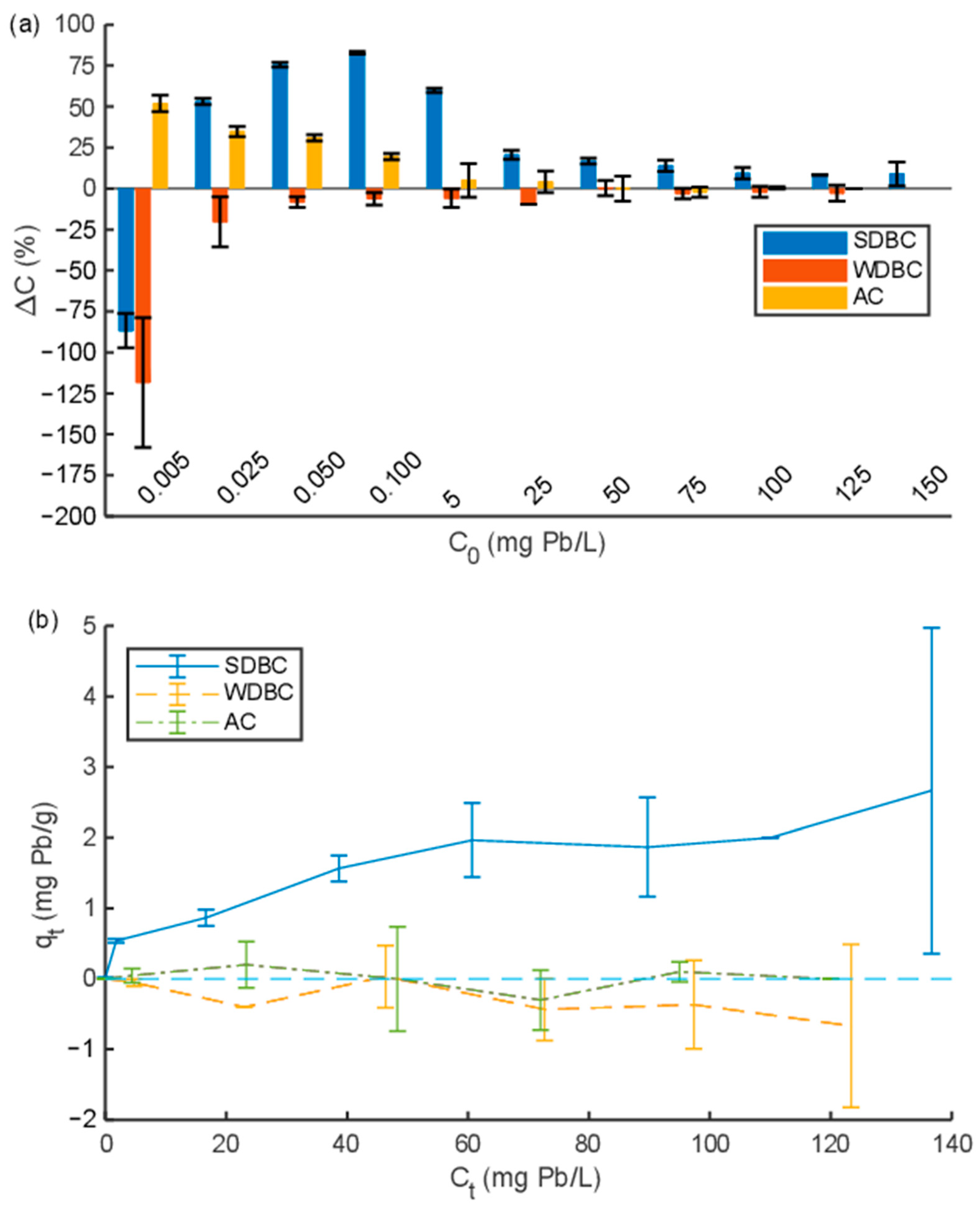
3.2.1. Pb2+ Sorption Isotherms: SDBC Compared to Reference Materials WDBC and AC
As seen in
Figure 1a, SDBC removed Pb
2+ at all concentrations except at the lowest concentration (0.005 mg/L), where Pb
2+ instead was released into the solution (the final concentration was higher than the initial concentration). The maximum Pb
2+ removal of SDBC was 83 ± 1% at an initial concentration of 0.1 mg/L. AC removed Pb
2+ at initial concentrations of 0.005–0.1 mg/L, while it did not significantly remove or release Pb
2+ at concentrations of 5 mg/L or higher. WDBC released Pb
2+ when the initial concentration was low, while at higher concentrations, it did not significantly release or remove Pb
2+.
