Laser-Induced Ignition and Combustion Behavior of Individual Graphite Microparticles in a Micro-Combustor
Abstract
1. Introduction
2. Materials and Methods
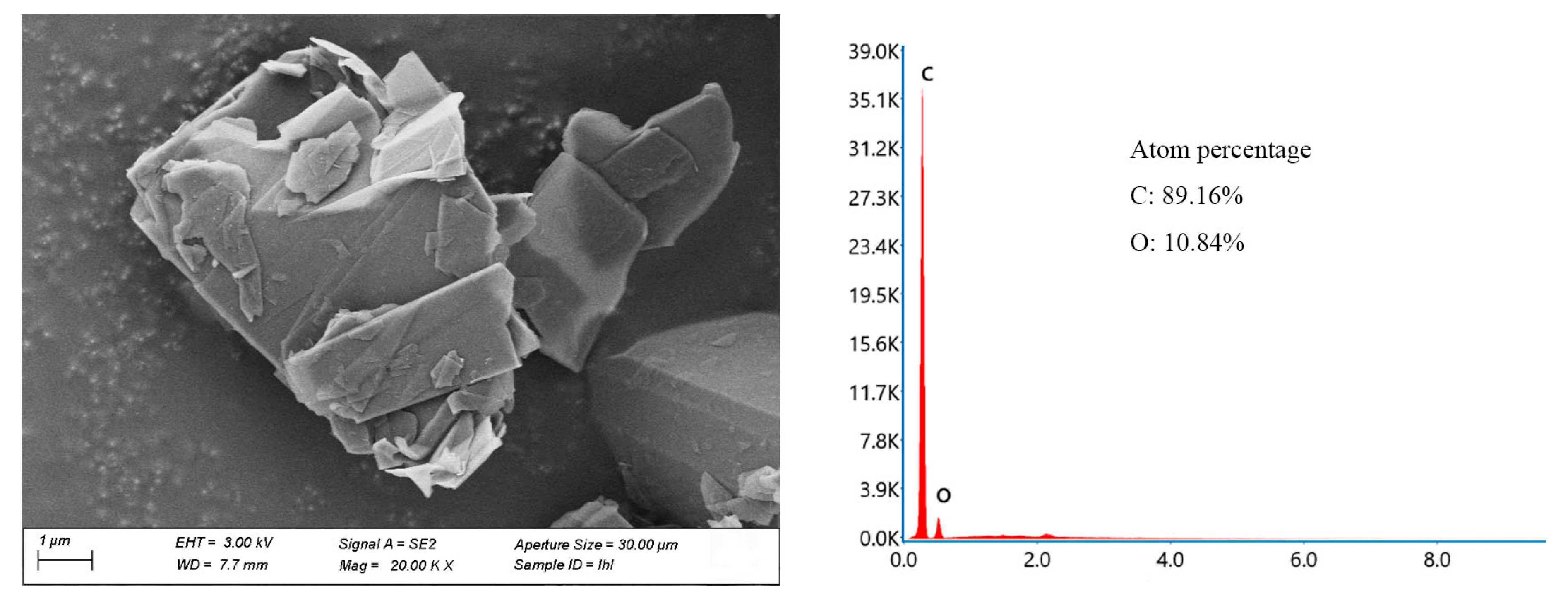
2.1. Preparation and Characterization
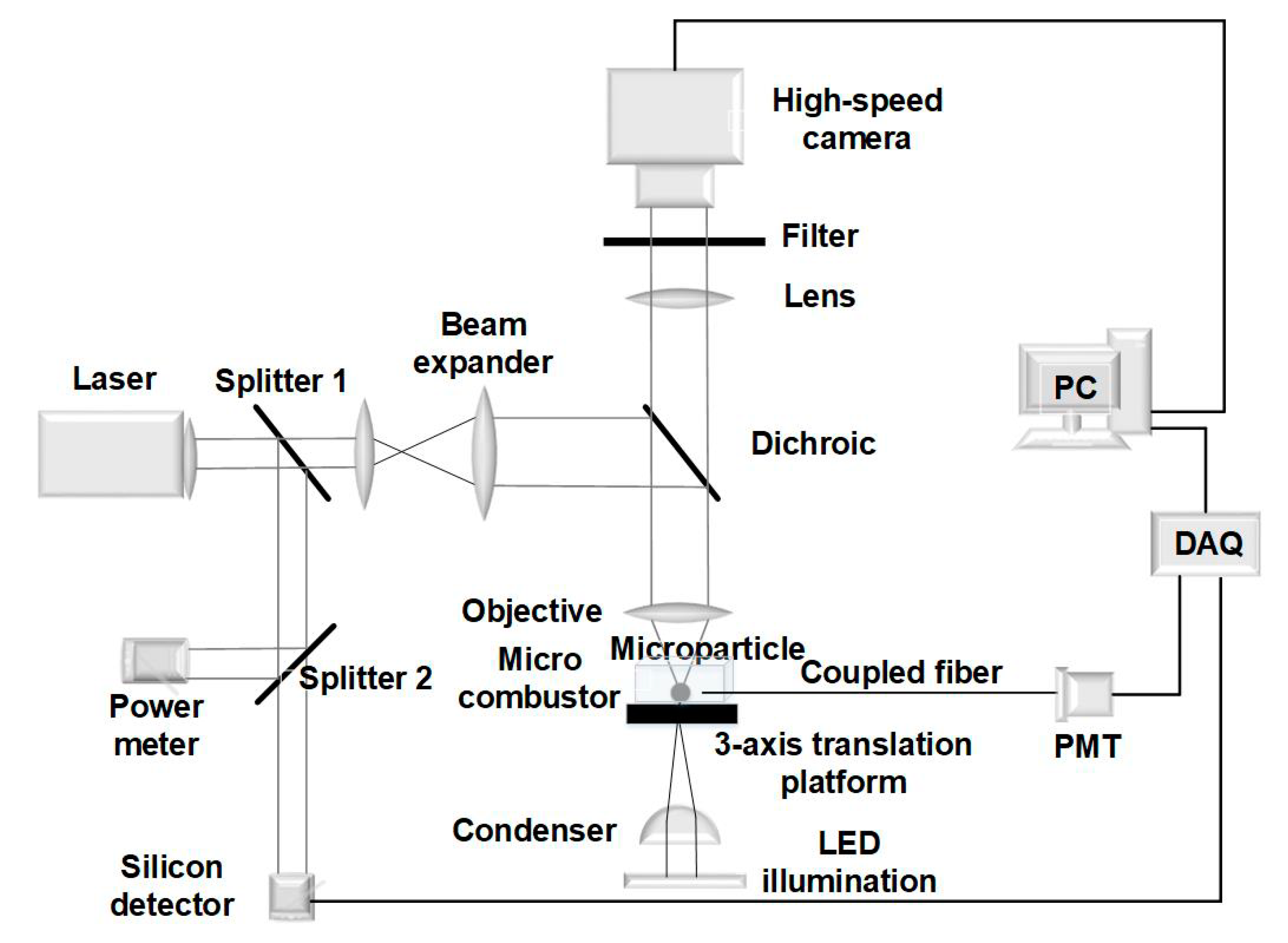
