3.1. Overview of Copper and Nickel–Copper Smelter Operations
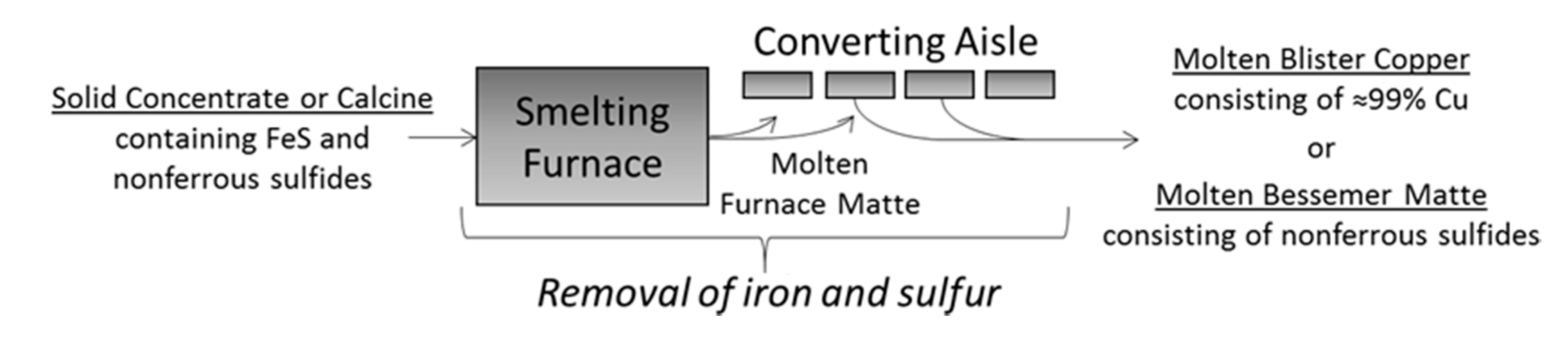
The copper pyrometallurgy process treats mineral concentrates to produce copper anodes that are, in turn, electrorefined to generate effectively pure (99.99% Cu) end-product cathodes. The concentrates are comprised of copper–iron sulfide minerals with particle sizes of generally less than 150 μm. Elemental compositions typically range from 25% to 30% Cu, 25% to 35% S, and 20% to 40% Fe, with the remainder made up of gangue material (oxides). The smelter targets the selective oxidation of Fe and S in order to retain Cu for the final product. Specifically, the iron is skimmed away as liquid slag and the sulfur is removed as SO
2 offgas, eventually resulting in molten copper [
13,
14]. This pyrometallurgical technique accounts for approximately 75% of the primary copper production worldwide [
15,
16], the majority of which is carried out using the conventional approach depicted in
Figure 3. In some cases, the incoming concentrates are subject to roasting reactions prior to being fed into the smelting operation; these roasted concentrates are known as calcines.
Within a copper smelter, the concentrate or calcine is smelted by exothermic reactions while controlling the oxygen mass balance, which produces an intermediate matte phase composed of copper, iron, and sulfur. This molten matte is then subject to further oxidation, which converts the matte into a molten metal product called blister copper (>98% purity). The subsequent liquid–state refinement (fire-refining) precedes the casting of a Cu anode product (~99.5% purity). The anodes are then transferred to an electrorefinery for a final upgrade to end-product Cu cathodes that are 99.99% pure; the electrorefinery also recovers gold, silver, and other valuable byproducts that are contained in the anodes. Nonetheless, smelting and converting are the central processes in a copper smelter, since they transform the mineral input into an initial metallic output [
14].
The smelting operation is a continuous process typically executed in one (or at certain large plants, two) large furnace(s). There are two main smelting technologies that induce similar chemical transformations but differ in the mechanism by which oxygen is delivered to the concentrate. Flash smelting, in which the oxidation of the concentrate takes place in a generally vertically mounted burner, currently accounts for roughly 45% of the world copper smelting capacity [
2,
15,
17]. Within a flash smelting furnace, the refractory-lined reaction shaft is like an industrial scale “drop tube” (
Figure 2). On the other hand, bath smelting technologies inject air or oxygen-enriched air into the molten bath either via a top-mounted lance (called top smelting lance or TSL technology) or via submerged tuyères; bath smelting technologies account for roughly 50% of smelting capacity, and this proportion has recently increasing due to new bath smelting technologies introduced in China [
15,
17].