The lack of sorption capacity of WDBC and the non-significant sorption capacity of AC at initial concentrations of 5 mg/L and higher was not anticipated, since previous works indicated that WDBC and AC may efficiently sorb Pb
2+. However, the results for AC are in relatively good agreement with data according to Sajjad et al. [
42]. They investigated the sorption capacity of commercial AC of the same type as was used in our experiments. The Pb
2+ removal at pH 2 according to Sajjad et al. [
42] was ~12% (read from figure) at an initial concentration in the range of 5–20 mg/L. The corresponding Pb
2+ removal in our experiments (initial pH of 2) was 5 ± 10% and 4 ± 6% (initial concentrations 5 and 25 mg/L, respectively). At an initial pH of 4 and 8, our results showed that the pH increase induced by the addition of AC resulted in almost complete Pb
2+ removal (see
Supplementary Materials Figure S4).
The release of Pb
2+ from WDBC used here was ~1 mg/kg (see
Table 5, at a dose of 5 g/L, pH 2), which indicates that this WDBC is not an appropriate sorbent when the initial metal concentration and pH are very low. However, other WDBCs may be more appropriate. WDBCs have previously been shown to sorb Pb
2+. Biochar derived from poplar wood, pinewood branches, oak wood, and pine wood showed sorption capacities of 3.6, 58, 2.6, and 4.1 mg/g, respectively (at initial pH of 4, 2, 5, and 5) [
43,
44,
45]. The cause of the inferior performance of WDBC in our experiments was not fully identified, though the low solution pH may be one cause. The literature data with respect to Pb
2+ removal capacity at solution pH 2 (pine wood/pine branch biochar) are not consistent; it was relatively high according to Abdel-Fattah et al. [
44] (58 mg/g), but only by a few percent (sorption capacity in milligrams per gram not specified) according to Mohan et al. [
45]. The pH of the sorbent may also be of importance; the WDBC pH according to Zhao et al. [
43] was 8.2, while the WDBC used here had a pH of only 5.6 (as given in
Table 5). An increased initial pH promotes sorption (and at initial pH 8, precipitation of lead hydroxides); at an initial pH of 4 and 8, the removal of Pb
2+ by WDBC investigated here was 20% (0.19 mg/g) and 88% (0.84 mg/g), respectively (see
Supplementary Materials Figure S4). Other factors of relevance for the sorption capacity may be the surface area, the initial Pb
2+ concentration in the sorbent, or the surface acidity. WDBCs investigated by Zhao et al. [
43], Abdel-Fattah et al. [
44], and Mohan et al. [
45] all had smaller surface areas but higher sorption capacities compared to the WDBC investigated here, which indicates that the surface area is not the crucial factor. Furthermore, it does not seem possible to directly infer the release of Pb
2+ based on the initial concentration in the WDBC; the initial Pb
2+ concentration in the WDBC used here (0.854 mg/kg) was lower compared to that reported in biochar derived from hickory woodchips (26 mg/kg) and pine needles (39 mg/kg) [
46]. Wu et al. [
46] detected no Pb
2+ release from hickory woodchip biochar at pH 2 (24 h contact time), while the Pb
2+ release from pine needle biochar at pH 2 was 8.76 mg/kg (dose of 20 g/L), i.e., the amount of Pb
2+ initially contained was not proportional to the release. Surface acidity (reflecting the number of acidic functional groups) was identified as a key factor for Pb
2+ sorption (of biochars derived from rice husk, olive pits, and wood chips) according to Campos and De la Rosa [
47]. However, the surface acidity was not investigated here.