2.2. Experimental Setup for Ignition and Combustion Test
3. Results and Discussion
3.1. Ignition
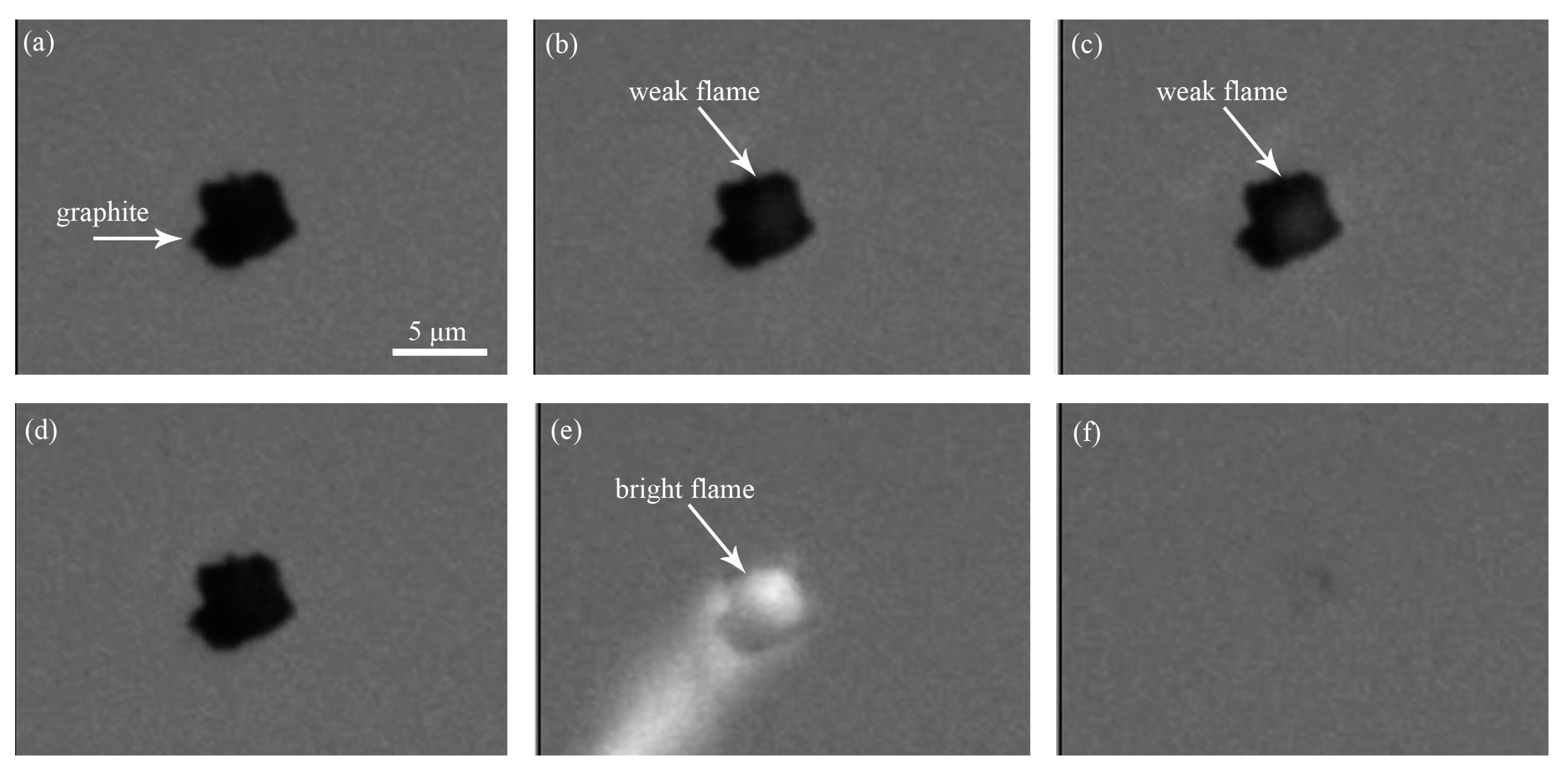
3.1.1. Ignition Mode
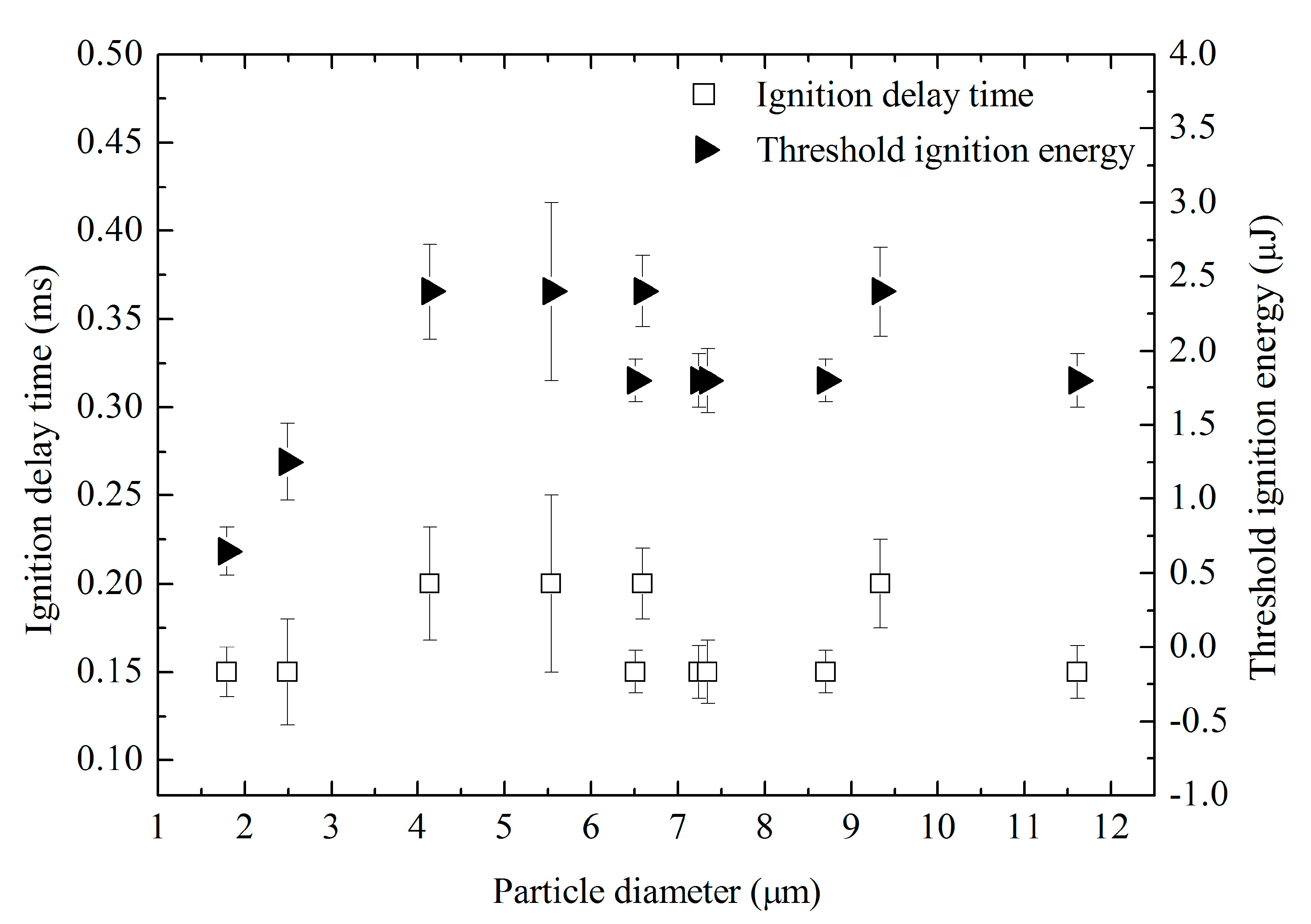
3.1.2. Ignition Delay Time and Threshold Energy
