Effect of Hydrogen Bond Donors and Acceptors on CO2 Absorption by Deep Eutectic Solvents
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental
2.2. Theoretical
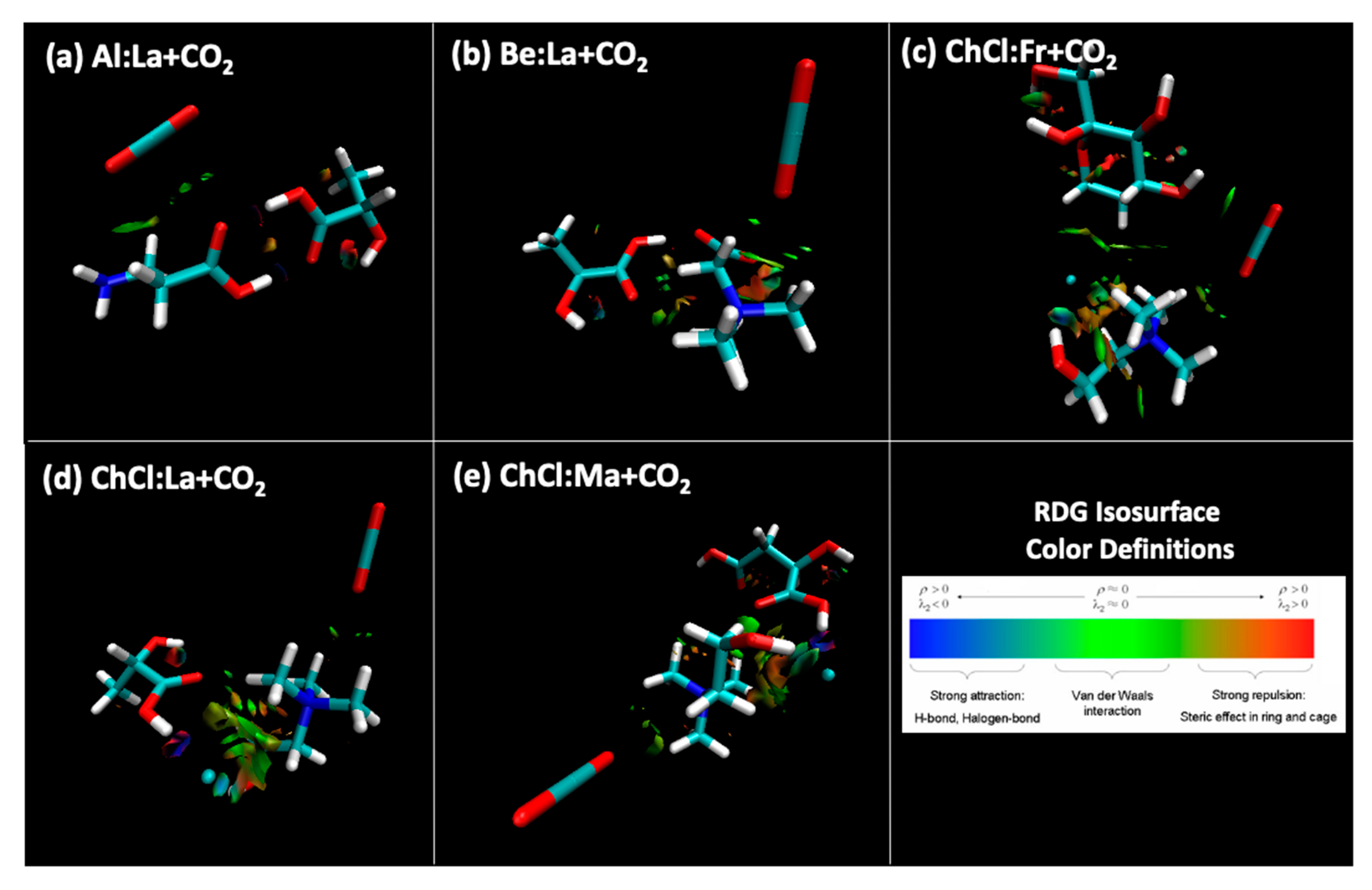
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Macário, I.P.E.; Oliveira, H.; Menezes, A.C.; Ventura, S.P.M.; Pereira, J.L.; Gonçalves, A.M.M.; Coutinho, J.A.P.; Gonçalves, F.J.M. Cytotoxicity profiling of deep eutectic solvents to human skin cells. Sci. Rep. 2019, 9, 3932. [Google Scholar] [CrossRef]
- Halder, A.K.; Cordeiro, M.N.D.S. Probing the Environmental Toxicity of Deep Eutectic Solvents and Their Components: An In Silico Modeling Approach. ACS Sustain. Chem. Eng. 2019, 7, 10649–10660. [Google Scholar] [CrossRef]
- Valencia-Marquez, D.; Flores-Tlacuahuac, A.; Vasquez-Medrano, R. An optimization approach for CO2 capture using ionic liquids. J. Clean. Prod. 2017, 168, 1652–1667. [Google Scholar] [CrossRef]
- Bi, Y.; Hu, Z.; Lin, X.; Ahmad, N.; Xu, J.; Xu, X. Efficient CO2 capture by a novel deep eutectic solvent through facile, one-pot synthesis with low energy consumption and feasible regeneration. Sci. Total Environ. 2020, 705, 135798. [Google Scholar] [CrossRef]
- Leron, R.B.; Li, M.-H. Solubility of carbon dioxide in a choline chloride–ethylene glycol based deep eutectic solvent. Thermochim. Acta 2013, 551, 14–19. [Google Scholar] [CrossRef]
- Sreedhar, I.; Nahar, T.; Venugopal, A.; Srinivas, B. Carbon capture by absorption–Path covered and ahead. Renew. Sustain. Energy Rev. 2017, 76, 1080–1107. [Google Scholar] [CrossRef]
- Mirza, N.; Mumford, K.; Wu, Y.; Mazhar, S.; Kentish, S.; Stevens, G. Improved Eutectic Based Solvents for Capturing Carbon Dioxide (CO2). Energy Procedia 2017, 114, 827–833. [Google Scholar] [CrossRef]
- Hjelmaas, S.; Storheim, E.; Flø, N.E.; Thorjussen, E.S.; Morken, A.K.; Faramarzi, L.; de Cazenove, T.; Hamborg, E.S. Results from MEA Amine Plant Corrosion Processes at the CO2 Technology Centre Mongstad. Energy Procedia 2017, 114, 1166–1178. [Google Scholar] [CrossRef]
- Husebye, J.; Brunsvold, A.L.; Roussanaly, S.; Zhang, X. Techno Economic Evaluation of Amine based CO2 Capture: Impact of CO2 Concentration and Steam Supply. Energy Procedia 2012, 23, 381–390. [Google Scholar] [CrossRef]
