Ramió-Pujol, S.; Ganigué, R.; Bañeras, L.; Colprim, J. Incubation at 25 °C prevents acid crash and enhances alcohol production in Clostridium carboxidivorans P7. Bioresour. Technol. 2015, 192, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Richter, H.; Molitor, B.; Wei, H.; Chen, W.; Aristilde, L.; Angenent, L.T. Ethanol production in syngas-fermenting: Clostridium ljungdahlii is controlled by thermodynamics rather than by enzyme expression. Energy Environ. Sci. 2016, 9, 2392–2399. [Google Scholar] [CrossRef]
- Allen, T.D.; Caldwell, M.E.; Lawson, P.A.; Huhnke, R.L.; Tanner, R.S. Alkalibaculum bacchi gen. nov., sp. nov., a CO-oxidizing, ethanol-producing acetogen isolated from livestock-impacted soil. Int. J. Syst. Evol. Microbiol. 2010, 60, 2483–2489. [Google Scholar] [CrossRef] [PubMed]
- Cardon, B.P.; Barker, H.A. Two New Amino-Acid-Fermenting Bacteria, Clostridium propionicum and Diplococcus glycinophilus. J. Bacteriol. 1946, 52, 629–634. [Google Scholar] [CrossRef]
- Liu, K.; Atiyeh, H.K.; Stevenson, B.S.; Tanner, R.S.; Wilkins, M.R.; Huhnke, R.L. Mixed culture syngas fermentation and conversion of carboxylic acids into alcohols. Bioresour. Technol. 2014, 152, 337–346. [Google Scholar] [CrossRef]
- Diender, M.; Stams, A.J.M.; Sousa, D.Z. Production of medium-chain fatty acids and higher alcohols by a synthetic co-culture grown on carbon monoxide or syngas. Biotechnol. Biofuels 2016, 9, 1–11. [Google Scholar] [CrossRef]
- Richter, H.; Molitor, B.; Diender, M.; Sousa, D.Z.; Angenent, L.T. A narrow pH range supports butanol, hexanol, and octanol production from syngas in a continuous co-culture of Clostridium ljungdahlii and Clostridium kluyveri with in-line product extraction. Front. Microbiol. 2016, 7, 1773. [Google Scholar] [CrossRef]
- Liew, F.; Koepke, M. Recombinant microorganisms make biodiesel. U.S. Patent 9,347,076, 21 June 2013. [Google Scholar]
- Liew, F.M.; Martin, M.E.; Tappel, R.C.; Heijstra, B.D.; Mihalcea, C.; Köpke, M. Gas Fermentation—A flexible platform for commercial scale production of low-carbon-fuels and chemicals from waste and renewable feedstocks. Front. Microbiol. 2016, 7, 694. [Google Scholar] [CrossRef]
- Abubackar, H.N.; Veiga, M.C.; Kennes, C. Biological conversion of carbon monoxide to ethanol: Effect of pH, gas pressure, reducing agent and yeast extract. Bioresour. Technol. 2012, 114, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Ahn, B.; Kim, Y.K. Growth enhancement of bioethanol-producing microbe Clostridium autoethanogenum by changing culture medium composition. Bioresour. Technol. Reports 2019, 6, 237–240. [Google Scholar] [CrossRef]
- Barik, S.; Prieto, S.; Harrison, S.B.; Clausen, E.C.; Gaddy, J.L. Biological production of alcohols from coal through indirect liquefaction - Scientific note. Appl. Biochem. Biotechnol. 1988, 18, 363–378. [Google Scholar] [CrossRef]
- Cotter, J.L.; Chinn, M.S.; Grunden, A.M. Ethanol and acetate production by Clostridium ljungdahlii and Clostridium autoethanogenum using resting cells. Bioprocess Biosyst. Eng. 2009, 32, 369–380. [Google Scholar] [CrossRef]
- Abubackar, H.N.; Veiga, M.C.; Kennes, C. Carbon monoxide fermentation to ethanol by Clostridium autoethanogenum in a bioreactor with no accumulation of acetic acid. Bioresour. Technol. 2015, 186, 122–127. [Google Scholar] [CrossRef]
- Saxena, J.; Tanner, R.S. Effect of trace metals on ethanol production from synthesis gas by the ethanologenic acetogen, Clostridium ragsdalei. J. Ind. Microbiol. Biotechnol. 2011, 38, 513–521. [Google Scholar] [CrossRef]
- Shen, Y.; Brown, R.C.; Wen, Z. Syngas fermentation by Clostridium carboxidivorans P7 in a horizontal rotating packed bed biofilm reactor with enhanced ethanol production. Appl. Energy 2017, 187, 585–594. [Google Scholar] [CrossRef]
- Li, D.; Meng, C.; Wu, G.; Xie, B.; Han, Y.; Guo, Y.; Song, C.; Gao, Z.; Huang, Z. Effects of zinc on the production of alcohol by Clostridium carboxidivorans P7 using model syngas. J. Ind. Microbiol. Biotechnol. 2018, 45, 61–69. [Google Scholar] [CrossRef]
- Sim, J.H.; Kamaruddin, A.H. Optimization of acetic acid production from synthesis gas by chemolithotrophic bacterium-Clostridium aceticum using statistical approach. Bioresour. Technol. 2008, 99, 2724–2735. [Google Scholar] [CrossRef]
- Abubackar, H.N.; Veiga, M.C.; Kennes, C. Biological conversion of carbon monoxide: Rich syngas or waste gases to bioethanol. Biofuels Bioprod. Biorefining 2011, 5, 93–114. [Google Scholar] [CrossRef]
