Abstract
Microalgae are photoautotrophic microorganisms capable of producing compounds with potential bioenergetic applications as an alternative energy source due to the imminent exhaustion of fossil fuels, their impact on the environment, and the constant population increase. The mass cultivation of these microorganisms requires high concentrations of nutrients, which is not profitable if analytical grade culture media are used. A viable alternative is the use of agro-industrial wastewater, due to the metabolic flexibility of these microorganisms and their ability to take advantage of the nutrients present in these substrates. For the reasons mentioned above, the effect of the cultivation in wastewater from cheese processing on the growth parameters and biomass composition of Scenedesmus sp. was evaluated, and its nutrient removal capacity determined. A high lipid concentration was obtained in the cultures with the dairy effluent (507.81 ± 19.09 mg g−1) compared to the standard culture medium, while the growth parameters remained similar to the control medium. Scenedesmus sp. achieved high percentages of nutrient assimilation of the wastewater used (88.41% and 97.07% for nitrogen and phosphorus, respectively). With the results obtained, the feasibility of cultivating microalgae in agro-industrial wastewater as an alternative culture medium that induces the accumulation of compounds with potential bioenergetic applications was verified.
1. Introduction
Fossil fuels are hydrocarbons generated millions of years ago that appear in solid (coal), liquid (oil), or gaseous (natural gas) forms, which come from the decomposition of organic matter [1,2]. These molecules are responsible for 86% of the energy produced worldwide [3]. However, they are the primary source of greenhouse gas emissions [4], making their use unsustainable. Due to their environmental impact, imminent depletion (40–60 years), and the increase in world energy demand (↑50% by 2030), other sustainable energy sources are being sought, such as solar, tidal, and biofuels, among others, considered as clean energies [5,6,7].
The latter comprise any fuel derived from biomass or its residues such as plants, animals, or microorganisms. Biofuels emerge as a profitable alternative to the regular consumption of fossil fuels; the most developed are bioethanol, biodiesel, and biogas, whose consumption reduces carbon emissions by between 30% and 50%. Biomethanol, bioethers, synthetic biofuels, biohydrogen, and vegetable oils are used less frequently [8]. The use of biofuels for energy production has many advantages; however, only 10% of the energy is obtained from biomass and waste [9]. These bioenergetic compounds include liquid, solid, and gaseous fuels [8] classified according to the source of production in the first, second, third, and fourth generation.
First-generation biofuels are produced from food or edible vegetable oils (>90% of the biodiesel produced worldwide) such as rapeseed, soybeans, sunflower, and palm, which are readily available crops. However, their mass production is not profitable due to the high costs of biodiesel production and competition with other important food crops for land use [10]. Second-generation biofuels are produced from agricultural residues (residual vegetable oil, straw, rice husk), forestry (wood, wood chips), and industrial and non-food crops, which require less land and water and reduce the problems of deforestation. Despite this, they are not abundant enough to cover world energy demand [11]. Third-generation biofuels are produced from algae and microorganisms such as bacteria, yeasts, cyanobacteria, and microalgae, which have higher yields than other biofuels (10 times more) and help in the consumption of atmospheric CO2, reducing environmental problems [12]. Biofuels produced by algae are profitable due to their high-efficiency rates (yield greater than 98,000 L ha−1 of biodiesel), they can supply a large part of the energy demand, they do not compromise food security, can be grown quickly (high growth rates), they are not toxic, and their cultivation does not need arable land [7,13].
Finally, fourth-generation biofuels result from the modification of photosynthetic microorganisms based on metabolic engineering, allowing easy extraction of microbial products, simplifying production stages, cost reduction, and performance improvement. Currently, the genetic manipulation of these microorganisms, such as microalgae, seeks a directed improvement of cellular activity that allows the generation of added value by-products, increasing the profitability of the process [14,15]. Microalgae have been prioritized as potential biofuel producers because they achieve yields between 15 and 300 times more than a traditional agricultural crop [7].
Microalgae can present with a eukaryotic cell organization (Chlorophyta, Bacillariophyta, Dinoflagellata) or prokaryote (Cyanophyta); however, cyanobacteria are not strictly within this classification, but they are considered for their high economic value. Microalgae can capture light energy and use it in organic molecules such as proteins, carbohydrates, or lipids through photosynthesis [16,17]. The cultivation of these microorganisms presents excellent advantages, such as high growth rates, high productivity, no requirement of agricultural land for their cultivation, short harvest cycles, ease of cultivation (few nutritional, environmental, and operational requirements), high lipid content, and high photosynthetic efficiency (4 times higher than plants). They are an inexhaustible-renewable resource, among others [18,19]. They also fulfill ecological functions such as global carbon sequestration (1.8 kg of CO2 kg−1 of dry biomass), fixation of atmospheric nitrogen, and production of more than 50% of global oxygen [17,18,19].
These microorganisms constitute a potential source of biomass for the production of different types of biofuels. In addition, they contain between 20% and 50% lipids (under stress conditions) in terms of the dry weight of the biomass [20]. Microalgal biomass production is assumed to be between 15 and 25 t ha−1 year−1, which would correspond to 4.5 and 7.5 t ha−1 year−1 of lipid production (cellular lipid percentage of 30%) [21]. In this way, current studies seek to intensify the growth rate and production of lipids. Microalgae, under stress conditions, can accumulate lipids and carbohydrates in higher concentrations, decreasing protein synthesis [22]. Some of the stress conditions evaluated are limitation of nutrients such as N, P, or S (C becomes triglycerides or fatty acids), the addition of metal ions (Fe+3, Mg+2, Ca+2), the addition of salts (EDTA), increase or decrease in light intensity, temperature, pH, and radiation [23,24].