- Davis, J.; Rochelle, G. Thermal degradation of monoethanolamine at stripper conditions. Energy Procedia 2009, 1, 327–333. [Google Scholar] [CrossRef]
- Luis, P. Use of monoethanolamine (MEA) for CO2 capture in a global scenario: Consequences and alternatives. Desalination 2016, 380, 93–99. [Google Scholar] [CrossRef]
- Abotaleb, A.; El-Naas, M.; Amhamed, A. Enhancing gas loading and reducing energy consumption in acid gasremoval systems: A simulation study based on real NGL plant data. J. Nat. Gas Sci. Eng. 2018, 55, 565–574. [Google Scholar] [CrossRef]
- Rozyyev, V.; Thirion, D.; Ullah, R.; Lee, J.; Jung, M.; Oh, H.; Atilhan, M.; Yavuz, C.T. High-capacity methane storage in flexible alkane-linked porous aromatic network polymers. Nat. Energy 2019, 4, 604–611. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef]
- Megías-Sayago, C.; Bingre, R.; Huang, L.; Lutzweiler, G.; Wang, Q.; Louis, B. CO2 Adsorption Capacities in Zeolites and Layered Double Hydroxide Materials. Front. Chem. 2019, 7, 551. [Google Scholar] [CrossRef]
- Titinchi, S.J.J. Chemically Modified Solid Adsorbents for CO2 Capture. Energy Procedia 2014, 8, 8153–8160. [Google Scholar] [CrossRef]
- Jiang, L.; Gonzalez-Diaz, A.; Ling-Chin, J.; Roskilly, A.P.; Smallbone, A.J. Post-combustion CO2 capture from a natural gas combined cycle power plant using activated carbon adsorption. Appl. Energy 2019, 245, 1–15. [Google Scholar] [CrossRef]
- Closmann, F.; Nguyen, T.; Rochelle, G.T. MDEA/Piperazine as a solvent for CO2 capture. Energy Procedia 2009, 1, 1351–1357. [Google Scholar] [CrossRef]
- Liu, J.; Li, X.; Zhang, Z.; Li, L.; Bi, Y.; Zhang, L. Promotion of CO2 capture performance using piperazine (PZ) and diethylenetriamine (DETA) bi-solvent blends. Greenh. Gases Sci. Technol. 2019, 9, 349–359. [Google Scholar] [CrossRef]
- Aghaie, M.; Rezaei, N.; Zendehboudi, S. A systematic review on CO2 capture with ionic liquids: Current status and future prospects. Renew. Sustain. Energy Rev. 2018, 96, 502–525. [Google Scholar] [CrossRef]
- Ramdin, M.; de Loos, T.W.; Vlugt, T.J.H. State-of-the-Art of CO2 Capture with Ionic Liquids. Ind. Eng. Chem. Res. 2012, 51, 8149–8177. [Google Scholar] [CrossRef]
- Chong, F.K.; Chemmangattuvalappil, N.; Foo, D.C.Y.; Atilhan, M.; Eljack, F.T. Ionic Liquid Mixture Design for Carbon Capture using Property Clustering Technique. Chem. Eng. Trans. 2015, 45, 1567–1572. [Google Scholar] [CrossRef]