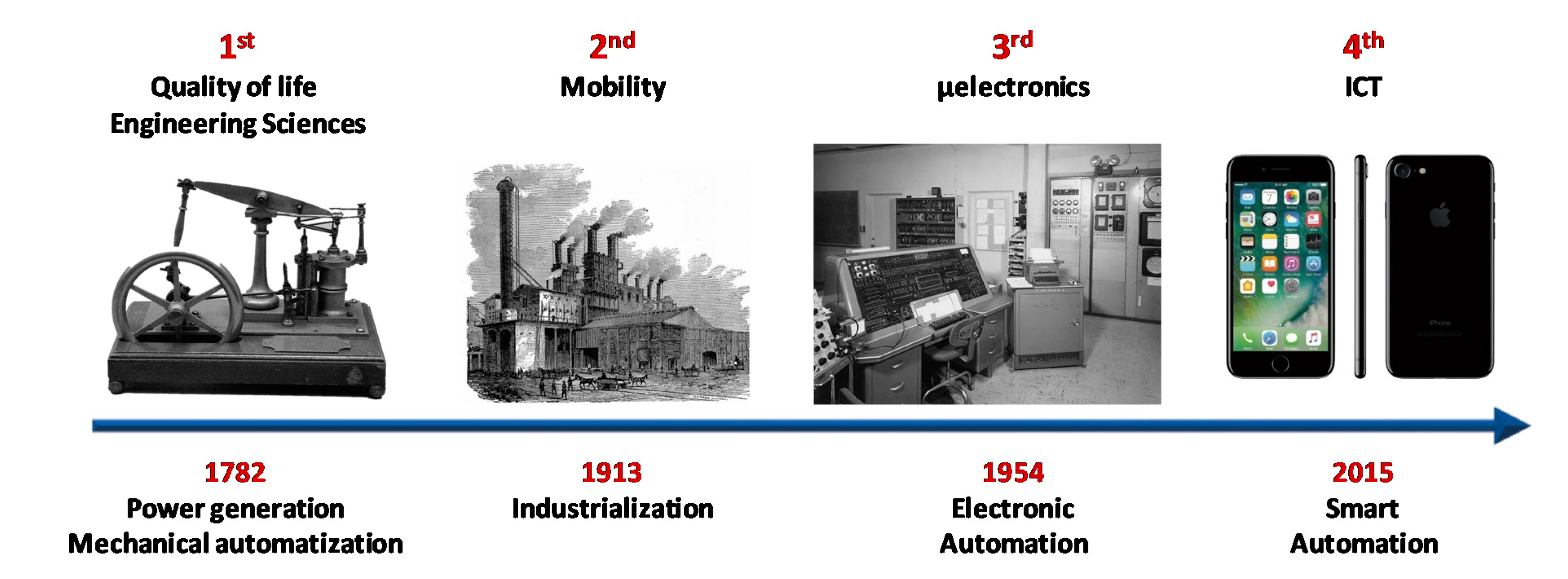
Peirce-Smith (PS) converting is the most longstanding and widely used technology in conventional copper smelters [
14] and is performed in discrete batches. PS converting is indeed a remnant of the second industrial revolution (
Figure 1) and was influenced by the 19th century developments of Sir Henry Bessemer in steelmaking [
16]. PS batch conversions are often carried out in parallel (
Figure 3) and may share a limited set of resources (e.g., oxygen and offgas handling capacities). The continuous-discrete contrast of smelting converting is central to conventional smelter dynamics, in which PS converting can be a major bottleneck in conventional copper smelters [
14]. Moreover, PS converting is also a feature of nickel–copper smelters (
Figure 3), noting the difference in the final discharge product (
Figure 3). Copper PS results in blister copper, whereas nickel–copper PS converting is simply to remove the iron and its associated sulfur; the resulting iron-free matte still contains considerable sulfur and is known as Bessemer matte, in honor of Sir Henry Bessemer.
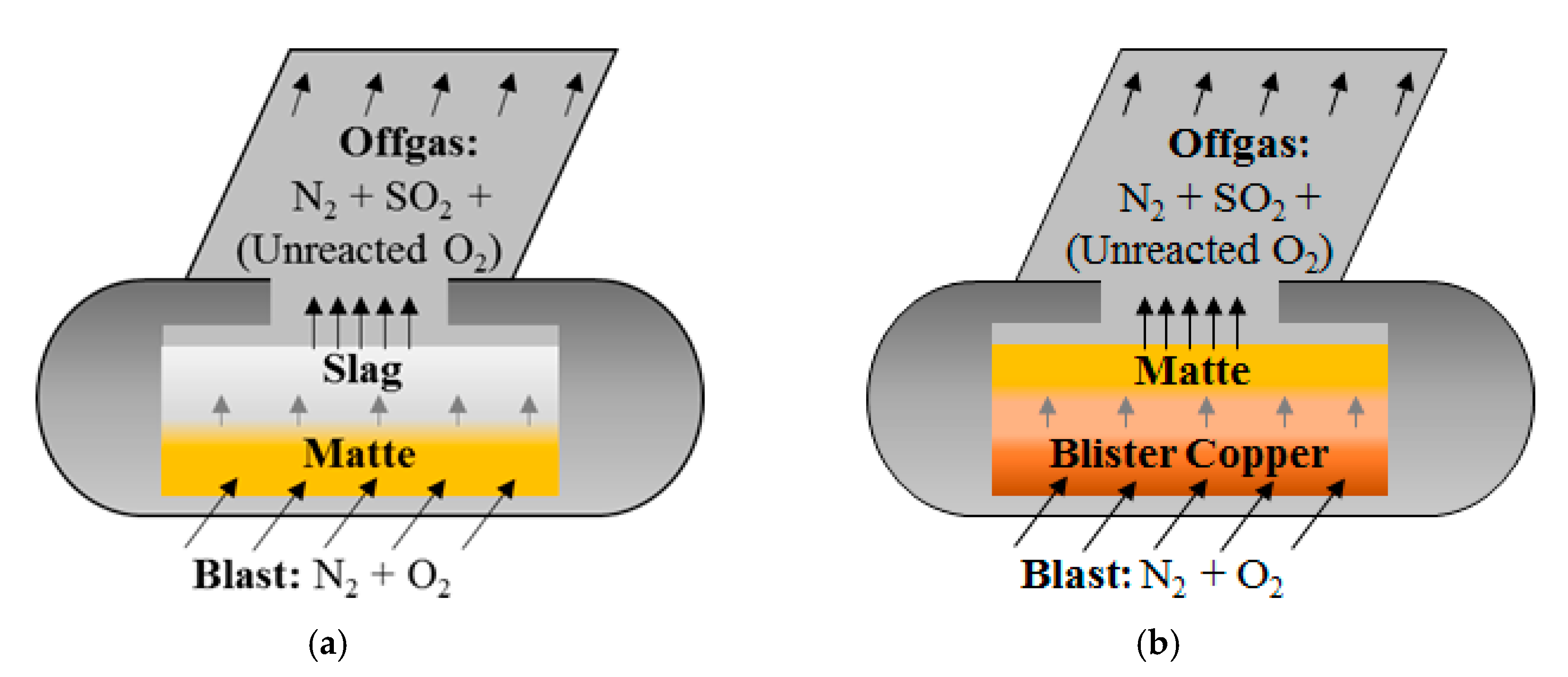
Smelting furnaces can generally produce, and hold, matte in excess of the converting capacity, which means that the smelting schedule can depend on the converting schedule. Given that smelting and converting are central to the overall smelter operation, all other critical functions at the smelter plant can also be restricted by the converting cycles. Each converting cycle begins when a fresh charge of matte (and possibly some amount of cold charge) is delivered to an empty converter and ends with the final discharge. The matte is subjected to pressurized blowing wherein oxygen-enriched air is blown into the melt, and N
2 and SO
2 are exhausted through a hood mounted on the vessel (
Figure 4). The offgas is captured in order to convert SO
2 into sulfuric acid; meanwhile, N
2 acts as a coolant in the process [
14]. Copper and nickel–copper smelters both apply the first stage of PS converting, which is called the slag-blow, producing an iron-rich slag that forms atop the denser matte (
Figure 4a). This stage may require intermittent pauses in order to skim away slag accumulation and replenish the vessel with fresh matte and cold charge. Once all of the slag is removed (<1% Fe in matte), copper smelters continue blowing the remaining matte; this final stage of converting is known as the copper-blow, as it results in the formation of blister copper that sinks to the bottom of the vessel (