The cultivation of these microorganisms on a large scale to obtain various metabolites of commercial interest generates a high demand for freshwater and nutrients, usually supplemented by chemically synthesized fertilizers due to the lower cost they present in relation to nutrients from formulated cultivation. However, industrial production with fertilizers is not profitable (>$400 t−1) if the costs of biomass production and processing are taken into account in the production of biofuels [25]. The use of agro-industrial wastewater emerges as a viable alternative in the cultivation of microalgae and cyanobacteria due to their outstanding capacity to adapt to various water bodies as sources of fresh, marine, brackish, and waste water, among others [26,27]. In this way, microalgae cultivation in wastewater is a profitable, highly productive, and ecological process (decreased eutrophication that causes low oxygen levels and contamination in freshwater bodies) [28,29].
Due to the advantages mentioned above, the cultivation of Scenedesmus sp. in wastewater from dairy industries is proposed in this study, evaluating mainly the production of lipids and carbohydrates. The growth, productivity, and biochemical composition of the biomass and the removal of nutrients by Scenedesmus sp. will also be determined.
This investigation was carried out to induce an increase in the lipid and carbohydrate content in the biomass of the microalgae Scenedesmus sp. using wastewater as a cultivation substrate for the production of metabolites of bioenergetic interest, and at the same time cleaning it, avoiding the serious environmental problems caused by wastewater being directly discharged into the water bodies.
2. Materials and Methods
2.1. Microorganism and Wastewater Characteristics
The microalgae Scenedesmus sp. (Figure 1) evaluated in this research was obtained from the Bank of microalgae and cyanobacterial strains of the Laboratorio de Biotecnología Microbiana (BiotemLab) of the Universidad de Guayaquil (Universidad de Guayaquil, Guayaquil, Guayas, Ecuador), coded as RJ 3009 and conserved in solid BG11 standard medium [30] at a temperature of 20 ± 2 °C, light intensity of 100.0 µmol photon m−2 s−1, and photoperiod 12 h light/12 h dark. Cultures of Scenedesmus sp. were acclimated to dairy industry wastewater by gradually adding increasing concentrations of cheese processing wastewater over several months, with an initial concentration of 10%.
Figure 1.
Light microscopic images (60x) of Scenedesmus sp.
The wastewater used as a culture medium was obtained from a dairy industry that produces cheeses and lactic derivatives. The dairy industry wastewater used in this research (DIWW) had the following characteristics: 2.22 mM total nitrogen (TN), 0.20 mM phosphate, 5.5 pH, 3500 mg O2 L−1 chemical oxygen demand (COD), and heavy metal concentrations less than 0.09 mg L−1, as shown in Table 1.

Table 1.
Chemical characteristics of the dairy industry wastewater (DIWW).
2.2. Culture Conditions
Scenedesmus sp. was cultivated in batch in triplicate in 150 mL photobioreactors (PBR) under a circadian illumination regime of 12 h light/12 h dark with a temperature of 30 °C (Figure 2), light intensity of 100.0 µmol photon m−2 s−1, and a continuous supply of CO2 pulses (1.5% v/v) to keep the pH stable (7.2 ± 0.2) and as a carbon source [31]. The cultures were inoculated with a concentration of 1.5 × 107 cell mL−1.
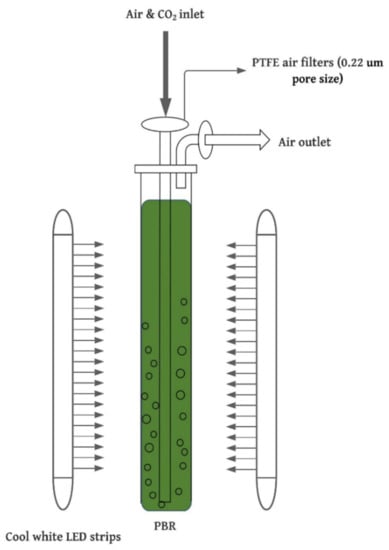
Figure 2.
Schematic diagram of the 150 mL lab-scale photobioreactor (PBR), used for Scenedesmus sp. cultivation. The air and CO2 inlet and air outlet were equipped with 0.22 µm PTFE (Polytetrafluoroethylene) filters (Sigma-Aldrich, Saint Louis, MO, USA).
For the control, the culture medium BG11 with the following composition was used: NaNO3 17.65 Mm, K2HPO4 0.23 mM, MgSO4·7H2O 0.30 mM, CaCl2·H2O 0.25 mM, citric acid 0.03 mM, ferric ammonium citrate 0.03 mM, EDTA 0.003 mM, Na2CO3 0.38 mM, H3BO3 0.046 mM, MnCl2·4H2O 0.009 mM, ZnSO4·7H2O 0.0008 mM, Na2MoO4·2H2O 0.0016 mM, CuSO4·5H2O 0.0003 mM, and Co(NO3)2·6H2O 0.0002 mM.
For the treatments, 100% dairy wastewater (DIWW) was used with a nutritional correction with the commercial fertilizer Bayfolan® (Bayer, Valencia, Spain) reaching a concentration of 20.80 mM of total nitrogen and 1.67 mM of PO43−.
2.3. Determination of Growth Parameters
Growth was evaluated by cell concentration (cell mL−1) every 24 h: 10 uL of culture was introduced into a Neubauer chamber, and cells from all squares were quantified in the central area.
Cell concentration was calculated using the following equation [32]:
where C is the cell concentration (cell mL−1), N the total number of cells counted in the area, and f the dilution factor.
The specific growth rate (µ:day−1) was determined at the end of the assay based on the values obtained from the cell concentration using the equation proposed by Arredondo et al. [33]:
where N1 and N2 are the cell density values at times t1 and t2, respectively.