- Shiflett, M.B.; Drew, D.W.; Cantini, R.A.; Yokozeki, A. Carbon Dioxide Capture Using Ionic Liquid 1-Butyl-3-methylimidazolium Acetate. Energy Fuels 2010, 24, 5781–5789. [Google Scholar] [CrossRef]
- García, G.; Atilhan, M.; Aparicio, S. Simultaneous CO2 and SO2 capture by using ionic liquids: A theoretical approach. Phys. Chem. Chem. Phys. 2017, 19, 5411–5422. [Google Scholar] [CrossRef] [PubMed]
- Rafat, A.; Atilhan, M.; Kahraman, R. Corrosion Behavior of Carbon Steel in CO2 Saturated Amine and Imidazolium-, Ammonium-, and Phosphonium-Based Ionic Liquid Solutions. Ind. Eng. Chem. Res. 2016, 55, 446–454. [Google Scholar] [CrossRef]
- Bhawna; Pandey, A.; Dhingra, D.; Pandey, S. Can common liquid polymers and surfactants capture CO2? J. Mol. Liq. 2019, 277, 594–605. [Google Scholar] [CrossRef]
- Zulfiqar, S.; Ilyas Sarwar, M.; Mecerreyes, D. Polymeric ionic liquids for CO2 capture and separation: Potential, progress and challenges. Polym. Chem. 2015, 6, 6435–6451. [Google Scholar] [CrossRef]
- Kupgan, G.; Abbott, L.J.; Hart, K.E.; Colina, C.M. Modeling Amorphous Microporous Polymers for CO2 Capture and Separations. Chem. Rev. 2018, 118, 5488–5538. [Google Scholar] [CrossRef]
- Zhang, J.; Chai, S.-H.; Qiao, Z.-A.; Mahurin, S.M.; Chen, J.; Fang, Y.; Wan, S.; Nelson, K.; Zhang, P.; Dai, S. Porous Liquids: A Promising Class of Media for Gas Separation. Angew. Chem. 2015, 127, 946–950. [Google Scholar] [CrossRef]
- Zhang, N.; Huang, Z.; Zhang, H.; Ma, J.; Jiang, B.; Zhang, L. Highly Efficient and Reversible CO2 Capture by Task-Specific Deep Eutectic Solvents. Ind. Eng. Chem. Res. 2019, 58, 13321–13329. [Google Scholar] [CrossRef]
- Trivedi, T.J.; Lee, J.H.; Lee, H.J.; Jeong, Y.K.; Choi, J.W. Deep eutectic solvents as attractive media for CO2 capture. Green Chem. 2016, 18, 2834–2842. [Google Scholar] [CrossRef]
- García, G.; Aparicio, S.; Ullah, R.; Atilhan, M. Deep Eutectic Solvents: Physicochemical Properties and Gas Separation Applications. Energy Fuels 2015, 29, 2616–2644. [Google Scholar] [CrossRef]
- Ren, H.; Lian, S.; Wang, X.; Zhang, Y.; Duan, E. Exploiting the hydrophilic role of natural deep eutectic solvents for greening CO2 capture. J. Clean. Prod. 2018, 193, 802–810. [Google Scholar] [CrossRef]