3.1.3. Effect of Particle Diameter on Ignition Delay and Threshold Energy
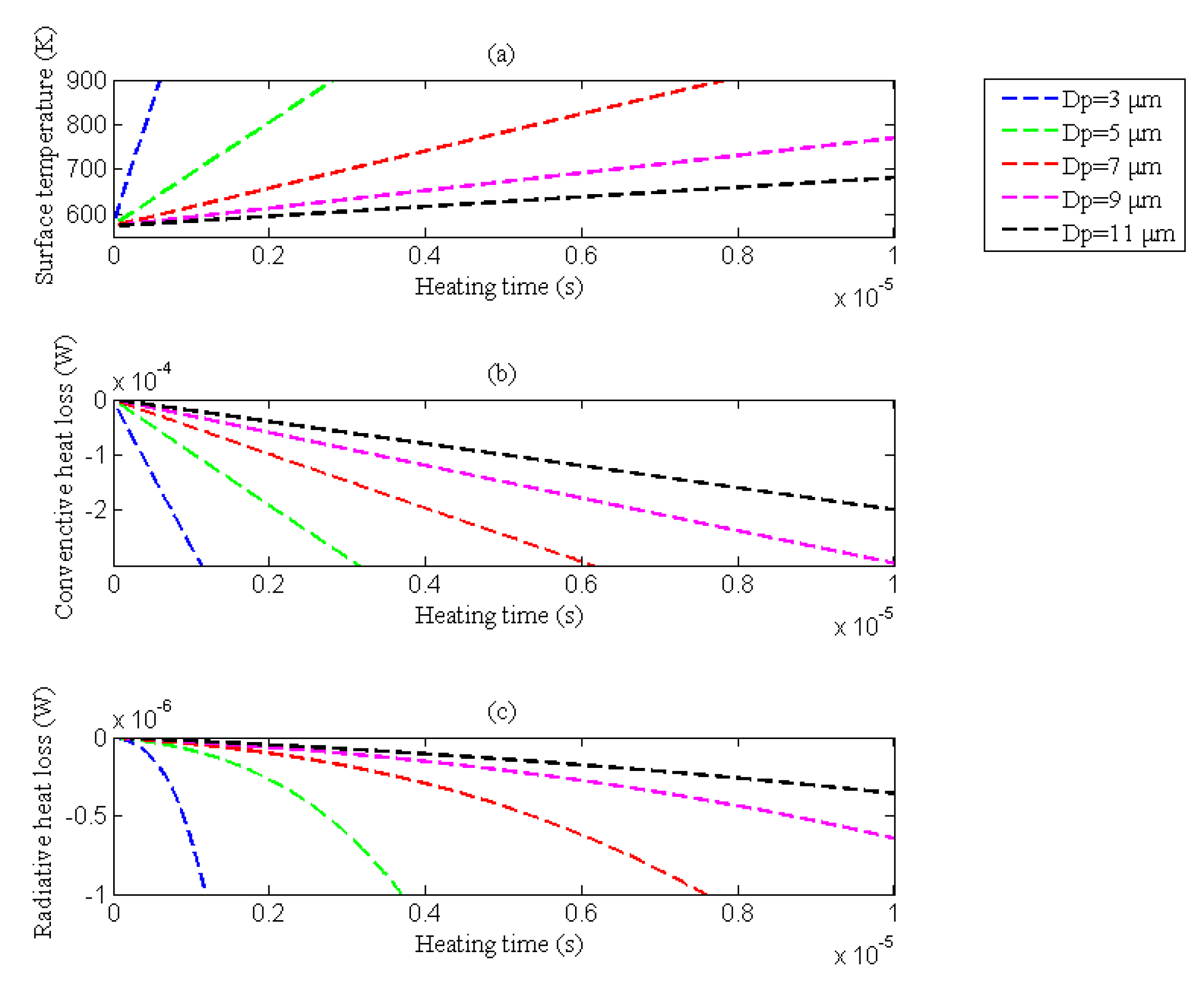
3.1.4. Influence of the Wall Heat Loss on the Ignition
3.2. Combustion
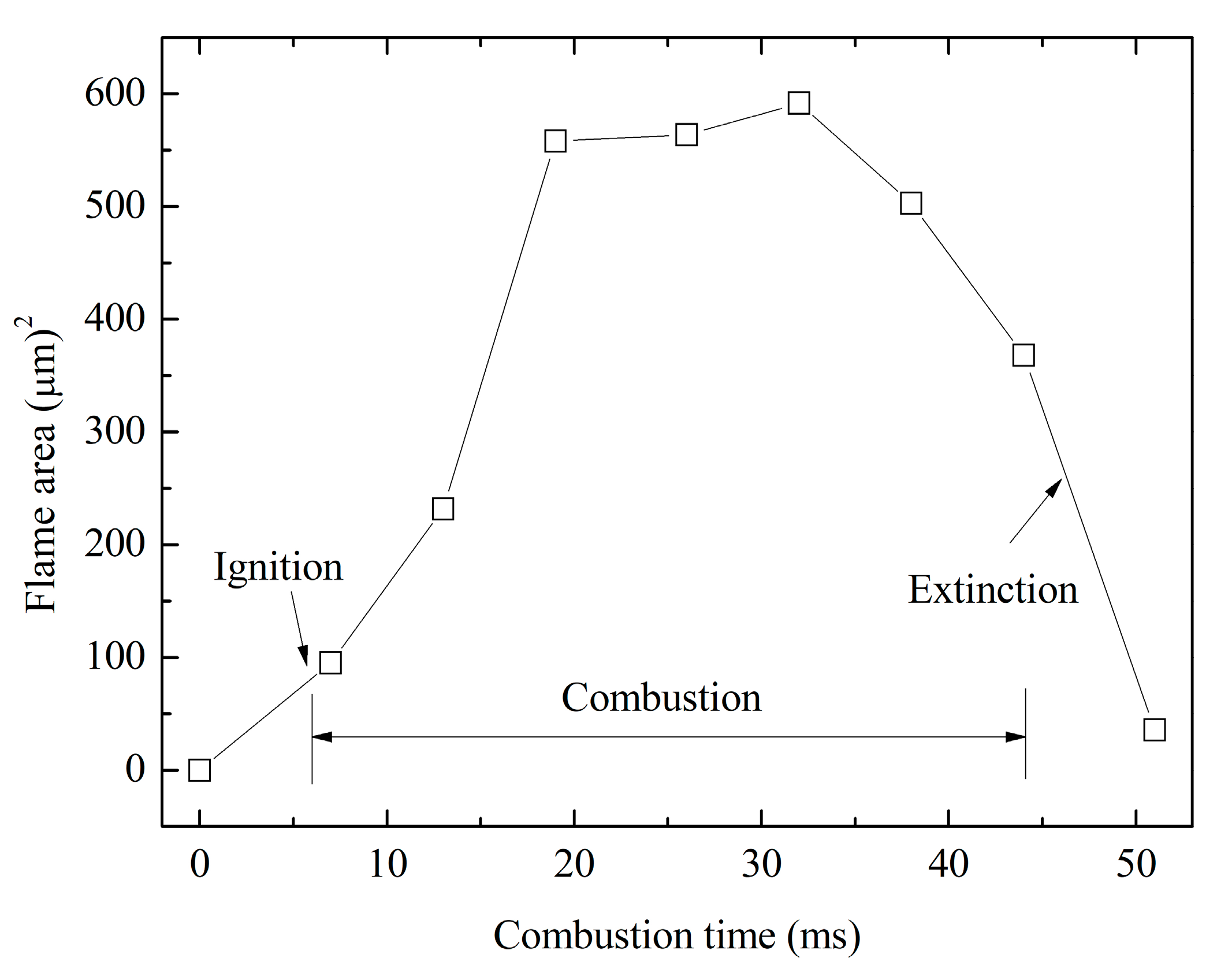
3.2.1. Combustion Stages
3.2.2. Flame Structure
3.2.3. Flame Front