µ = ln (N2 − N1)/t2 − t1
The dry biomass weight was determined using the protocol proposed by Zhu and Lee [34], which consists of the use of fiberglass filters of 47 mm diameter and a pore diameter of 0.7 µm (Whatman® GF/F). Previously, the filters were dried at 90 °C for 24 h in an oven, placed in a desiccator with SiO2 to avoid humidity, and weighed on an analytical balance. 5 mL of Scenedesmus sp. culture was filtered with the addition of 0.5 mM ammonium formate (HCO2NH4) to remove the salts from the culture medium and the wastewater. The biomass filters were dried at 80 °C for 24 h and weighed on an analytical balance. The dry weight corresponds to the difference between the weight of the filter with the dry biomass and the weight of the dry filter. In addition, the dry weight value of the suspended solids in the wastewater was subtracted. The biomass productivity (g L−1 day−1) was calculated using the following equation:
where X1 and X2 were the biomass concentrations (g L−1) on days t1 (start of assay) and t2 (end of the assay).
Pb = (X2 − X1)/t2 − t1
2.4. Determination of Biochemical Composition
The concentration of carbohydrates in the biomass was carried out by the Sulfuric Phenol method proposed by Dubois et al. [35]: 1 mL of centrifuged culture was used. The pellet was re-suspended in 1 mL of 1N NaOH. The pellet was shaken in the vortex, sonicated at 20 kHz and 4 °C for 20 min and centrifuged at 17,100× g, 4 °C for 15 min. From the obtained supernatant, 100 µL of each sample was transferred in triplicate, and 900 µL of distilled water, 25 µL of 80% phenol, and 2.5 mL of concentrated H2SO4 were added and shaken in the vortex. It was allowed to cool to room temperature for 30 min and was measured by spectrophotometry at 485 nm, using glucose as standard.
The protein content of the biomass was determined by the protocol of Lowry et al. [36] modified by Herbert et al. [37], where 2 mL of 1N NaOH are added to 6 mg of dry biomass, sonicated at 20 kHz and 4 °C for 15 min, followed by a water bath at 95–100 °C for 45 min. All samples were allowed to cool to room temperature and centrifuged at 17,100× g and 4 °C for 15 min. From the obtained supernatant, 100 µL of each sample was transferred in triplicate, 400 µL of distilled water, 300 µL of 1N NaOH, and 2 mL of saturated Cu-Tartrate solution were added, then it was mixed in the vortex and 10 min of reaction was expected. 400 µL of Folin Ciocalteu diluted v/v was added and left for 30 min at room temperature. Protein concentration was measured by spectrophotometry at 750 nm, using bovine serum albumin as standard.
The lipid concentration of the biomass was determined by the method of Bligh and Dyer [38]: 3 mL of methanol and 1.5 mL of chloroform v/v in a 2: 1 ratio were added to 5 mg of dry biomass. It was then sonicated at 4 °C at 20 kHz for 20 min, followed by a water bath at 50 °C for 30 min. The samples were allowed to cool to room temperature and centrifuged at 17,100× g and 4 °C for 10 min. 1.5 mL of chloroform and 1.5 mL of distilled water were added to the supernatant and shaken in the vortex. It was centrifuged again at 17,100× g and 4 °C for 5 min. To the organic phase previously separated from the upper aqueous phase, 0.5 mL of acetone was added (remaining substances were removed), evaporated with a nitrogen flow, and re-suspended in 1 mL of chloroform. The lipid concentration was determined by spectrophotometry using the Marsh and Weinstein [39] simple carbonization method: 100 µL of each re-suspended sample was transferred in triplicate to glass tubes, 2.5 mL of concentrated H2SO4 was added, and it was brought to carbonization (200 °C, 15 min). Finally, the tubes were allowed to cool to room temperature, and 3 mL of distilled water was added, to determine the absorbance at 375 nm by spectrophotometry, using palmitic acid as standard.
2.5. Determination of the Lipid Profile
The fatty acid methyl esters (FAMEs) profile of Scenedesmus sp. grown in dairy wastewater was determined using the following methodologies: fat content by the extraction method with a mixture of ethers (Method 989.05) from AOAC [40], concentration and composition of saturated fatty acids (myristic, palmitic, stearic, arachidic), monounsaturated (oleic) and polyunsaturated (linoleic, linolenic, EPA, DHA) by AOAC Method 963.22 [41] by gas chromatography and the content of trans fats by AOAC Method 996.06 [42] which involves the use of gas chromatography after extraction with acid/alkaline hydrolysis and mixed ethers.
2.6. Nutrient Removal and Wastewater Characterization
The total nitrogen concentration was determined by an adaptation of the persulfate digestion method proposed by D’Elia and Steudler [43], and the PO43− content by the vanadate-molybdate method of Tandon et al. [44]. These concentrations were measured by the Lovibond MD600/MaxiDirect photometer (Lovibond®, Dortmund, North Rhine-Westphalia, Germany) based on the established protocols.
The characterization of the wastewater used in the test was carried out by determining the pH, chemical oxygen demand (COD), and the concentration of TN, PO43−, and heavy metals (As, Cd, Hg, Pb). The COD expressed in mg O2 L−1 was determined by an adaptation of the dichromate-H2SO4 method of APHA [45] measured by the Lovibond MD600 photometer based on respective protocols (Method M132). Mercury concentration was determined by applying the Evans, Johnson, and Leah protocol [46] by hot plate digestion with concentrated HNO3 and cold vapor atomic absorption spectrophotometry. Cadmium and lead were determined following AOAC Method 999.10 [47], using microwave digestion with HNO3 and H2O2 under pressure followed by atomic absorption spectroscopy in a graphite furnace. The arsenic concentration was determined using the programmed three-stage digestion technique with HNO3 and atomic absorption spectrophotometry with a graphite furnace [48].