- Kundiyana, D.K.; Huhnke, R.L.; Maddipati, P.; Atiyeh, H.K.; Wilkins, M.R. Feasibility of incorporating cotton seed extract in Clostridium strain P11 fermentation medium during synthesis gas fermentation. Bioresour. Technol. 2010, 101, 9673–9680. [Google Scholar] [CrossRef] [PubMed]
- Maddipati, P.; Atiyeh, H.K.; Bellmer, D.D.; Huhnke, R.L. Ethanol production from syngas by Clostridium strain P11 using corn steep liquor as a nutrient replacement to yeast extract. Bioresour. Technol. 2011, 102, 6494–6501. [Google Scholar] [CrossRef] [PubMed]
- Saxena, J.; Tanner, R.S. Optimization of a corn steep medium for production of ethanol from synthesis gas fermentation by Clostridium ragsdalei. World J. Microbiol. Biotechnol. 2012, 28, 1553–1561. [Google Scholar] [CrossRef]
- Ramachandriya, K.D.; Kundiyana, D.K.; Wilkins, M.R.; Terrill, J.B.; Atiyeh, H.K.; Huhnke, R.L. Carbon dioxide conversion to fuels and chemicals using a hybrid green process. Appl. Energy 2013, 112, 289–299. [Google Scholar] [CrossRef]
- Shen, S.; Gu, Y.; Chai, C.; Jiang, W.; Zhuang, Y.; Wang, Y. Enhanced alcohol titre and ratio in carbon monoxide-rich off-gas fermentation of Clostridium carboxidivorans through combination of trace metals optimization with variable-temperature cultivation. Bioresour. Technol. 2017, 239, 236–243. [Google Scholar] [CrossRef]
- Fernández-Naveira, Á.; Abubackar, H.N.; Veiga, M.C.; Kennes, C. Efficient butanol-ethanol (B-E) production from carbon monoxide fermentation by Clostridium carboxidivorans. Appl. Microbiol. Biotechnol. 2016, 100, 3361–3370. [Google Scholar] [CrossRef]
- Arslan, K.; Bayar, B.; Nalakath Abubackar, H.; Veiga, M.C.; Kennes, C. Solventogenesis in Clostridium aceticum producing high concentrations of ethanol from syngas. Bioresour. Technol. 2019, 292. [Google Scholar] [CrossRef]
- Abubackar, H.N.; Bengelsdorf, F.R.; Dürre, P.; Veiga, M.C.; Kennes, C. Improved operating strategy for continuous fermentation of carbon monoxide to fuel-ethanol by clostridia. Appl. Energy 2016, 169, 210–217. [Google Scholar] [CrossRef]
- Martin, M.E.; Richter, H.; Saha, S.; Angenent, L.T. Traits of selected Clostridium strains for syngas fermentation to ethanol. Biotechnol. Bioeng. 2016, 113, 531–539. [Google Scholar] [CrossRef]
- Abubackar, H.N.; Veiga, M.C.; Kennes, C. Production of acids and alcohols from syngas in a two-stage continuous fermentation process. Bioresour. Technol. 2018, 253, 227–234. [Google Scholar] [CrossRef]
- Richter, H.; Martin, M.E.; Angenent, L.T. A two-stage continuous fermentation system for conversion of syngas into ethanol. Energies 2013, 6, 3987–4000. [Google Scholar] [CrossRef]
- Hurst, K.M.; Lewis, R.S. Carbon monoxide partial pressure effects on the metabolic process of syngas fermentation. Biochem. Eng. J. 2010, 48, 159–165. [Google Scholar] [CrossRef]
- Sim, J.H.; Kamaruddin, A.H.; Long, W.S.; Najafpour, G. Clostridium aceticum-A potential organism in catalyzing carbon monoxide to acetic acid: Application of response surface methodology. Enzyme Microb. Technol. 2007, 40, 1234–1243. [Google Scholar] [CrossRef]
- Mayer, A.; Schädler, T.; Trunz, S.; Stelzer, T.; Weuster-Botz, D. Carbon monoxide conversion with Clostridium aceticum. Biotechnol. Bioeng. 2018, 115, 2740–2750. [Google Scholar] [CrossRef] [PubMed]
- Demler, M.; Weuster-Botz, D. Reaction engineering analysis of hydrogenotrophic production of acetic acid by Acetobacterium woodii. Biotechnol. Bioeng. 2011, 108, 470–474. [Google Scholar] [CrossRef] [PubMed]
- Najafpour, G.; Younesi, H. Ethanol and acetate synthesis from waste gas using batch culture of Clostridium ljungdahlii. Enzyme Microb. Technol. 2006, 38, 223–228. [Google Scholar] [CrossRef]
- Klasson, K.T.; Ackerson, M.D.; Clausen, E.C.; Gaddy, J.L. Bioreactor design for synthesis gas fermentations. Fuel 1991, 70, 605–614. [Google Scholar] [CrossRef]
- Mohammadi, M.; Younesi, H.; Najafpour, G.; Mohamed, A.R. Sustainable ethanol fermentation from synthesis gas by Clostridium ljungdahlii in a continuous stirred tank bioreactor. J. Chem. Technol. Biotechnol. 2012, 87, 837–843. [Google Scholar] [CrossRef]
- Younesi, H.; Najafpour, G.; Mohamed, A.R. Liquid fuel production from synthesis gas via fermentation process in a continuous tank bioreactor (CSTBR) using Clostridium ljungdahlii. Iran. J. Biotechnol. 2006, 4, 45–53. [Google Scholar]
- Methods for Increasing the Production of Ethanol from Microbial Fermentation. U.S. Patent Application No. 20030211585, 23 October 2007. Available online: https://patents.google.com/patent/US20030211585A1/en (accessed on 1 November 2020).