SDBC had a higher Pb
2+ removal capacity than WDBC and AC at all concentrations, except at 0.005 mg/L, where AC had the highest removal capacity (52 ± 5%). The decreasing removal capacity of SDBC at initial concentrations lower than 0.05 mg/L was due to the initial content of Pb in the biochar, as given in
Table 5. WDBC released similar amounts of Pb as SDBC, which is unexpected since the initial concentration of Pb in WDBC was less than one hundredth compared to SDBC. The initial concentration of Pb in AC was similar to that in WDBC, but the release of Pb was much lower. Thus, the strength of the sorption or binding of Pb seems to differ between these sorbents. In contrast, the relative release of Ni from SDBC, WDBC, and AC seems to be in agreement with the initial concentrations in the different sorbents (based on the data in
Table 5). This shows that the release of metals was not proportional to the initial metal concentration in the sorbents, but the relative amount of metals released varied both depending on sorbent properties and between different metals. The sorbent properties that govern metal release may be, e.g., the number and type of surface functional groups and the amount of anion salts such as phosphates and carbonates (with which the heavy metals may precipitate) in the sorbent (the relevance of functional groups and anion salts are further discussed below but were not measured here). Similar to what was found here, the amount of metals released by SDBC in standard toxicity characteristic leaching procedure (TCLP) tests according to Ho et al. [
25] and Wu et al. [
46] differed even though the initial metal concentrations in SDBC were similar.
An explanation for the higher removal capacity of SDBC could be that it likely contains more phosphates and carbonates compared to the other sorbents, which is beneficial for the precipitation of metal phosphates and carbonates [
25]. A further contribution may be the accumulation of exchangeable alkali metals in SDBC during sludge pyrolysis [
14], which was confirmed by the low VM content (high ash content) of SDBC compared to the other sorbents. The higher surface area of AC and WDBC compared to SDBC evidently did not result in higher sorption capacity, in agreement with observations in previous studies [
32,
41]. This suggests that surface area in itself is not a good basis for judging the metal sorption capacity of carbonous sorbents when the sorbents have different origins or were produced under different conditions. This also confirms that surface chemistry is of great importance for metal sorption potential.