The assimilation of nutrients and COD removal were calculated using the percentage difference between the concentrations of nitrogen, PO43−, and COD at the beginning and end of the assay (supernatant).
2.7. Morphological Changes
Cell size measurements (length, width) were made with Digital Image System Software (Digital Imaging Systems®, Buckinghamshire, England) while cell area and volume were determined by applying the following equations [49]:
where r1 and r2 correspond to half of the values of length and width of the cell (a: length b: width).
A = π r1 r2
V = π a2 b/3
2.8. Statistic Analysis
The statistical and graphical analyses were conducted using Prism program version 8.3.0. (GraphPad Software, Inc®, San Diego, CA, USA). All the data obtained when evaluating the differences between the control and the treatments were analyzed using one-way analysis of variance (ANOVA) and post hoc Tukey multiple comparisons test with a significance level of 95% (p ≤ 0.05).
3. Results
The current demand for bioenergy generation that is clean, profitable, and productive has increased the study of microorganisms to produce compounds for energetic use such as bioethanol, biodiesel, biogas, among others. To achieve the research objectives, growth was determined by the daily cell density during the 11 days of culture and the dry weight of the biomass. At the end of the process, the biochemical composition of the biomass was evaluated to determine the increase in metabolites for bioenergetic applications.
3.1. Determination of Growth Parameters
The culture of Scenedesmus sp. for 11 days is shown in Figure 3, where the maximum cell density reached by the culture at 100% dairy industry wastewater is observed (4.4 × 107 ± 1.4 × 106 cells mL−1). These results did not register significant differences from the controls where a maximum cell concentration of 4.3 × 107 ± 3.4 × 106 cells mL−1 was obtained. These are expected results since the stress to which the microalgae were subjected to wastewater culture reduces cell growth. However, the results obtained when calculating the specific growth rate did show significant differences between controls and treatments, with 0.51 ± 0.06 day−1 and 0.54 ± 0.14 day−1, respectively, as shown in Figure 4.
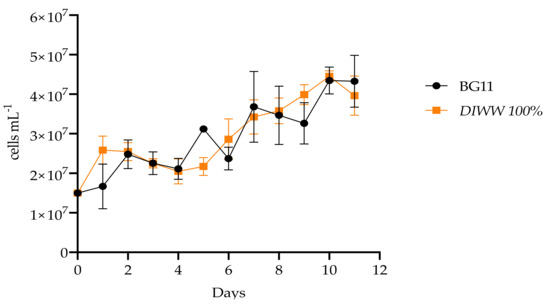
Figure 3.
Cell density of the batch culture of Scenedesmus sp. in BG11 standard medium and dairy industry wastewater (n = 3).
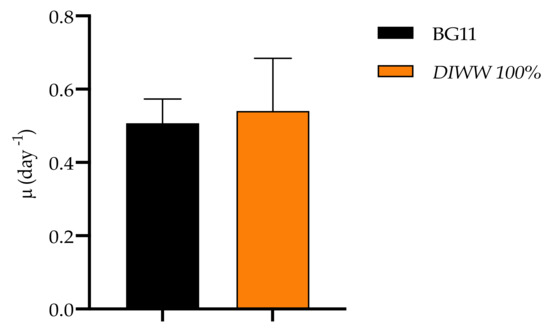
Figure 4.
Specific growth rate of Scenedesmus sp. obtained in standard BG11 culture medium compared to the medium with the dairy industry wastewater (n = 3).
The biomass productivity determined based on the dry weight data does not show significant differences between controls and treatments with 1.84 ± 0.93 g L−1 day−1 and 1.75 ± 0.60 g L−1 day−1, respectively (Table 2, Figure 5).

Table 2.
Kinetic parameters of Scenedesmus sp. grown in batch for 11 days of cultivation. Xm: maximum cell concentration obtained. µ: specific growth rate. Qx: biomass productivity.
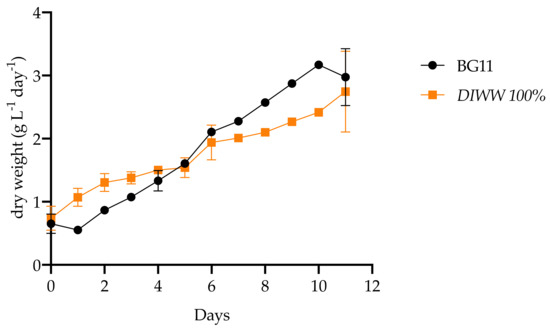
Figure 5.
Biomass productivity obtained with Scenedesmus sp. in BG11 culture medium and wastewater from the dairy industry (n = 3).
3.2. Determination of the Composition of Biomass
Microalgae in standard growth conditions are characterized by lipid concentrations of 10–30% and carbohydrates of 5–30% of their composition [50], making them potential candidates to produce these compounds under stress conditions that induce their increase.
In this research, the cultivation of Scenedesmus sp. in dairy industry wastewater (DIWW) gave promising results compared to the standard BG11 culture medium in terms of compounds with potential use in bioenergy, reaching values of 276.96 ± 20.46 mg g−1 for proteins, 304.09 ± 31.65 mg g−1 for carbohydrates, and 350.81 ± 33.05 mg g−1 for lipids in the control; and 198.36 ± 12.73 mg g−1 for protein concentration, 268.51 ± 24.52 mg g−1 for carbohydrates, and 507.81 ± 19.09 mg g−1 for lipid production in the treatment. These values indicate that the biomass of Scenedesmus sp. cultivated in dairy industry wastewater reaches approximate percentages of 20%, 27%, and 51% of proteins, carbohydrates, and lipids, respectively.