3.2.4. Crystalline Structure of Graphite after Combustion
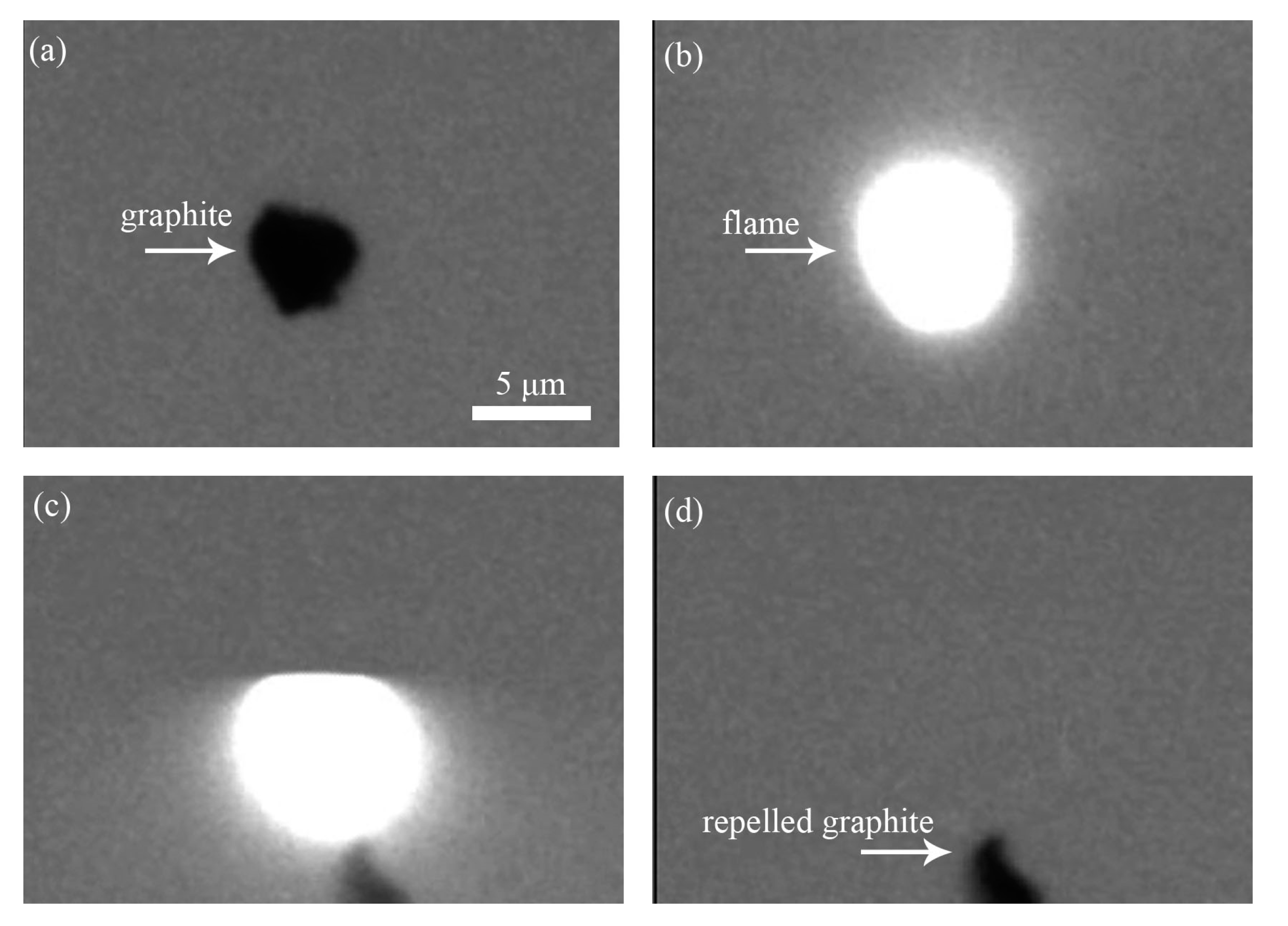
3.3. Photophoresis
3.4. Repetitive Extinction and Reignition
4. Conclusions
- (1)
- The micron-sized graphite particles scattered in the micro-combustor were heterogeneously ignited by laser. The particle diameter scarcely influenced the threshold ignition energy and the ignition delay time. After combustion, the crystalline structure of graphite had the same pattern as the unburned graphite.
- (2)
- The combustion radiation intensity first increased, then abruptly decreased, and again increased and oscillated to become abruptly extinct. The envelop flame front diffused quickly, and the flame became brighter. After oscillating combustion, the envelop flame front suppressed. The extension and suppression velocities of the flame were almost equivalent.