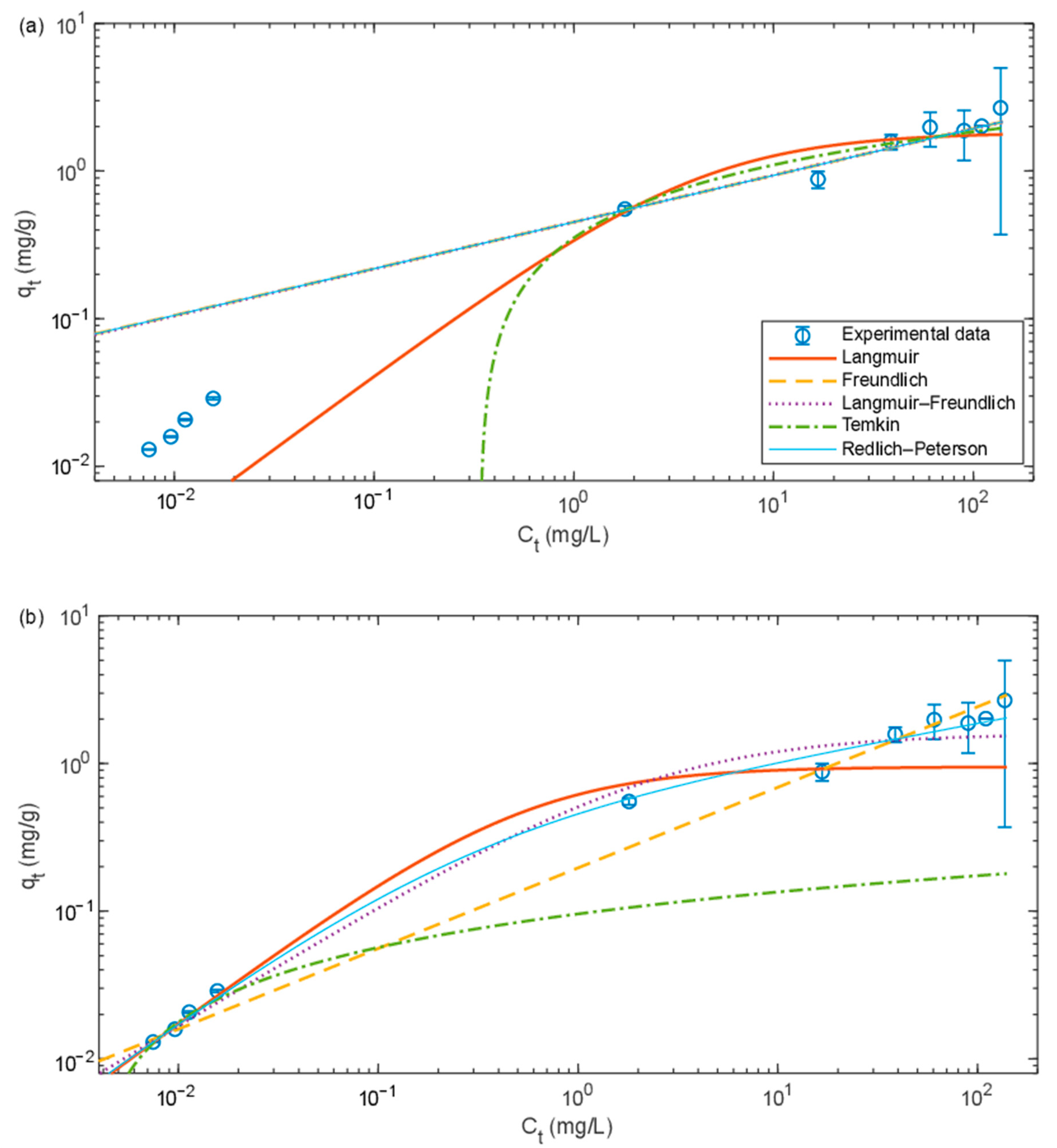
The initial amount of sorbed metal on SDBC (
) was calculated to represent 1.37 × 10
−2 mg/g (a linear relationship between
and
(
= 1.81
) was assumed to be valid up to
= 0.015 mg/L (
0.1 mg/L), R
2 = 0.98). SDBC reached a maximum Pb
2+ sorption capacity of approximately 2 mg/g at a final concentration of ~60 mg/L (initial concentrations of 75 mg Pb/L and above) (see
Figure 1b). The kinetic experiments later revealed that the duration of the isotherm experiment (24 h) was not sufficient to reach equilibrium for sorption to SDBC, which implies that the real maximum sorption capacity should be slightly higher than what was observed in the isotherm experiment.
The maximum Pb2+ sorption capacity of AC was lower compared to SDBC—only (3.5 ± 0.4) × 10−3 mg/g—at an initial concentration of 0.1 mg/L ( for AC was very close to zero). At higher initial concentrations, the difference between the initial and final Pb2+ concentrations was small and the measurement uncertainty was therefore high.
The final pH was 2.5–2.8 for SDBC, 2.1–2.2 for WDBC, and 2.2–2.3 for AC. The low pH resulted in a lower maximum sorption capacity of Pb
2+ compared to previous studies of Pb
2+ sorption to sludge-derived biochar [
15,
24,
25]. According to Ho et al. [
24], biochar derived from anaerobically digested sludge could sorb 50 mg Pb/g at pH 5, but only approximately 3 mg/g at an initial pH of 2 (similar final pH)—the latter value is similar to our findings. Ho et al. [
24] showed that the main sorption processes at pH 5 were precipitation and ion exchange; these processes are favored by an increased pH.
As already mentioned, the SDBC was pyrolyzed at 550–600 °C with a 16–30 min duration. SDBC produced from the same feedstock but under different conditions of pyrolysis could have a different sorption capacity. Several studies on SDBC have indicated that a high pyrolysis temperature might result in a decreased sorption capacity, despite the increased surface area. This is attributed to the destruction of organic functional groups, e.g., carboxyl and hydroxyl groups [
24,
48]. Zhang et al. [
15] found that SDBC pyrolyzed at 400 °C for 2 h had a higher sorption capacity for Pb
2+ and Cr
4+ compared to SDBC pyrolyzed at 500 and 600 °C for 1 h. In contrast, Chen et al. [
14] found that a pyrolysis temperature of 800–900 °C provided higher removal capacity for Cd
2+ compared to SDBC produced at a lower pyrolysis temperature, even though the increased pyrolysis temperature was shown to decrease the number of surface functional groups.