These results show that the biomass composition of Scenedesmus sp. is modified when cultivated in dairy effluents, a substrate that acts as a physiological stress factor since it tends to increase lipid concentrations and decrease protein content as shown in Figure 6.
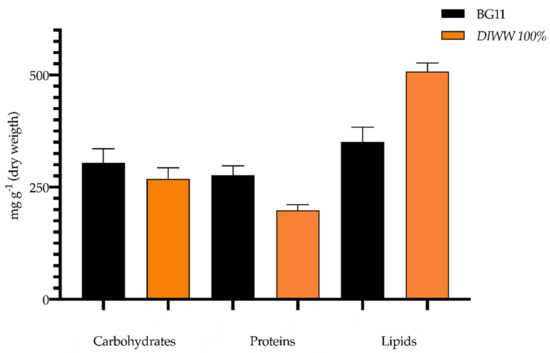
Figure 6.
Biomass composition of Scenedesmus sp. grown in standard BG11 culture medium and dairy industry wastewater (n = 3).
3.3. Determination of the Lipid Profile
The analysis of fatty acid methyl esters (FAMEs) produced by Scenedesmus sp. (RJ 3009) grown in dairy effluents shows dominance of polyunsaturated fatty acids (44%), followed by saturated fatty acids (29%) and, in a lower concentration, fatty acids monounsaturated (21%). A higher proportion of linolenic, linoleic, and palmitic acid was obtained: 24%, 20%, and 19% of the total lipid profile, respectively (See Table 3).

Table 3.
Fatty acid composition of Scenedesmus sp. grown in 100% dairy industry wastewater.
3.4. Morphological Changes
The culture in the dairy industry wastewater caused a substrate-induced modification in the size of the cells compared to the control. The same was observed concerning the area and cell volume (see Table 4).

Table 4.
Cell morphological changes in Scenedesmus sp. in cultures with dairy wastewater compared to standard medium BG11.
These values show an increase of 1.78 and 1.46 times the cell area and volume in the treatments compared to the control, respectively.
3.5. Nutrient Removal
Microalgae have the ability to use nutrients from wastewater as culture substrates for growth, which gives an additional advantage to growing these microorganisms for wastewater treatment.
In the cultivation of Scenedesmus sp. with 100% dairy industry wastewater, removal percentages of 88.4% for total nitrogen, 97.1% for PO43−, and 89.3% for COD were obtained (Table 5).

Table 5.
Nutrient assimilation and chemical oxygen demand (COD) removal percentages for Scenedesmus sp. grown in 100% dairy industry wastewater.
4. Discussion
Wastewater as an alternative culture medium induces the modification of the biochemical composition of the biomass of microalgae and cyanobacteria, which depends on the type of sewage used since they are generally toxic environments for microalgae and can cause some sensitivity in these microorganisms. However, it has been shown that microalgae can acclimatize to a series of stress conditions such as variations in salinity, light or temperature, the influence of heavy metals, nutrient deficiency, among others [51]. Microalgae are potential biological systems for treating various wastewater sources due to their metabolic flexibility [52]. Concerning heavy metals, it has been shown that microalgae have developed mechanisms to tolerate the toxicity of these elements, which constitutes an advantage in wastewater bioremediation processes. However, this will depend on the metabolism and resistance of the strain used [53]. The wastewater sample used in this investigation contains heavy metals concentrations within the permissible range except for mercury, which exceeds the limit by 44.5 times, as observed in Table 6.

Table 6.
Recommended limits of heavy metals in wastewater [54,55].
The results obtained in this research show that agro-industrial wastewater can be used as a culture substrate for the microalgae Scenedesmus sp., which is reflected in the increase in cell density similar to that obtained with the standard culture medium. This result is consistent with the research by Nagi et al. [56] and Koreivienė et al. [57], who used low initial cell concentrations and obtained an increase in cell density of up to 7.5 and 3600 times, respectively.
The growth of microalgae in dairy wastewater will depend on the metabolic capacity of the selected strains and the particular characteristics of the wastewater used as a culture substrate. Lu et al. [58] evaluated the growth of Chlorella sp. in wastewater from raw dairy products and observed after eight days that this strain is capable of adapting rapidly to the effluent used. This is evidenced in the thirteen-fold increase in the biomass concentration compared to the initial inoculum. This result agrees with what was obtained by Marazzi et al. [59], who demonstrated that dairy wastewater from whey processing, characterized by high contents of COD and organic nitrogen, allows the growth of Scenedesmus acuminatus, with percentages of removal of nutrients greater than 65%. Similar results obtained with other strains show that the native microalgae Scenedesmus sp. (RJ 3009) can adapt to dairy wastewater as an alternative growth medium, sustainable and profitable for culture at scale.
The growth rate of these microorganisms grown in agro-industrial wastewater generally correlates with the concentration of N and P in the wastewater [60]. The specific growth rate in our research was similar in cultures maintained with DIWW and cultures with standard medium (BG11). This result agrees with the work carried out by Jebali et al. [61] and Sweiss [62]. They demonstrated that strains that adapt to wastewater have better growth and show a high percentage of reduction in wastewater organic load. Hena et al. [63] used isolated strains from urban wastewater and evaluated their cultivation in municipal wastewater from a treatment plant, with Scenedesmus sp. LS40 obtained a specific growth rate of 0.468 day−1 and a lipid accumulation percentage of 29.4% in dry biomass, growth results similar to this investigation, but with lower lipid concentrations than our research. Ling et al. [64] isolated the microalgae Scenedesmus obliquus from wastewater from a dairy farm and later used it in municipal wastewater treatment, where they obtained a maximum specific growth rate of 0.48 day−1 with high removal percentages of ammonium and phosphate; however, the lipid content only reached 19.7% of the dry weight of the biomass. Wu et al. [65] evaluated the culture of Scenedesmus sp. in urban wastewater with different N:P ratios obtaining values for the specific growth rate in the range of 0.14–0.62 day−1; the highest values for this parameter were with high N:P ratios.