- (3)
- During combustion, the graphite particle suffered the negative photophoretic force and moved far from the focal point of the laser, resulting in extinction.
- (4)
- The heat loss of the micro-combustor wall had a significant effect on the ignition and combustion. The flame bifurcations, weak flame, repetitive extinction and reignition of the graphite in limited space could be clearly recognized.
Author Contributions
Funding
Conflicts of Interest
References
- Smith, I.W. The combustion rates of coal chars: A review. Symp. (Int.) Combust. 1982, 19, 1045–1065. [Google Scholar] [CrossRef]
- Abuelnuor, A.A.A.; Wahid, M.A.; Hosseini, S.E.; Saat, A.; Saqr, K.M.; Sait, H.H.; Osman, M. Characteristics of biomass in flameless combustion: A review. Renew. Sustain. Energy Rev. 2014, 33, 363–370. [Google Scholar] [CrossRef]
- Zhou, H.; Meng, A.H.; Long, Y.Q.; Li, Q.H.; Zhang, Y.G. An overview of characteristics of municipal solid waste fuel in China: Physical, chemical composition and heating value. Renew. Sustain. Energy Rev. 2014, 36, 107–122. [Google Scholar] [CrossRef]
- Thomas, J.M. Reactivity of carbon: Some current problems and trends. Carbon 1970, 8, 413–421. [Google Scholar] [CrossRef]
- Valerio, C.; Luigi, P.; Sandro, P. Ignition and combustion of individual, levitated char particles. Combust. Flame 1995, 103, 181–193. [Google Scholar]
- Solís-Fernández, P.; Paredes, J.I.; Cosío, A.; Martínez-Alonso, A.; Tascón, J.M.D. A comparison between physically and chemically driven etching in the oxidation of graphite surfaces. J. Colloid Interface Sci. 2010, 344, 451–459. [Google Scholar] [CrossRef]
- Stevens, F.; Beebe, T.P. Computer modeling of graphite oxidation: Differences between monolayer and multilayer etching. Comput. Chem. 1999, 23, 175–183. [Google Scholar] [CrossRef]
- Qu, M.; Ishigaki, M.; Tokuda, M. Ignition and combustion of laser-heated pulverized coal. Fuel 1996, 75, 1155–1160. [Google Scholar] [CrossRef]
- Makino, A.; Namikiri, T.; Kimura, K. Combustion rates of graphite rods in the forward stagnation field with high-temperature airflow. Combust. Flame 2003, 132, 743–753. [Google Scholar] [CrossRef]
- Stauch, R.; Maas, U. Transient detailed numerical simulation of the combustion of carbon particles. Int. J. Heat Mass Tranf. 2009, 52, 4584–4591. [Google Scholar] [CrossRef]
- Makino, A.; Kato, I.; Senba, M.; Fujizaki, H.; Araki, N. Flame structure and combustion rate of burning graphite in the stagnation flow. Symp. (Int.) Combust. 1996, 26, 3067–3074. [Google Scholar] [CrossRef]
- Makino, A.; Fujizaki, H.; Araki, N. Combustion Rate of Burning Graphite in a Stagnation Flow of Water Vapor. Combust. Flame 1998, 113, 258–263. [Google Scholar] [CrossRef]
- Makino, A.; Umehara, N. Combustion rates of graphite rods in the forward stagnation field of the high-temperature, humid airflow. Proc. Combust. Inst. 2007, 31, 1873–1880. [Google Scholar] [CrossRef]
- Maruta, K. Micro and mesoscale combustion. Proc. Combust. Inst. 2011, 33, 125–150. [Google Scholar] [CrossRef]
- Ju, Y.G.; Maruta, K. Microscale combustion: Technology development and fundamental research. Prog. Energy Combust. Sci. 2011, 37, 669–715. [Google Scholar] [CrossRef]
- Delhaes, P. Graphite and Precursors; CRC Press: Boca Raton, FL, USA, 2001; pp. 40–50. ISBN 90-5699-228-7. [Google Scholar]
- Li, S.J.; Huang, X.F. The manipulation and combustion of carbon-based micro particles by optical tweezers. Int. J. Optomechatron. 2015, 9, 35–47. [Google Scholar] [CrossRef]
- Li, S.; Huang, X.; Zhou, D. Experiments and Numerical Calculations on Laser-Induced Ignition of Single Micron-Sized Aluminum Fuel Particle. Propellants Explos. Pyrotech. 2017, 42, 523–531. [Google Scholar] [CrossRef]