Figure 4b). The copper-blow does not produce any more slag and, therefore, does not require intermittent skimming. Nickel–copper smelters, however, only apply the slag-blow (
Figure 4a), not the copper-blow. In either context, the cycle is complete when all of the matte is converted to the correct endpoint and discharged (
Figure 3).
In addition to scheduling constraints, various process parameters are also subject to statistical variations, including the chemical composition of incoming plant feed, matte grade, furnace performance, and converter cycle times, among other global factors. It is critical to measure, model, and simulate such uncertainties in order to streamline and support the decision-making processes in the design, development, and operation stages of industrial systems.
3.2. Detailing of Smelter Dynamics Within Discrete Event Simulation
In a previous work, Navarra et al. [
18,
19] incorporated thermochemical equilibria within a discrete event simulation (DES); it was suggested generally that the hybridization of time-adaptive finite differences (TAFD) and DES is a suitable paradigm for multiphase smelter reengineering projects [
20]. Within copper and nickel–copper smelters, thermochemical equilibria determine the iron-speciation of smelting and converting slags, as described in the following section. However, the early phases of a smelter reengineering project can assume fixed molar ratios of iron and oxygen.
Assuming that the smelter feed is composed mainly of iron, sulfur, and copper, a smelter accomplishes the following unbalanced reaction:
In which FeO
x represents a mixture of wustite FeO and magnetite Fe
3O
4, such that
x = 1 and
x = 1.25 corresponds to pure wustite and magnetite, respectively. For simplicity,
x can be fixed to 1, although typical values can range between 1.0 and 1.1, depending on the nature and quantity of the flux and the monitoring and control of the process itself; in particular, a low level of magnetite in slag is desirable, which is associated with low slag viscosity. In practice, the flux is predominantly silica SiO
2, but certain smelters include varying quantities of CaO and other stable oxides; CaO is especially common in continuous converting [
21], which is an alternative to the conventional PS converting [
16]. The SO
2 is captured for sulfuric acid production, and the blister copper is subject to fire refining prior to being cast into anodes that undergo electrolytic refining. A similar reaction can describe nickel–copper smelters:
The subsequent processing of Bessemer matte depends on the given nickel–copper plant. Equation (1a,b) provide more detail to
Figure 3 for copper and nickel–copper smelters, respectively.