- Mulia, K.; Putri, S.; Krisanti, E.; Nasruddin. Natural deep eutectic solvents (NADES) as green solvents for carbon dioxide capture. AIP Conf. Proc. 2017, 1823, 020022. [Google Scholar] [CrossRef]
- Amhamed, A.; Atilhan, M.; Berdiyorov, G. Permeabilities of CO2, H2S and CH4 through Choline-Based Ionic Liquids: Atomistic-Scale Simulations. Molecules 2019, 24, 2014. [Google Scholar] [CrossRef]
- Ma, C.; Sarmad, S.; Mikkola, J.-P.; Ji, X. Development of Low-Cost Deep Eutectic Solvents for CO2 Capture. Energy Procedia 2017, 142, 3320–3325. [Google Scholar] [CrossRef]
- Cruz, H.; Jordão, N.; Branco, L.C. Deep eutectic solvents (DESs) as low-cost and green electrolytes for electrochromic devices. Green Chem. 2017, 19, 1653–1658. [Google Scholar] [CrossRef]
- Ullah, R.; Atilhan, M.; Anaya, B.; Khraisheh, M.; García, G.; ElKhattat, A.; Tariq, M.; Aparicio, S. A detailed study of cholinium chloride and levulinic acid deep eutectic solvent system for CO2 capture via experimental and molecular simulation approaches. Phys. Chem. Chem. Phys. 2015, 17, 20941–20960. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, H.; Sun, Y.; Zeng, S.; Zhang, X.; Nie, Y.; Zhang, S.; Ji, X. Screening Deep Eutectic Solvents for CO2 Capture with COSMO-RS. Front. Chem. 2020, 8, 82. [Google Scholar] [CrossRef]
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural Deep Eutectic Solvents–Solvents for the 21st Century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071. [Google Scholar] [CrossRef]
- Isaifan, R.J.; Amhamed, A. Review on Carbon Dioxide Absorption by Choline Chloride/Urea Deep Eutectic Solvents. Adv. Chem. 2018, 2018, 2675659. [Google Scholar] [CrossRef]
- Gu, Y.; Hou, Y.; Ren, S.; Sun, Y.; Wu, W. Hydrophobic Functional Deep Eutectic Solvents Used for Efficient and Reversible Capture of CO2. ACS Omega 2020, 5, 6809–6816. [Google Scholar] [CrossRef] [PubMed]
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R.K. Deep Eutectic Solvents Formed between Choline Chloride and Carboxylic Acids: Versatile Alternatives to Ionic Liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147. [Google Scholar] [CrossRef] [PubMed]
- Sze, L.L.; Pandey, S.; Ravula, S.; Pandey, S.; Zhao, H.; Baker, G.A.; Baker, S.N. Ternary Deep Eutectic Solvents Tasked for Carbon Dioxide Capture. ACS Sustain. Chem. Eng. 2014, 2, 2117–2123. [Google Scholar] [CrossRef]