Productivity is also influenced by the microalgal strain used and the characteristics of the environment where it grows (Table 7). Hena et al. [66] reported productivity of 0.20 g L−1 day−1 for Chlorella saccharophila and 0.21 g L−1 day−1 for Scenedesmus sp. in untreated wastewater from dairy farms, with lipid concentrations of 21.82 and 13.64%, respectively. Kuo et al. [67] cultivated Chlorella sp. in different concentrations of piggery wastewater (PWW) (0%, 25%, 50%, 75%, and 100%) which was previously sterilized and centrifuged. They obtained productivity of 0.68 g L−1 day−1 in the treatment with 100% PWW; however, the highest production of lipids was at 25% PWW, with 29.3%. In a more extensive study, Jeong and Jang [68] evaluated the lipid production of ten chlorophyte microalgae isolated from wastewater and an urban water treatment plant. The microalgae were cultivated in the Bold basal medium, and results showed a varied concentration of lipids. The lowest lipid content was 24.0% in Micractinium pusillum and the highest with 46.9% in Chlorella sorokiniana. We must emphasize that the lipid concentrations of Scenedesmus sp. grown in DIWW in our work are significantly higher (50.78%) than the studies mentioned above. This last characteristic, added to the high productivity obtained (1.75 g L−1 day−1), makes these microalgae an interesting alternative biodiesel production source.

Table 7.
Biomass productivity and lipid concentration of microalgae grown in wastewater.
Scenedesmus has been widely studied concerning its production and composition of biomass in cultures with wastewater. Shen et al. [69] evaluated the municipal wastewater treatment (MWW) with S. obliquus, reaching a productivity of 0.578 g L−1 d−1, and the composition of the dry biomass presented 19% of proteins, 64% of carbohydrates, and 17% of lipids. However, Ansari et al. [70] with the same species and wastewater (S. obliquus, MWW), obtained lower productivity, 0.085 g L−1 d−1, and the composition of the dry biomass was 28.5% protein, 27.5% carbohydrates, and 26.5% lipids. The difference between both studies is that Shen et al. supplemented the culture with CO2, and it is due to this that they had a high productivity, and percentage of carbohydrates (64%).
The results in terms of productivity and biomass composition of Scenedesmus spp. grown with standard media are varied. Thus, regarding S. obliquus grown in a one-liter PBR, with the standard Detmer culture medium, the cultures were supplemented with 2.5% CO2, the maximum productivity reached was 0.573 g L−1 d−1, and the composition of the biomass presented the following characteristics: 35.48%, 38.81%, and 8.94% for proteins, carbohydrates and lipids, respectively [71]. However, for S. obtusiusculus grown in the same system (PBR), with the standard culture medium BG11, with the supplement of CO2 5.0%, the cultures reached productivity of 0.500 g L−1 d−1, and the composition of the biomass presented the following profile: proteins 25%, carbohydrates 28%, and lipids 38% [72].
The content and composition of fatty acids in microalgae are influenced by culture conditions, directly affecting the concentration of FAMEs. In general, the composition of the fatty acids of microalgae grown in wastewater shows the dominance of unsaturated fatty acids (monounsaturated, polyunsaturated) and, in less concentration, saturated fatty acids. This is evidenced in investigations where 77% and 23% of unsaturated and saturated esters [73] have been obtained in a culture of Chlorella vulgaris in dairy wastewater effluents, 63% and 37% [66] with a microalgae-cyanobacteria consortium cultivated in wastewater from a dairy farm, 51% and 42% [74] with Scenedesmus ecornis using fertilizer plant wastewater as culture substrate, 51% and 44% [75] with Scenedesmus cultivated in 100% synthetic urban wastewater, and 65% and 29% obtained in this research (Scenedesmus sp.) in 100% DIWW. Good quality biodiesel is mainly composed of C14–C18 fatty acids, as obtained in this study where short-chain fatty acids reached high concentrations, represented by palmitic, linoleic, and linolenic acids. However, European Standard 14214 [76] determined that for quality biodiesel for vehicles, the maximum concentration of linolenic acid (C18:3) is 12%, a value that is two times lower than that obtained with Scenedesmus sp. (RJ 3009). Most of the microalgae oils do not comply with this standard due to polyunsaturated fatty acids in a higher proportion than the degree of unsaturation of this type of lipid compound. Despite this inconvenience, studies affirm that establishing different growing conditions, partial catalytic hydrogenation processes of the oil, mixtures with lipid substances from other raw materials and lipid extraction from wet biomass can reduce oxidative stability problems, inadequate cold flow control, and unsaturation levels, making the use of microalgal oil as a source of biodiesel viable [77,78].
The microalgae Scenedesmus sp. can grow in various types of sewage and accumulate lipids intracellularly, and this was verified in our research with the high values of productivity and lipid concentration obtained in the cultures maintained in the DIWW. However, the morphology of cells is altered by their tendency to accumulate lipids and carbohydrates in organelles as energy stores [79]. These morphological changes are evidenced in the increase in the surface-volume values, where the cells become spherical for better absorption of light and nutrients [80] (Figure 7).