Depending on the scope and phase of the project,
x can be regarded as a single global value for the entire smelter or as distinct values for the smelting furnace(s) and converters. Equation (1a) can thus be rewritten
For copper smelters, which decompose the global x into xS and xC, characterize the slag of the smelting furnace. A similar decomposition of the global x could be applied to Equation (1b) in the case of nickel–copper smelters.
Moreover, the individual slag-blow segments of PS cycles can each be assigned appropriate
x values. Therefore, Equation (2) could be further detailed as:
In which
xS characterizes the slag of the smelting furnace, and, depending on the level of detail, x
Ci can characterize the types of converter cycles or can characterize the individual types of slag-blow segments, for
i = 1 to
n. For example,
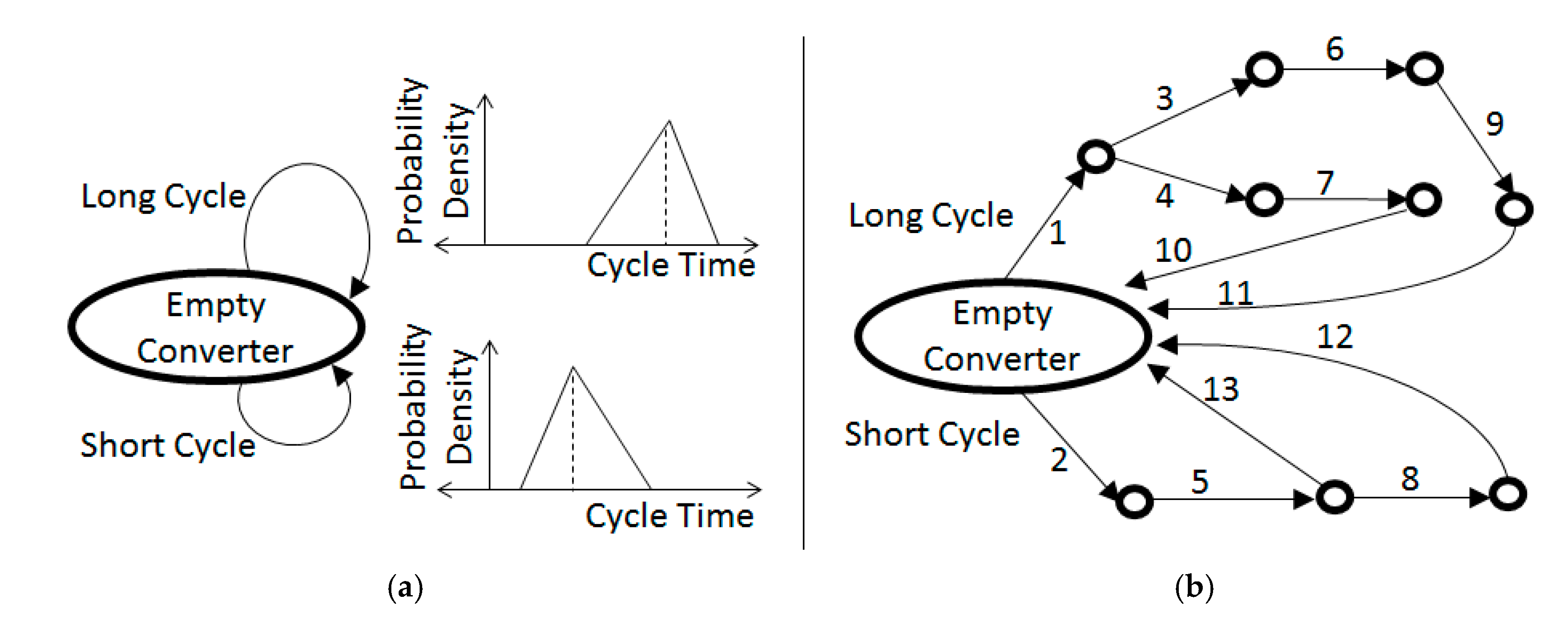
Figure 5a shows an action graph that occurs within a smelter that practices two kinds of converter cycles, long and short; hence,
n = 2.
Figure 5b shows a more detailed representation, which considers 13 kinds of blow segments. (The slag-blow segments are punctuated with charging and skimming actions, although these are not explicitly shown in
Figure 5b). For a conventional copper smelter, actions 1–9 describe slag-blow segments (
Figure 4a); hence,
n = 9, and the remaining actions 10–13 represent copper-blow segments (
Figure 4b) that complete the cycle as a batch of blister copper is discharged. In the case of a nickel–copper smelter, all of the arcs represent slag-blow segments; hence,
n = 13, noting that the discharge is the so-called Bessemer matte (Ni, Cu, Co and S) that is described in
Figure 3 and Equation (1b).