Some of the blank samples of ultrapure water were found to contain small amounts of Pb2+, i.e., <0.3 μg/L. The concentrations of Ni2+, Cd2+, Cr3+, Cu2+, and Zn2+ were below the detection limits though. The Pb2+ found was likely (unintentionally) added during sample preparation or filtering. The detected Pb was estimated to contribute a maximum of 4%–10% of the final concentration in the samples of lowest initial metal concentrations and was disregarded in the calculations.
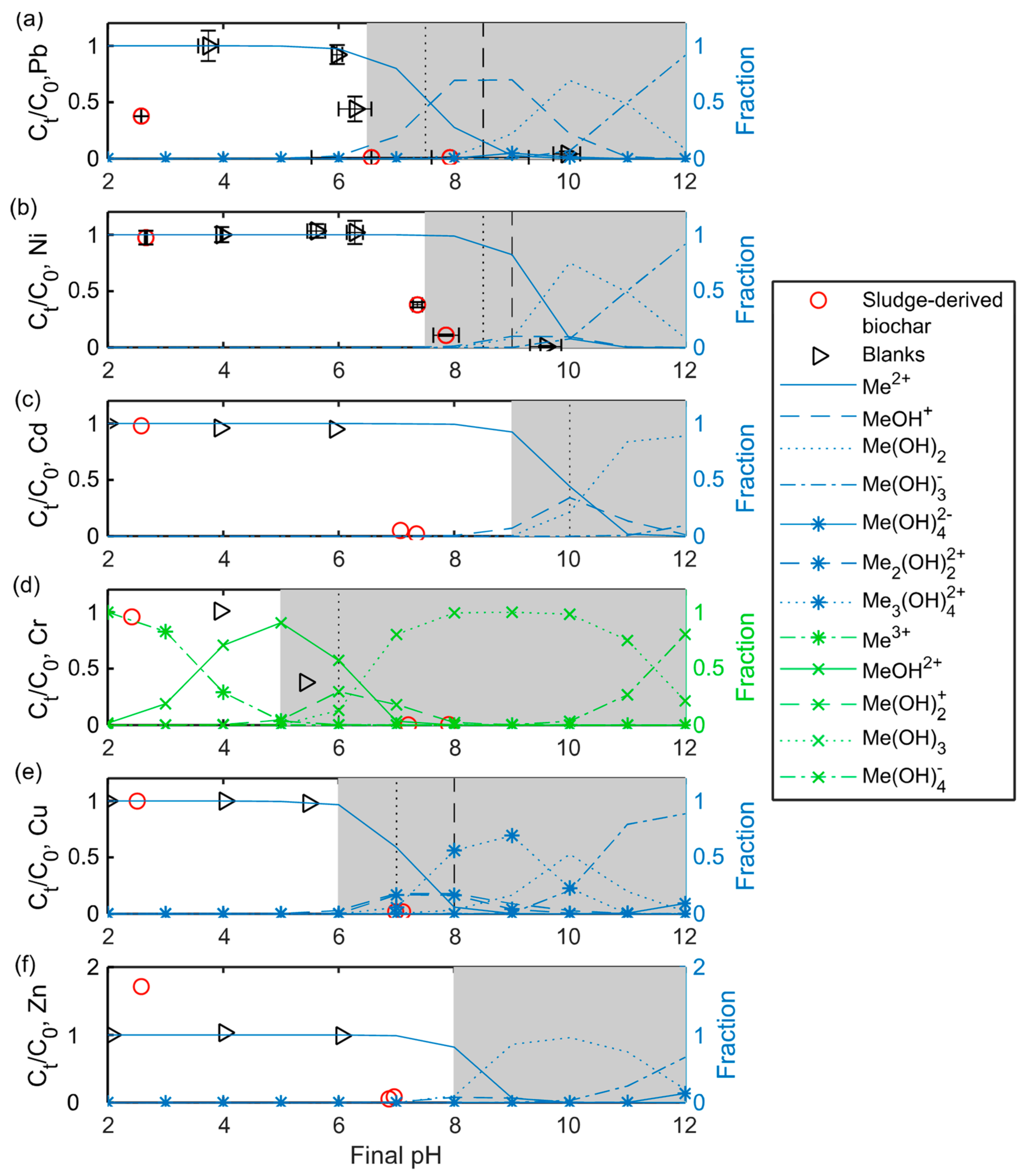
3.2.4. Effect of pH on Pb2+, Ni2+, Cd2+, Cr3+, Cu2+, and Zn2+ Sorption to SDBC
SDBC caused an increase in the sample pH (regardless of initial pH), with a maximum final pH of approximately 8 (see
Supplementary Materials Figure S5). This pH increase may have derived from the release of alkalis such as Ca
2+ [
51].