- Acharya, B.; Dutta, A.; Basu, P. Ethanol production by syngas fermentation in a continuous stirred tank bioreactor using Clostridium ljungdahlii. Biofuels 2019, 10, 221–237. [Google Scholar] [CrossRef]
- Ahmed, A.; Cateni, B.G.; Huhnke, R.L.; Lewis, R.S. Effects of biomass-generated producer gas constituents on cell growth, product distribution and hydrogenase activity of Clostridium carboxidivorans P7T. Biomass Bioenergy 2006, 30, 665–672. [Google Scholar] [CrossRef]
- Ukpong, M.N.; Atiyeh, H.K.; De Lorme, M.J.M.; Liu, K.; Zhu, X.; Tanner, R.S.; Wilkins, M.R.; Stevenson, B.S. Physiological response of Clostridium carboxidivorans during conversion of synthesis gas to solvents in a gas-fed bioreactor. Biotechnol. Bioeng. 2012, 109, 2720–2728. [Google Scholar] [CrossRef]
- Heffernan, J.K.; Valgepea, K.; de Souza Pinto Lemgruber, R.; Casini, I.; Plan, M.; Tappel, R.; Simpson, S.D.; Köpke, M.; Nielsen, L.K.; Marcellin, E. Enhancing CO2-Valorization Using Clostridium autoethanogenum for Sustainable Fuel and Chemicals Production. Front. Bioeng. Biotechnol. 2020, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Atiyeh, H.K.; Zhang, H.; Tanner, R.S.; Huhnke, R.L. Enhanced ethanol production from syngas by Clostridium ragsdalei in continuous stirred tank reactor using medium with poultry litter biochar. Appl. Energy 2019, 236, 1269–1279. [Google Scholar] [CrossRef]
- Kundiyana, D.K.; Huhnke, R.L.; Wilkins, M.R. Syngas fermentation in a 100-L pilot scale fermentor: Design and process considerations. J. Biosci. Bioeng. 2010, 109, 492–498. [Google Scholar] [CrossRef]
- Asimakopoulos, K.; Gavala, H.N.; Skiadas, I.V. Reactor systems for syngas fermentation processes: A review. Chem. Eng. J. 2018, 348, 732–744. [Google Scholar] [CrossRef]
- Datar, R.P.; Shenkman, R.M.; Cateni, B.G.; Huhnke, R.L.; Lewis, R.S. Fermentation of biomass-generated producer gas to ethanol. Biotechnol. Bioeng. 2004, 86, 587–594. [Google Scholar] [CrossRef]
- Rajagopalan, S.; Datar, R.P.; Lewis, R.S. Formation of ethanol from carbon monoxide via a new microbial catalyst. Biomass Bioenergy 2002, 23, 487–493. [Google Scholar] [CrossRef]
- Devarapalli, M.; Atiyeh, H.K.; Phillips, J.R.; Lewis, R.S.; Huhnke, R.L. Ethanol production during semi-continuous syngas fermentation in a trickle bed reactor using Clostridium ragsdalei. Bioresour. Technol. 2016, 209, 56–65. [Google Scholar] [CrossRef]
- Biological Production of Ethanol from Waste Gases with Clostridium ljungdahlii. U.S. Patent 6136577, 24 October 2000. Available online: https://patents.google.com/patent/US6136577A/en (accessed on 1 November 2020).
- Riegler, P.; Bieringer, E.; Chrusciel, T.; Stärz, M.; Löwe, H.; Weuster-Botz, D. Continuous conversion of CO2/H2 with Clostridium aceticum in biofilm reactors. Bioresour. Technol. 2019, 291, 121760. [Google Scholar] [CrossRef]
- Yasin, M.; Jeong, Y.; Park, S.; Jeong, J.; Lee, E.Y.; Lovitt, R.W.; Kim, B.H.; Lee, J.; Chang, I.S. Microbial synthesis gas utilization and ways to resolve kinetic and mass-transfer limitations. Bioresour. Technol. 2015, 177, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Modular Membrane Supported Bioreactor for Conversion of Syngas Components to Liquid Products. U.S. Patent 8,828,692, 9 September 2014.
- Datta, R.; Tsai, S.-P.; Basu, R.; Yoon, S.-H. Membrane Supported Bioreactor for Conversion of Syngas Components to Liquid Products 2009. U.S. Patent Application No. 20090017514, 15 January 2009. [Google Scholar]
- Lee, P. Syngas fermentation to ethanol using innovative hollow fiber membrane. Grad. Diss. 2010. [Google Scholar] [CrossRef][Green Version]
- Shen, Y.; Brown, R.; Wen, Z. Syngas fermentation of Clostridium carboxidivoran P7 in a hollow fiber membrane biofilm reactor: Evaluating the mass transfer coefficient and ethanol production performance. Biochem. Eng. J. 2014, 85, 21–29. [Google Scholar] [CrossRef]
- Kantzow, C.; Mayer, A.; Weuster-Botz, D. Continuous gas fermentation by Acetobacterium woodii in a submerged membrane reactor with full cell retention. J. Biotechnol. 2015, 212, 11–18. [Google Scholar] [CrossRef]
- Roy, S.; Bauer, T.; Al-Dahhan, M.; Lehner, P.; Turek, T. Monoliths as multiphase reactors: A review. AIChE J. 2004, 50, 2918–2938. [Google Scholar] [CrossRef]
- Shen, Y.; Brown, R.; Wen, Z. Enhancing mass transfer and ethanol production in syngas fermentation of Clostridium carboxidivorans P7 through a monolithic biofilm reactor. Appl. Energy 2014, 136, 68–76. [Google Scholar] [CrossRef]
- Devarapalli, M.; Lewis, R.S.; Atiyeh, H.K. Continuous ethanol production from synthesis gas by Clostridium ragsdalei in a trickle-bed reactor. Fermentation 2017, 3, 23. [Google Scholar] [CrossRef]
- Daniell, J.; Köpke, M.; Simpson, S.D. Commercial biomass syngas fermentation. Energies 2012, 5, 5372–5417. [Google Scholar] [CrossRef]
- Trevethick, S.R.; Bromley, J.C.; Simpson, S.D.; Khosla, V.; Lanza Tech New Zealand Ltd. Fermentation of Gaseous Substrates. U.S. Patent 8,178,330, 15 May 2012. [Google Scholar]
- Li, X.; Cossey, B.J.; Trevethick, S.R.; LanzaTech New Zealand Ltd. Fermentation of Gaseous Substrates. U.S. Patent 9,617,509, 11 April 2017. [Google Scholar]
- Fermentation of Gaseous Substrates. U.S. Patent 9617509B2. Available online: https://patents.google.com/patent/US9617509B2/en (accessed on 21 November 2020).