The decision to apply one segment versus another (e.g., segment 3 versus 4) would depend on the state of the plant, to the extent that the state variables can monitored with the available sensors. Even if the resulting slag compositions for the different cycles (
Figure 5a) or blow segments (
Figure 5b) are relatively consistent, it may be unclear how frequently each cycle or segment will be applied, e.g., depending on how often certain plant conditions occur. A global mass balance based on Equation (3) requires an estimation of how often each of the different cycles (
Figure 5a) or segments (
Figure 5b) are applied; such estimations are the result of DES computations, as described below.
The broad distributions of
Figure 5a approximate the combined effects of narrower distributions that would characterize the individual segments of
Figure 5b. A proposed technological change within the smelter (e.g., installation of new sensors) may require a rethinking of the slag-blow and the definition of new action graphs. Depending on the project, it may be necessary to further decompose the actions of
Figure 5b into sub-actions and sub-sub-actions, possibly including thermochemical modeling [
22,
23] or computational fluid dynamics [
24]. This decomposition may be essential in order to properly simulate the system with and without the technological change, thereby evaluating the benefit of the proposed change. In the case of sensors, it is necessary to simulate how the additional information will be incorporated into the decisions and operational actions of the smelter, thereby computing the value of these better-informed decisions and actions.
In many reengineering projects, the phenomena that occur within the smelter may be less important than the phenomena that occur outside of the smelter. For instance, the DES model of the Hernán Videla Lira (HVL) Smelter developed by Navarra et al. [
25] focuses on the smelter-wide response to changing meteorological conditions and has a comparatively simple representation of converter cycles, similar to
Figure 5a. The HVL Smelter considers distinct categories of meteorological conditions—normal, unfavorable, and extreme—to describe the potential for the surrounding atmosphere to disperse the SO
2 effluent. If the smelter is running in its normal operational mode when the unfavorable meteorological conditions emerge, there is a so-called “environmental incident”. The model of Navarra et al. [
25] computes the trade-off between production and environmental risk. Moreover, this model quantifies the improved trade-off that can result from a more accurate array of meteorological sensors.
DES development is a means to extend the static mass balances, to detail the critical phenomena that are driving and/or constraining a particular phase of an engineering project. The simulated events can dynamically affect the mass balances that are detailed throughout the model.
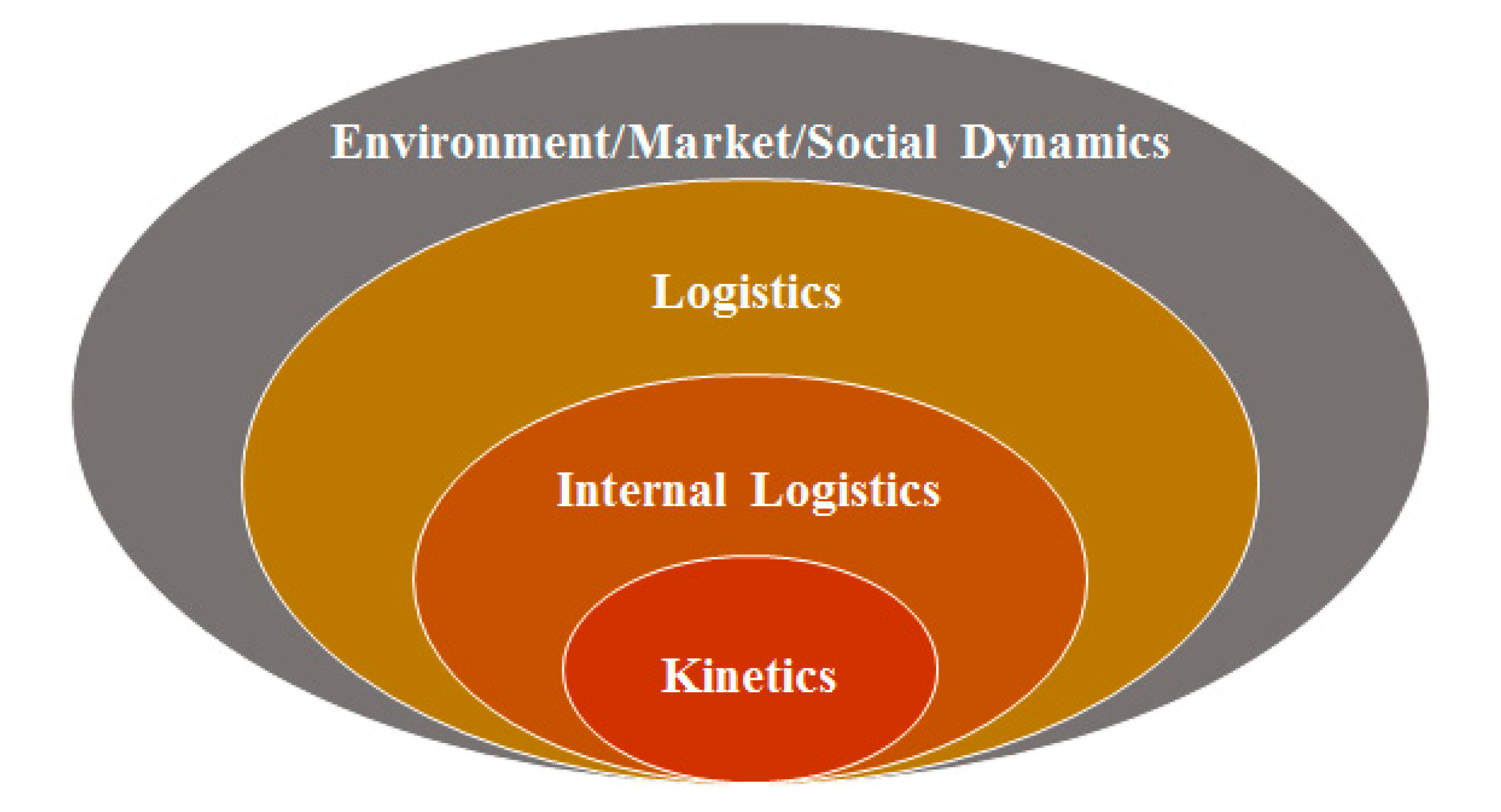