Figure 5 shows the final concentrations of Pb
2+, Ni
2+, Cd
2+, Cr
3+, Cu
2+, and Zn
2+ for the pH experiments in relation to the final pH of the solution.
SDBC showed the potential to sorb over 90% of Cd2+ and Zn2+ at a final pH of approximately 7, which indicates that precipitation is not a dominating mechanism, since Cd2+ and Zn2+ should be prevalent as metal ions below ~9 and 8, respectively. Instead, sorption processes such as ion exchange and surface complexation are likely involved, together with electrostatic interaction. At an initial pH of 2 (final pH of ~2.5), SDBC released Zn2+, which caused an increase in the sample Zn2+ concentration of ~70%. Pb2+ was partly sorbed at a final pH of <3. At a higher pH (from final pH ~6.5 and above), Pb was removed to almost 100%, which might partly have been caused by precipitation, since lead hydroxides could start to form from a final pH of approximately 6.5.
Cr3+ and Cu2+ were removed to almost 100% at a final pH of approximately 7 and above. This removal could partly or entirely be attributed to precipitation, since Cr3+ and Cu2+ could precipitate from a final pH of approximately 5 and 6, respectively. The Ni2+ removal of more than 50% at a final pH of approximately 7.5 could partly be attributed to precipitation, since Ni2+ precipitation could occur from approximately pH 7.5.
The speciation of heavy metals was not directly investigated here. However, it should be noted that the speciation of heavy metals may not only be affected by the pH in the solution, but also by other factors such as redox potential. Wongrod et al. [
52] showed that the oxidation of As
3+ to As
5+ (redox potential under standard conditions: +0.56 V) occurred after the addition of SDBC. Reduction may also occur, as Cr
6+ is easily reduced to Cr
3+ (redox potential under standard conditions: Above +1.3) [
30]. In the case of the metals investigated here, redox reactions are most likely to be of importance in the case of Cu
2+, as this metal has the highest standard reduction potential (the standard reduction potentials fall in the order of: +0.34, −0.13, −0.25, −0.40, −0.74, and −0.76 for Cu
2+, Pb
2+, Ni
2+, Cd
2+, Cr
3+, and Zn
2+, respectively). El-Naggar et al. [
53] investigated heavy metal mobility in soil amended with rice hull biochar under fluctuating redox conditions and found that an increase in redox potential resulted in larger fraction of dissolved heavy metals (Cd, Cu, Ni, and Zn). The dissolution was associated with a decline in pH. Oxidation of sulfides and increased concentrations of dissolved aromatic organic compounds at high redox potentials was given as other possible explanations of the increased solubility of heavy metals under high redox potential.