- Aemetis Completes Operation of Cellulosic Ethanol demo Unit; 12 Mgpy Plant for 2019-Green Car Congress. Available online: https://www.greencarcongress.com/2018/03/20180309-aemetis.html (accessed on 1 November 2020).
- Anis, S.; Zainal, Z.A. Tar reduction in biomass producer gas via mechanical, catalytic and thermal methods: A review. Renew. Sustain. Energy Rev. 2011, 15, 2355–2377. [Google Scholar] [CrossRef]
- Xu, D.; Lewis, R.S. Syngas fermentation to biofuels: Effects of ammonia impurity in raw syngas on hydrogenase activity. Biomass Bioenergy 2012, 45, 303–310. [Google Scholar] [CrossRef]
- Ahmed, A.; Lewis, R.S. Fermentation of biomass-generated synthesis gas: Effects of nitric oxide. Biotechnol. Bioeng. 2007, 97, 1080–1086. [Google Scholar] [CrossRef]
- Oswald, F.; Zwick, M.; Omar, O.; Hotz, E.N.; Neumann, A. Growth and product formation of Clostridium ljungdahlii in presence of cyanide. Front. Microbiol. 2018, 9, 1–12. [Google Scholar] [CrossRef]
- Asadullah, M. Biomass gasification gas cleaning for downstream applications: A comparative critical review. Renew. Sustain. Energy Rev. 2014, 40, 118–132. [Google Scholar] [CrossRef]
- Kumar, A.; Jones, D.D.; Hanna, M.A. Thermochemical biomass gasification: A review of the current status of the technology. Energies 2009, 2, 556–581. [Google Scholar] [CrossRef]
- Huber, G.W.; Iborra, S.; Corma, A. Synthesis of transportation fuels from biomass: Chemistry, catalysts, and engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef]
- Woolcock, P.J.; Brown, R.C. A review of cleaning technologies for biomass-derived syngas. Biomass Bioenergy 2013, 52, 54–84. [Google Scholar] [CrossRef]
- Abdoulmoumine, N.; Adhikari, S.; Kulkarni, A.; Chattanathan, S. A review on biomass gasification syngas cleanup. Appl. Energy 2015, 155, 294–307. [Google Scholar] [CrossRef]
- Zwart, R.W.R. Gas cleaning downstream biomass gasification. Sent. Energy Res. Center Neth. 2009. [Google Scholar] [CrossRef]
- Vasudevan, D.; Richter, H.; Angenent, L.T. Upgrading dilute ethanol from syngas fermentation to n-caproate with reactor microbiomes. Bioresour. Technol. 2014, 151, 378–382. [Google Scholar] [CrossRef]
- Oswald, F.; Dörsam, S.; Veith, N.; Zwick, M.; Neumann, A.; Ochsenreither, K.; Syldatk, C. Sequential mixed cultures: From syngas to malic acid. Front. Microbiol. 2016, 7, 1–12. [Google Scholar] [CrossRef]
- Lagoa-Costa, B.; Abubackar, H.N.; Fernández-Romasanta, M.; Kennes, C.; Veiga, M.C. Integrated bioconversion of syngas into bioethanol and biopolymers. Bioresour. Technol. 2017, 239, 244–249. [Google Scholar] [CrossRef]
- Perez, J.M.; Richter, H.; Loftus, S.E.; Angenent, L.T. Biocatalytic reduction of short-chain carboxylic acids into their corresponding alcohols with syngas fermentation. Biotechnol. Bioeng. 2013, 110, 1066–1077. [Google Scholar] [CrossRef]
- Isom, C.E.; Nanny, M.A.; Tanner, R.S. Improved conversion efficiencies for n-fatty acid reduction to primary alcohols by the solventogenic acetogen “Clostridium ragsdalei”. J. Ind. Microbiol. Biotechnol. 2015, 42, 29–38. [Google Scholar] [CrossRef]
- Hwang, H.W.; Yoon, J.; Min, K.; Kim, M.S.; Kim, S.J.; Cho, D.H.; Susila, H.; Na, J.G.; Oh, M.K.; Kim, Y.H. Two-stage bioconversion of carbon monoxide to biopolymers via formate as an intermediate. Chem. Eng. J. 2020, 389, 124394. [Google Scholar] [CrossRef]
- Diender, M.; Stams, A.J.M.; Sousa, D.Z. Pathways and bioenergetics of anaerobic carbon monoxide fermentation. Front. Microbiol. 2015, 6, 1–18. [Google Scholar] [CrossRef]
- Choi, D.; Chipman, D.C.; Bents, S.C.; Brown, R.C. A techno-economic analysis of polyhydroxyalkanoate and hydrogen production from syngas fermentation of gasified biomass. Appl. Biochem. Biotechnol. 2010, 160, 1032–1046. [Google Scholar] [CrossRef]
- Kerby, R.L.; Hong, S.S.; Ensign, S.A.; Coppoc, L.J.; Ludden, P.W.; Roberts, G.P. Genetic and physiological characterization of the Rhodospirillum rubrum carbon monoxide dehydrogenase system. J. Bacteriol. 1992, 174, 5284–5294. [Google Scholar] [CrossRef]