- Sarmad, S.; Mikkola, J.-P.; Ji, X. Carbon Dioxide Capture with Ionic Liquids and Deep Eutectic Solvents: A New Generation of Sorbents. ChemSusChem 2017, 10, 324–352. [Google Scholar] [CrossRef]
- García, G.; Atilhan, M.; Aparicio, S. Interfacial Properties of Deep Eutectic Solvents Regarding to CO2 Capture. J. Phys. Chem. C 2015, 119, 21413–21425. [Google Scholar] [CrossRef]
- Zubeir, L.F.; van Osch, D.J.G.P.; Rocha, M.A.A.; Banat, F.; Kroon, M.C. Carbon Dioxide Solubilities in Decanoic Acid-Based Hydrophobic Deep Eutectic Solvents. J. Chem. Eng. Data 2018, 63, 913–919. [Google Scholar] [CrossRef]
- Haider, M.B.; Jha, D.; Marriyappan Sivagnanam, B.; Kumar, R. Thermodynamic and Kinetic Studies of CO2 Capture by Glycol and Amine-Based Deep Eutectic Solvents. J. Chem. Eng. Data 2018, 63, 2671–2680. [Google Scholar] [CrossRef]
- Altamash, T.; Amhamed, A.I.; Aparicio, S.; Atilhan, M. Combined Experimental and Theoretical Study on High Pressure Methane Solubility in Natural Deep Eutectic Solvents. Ind. Eng. Chem. Res. 2019, 58, 8097–8111. [Google Scholar] [CrossRef]
- Altamash, T.; Nasser, M.S.; Elhamarnah, Y.; Magzoub, M.; Ullah, R.; Qiblawey, H.; Aparicio, S.; Atilhan, M. Gas solubility and rheological behavior study of betaine and alanine based natural deep eutectic solvents (NADES). J. Mol. Liq. 2018, 256, 286–295. [Google Scholar] [CrossRef]
- Altamash, T.; Nasser, M.S.; Elhamarnah, Y.; Magzoub, M.; Ullah, R.; Anaya, B.; Aparicio, S.; Atilhan, M. Gas Solubility and Rheological Behavior of Natural Deep Eutectic Solvents (NADES) via Combined Experimental and Molecular Simulation Techniques. ChemistrySelect 2017, 2, 7278–7295. [Google Scholar] [CrossRef]
- Ebner, A.D.; Gray, M.L.; Chisholm, N.G.; Black, Q.T.; Mumford, D.D.; Nicholson, M.A.; Ritter, J.A. Suitability of a Solid Amine Sorbent for CO2 Capture by Pressure Swing Adsorption. Ind. Eng. Chem. Res. 2011, 50, 5634–5641. [Google Scholar] [CrossRef]