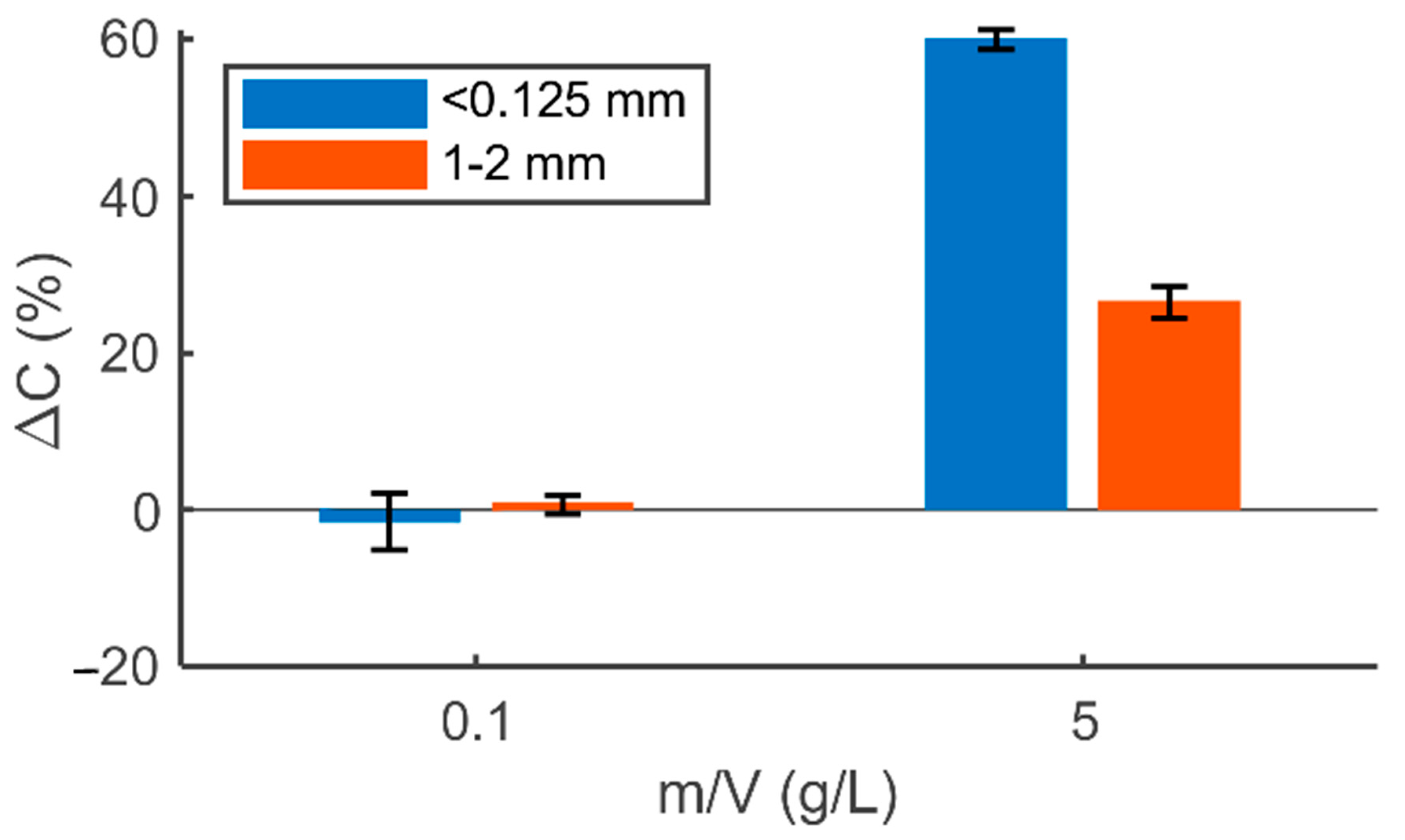
When sorbents were added to ultrapure water at an initial pH of 2, the release of Pb, Ni Cd, Cr, Cu, and Zn occurred (as given in
Table 5). At an initial pH of 4, the release of Pb
2+ from SDBC decreased to 3.6 × 10
−4 mg/L, and no release of Pb
2+ was detected at an initial pH of 8 (see
Supplementary Materials Table S1). These results agree with the findings of Wu et al. [
46], who observed a higher release of Pb when samples were acidified to pH 2–4. The release of Pb
2+ per gram of SDBC added was higher at a lower sorbent dose, with the release of 1.2 × 10
−3 mg/g at a dose of 5 g/L and 4.0 × 10
−2 mg/g at a dose of 0.1 g/L (pH 2, sorbent size interval <0.125 mm, and contact time 24 h).
The release of Zn
2+ was similar to that found by Ho et al. [
24], who performed acid leaching tests on sludge-derived biochar and detected resulting Zn concentrations of 4.9 mg/L. Similar to our findings, Ho et al. [
24] detected low concentrations of Pb, Ni, Cd, Cr, and Cu after the acid leaching tests.