- Do, Y.S.; Smeenk, J.; Broer, K.M.; Kisting, C.J.; Brown, R.; Heindel, T.J.; Bobik, T.A.; DiSpirito, A.A. Growth of Rhodospirillum rubrum on synthesis gas: Conversion of CO to H2 and poly-β-hydroxyalkanoate. Biotechnol. Bioeng. 2007, 97, 279–286. [Google Scholar] [CrossRef]
- Najafpour, G.D.; Younesi, H. Bioconversion of synthesis gas to hydrogen using a light-dependent photosynthetic bacterium, Rhodospirillum rubrum. World J. Microbiol. Biotechnol. 2007, 23, 275–284. [Google Scholar] [CrossRef]
- Najafpour, G.; Younesi, H.; Mohamed, A.R. Effect of organic substrate on hydrogen production from synthesis gas using Rhodospirillum rubrum, in batch culture. Biochem. Eng. J. 2004, 21, 123–130. [Google Scholar] [CrossRef]
- Revelles, O.; Tarazona, N.; García, J.L.; Prieto, M.A. Carbon roadmap from syngas to polyhydroxyalkanoates in Rhodospirillum rubrum. Environ. Microbiol. 2016, 18, 708–720. [Google Scholar] [CrossRef] [PubMed]
- Brandl, H.; Knee, E.J.; Fuller, R.C.; Gross, R.A.; Lenz, R.W. Ability of the phototrophic bacterium Rhodospirillum rubrum to produce various poly (β-hydroxyalkanoates): Potential sources for biodegradable polyesters. Int. J. Biol. Macromol. 1989, 11, 49–55. [Google Scholar] [CrossRef]
- Karmann, S.; Panke, S.; Zinn, M. Fed-batch cultivations of rhodospirillum rubrum under multiple nutrient-limited growth conditions on syngas as a novel option to produce poly(3-hydroxybutyrate) (PHB). Front. Bioeng. Biotechnol. 2019, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Najafpour, G.; Younesi, H.; Mohamed, A.R. Continuous hydrogen production via fermentation of synthesis gas. Petrol Coal. Pet. Coal 2013, 45, 154–158. [Google Scholar]
- Younesi, H.; Najafpour, G.; Ku Ismail, K.S.; Mohamed, A.R.; Kamaruddin, A.H. Biohydrogen production in a continuous stirred tank bioreactor from synthesis gas by anaerobic photosynthetic bacterium: Rhodopirillum rubrum. Bioresour. Technol. 2008, 99, 2612–2619. [Google Scholar] [CrossRef]
- Sugimoto, T.; Tsuge, T.; Tanaka, K.; Ishizaki, A. Control of acetic acid concentration by pH-Stat continuous substrate feeding in heterotrophic culture phase of two-stage cultivation of Alcaligenes eutrophus for production of P(3HB) from CO2, H2, and O2 under non-explosive conditions. Biotechnol. Bioeng. 1999, 62, 625–631. [Google Scholar] [CrossRef]
- Ishizaki, A.; Tanaka, K.; Taga, N. Microbial production of poly-D-3-hydroxybutyrate from CO2x. Appl. Microbiol. Biotechnol. 2001, 57, 6–12. [Google Scholar]
- Ishizaki, A.; Taga, N.; Takeshita, T.; Sugimoto, T.; Tsuge, T.; Tanaka, K. Microbial Production of Biodegradable Plastics from Carbon Dioxide and Agricultural Waste Material. ACS Symp. Ser. 1997, 666, 294–306. [Google Scholar]
- Taga, N.; Tanaka, K.; Ishizaki, A. Effects of rheological change by addition of carboxymethylcellulose in culture media of an air-lift fermentor on poly-D-3-hydroxybutyric acid productivity in autotrophic culture of hydrogen-oxidizing bacterium, alcaligenes eutrophus. Biotechnol. Bioeng. 1997, 53, 529–533. [Google Scholar] [CrossRef]
- Volova, T.G.; Kalacheva, G.S.; Altukhova, O.V. Autotrophic synthesis of polyhydroxyalkanoates by the bacteria Ralstonia eutropha in the presence of carbon monoxide. Appl. Microbiol. Biotechnol. 2002, 58, 675–678. [Google Scholar]
- Heinrich, D.; Raberg, M.; Steinbüchel, A. Studies on the aerobic utilization of synthesis gas (syngas) by wild type and recombinant strains of Ralstonia eutropha H16. Microb. Biotechnol. 2018, 11, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Fuhrmann, S.; Ferner, M.; Jeffke, T.; Henne, A.; Gottschalk, G.; Meyer, O. Complete nucleotide sequence of the circular megaplasmid pHCG3 of Oligotropha carboxidovorans: Function in the chemolithoautotrophic utilization of CO, H2 and CO2. Gene 2003, 322, 67–75. [Google Scholar] [CrossRef]
- Paul, D.; Bridges, S.M.; Burgess, S.C.; Dandass, Y.S.; Lawrence, M.L. Complete genome and comparative analysis of the chemolithoautotrophic bacterium Oligotropha carboxidovorans OM5. BMC Genom. 2010, 11, 511. [Google Scholar] [CrossRef][Green Version]
- “SYNPOL.”. Available online: http://www.synpol.org/ (accessed on 21 November 2020).