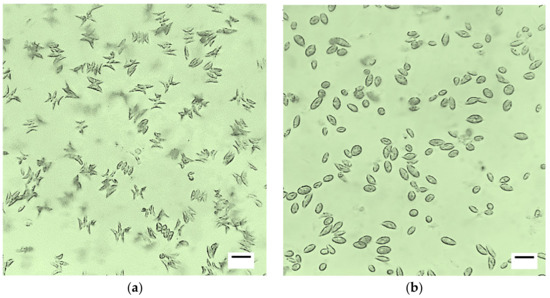
Figure 7.
Light microscopes images (40x) of Scenedesmus sp. in different culture conditions (a) Scenedesmus sp. in BG11 standard culture medium. (b) Scenedesmus sp. in dairy industry wastewater [81]. Scale: 20 µm.
It is proven that microalgae can increase biomass production, while greater availability of nutrients exists in the environment. Tuantet et al. [82] used a mixture of male and female human urine characterized by high nitrogen and phosphorus contents as a culture substrate for Chlorella sorokiniana. The authors obtained productivity of 9.3 g L−1 day−1 and removal efficiencies of 60% for nitrogen and 100% for phosphorus. The productivity value achieved is due to the high concentrations of nitrogen and phosphorus in the urine used, promoting the growth of these microorganisms.
The content of organic compounds in agro-industrial wastewater, availability of nutrients, and cultivation conditions, influence the composition of the biomass of the microalgae cultivated in this type of substrate [83,84]. In this research, cultivation in the wastewater of the dairy industry influenced the metabolism of Scenedesmus sp., directing the flow of carbon fixed by photosynthesis to the synthesis of lipids in higher concentration (50.78% lipids) compared to the cultures maintained with the standard culture medium (35.08%). These results are similar to those obtained by Shen et al. [85], where they used the microalgae Botryococcus sp. isolated from a domestic wastewater treatment plant. This microalga was cultivated in synthetic wastewater, where it obtained a lipid production of 61.7% and nutrient removal of 64.5% of nitrogen and 89.8% of phosphorus. Botryococcus sp. obtained a high concentration of lipids explained by the heterotrophic culture conditions to which it was subjected (glucose 10 g L−1, absence of light). However, the nutrient removal efficiency was lower compared to our research.
Shen et al. [86] used wastewater from a pig farm with the previous filtration of solid particles to cultivate a different microalgae strain in lipid production. The Chlorella vulgaris microalgae obtained a lipid concentration of 38.6% and high percentages of nutrient removal (96% nitrogen removal and 99.8% phosphorus removal). These results suggest that the microalgae used has a high capacity for removing nutrients from wastewater for the production of biofuels; however, the pre-treatment applied reduces the profitability of the process and increases production costs.
The efficiency of nutrient removal by microalgae is directly related to their ability to use inorganic nitrogen and phosphorus for their growth [87], which will depend on the strain used and the availability of nutrients in the wastewater. In the present investigation, nutrient removal percentages higher than 85% were obtained for nitrogen and phosphorus, making the use of Scenedesmus sp. viable for the treatment of this type of effluent. These results can be compared with those obtained by Gupta et al. [88], who used the Scenedesmus obliquus strain in different concentrations of municipal wastewater previously filtered and aerated for eight hours, obtaining removal percentages of 98% nitrogen and 97% phosphorus at 15 days when cultivating the microalgae at 100% sewage; however, lipid production only reached 23% of the biomass composition. Although the strain used reached percentages of nutrient removal efficiency similar to those of the present investigation, a pre-treatment was carried out on the wastewater used, which reduces its profitability in possible industrial applications.
On the other hand, the chemical oxygen demand is an important parameter related to the amount of organic matter susceptible to oxidation processes, closely related to eutrophication and contamination in the environment. Ding et al. [89] using Chlorella culture with different percentages of milk residual water (5%, 10%, 20%) obtained removal rates of 84.2%, 87.9%, and 89.7% of COD, while Randi et al. [90] in the cultivation of Chlorella vulgaris and Anabaena ambigua in previously filtered dairy effluents obtained removal percentages of 96.36% and 95.9%, respectively. This is similar to that reported by Brar et al. [91], which shows a removal of 87.5% in cultures of C. pyrenoidosa in 75% of dairy wastewater; however, this percentage of COD reduction is achieved after 25 days of culture. The result agrees with that obtained with Scenedesmus sp., evidencing that microalgae can reduce COD in agro-industrial effluents. However, the present work shows advantages when using 100% of the residual, with high COD concentrations and no pretreatment process, as a cultivation substrate. It is worth mentioning that a high COD removal can be obtained by using microalgal consortia, increasing the efficiency of nutrient assimilation, as reported by Hena et al. [63]. They observed that the use of a consortium of microalgae and cyanobacteria represented by Chlorella, Ankistrodesmus, Chlamydomonas, and Scenedesmus reached 98.8% COD removal.
5. Conclusions
Agro-industrial wastewater is a sustainable alternative for the cultivation of microalgae for bioenergetic applications to use the nutrients present in these substrates for the accumulation of energy compounds and, at the same time, purify them, avoiding contamination of freshwater sources.
The native microalgae Scenedesmus can be grown in dairy industry wastewater with similar culture results to standard BG11 medium concerning productivity and growth. Scenedesmus sp. could increase its concentration of lipids in this culture system without presenting a significant decrease in cell density, diverting the carbon flux to synthesize these compounds.
The cultivation of Scenedesmus sp. in agro-industrial wastewater constitutes a profitable and sustainable biotechnological alternative for obtaining biomass with the high added value of commercial interest.