Figure 6 makes the distinction between the events that occur outside of the smelter and within the smelter, which constitute the external and internal logistics, respectively. There is a further distinction between the logistical coordination of smelter equipment (furnaces, ladles, cranes, etc.) and the kinetics that occur within the equipment. In general, the state variables that describe the system, and the events that would alter these variables, can be positioned within the concentric ellipses of
Figure 6. The incorporation of variables and events within a DES model must be guided by the scope and the phase of the engineering project. For example, it is not recommended to detail individual crane movements, unless the particular project would benefit from a comprehension of this aspect [
26]. Likewise, it is not recommended to detail particular equipment breakdown events, unless the project would benefit from a comprehension of this aspect [
27].
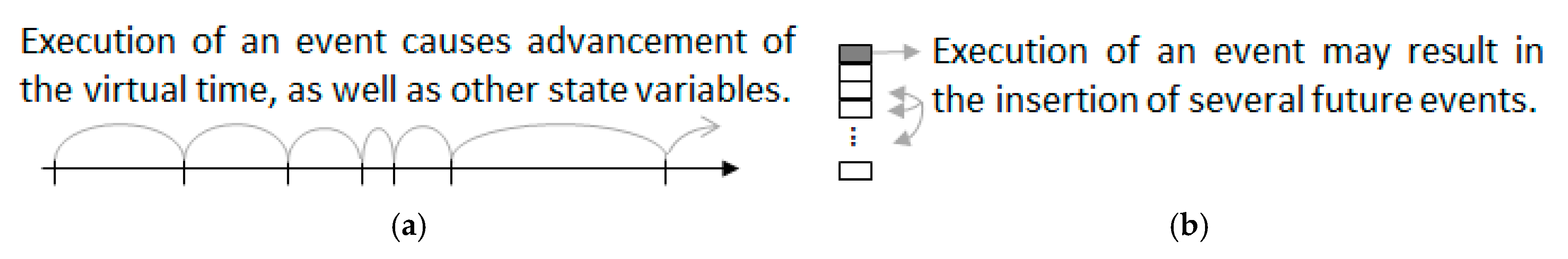
The computational efficiency of DES is due to adaptive time stepping, as the virtual clock advances from one event to the next without explicitly representing the dynamics that occur between events (
Figure 7a). The sequencing of events is governed by the future event list (
Figure 7b). Within this scheme, a prolonged activity is represented by a sequence of events, including starting and ending the activity. For example, a basic representation of converter cycles described by
Figure 5a may include only two events: the start and end of the cycle. A more detailed representation (e.g.,
Figure 5b) may include several intervening events to represent individual slag- and copper-blow segments, as well as the intervening skimming, charging, actions of the operators, etc. with the level of details that correspond to the given project. Incidentally, DES applies random number generation to determine the duration and outcome of the activities and is thus a form of Monte Carlo simulation [
28]; the distributions and action paths illustrated in
Figure 5 can be incorporated into the framework.
Moreover, the simultaneous operation of several converters in unison with other logistical phenomena is integrated into one single future event list (
Figure 7b); hence, a system-wide representation. Periods of time with relatively few events are computed relatively quickly, thereby focusing the computational efforts on periods of time that are more heavily packed with activity. This time-adaptive aspect of DES allows the simulation of thousands of operating days within minutes.
A DES framework can include operational criteria that determine the action pathway of converter cycles, allowing the computation of frequency confidence intervals. Following the example of
Figure 5a, the average frequency of short cycles may be between 2.8 and 3.2 cycles/day with 95% confidence and that of long cycles may be between 0.9 and 1.2 cycles/day with 95% confidence; this result will allow a mass balance based on Equation (3), given the data about the matte that are charged within each cycle and the corresponding flux and oxygen requirements. In a slightly more detailed representation, the DES framework may include the criteria that would determine the more detailed action paths of
Figure 5b.