| Feedstock | Syngas Composition (% v/v) | Gasifier Type | Gasification Conditions | Reference | |||
|---|---|---|---|---|---|---|---|
| H2 | CO | CO2 | CH4 | ||||
| Mesquite wood | 1.6–3.0 | 13.0–21.0 | 11.0–25.0 | 1.0–1.5 | Fixed bed gasifier | GA: air; T: 782 °C; ER: 2.70 | [38] |
| Juniper wood | 2.5–3.5 | 21.0–25.0 | 9.0–12.0 | 1.5–1.8 | Fixed bed gasifier | GA: air; T: 713 °C; ER: 2.70 | [38] |
| Pine wood | 30.5 | 52.8 | 14.7 | 2.0 | Downdraft fixed bed gasifier | GA: steam; T: 900 °C; ER: N.A. | [39] |
| Oak wood | 18.0 | 21.0 | 12.0 | 2.0 | Downdraft fixed bed gasifier | GA: air; T: N.A.; ER: N.A. | [40] |
| Poplar wood | 45.5 | 23.1 | 20.8 | 8.6 | Rotary kiln reactor | GA: steam; T: 1500 °C; ER: N.A. | [41] |
| Eucalyptus wood | 10.7 | 20.2 | 9.1 | 8.6 | Downdraft fixed bed gasifier | GA: air; T: 865 °C; ER: 0.31 | [42] |
| Coffee wood | 12.4 | 14.0 | 10.4 | 6.5 | Downdraft fixed bed gasifier | GA: air; T: 813 °C; ER: 0.32 | [42] |
| Rubber wood | 6.0–8.0 | 10.0–14.0 | 16.0–18.0 | N.A. | Bubbling fluidized bed gasifier | GA: air; T: 750–900 °C; ER: 0.38 | [43] |
| Oil palm wood | 60.0–70.0 | 10.0–30.0 | 20.0–50.0 | 5.0–10.0 | N.A. | GA: steam; T: 800 °C; ER: N.A. | [44] |
| Spruce wood | 10.7 | 25.9 | 9.7 | 3.8 | Fixed bed reactor | GA: air; T: 800 °C; ER: N.A. | [45] |
| Wood residue | 42.5 | 23.0 | 18.1 | 11.5 | Fluidized bed gasifier | GA: air; T: 823 °C; ER: 0.17 | [46] |
| Vermont wood a | 28.6 | 23.5 | 24.0 | 15.5 | Fluidized bed gasifier | GA: steam; T: 600–710 °C; ER: N.A. | [47] |
| Wood residue b | 26.2–28.0 | 50.0–60.3 | 12.7–23.3 | 0.9–1.8 | Entrained flow gasifier | GA: oxygen; T: 1200–1500 °C; ER: 0.44 | [48] |
| SRF wood c | 15.7–16.5 | 15.9–17.2 | 14.3–15.1 | 2.6–2.7 | Downdraft fixed bed reactor | GA: air; T: 650–800 °C; ER: 0.25–0.26 | [49] |
| Wood waste d | 9.4–14.8 | 15.1–19.4 | 11.0–15.8 | 3.2–4.3 | Bubbling fluidized bed gasifier | GA: air/air and steam mixture; T: 850 °C; ER: 0.20–0.29 | [50] |
| Feedstock | Syngas Composition (% v/v) | Gasifier Type | Gasification Conditions | Ref. | |||
|---|---|---|---|---|---|---|---|
| H2 | CO | CO2 | CH4 | ||||
| Corn straw | 48.5 | 33.9 | 12.2 | 5.3 | N.A. | GA: N.A.; T: 750–900 °C; ER: N.A. | [51] |
| Wheat straw | 25.4 | 27.5 | 22.0 | 16.3 | Fluidized bed gasifier | GA: steam; T: 600–710 °C; ER: N.A. | [47] |
| Rice husk | 5.0–8.0 | 16.0–21.0 | 15.0–16.0 | 46.0 | Fluidized bed gasifier | GA: air; T: 700–800 °C; ER: 0.18–0.27 | [52] |
| Coffee husk | 6.6 | 13.8 | 12.1 | 14.8 | Downdraft fixed bed gasifier | GA: air; T: 669 °C; ER: 0.12 | [42] |
| Coconut coir | 7.0–21.4 | 18.6–20.3 | 19.1–21.3 | 6.1–9.0 | Entrained flow reactor | GA: air; T: 726–941 °C; ER: 0.21–0.30 | [53] |
| Groundnut shells | 13.8 | 13.0 | 13.5 | 5.7 | Bubbling fluidized bed gasifier | GA: air; T: 714.4 °C; ER: 0.31 | [54] |
| Almond shells | 34.2–39.6 | 17.8–23.2 | 10.7–16.8 | N.A. | Bubbling fluidized bed reactor | GA: N.A.; T: 820 °C; ER: N.A. | [55] |
| Hazelnut shells | 11.1–14.7 | 8.6–20.7 | 9.5–16.3 | 1.4–2.5 | Downdraft fixed bed gasifier | GA: air; T: 1000–1050 °C; ER: N.A. | [56] |
| Hay | 8.8 | 19.7 | 14.4 | 3.0 | Fixed bed reactor | GA: air; T: 800 °C; ER: N.A. | [45] |
| Corn stover | 26.9 | 24.7 | 23.7 | 15.3 | Fluidized bed gasifier | GA: steam; T: 600–710 °C; ER: N.A. | [47] |
| Olive kernels | 5.4–9.3 | 6.9–8.6 | 19.0–21.7 | 1.8–3.0 | Circulating fluidized bed gasifier | GA: air; T: 800 °C; ER: 0.4–0.7 | [57] |
| Vine pruning | 17.1–18.4 | 21.3–21.7 | 11.3–13.0 | 2.1–2.6 | Downdraft fixed bed reactor | GA: air; T: N.A.; ER: 0.26 | [58] |
| Corncobs | 17.3 | 22.6 | 12.0 | 1.98 | Downdraft fixed bed reactor | GA: air; T: N.A.; ER: 0.28 | [59] |
| Citrus peels | 60.0–65.0 | 15.0–25.0 | 15.0–23.0 | <5.0 | Fixed bed gasifier | GA: steam; T: 750 °C; ER: N.A. | [60] |
| Posidonia oceanica | 11.8–24.9 | 4.1–12.7 | 14.1–20.0 | 2.0–3.0 | Fluidized bed gasifier | GA: air; T: 750 °C; ER: 0.3 | [61] |
| Empty fruit brunch | 12.9–13.5 | 17.0–17.4 | 13.7–14.5 | 1.5–1.9 | Downdraft fixed bed gasifier | GA: air; T: 650–825 °C; ER: N.A. | [62] |