- Karadas, F.; Yavuz, C.T.; Zulfiqar, S.; Aparicio, S.; Stucky, G.D.; Atilhan, M. CO2 Adsorption Studies on Hydroxy Metal Carbonates M(CO3)x(OH)y (M = Zn, Zn–Mg, Mg, Mg–Cu, Cu, Ni, and Pb) at High Pressures up to 175 bar. Langmuir 2011, 27, 10642–10647. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.; De Castro, P.; Echenique, P.; Estrada, J.; Hanwell, M.D.; Murray-Rust, P.; Sherwood, P.; Thomas, J.; Townsend, J. The Quixote project: Collaborative and Open Quantum Chemistry data management in the Internet age. J. Cheminform. 2011, 3, 38. [Google Scholar] [CrossRef] [PubMed]
- Neese, F. The ORCA program system. WIREs Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Simon, S.; Duran, M.; Dannenberg, J.J. How does basis set superposition error change the potential surfaces for hydrogen-bonded dimers? J. Chem. Phys. 1996, 105, 11024–11031. [Google Scholar] [CrossRef]
- Bader, R.F.W. Atoms in Molecules: A Quantum Theory; Clarendon Press: Oxford, UK, 1994. [Google Scholar]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Aparicio, S.; Yavuz, C.T.; Atilhan, M. Molecular Insights into Benzimidazole-Linked Polymer Interactions with Carbon Dioxide and Nitrogen. ChemistrySelect 2018, 3, 3691–3701. [Google Scholar] [CrossRef]
- Xiao, J.; Zhao, Y.-P.; Fan, X.; Cao, J.-P.; Kang, G.-J.; Zhao, W.; Wei, X.-Y. Hydrogen bonding interactions between the organic oxygen/nitrogen monomers of lignite and water molecules: A DFT and AIM study. Fuel Process. Technol. 2017, 168, 58–64. [Google Scholar] [CrossRef]
- Anbu, V.; Vijayalakshmi, K.A.; Karunathan, R.; Stephen, A.D.; Nidhin, P.V. Explosives properties of high energetic trinitrophenyl nitramide molecules: A DFT and AIM analysis. Arab. J. Chem. 2019, 12, 621–632. [Google Scholar] [CrossRef]
- Johnson, E.R.; Keinan, S.; Mori-Sánchez, P.; Contreras-García, J.; Cohen, A.J.; Yang, W. Revealing Noncovalent Interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef] [PubMed]
- Abranches, D.O.; Silva, L.P.; Martins, M.A.R.; Pinho, S.P.; Coutinho, J.A.P. Understanding the Formation of Deep Eutectic Solvents: Betaine as a Universal Hydrogen Bond Acceptor. ChemSusChem 2020, 13, 4916–4921. [Google Scholar] [CrossRef] [PubMed]
- Crespo, E.A.; Costa, J.M.L.; Palma, A.M.; Soares, B.; Martín, M.C.; Segovia, J.J.; Carvalho, P.J.; Coutinho, J.A.P. Thermodynamic characterization of deep eutectic solvents at high pressures. Fluid Phase Equilibria 2019, 500, 112249. [Google Scholar] [CrossRef]