Standard DES frameworks do not explicitly represent the dynamics that occur between events. However, a linearly dynamic state variable can be represented as a combination of discretely dynamic state variables. For instance, the mass of feed stockpile
k may be computed at a time
t, as
in which
and
are the mass and rate change of
k that were computed at the previous event, which occurred at time
tPrevious. Thus, each feed
k would require two discretely dynamic variables (
and
), in addition to the
tPrevious variable that remembers the time of the previous event. Equation (4) can used in simulations that consider alternating modes of operation that control feed blends in response to imbalances in incoming concentrates [
29].
Other linearly continuous variables can be implemented in a manner similar to Equation (4), representing each of these continuous variables as two discrete variables: level and rate (e.g.,
and
). Considering the DES representation of time (
Figure 7), this constitutes a time-adaptive finite difference (TAFD) scheme. However, a full representation of the continuous dynamics requires the detection of threshold-crossing events, as described in
Section 3.4. These threshold-crossing events are especially important in assessing the installation of sensors whose role may be to signal the need for corrective actions precisely when critical thresholds are crossed.
3.3. Slag Iron Speciation and Other Thermochemical Considerations
The iron oxide speciation, i.e., the balance between FeO and Fe
3O
4, can be quantified as the oxygen-to-iron ratio
x presented in Equations (1)–(3). Indeed,
x represents a degree of freedom that must be resolved in order to complete the mass balance. This degree of freedom can also be expressed as the ratio of ferric to ferrous ions within the slag, α = Fe
3+/Fe
2+, often called the degree of oxidation. The homeomorphic relationship between
x and
α is given by
Equation (1a) can thus be rewritten as
similar to Equation (1b), in which the minimum
α = 0 corresponds to pure wustite FeO and
α = 2 corresponds to pure magnetite Fe
3O
4. Equation (6) can be further detailed in a similar manner as Equations (2) and (3) by assigning appropriate subscripts to
α, as in [
20].
In the modeling of slag chemistry, α is preferred over x to avoid ambiguity between the reactive oxygen of the blast and the inert oxygen that is strongly bonded within the flux (i.e., within the SiO2, CaO, etc.). For instance, the role of SiO2 flux is made more evident by expressing the wustite as a component within a fayalite matrix FeO·2SiO2; hence, the balance of FeO versus Fe3O4 is considered as FeO·2SiO2 and Fe3O4. In practice, SiO2 is added into the slag in proportions that surpass the stoichiometry of fayalite and may be accompanied by other stable oxides. Under matte-processing conditions, the stable molecules SiO2, CaO, etc. can be regarded as if they were indivisible atoms. Most notably, the strongly bonded oxygen is not explicitly represented in Equations (1)–(3) and is not taken into account in x; these equations only explicitly consider the blast oxygen. The degree of oxidation α considers only the iron species isolated from any mention of the blast and flux oxygen.
Within Equation (6) and its nickel–copper equivalent, the slag-blow reaction can be isolated and balanced as:
which applies to both the smelting and converting furnaces for both copper and nickel–copper smelters. Indeed, the melting of the feed of Equation (6) results in molten matte that is a mixture of FeS and Cu
2S; in the case of nickel–copper smelters, the matte will also contain nickel and cobalt sulfides [
18], but Equation (7) is still correct. The incoming blast includes N
2, as well as O
2 (see
Figure 4). As the N
2 passes through the bath and is exhausted into the offgas, along with the SO
2, it carries away sensible heat and is a critical consideration in controlling the bath temperature.
To resolve the degree of freedom
α (or equivalently
x), the equilibrium between iron oxide species can be expressed as
having enthalpy and entropy values Δ
H0 = 622,549 J/mol and Δ
S0 = 342.64 J/mol K, respectively, which can be obtained from HSC Chemistry
TM. The corresponding Gibbs free energy balance is
which is set to zero to assume equilibrium.
R is the ideal gas constant,
T is the bath temperature, and
aij is the activity of species
i within phase
j. The activity of SO
2 in the offgas is taken to be the partial pressure
pSO2,Offgas.
Within Equation (9), the activities (
aFeS,Matte,
aFeO,Slag, and
aFe3O4,Slag) can be re-expressed in terms of
α,
T, and the operational parameters. The usual parameters include the oxygen enrichment of the blast
φ and the silica–iron mass ratio
r = (
mSiO2,Slag/
mFe,Slag), which are considered in
Section 4. Empirical measurements relate the activities
aij to their respective mole fractions
Xij. In particular, the classic model of Goto [
30,
31] is validated for smelting and converting, in both the copper and nickel–copper contexts [
32], and is the subject of
Appendix A.
Iron speciation computations are simpler for smelting furnaces than for converting, since the smelting bath temperature can usually be treated as if it were at a steady state and is approximately uniform and constant. Under this simplification, α can be resolved through an application of Newton’s Method [
18,
19]:
or, in case
T is not constant,
which is a two-variable form of Newton’s Method, in which (
T(k),
α(k)) denote the results of the
kth Newton iteration. The righthand sides of Equations (10) and (11) include proxy functions,
fG and
fH, and their derivatives, which are all evaluated at the preceding values (
T(k−1), α
(k−1)), considering (
T(0),
α(0)) = (1473 K, 0.15) as typical starting values. The proxy function
fG must be formulated so that
fG = 0 when the Gibbs free energy balance of Equation (9) is satisfied, i.e., when Δ
G = 0.