| Sugarcane bagasse | 7.4–8.0 | 8.0–12.9 | 15.9–18.7 | 1.4–2.5 | Cyclone gasifier | GA: air; T: 600–950 °C; ER: 0.18–0.25 | [63] |
| Sewage sludge | 5.1–8.1 | 19.5–31.6 | 13.3–16.5 | 0.9–1.5 | Fixed-bed gasifier | GA: air; T: 650–1100 °C; ER: 0.12–0.27 | [64] |
| Miscanthus X giganteus | 8.6 | 16.4 | 14.0 | 4.4 | Bubbling fluidized bed reactor | GA: air; T: 800 °C; ER: 0.21 | [65] |
| Switchgrass (Panicum vigatum) | 23.5 | 33.2 | 19.4 | 17.0 | Fluidized bed gasifier | GA: steam; T: 600–710 °C; ER: N.A. | [47] |
| Thistle (Cynara cardunculus L.) | 36.6 | 8.5 | 50.4 | 4.5 | Circulating fluidized bed gasifier | GA: steam and oxygen; T: 750 °C; ER: 0.3 | [66] |
| Wheatgrass (Elytrigia elongata) | 10.8 | 12.3 | 16.5 | 5.3 | Bubbling fluidized bed gasifier | GA: oxygen-enriched air; T: 800 °C; ER: N.A. | [67] |
| Reactor Configuration | Syngas Composition | Operative Conditions | Microorganism | Products | Products Concentration-Productivity | Reference |
|---|---|---|---|---|---|---|
| CSTR | 19% CO, 77% H2, 4% CH4 | T: 38 °C pH: 5.0 Agitation rate: 1000 rpm | C. ljungdahlii | Ethanol | 10.00 g/L 6.70 g/L/day | [134] |
| CSTR (2 L) | 55% CO, 20% H2, 10% CO2, 15% Ar | T: 37 °C pHi: 6.8 Agitation rate: 500 rpm GFR: 14 mL/min KLa: 135 h−1 | C. ljungdahlii | Ethanol Acetate | 6.50 g/L 5.43 g/L | [132,133] |
| CSTR (3 L) | 60% CO, 35% H2, 5% CO2 | T: 37 °C pH: 4.0–4.8 Agitation rate: 300–500 rpm GFR: 5–15 mL/min LFR: 0.25–0.75 mL/min KLa: 34.02 h−1 | C. ljungdahlii | Ethanol Acetate | 3.75 g/L 14.97 g/L | [135] |
| CSTR (7.5 L) | 16.5% CO, 15.5% CO2, 5% H2, 56% N2 | T: 37 °C pHi: 5.9 to pHf: 5.3 Agitation rate: 400 rpm | C. carboxidivorans | Ethanol Acetate | N.A. N.A. | [136] |
| CSTR (7.5 L) | 20% CO, 5% H2, 15% CO2, 60% N2 | T: 37 °C pHi: 5.7 Agitation rate: 150 rpm GFR: 10 standard L/min | C. carboxidivorans | Ethanol Butanol | 1.48–2.82 g/L 35.00–65.00 mM/g of cells/day 0.33–0.53 g/L | [137] |
| CSTR | 2% CO, 65% H2, 23% CO2, 10% Ar | T: 37 °C pH: 5.0 Agitation rate: 800 rpm GFR: 30 mL/min D: 0.5 day−1 | C. autoethanogenum | Ethanol Acetate | 9.69 g/L 5.97 g/L | [138] |
| CSTR (3 L) | 40% CO, 30% H2, 30% CO2 | T: 37 °C pHi: 5.9–4.8 Agitation rate: 200–300 rpm GFR: 1.3–1.8 mmol/min | C. ragsdalei | Ethanol | 11.00 g/L | [139] |
| CSTR (100 L) | 5% H2, 15% CO2, 20% CO, 60% N2 | T: 37 °C pHi: 5.9 to pHf: 4.7 Agitation rate: 150 rpm GFR: 0.9 standard L/min | C. ragsdalei | Ethanol Acetate 2-Propanol 1-Butanol | 25.26 g/L 4.82 g/L 8.86 g/L 0.47 g/L | [140] |
| BCR (4.5 L) | 25% CO, 15% CO2, 60% N2 | T: 37 °C pH: 5.8 GFR: 200 ccm LFR: 200–300 mL/min D: 0.026 h−1 | C. carboxidivorans | Ethanol Acetate Butanol | N.A. N.A. N.A. | [143] |
| BCR (4 L) | 14.7% CO, 4.4% H2, 16.5% CO2, 56.8% N2, 4.2% CH2, 2.4% C2H4, 0.8% C2H6 | T: 37 °C pHi: 5.8–5.9 GFR: 180 ccm LFR: 1.5 mL/min | C. carboxidivorans | Ethanol Acetate Butanol Butyrate | 1.60 g/L N.A. N.A. N.A. | [142] |
| TBR (1 L) | 38% CO, 28.5% H2, 28.5% CO2, 5% N2 | T: 37 °C pH: 5.8 GFR: 2.3 or 4.6 sccm LFR: 200, 500, 700 mL/min (semi-continuous mode) | C. ragsdalei | Ethanol Acetate | 5.70 g/L and 37.00 mg/L/h 12.30 g/L | [144] |
| TBR (1 L) | 38% CO, 28.5% H2, 28.5% CO2, 5% N2 | T: 37 °C pHi: 5.8 GFR: 2.8–18.9 sccm LFR: 200–500 mL/min D: 0.012 h−1 (continuous mode) | C. ragsdalei | Ethanol Acetate | 13.20 g/L and 158.00 mg/L/h 3.30 g/L | [155] |
| HFMBR | 40% CO, 30% H2, 30% CO2 | T: 37 °C pHi: 5.9 to pHf: 4.5 Agitation rate: 100 rpm GFR: 60 std L/min LFR: 180 mL/min | C. ragsdalei | Ethanol | 10.00 g/L | [149] |
| HFMBR | 50% CO, 30% H2, 20% CO2 | T: 35 °C pH: 5.0GFR: 250 mL/min KLa: 385.2 h−1 | C. ljungdahlii | Ethanol Acetate | 6.00 g/L 3.00 g/L | [150] |
| HFMBR (8 L) | 20% CO, 5% H2, 15% CO2, 60% N2 | T: 37 °C pHi: 6.0 to pHf: 4.5–5.5 Agitation rate: 200 rpm GFR: 50–300 mL/min LFR: 50–200 mL/min KLa: 1096.2 h−1 | C. carboxidivorans | Ethanol Acetate | 23.93 g/L and 3.44 g/L/day 5.00 g/L | [151] |
| CSTR (1 L) with a submerged HFMs module | 40% H2, 17% CO2, 43% N2 | T: 30 °C pH: 7.0 Agitation rate: 1200 rpm GFR: 30 L/h D: 0.35 h−1 | A. woodii | Acetate | 17.60 g/L and 148.00 g/L/day | [152] |