- Wang, J.; Cheng, H.; Song, Z.; Chen, L.; Deng, L.; Qi, Z. Carbon Dioxide Solubility in Phosphonium-Based Deep Eutectic Solvents: An Experimental and Molecular Dynamics Study. Ind. Eng. Chem. Res. 2019, 58, 17514–17523. [Google Scholar] [CrossRef]
- Altamash, T.; Atilhan, M.; Aliyan, A.; Ullah, R.; Garcia, G.; Aparicio, M. Insights into choline chloride-phenylacetic acid deep eutectic solvent for CO2 absorption. RSC Adv. 2016, 6, 109201–109210. [Google Scholar] [CrossRef]
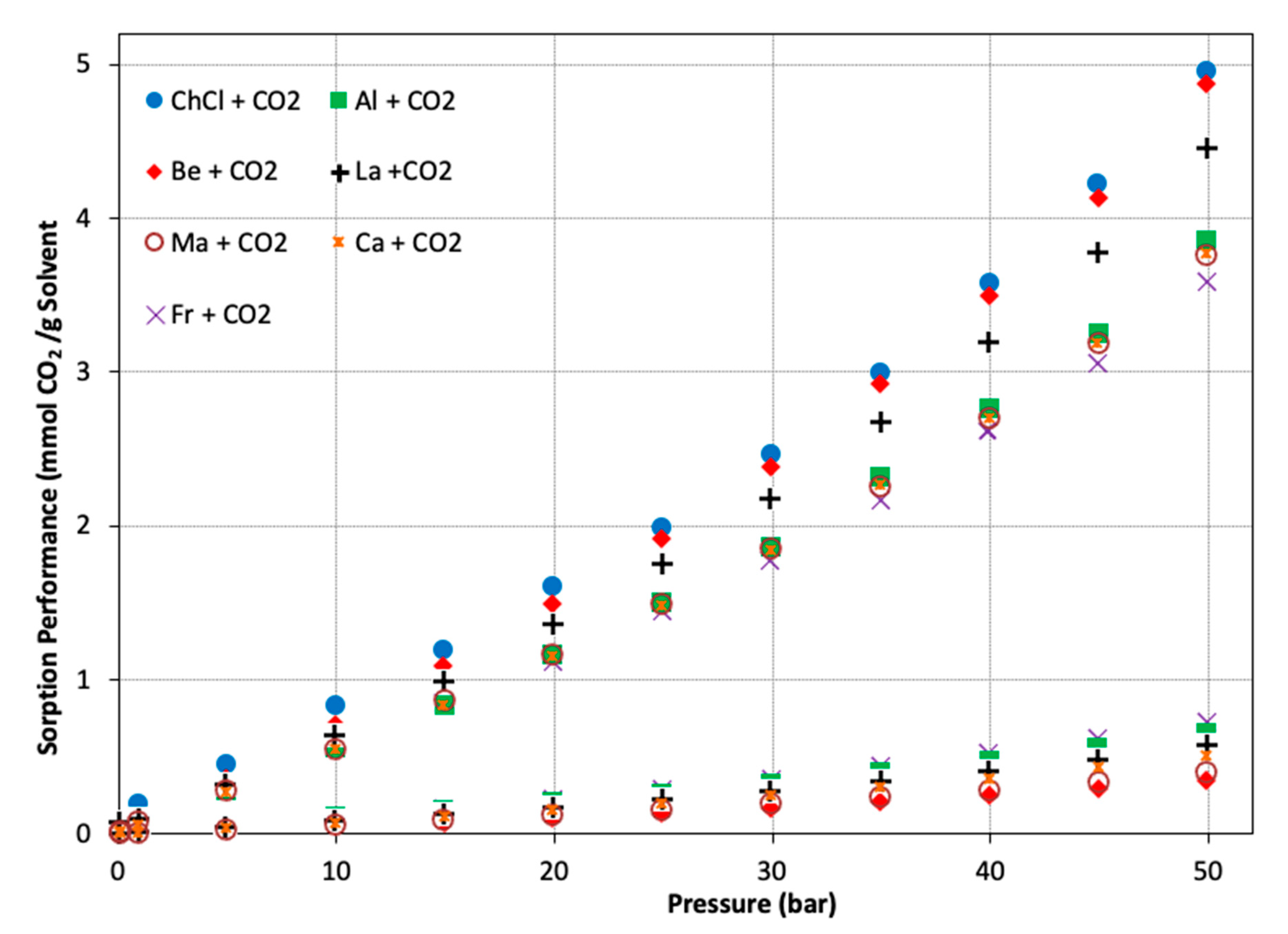
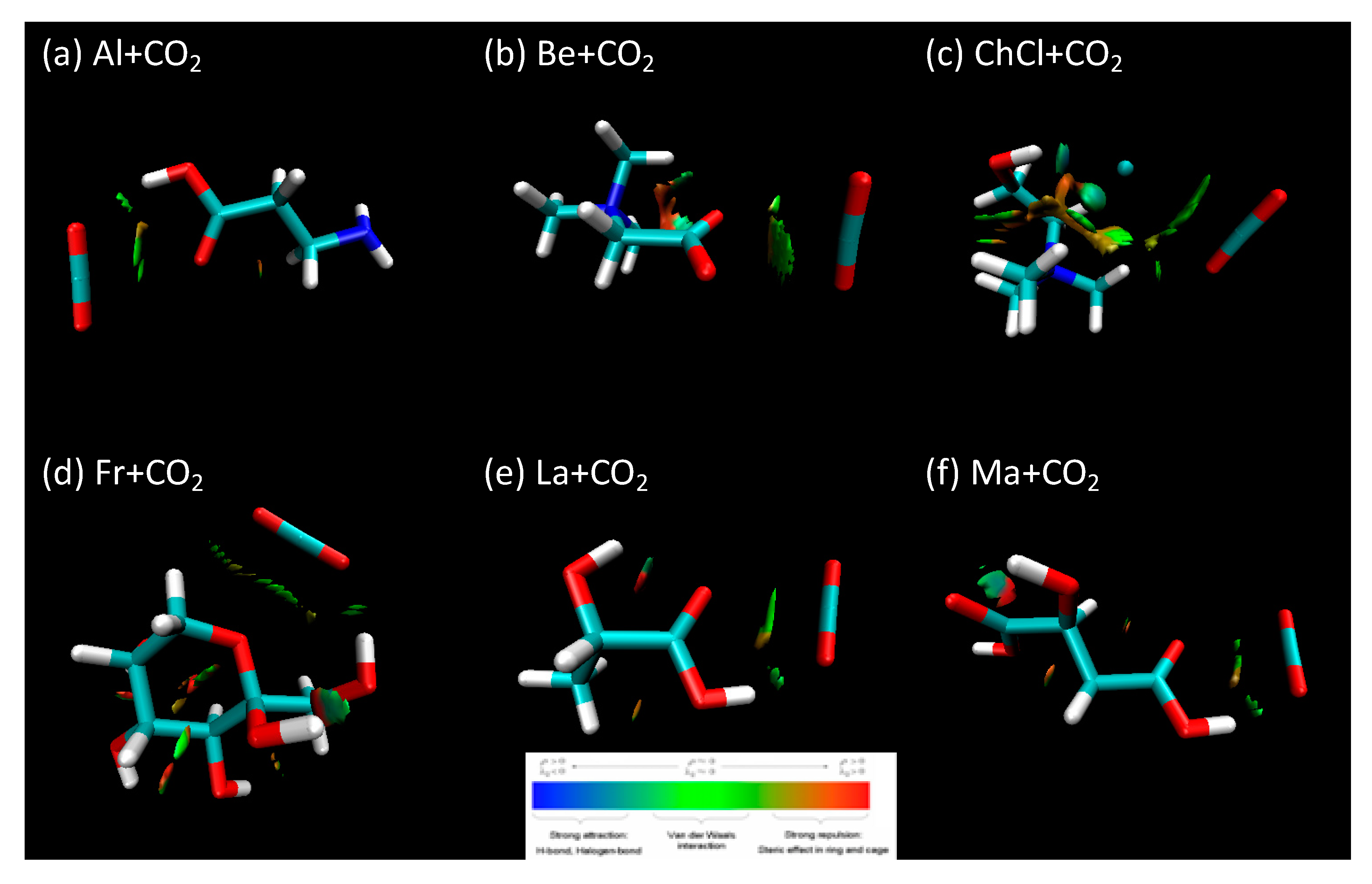
| Hydrogen Bond Donor (HBD) | 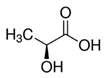 Lactic Acid | 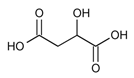 Malic Acid | 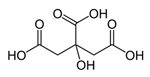 Malic Acid | 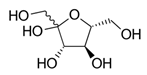 Fructose |
| Hydrogen Bond Acceptor (HBA) | 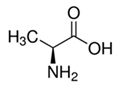 Alanine | 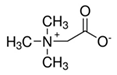 Betaine | 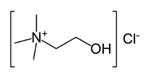 Choline Chloride | |
| Structure | Sorption (mmol CO2/g) | Sorption Comparison | Binding Energy (eV) | Binding Comparison |
|---|---|---|---|---|
| ChCl + CO2 | 4.96 | HBA > NADES > HBD | −0.137 | HBA > HBD |
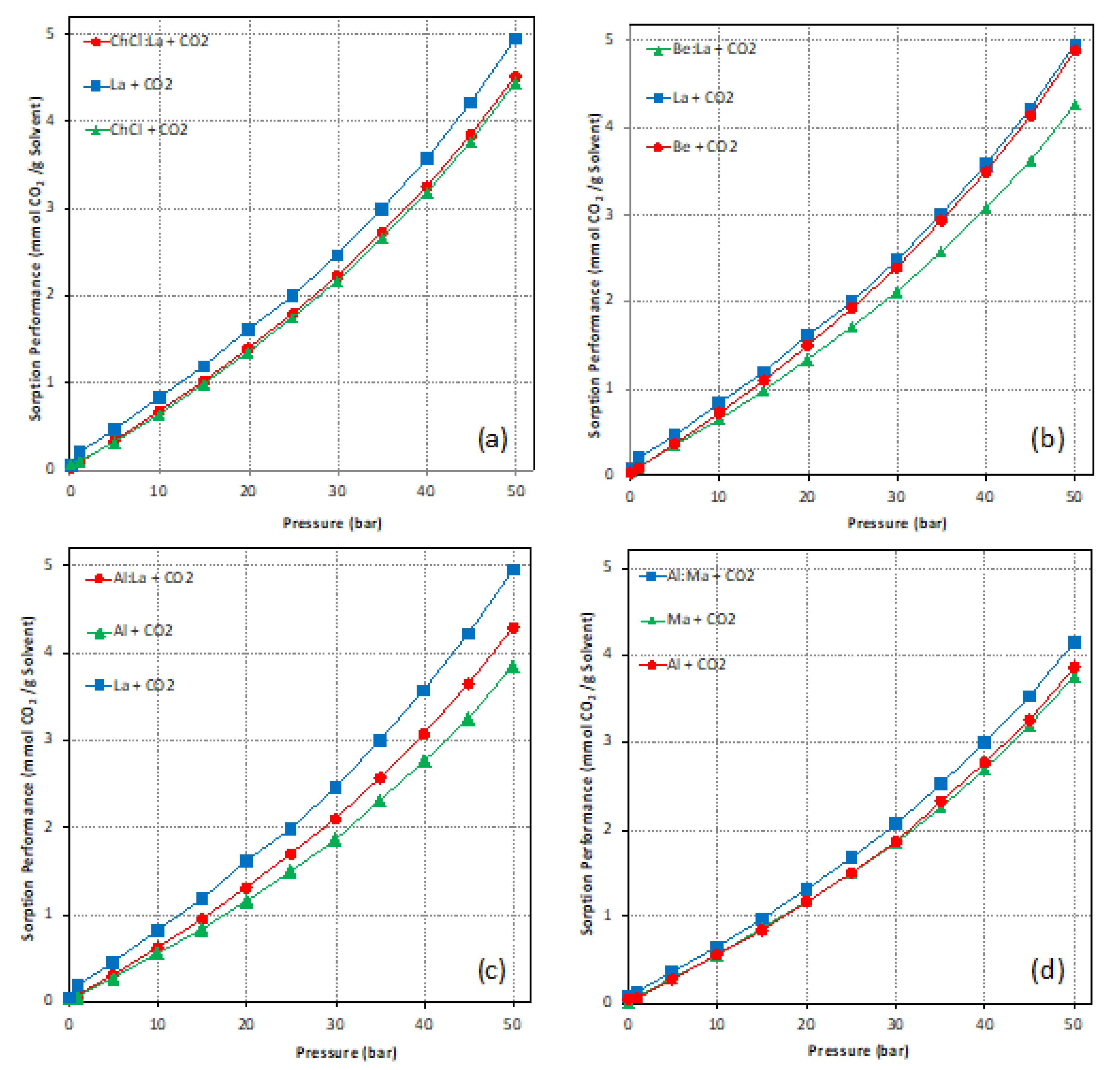
| ChCl:La + CO2 | 4.52 | 1.433 | ||
| La + CO2 | 4.46 | −0.122 | ||
| Be + CO2 | 4.88 | HBA > HBD > NADES | −0.112 | HBD > HBA |
| Be:La + CO2 | 4.26 | 1.292 | ||
| La + CO2 | 4.46 | −0.122 | ||
| Al + CO2 | 3.86 | HBD > NADES > HBA | −0.119 | HBA ~ HBD |
| Al:La + CO2 | 4.30 | 0.828 | ||
| La + CO2 | 4.46 | −0.122 | ||
| Al + CO2 | 3.86 | NADES > HBA > HBD | −0.119 | HBA > HBD |
| Al:Ma + CO2 | 4.14 | N/A | ||
| Ma + CO2 | 3.76 | −0.104 | ||
| ChCl + CO2 | 4.96 | HBA > NADES > HBD | −0.137 | HBA > HBD |
| ChCl:Fr + CO2 | 4.24 | 2.199 | ||
| Fr + CO2 | 3.58 | −0.101 | ||
| ChCl + CO2 | 4.96 | HBA > NADES > HBD | −0.137 | HBA > HBD |
| ChCl:Ma + CO2 | 4.22 | 1.514 | ||
| Ma + CO2 | 3.76 | −0.104 |
| Group | Structure | Energy (eV) |
|---|---|---|
| NADES | Al:La | −18,157.79 |
| Be:La | −20,295.39 | |
| ChCl:Fr | −40,166.78 | |
| ChCl:La | −30,819.46 | |
| ChCl:Ma | −35,950.19 | |
| Gas | CO2 | −5,131.10 |
| HBD | La | −9,348.54 |
| Fr | −18,695.51 | |
| Ma | −14,479.15 | |
| HBA | Al | −8,807.68 |
| Be | −10,944.60 | |
| ChCl | −21,468.77 |
| Group | Structure | Energy (eV) | Binding Energy (eV) | Average Binding Energy (eV) |
|---|---|---|---|---|