- Huang, X.; Li, S.; Zheng, X.; Yang, S.; Guo, Y. Combustion Mechanism of a Novel Energetic Fuel Candidate Based on Amine Metal Borohydrides. Energy Fuel 2016, 30, 1383–1389. [Google Scholar] [CrossRef]
- Essenhigh, R.H.; Misra, M.K.; Shaw, D.W. Ignition of coal particle: A review. Combust. Flame 1989, 77, 3–30. [Google Scholar] [CrossRef]
- Effron, E.; Hoelscher, H.E. Graphite oxidation at low temperature. AIChE J. 1964, 10, 388–392. [Google Scholar] [CrossRef]
- Gulbransen, E.A.; Andrew, K.F.; Brassart, F.A. The Oxidation of Graphite at Temperatures of 600° to 1500 °C and at Pressures of 2 to 76 Torr of Oxygen. J. Electrochem. Soc. 1963, 110, 476–483. [Google Scholar] [CrossRef]
- Arthur, J.R. Reactions between carbon and oxygen. Trans. Faraday Soc. 1951, 47, 164–178. [Google Scholar] [CrossRef]
- Hou, F.; Li, S.; Wang, Y.; Huang, X. Laser-Induced Ignition and Combustion of Individual Aluminum Particles below 10 μm by Microscopic High-Speed Cinematography. Processes 2020, 8, 280. [Google Scholar] [CrossRef]
- Richecoeur, F.; Kyritsis, D.C. Experimental study of flame stabilization in low Reynolds and Dean number flows in curved mesoscale ducts. Proc. Combust. Inst. 2005, 30, 2419–2427. [Google Scholar] [CrossRef]
- Miesse, C.; Masel, R.I.; Short, M.; Shannon, M.A. Diffusion flame instabilities in a 0.75 mm non-premixed microburner. Proc. Combust. Inst. 2005, 30, 2499–2507. [Google Scholar] [CrossRef]
- Xu, B.; Ju, Y. Studies on non-premixed flame streets in a mesoscale channel. Proc. Combust. Inst. 2009, 32, 1375–1382. [Google Scholar] [CrossRef]
- Snead, L.; Burchell, T. Oxidation of High-Quality Graphite for IFE. In Proceedings of the 3rd Laser IFE Program Workshop, San Diego, CA, USA, 13–14 November 2001; pp. 1–9. [Google Scholar]
- Jovanovic, O. Photophoresis-Light induced motion of particles suspended in gas. J. Quant. Spectrosc. Radiat. Transf. 2009, 110, 889–901. [Google Scholar] [CrossRef]
- Wurm, G.; Krauss, O. Experiments on negative photophoresis and application to the atmosphere. Atmos. Environ. 2008, 42, 2682–2690. [Google Scholar] [CrossRef]
- Mikami, M.; Kojima, N. An experimental and modeling study on stochastic aspects of microexplosion of binary-fuel droplets. Proc. Combust. Inst. 2002, 29, 551–559. [Google Scholar] [CrossRef]
- Tsuboi, Y.; Yokomori, T.; Maruta, K. Lower limit of weak flame in a heated channel. Proc. Combust. Inst. 2009, 32, 3075–3081. [Google Scholar] [CrossRef]
- Oshibe, H.; Nakamura, H.; Tezuka, T.; Hasegawa, S.; Maruta, K. Stabilized three stage oxidation of DME/air mixture in a micro flow reactor with a controlled temperature profile. Combust. Flame 2010, 157, 1572–1580. [Google Scholar] [CrossRef]
- Kim, K.T.; Lee, D.H.; Kwon, S. Effects of thermal and chemical surface-flame interaction on flame quenching. Combust. Flame 2006, 146, 19–28. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhang, M.; Chang, S.; Li, S.; Huang, X. Laser-Induced Ignition and Combustion Behavior of Individual Graphite Microparticles in a Micro-Combustor. Processes 2020, 8, 1493. https://doi.org/10.3390/pr8111493
Wang Y, Zhang M, Chang S, Li S, Huang X. Laser-Induced Ignition and Combustion Behavior of Individual Graphite Microparticles in a Micro-Combustor. Processes. 2020; 8(11):1493. https://doi.org/10.3390/pr8111493
Chicago/Turabian StyleWang, Yue, Minqi Zhang, Shuhang Chang, Shengji Li, and Xuefeng Huang. 2020. "Laser-Induced Ignition and Combustion Behavior of Individual Graphite Microparticles in a Micro-Combustor" Processes 8, no. 11: 1493. https://doi.org/10.3390/pr8111493
APA StyleWang, Y., Zhang, M., Chang, S., Li, S., & Huang, X. (2020). Laser-Induced Ignition and Combustion Behavior of Individual Graphite Microparticles in a Micro-Combustor. Processes, 8(11), 1493. https://doi.org/10.3390/pr8111493