Appendix A presents a formulation of
fG that is based on the classic Goto model [
30,
31]. Likewise,
fH is formulated such that
fH = 0 when the heat balance is satisfied [
19].
Following the results of
Appendix A, it is relatively simple to program Equations (10) and (11) into a simulation platform, thereby relating slag chemistry to the wustite–magnetite balance and, indeed, to the overall mass balance. Depending on the project, Goto’s model may be an appropriate starting point, although it does not consider olivine slags [
21], nor does it consider the transport of minor elements. In practice, it is preferable to have more wustite than magnetite, since the latter increases the slag viscosity and the entrainment of matte into the slag [
21,
33]. The modification of slag chemistry through flux additions affects the migration of minor elements [
22,
23], possibly at the expense of having higher slag viscosity [
21].
There is an interest to develop DES platforms that draw upon state-of-the-art thermochemical databases [
19] to assist in the retrieval of valuable elements such as gold, silver, and platinum and the handling of deleterious elements such as arsenic, bismuth, and antimony. The authors suspect that the partition of trace elements can be efficiently computed as a function of the main elements a posteriori when Equations (10) and (11) converge. This is an area of future research, and the resulting platforms would support smelter-wide strategies for the processing increasingly problematic feeds. Yet, in reality, for similar apparent conditions like matte grade, temperature, etc., the balance of FeO versus Fe
3O
4 can depend on various parameters, including flux quality, refractory wear, and amount of charge (hence, affecting mixing), and so, an empirical approach to speciation may be more effective.
3.4. Estimation of Threshold Crossing Times Using Time-Adaptive Finite Differences
Time-adaptive finite differences are an important feature of our smelter DES frameworks, allowing the correct placement of threshold-crossing events within the future event list (
Figure 8). In the approach of Navarra et al. [
18,
19], the Newton iterations of Equations (10) and (11) are nested within the Runge-Kutta-Fehlberg (RKF) method, which is a well-known time-adaptive finite difference (TAFD) scheme [
34,
35]. This method is a combination of finite difference schemes based on fourth and fifth-order Taylor expansions [
34]; an attempted timestep is only accepted if the fourth and fifth order estimates of
T and α are within an acceptable error tolerance; otherwise, a smaller timestep is attempted. Moreover, each attempted timestep uses the previous results of
T and α as a starting point for the Newton iterations, so that convergence is faster, thus making the computation more efficient.
The efficiency of DES depends on this time-adaptive aspect to limit the computational effort devoted to dynamics that occur between the discrete events (
Figure 7). Alternatives to the approach of Navarra et al. [
18,
19] include the use of cubic Hermite spline interpolation [
36,
37] and Richardson extrapolation [
38]. These techniques can be adapted to isolate the threshold crossing event of a state variable and improve the accuracy with which we can find the time at which it crosses. In fact, it is important to recognize that both the efficiency and accuracy of the method rely on an accurate time estimate for threshold crossing. In our case, the threshold is first contained in the interval bounded by the two timesteps bracketing an evaluation of the state variable below and above the threshold. This precisely guarantees we have access to values already computed at both of the upper and lower estimates (as a result of the RKF attempt), which includes state variable values, as well as their time derivatives (by direct evaluation if the right-hand side at the state variable values). With this data, we constructed a cubic Hermite spline, which is, therefore, a valid fourth-order interpolation of the solution over this interval. Since the Hermite spline is, in fact, a cubic polynomial, we can compute analytically the crossing time as a simple root-finding problem. We also note that this approach is robust, since the data at the end of the interval provided need not be exact. In fact, if the state variable data has a fourth-order accurate truncation error (e.g., by only using a third order Runge-Kutta integrator) and the derivatives are third-order accurate, the interpolant is guaranteed to be fourth-order accurate [
37]. Further, while this approach is tailored to the RKF used here, it may be expanded by considering Hermite quintics if a further need for accuracy is required.
While conventional DES frameworks support basic representations of smelter logistics, a hybridization with time-adaptive finite differences supports a detailed representation of the kinetics that occur within the individual unit operations. In particular, the detailed representation of individual slag-blow segments may require a dynamic simulation of the evolving thermochemical states of matte and slag. Moreover, the DES-TAFD hybridization allows the simulation of several simultaneous converting cycles, in conjunction with the intervening actions involving cranes and ladles, the delivery of a cold charge, and other phenomena. Nonetheless, the explicit representation of slag-blow segments should only be implemented if it is beneficial to the particular engineering project. Beyond the internal dynamics of smelter, a project may require an explicit representation of market-related or environmental phenomena (
Figure 6). The hybridization of DES and TAFD is indeed capable of linking detailed representations of diverse aspects throughout the smelter and beyond, whose coordination may be critical to the sustainability of the smelter. Furthermore, aging smelters will not be sustainable unless they can successfully modernize their operational practices, benefitting from sensors and other novel technology.