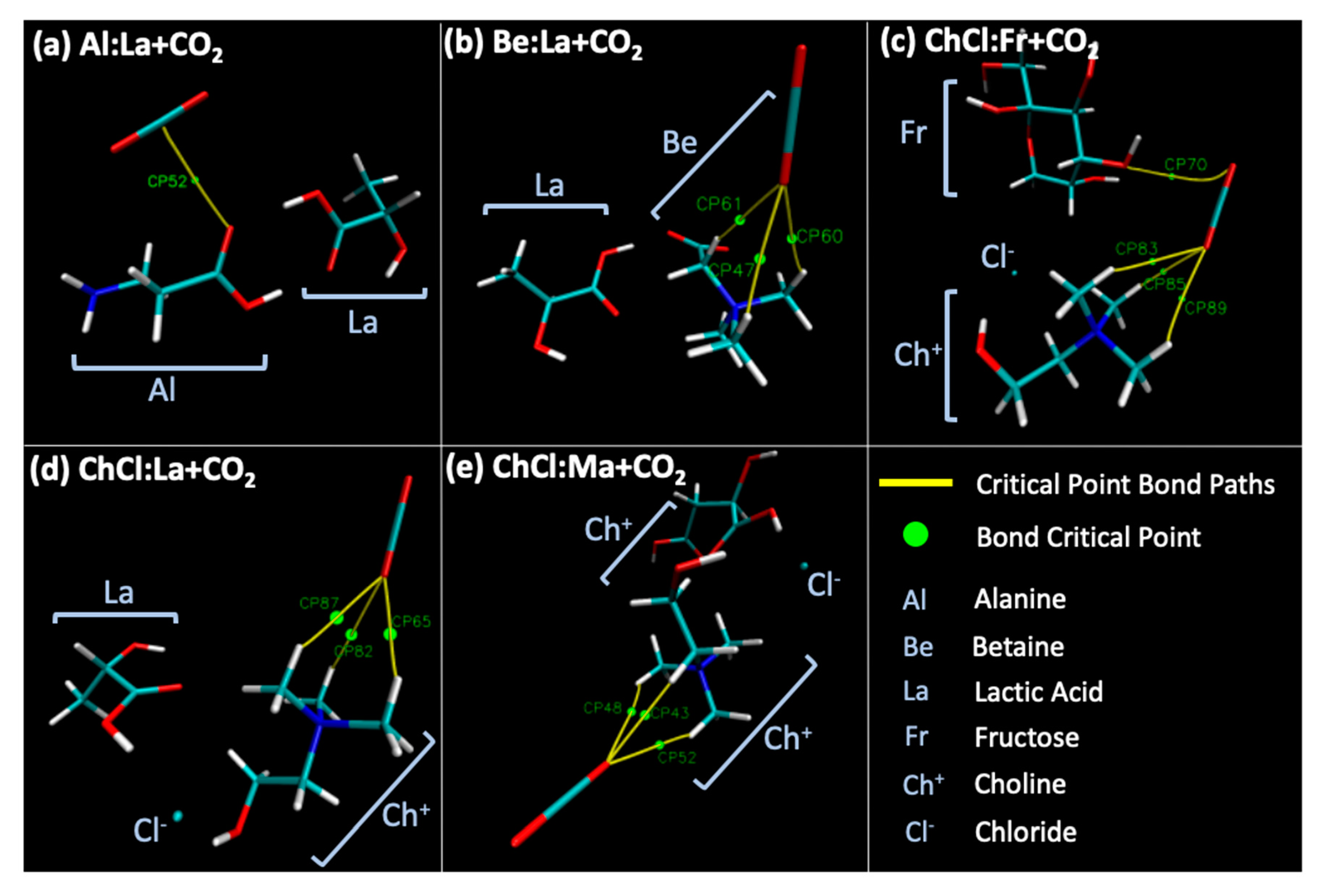
| NADES + CO2 | Al:La + CO2_p1 | −23,288.051 | 0.840 | 0.828 |
| Al:La + CO2_p2 | −23,288.072 | 0.819 | ||
| Al:La + CO2_p3 | −23,288.065 | 0.826 | ||
| Be:La + CO2_p1 | −25,425.242 | 1.248 | 1.292 | |
| Be:La + CO2_p2 | −25,425.200 | 1.289 | ||
| Be:La + CO2_p3 | −25,425.150 | 1.339 | ||
| ChCl:Fr + CO2_p1 | −45,295.714 | 2.167 | 2.199 | |
| ChCl:Fr + CO2_p2 | −45,295.679 | 2.202 | ||
| ChCl:Fr + CO2_p3 | −45,295.654 | 2.227 | ||
| ChCl:La + CO2_p1 | −35,949.184 | 1.370 | 1.433 | |
| ChCl:La + CO2_p2 | −35,949.103 | 1.451 | ||
| ChCl:La + CO2_p3 | −35,949.075 | 1.478 | ||
| ChCl:Ma + CO2_p1 | −41,079.907 | 1.379 | 1.514 | |
| ChCl:Ma + CO2_p2 | −41,079.733 | 1.553 | ||
| ChCl:Ma + CO2_p3 | −41,079.676 | 1.610 | ||
| HBA + CO2 | Al + CO2_p1 | −13,938.915 | −0.138 | −0.119 |
| Al + CO2_p2 | −13,938.876 | −0.099 | ||
| Be + CO2_p1 | −16,075.871 | −0.173 | −0.112 | |
| Be + CO2_p2 | −16,075.749 | −0.051 | ||
| ChCl + CO2_P1 | −26,600.028 | −0.158 | −0.137 | |
| ChCl + CO2_P2 | −26,599.997 | −0.127 | ||
| ChCl + CO2_P3 | −26,599.996 | −0.126 | ||
| HBD + CO2 | Fr + CO2_p1 | −23,826.696 | −0.093 | −0.101 |
| Fr + CO2_p2 | −23,826.712 | −0.108 | ||
| La + CO2_p1 | −14,479.781 | −0.145 | −0.122 | |
| La + CO2_p2 | −14,479.736 | −0.099 | ||
| Ma + CO2_p1 | −19,610.352 | −0.101 | −0.104 | |
| Ma + CO2_p2 | −19,610.358 | −0.106 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altamash, T.; Amhamed, A.; Aparicio, S.; Atilhan, M. Effect of Hydrogen Bond Donors and Acceptors on CO2 Absorption by Deep Eutectic Solvents. Processes 2020, 8, 1533. https://doi.org/10.3390/pr8121533
Altamash T, Amhamed A, Aparicio S, Atilhan M. Effect of Hydrogen Bond Donors and Acceptors on CO2 Absorption by Deep Eutectic Solvents. Processes. 2020; 8(12):1533. https://doi.org/10.3390/pr8121533
Chicago/Turabian StyleAltamash, Tausif, Abdulkarem Amhamed, Santiago Aparicio, and Mert Atilhan. 2020. "Effect of Hydrogen Bond Donors and Acceptors on CO2 Absorption by Deep Eutectic Solvents" Processes 8, no. 12: 1533. https://doi.org/10.3390/pr8121533
APA StyleAltamash, T., Amhamed, A., Aparicio, S., & Atilhan, M. (2020). Effect of Hydrogen Bond Donors and Acceptors on CO2 Absorption by Deep Eutectic Solvents. Processes, 8(12), 1533. https://doi.org/10.3390/pr8121533