| MLBR (8 L) | 20% CO, 5% H2, 15% CO2, 60% N2 | T: 37 °C pHi: 6.0 to pHf: 4.5–5.5 GFR: 50–300 mL/min LFR: 200–500 mL/min D: 0.48 day−1 KLa: 50–550 h−1 | C. carboxidivorans | Ethanol Acetate | 4.89 g/L and 2.35 g/L/day 3.05 g/L and 1.46 g/L/day | [154] |
| Methods | Brief Description | Impurities Removed | Reference | |
|---|---|---|---|---|
| Physical methods | Cyclone separators | A centrifugal force is applied to separate solids and aerosols from the gas. | Large particles (>5 µm diameter), tars | [161,166,168,169] |
| Electrostatic precipitators (ESPs) | Dust particles and droplets of tar attach to gas ions produced in a corona discharge. Particles and droplets become charged and precipitate due to an applied electric field. | Particulate a, tars | [161,166,168,169] | |
| Rotating particle separators (RPSs) | It is made of a rotating cylinder, where (i) tars are condensed and then the droplets are removed or (ii) a solvent is injected and then the saturated solvent is taken out. | Particulate a, tars | [161] | |
| Filtration | Tars, dust, and particles are blocked on the filter surface. Different types of filters can be used, such as fabric filters, ceramic filters, activated carbon-based absorbers, sandbed filters, and catalytic filters. | Particulate a (tar elimination is not efficient) | [161,168,169] | |
| Wet scrubber (water, RME, OLGA scrubber b) | It is composed of a gas cooler, fine tar mist separator, and occasionally, a solid particle separator. Impurities elimination occurs via condensation, precipitation, diffusion, solubility, and absorption. | Tars, NH3, HCl | [166,169] | |
| Rectisol wash | This process takes place at a temperature below −40 °C and at high pressure. Methanol is used as a solvent due to its ability to allow for physical absorption of NH3 and HCN, but also of H2S and CO2. | NH3, HCN, H2S, and CO2 | [168,169] | |
| Absorption | It is a physical phenomenon consisting of the penetration of a substance into a solid (polyethylene glycol; oxides of Fe, Mn, Zn, Cu, and Ca; molecular sieves) or liquid body (alkaline solution). | H2S | [168,169] | |
| Membrane permeation | It is the separation of individual compounds on the basis of the difference in their rates of permeation through a thin membrane barrier. | H2S | [170] | |
| Thermal methods | Thermal cracking | It consists of a high-temperature (>1000 °C) treatment, during which heavy tar compounds are decomposed into lighter compounds. | Tars | [161,168,169] |
| Catalytic methods | Hot catalytic gas conditioning | Catalytic strategies provide the possibility to transform the impurities into useful gas compounds, such as CO and H2. Catalysts can be natural minerals (calcined rocks, such as calcined dolomite, magnesite, and calcite; olivine; clay materials; iron ores) or metallic and metal oxide synthetic catalysts (alkali metal-based, activated alumina, and transition metal-based catalysts, such as Pt, Zr, Rh, Ru, Fe, and Ni). | Tars, NH3, NOx, N2O, H2S | [161,168,169] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciliberti, C.; Biundo, A.; Albergo, R.; Agrimi, G.; Braccio, G.; de Bari, I.; Pisano, I. Syngas Derived from Lignocellulosic Biomass Gasification as an Alternative Resource for Innovative Bioprocesses. Processes 2020, 8, 1567. https://doi.org/10.3390/pr8121567
Ciliberti C, Biundo A, Albergo R, Agrimi G, Braccio G, de Bari I, Pisano I. Syngas Derived from Lignocellulosic Biomass Gasification as an Alternative Resource for Innovative Bioprocesses. Processes. 2020; 8(12):1567. https://doi.org/10.3390/pr8121567
Chicago/Turabian StyleCiliberti, Cosetta, Antonino Biundo, Roberto Albergo, Gennaro Agrimi, Giacobbe Braccio, Isabella de Bari, and Isabella Pisano. 2020. "Syngas Derived from Lignocellulosic Biomass Gasification as an Alternative Resource for Innovative Bioprocesses" Processes 8, no. 12: 1567. https://doi.org/10.3390/pr8121567
APA StyleCiliberti, C., Biundo, A., Albergo, R., Agrimi, G., Braccio, G., de Bari, I., & Pisano, I. (2020). Syngas Derived from Lignocellulosic Biomass Gasification as an Alternative Resource for Innovative Bioprocesses. Processes, 8(12), 1567. https://doi.org/10.3390/pr8121567