Author Contributions
X.Á., I.M., and M.-E.V. conceived and designed the experiments; I.M., A.C., and M.-E.V. performed the experiments; X.Á., I.M., and M.-E.V. analyzed the data; I.M. and X.Á. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This work has been supported by the Internal Competitive Fund (FCI) of the University of Guayaquil, through resolution No. R-CIFI-UG-S011-233-21-06-2019.
Acknowledgments
We thank the team of the Microalgae and Cyanobacteria Biotechnology area for the technical support during the project. The wastewater from the dairy industry was kindly provided by Gean Rossi (Quito), Ecuador.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Emsbo-Mattingly, S.D.; Litman, E. Polycyclic aromatic hydrocarbon homolog and isomer fingerprinting. In Standard Handbook Oil Spill Environmental Forensics, 2nd ed.; Stout, S., Wang, Z., Eds.; Academic Press: Cambridge, UK, 2016; pp. 255–312. [Google Scholar] [CrossRef]
- Kiang, Y. Fuel Property Estimation and Combustion Process Characterization. Conventional Fuels, Biomass, Biocarbon, Waste Fuels, Refuse Derived Fuel, and Other Alternative Fuels, 1st ed.; Academic Press: Cambridge, UK, 2018. [Google Scholar]
- Gautam, P.; Kumar, S.; Lokhandwala, S. Energy-Aware Intelligence in Megacities. In Current Developments in Biotechnology and Bioengineering. Waste Treatment Processes for Energy Generation, 1st ed.; Kumar, S., Kumar, R., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 211–238. [Google Scholar] [CrossRef]
- Howarth, R.W.; Santoro, R.; Ingraffea, A. Methane and the greenhouse-gas footprint of natural gas from shale formations. Clim. Chang. 2011, 106, 679–690. [Google Scholar] [CrossRef]
- Shafiee, S.; Topal, E. When will fossil fuel reserves be diminished? Energy Policy 2009, 37, 181–189. [Google Scholar] [CrossRef]
- Canté, F. Renta negativa y decrecimiento económico. Apunt. CENES 2018, 37, 53–74. [Google Scholar] [CrossRef]
- Rather, M.A.; Bano, P. Third Generation Biofuels: A Promising Alternate Energy Source. In Integrating Green Chemistry and Sustainable Engineering; Wiley & Sons: Hoboken, NJ, USA, 2019; pp. 1–21. [Google Scholar]
- Datta, A.; Hossain, A.; Roy, S. An Overview on Biofuels and Their Advantages and Disadvantages. Asian J. Chem. 2019, 31, 1851–1858. [Google Scholar] [CrossRef]
- International Energy Agency. World Energy Outlook 2007: China and India Insights; OECD Publishing: Paris, France, 2007. [Google Scholar] [CrossRef]
- Naqvi, M.; Yan, J. First-Generation Biofuels. In Handbook of Clean Energy Systems; Wiley & Sons: Hoboken, NJ, USA, 2015; Volume 1, pp. 1–18. [Google Scholar]
- Raghavendra, H.; Mishra, S.; Upashe, S.P.; Floriano, J.F. Research and Production of Second-Generation Biofuels. In Bioprocessing for Biomolecules Production, 1st ed.; Molina, G., Gupta, V., Singh, B., Gathergood, N., Eds.; Wiley & Sons: Hoboken, NJ, USA, 2019; pp. 383–400. [Google Scholar]
- Richmond, A. Biological Principles of Mass Cultivation. In Handbook of Microalgae Culture: Biotechnology and Applied Phycology, 1st ed.; Richmond, A., Ed.; Blackwell Publishing: Hoboken, NJ, USA, 2004; pp. 125–177. [Google Scholar]
- Chisti, Y. Biodiesel from microalgae beats bioethanol. Trends Biotechnol. 2008, 26, 126–131. [Google Scholar] [CrossRef]
- Moravvej, Z.; Makarem, M.A.; Rahimpour, M.R. The fourth generation of biofuel. In Second and Third Generation of Feedstocks, 1st ed.; Basile, A., Dalena, F., Eds.; Elsevier: Amsterdam, The Netherlands; Oxford, UK; Cambridge, MA, USA, 2019; pp. 557–597. [Google Scholar] [CrossRef]
- Abdullah, B.; Muhammad, S.A.F.S.; Shokravi, Z.; Ismail, S.; Kassim, K.A.; Mahmood, A.N.; Aziz, M.A. Fourth generation biofuel: A review on risks and mitigation strategies. Renew. Sustain. Energy Rev. 2019, 107, 37–50. [Google Scholar] [CrossRef]
- Randrianarison, G.; Ashraf, M.A. Microalgae: A potential plant for energy production. Geol. Ecol. Landsc. 2017, 1, 104–120. [Google Scholar] [CrossRef]
- Zullaikah, S.; Utomo, A.T.; Yasmin, M.; Ong, L.K.; Ju, Y.-H. Ecofuel conversion technology of inedible lipid feedstocks to renewable fuel. In Advances in Eco-Fuels for a Sustainable Environment, 1st ed.; Kalam Azad, A., Ed.; Woodhead Publishing: Cambridge, MA, USA, 2019; pp. 237–276. [Google Scholar]
- Chapman, R.L. Algae: The world’s most important “plants”—An introduction. Mitig. Adapt. Strat. Glob. Chang. 2013, 18, 5–12. [Google Scholar] [CrossRef]
- Nascimento, M.D.; Rizza, L.S.; Di Palma, A.A.; Dublan, M.D.L.A.; Salerno, G.L.; Rubio, L.M.; Curatti, L. Cyanobacterial biological nitrogen fixation as a sustainable nitrogen fertilizer for the production of microalgal oil. Algal Res. 2015, 12, 142–148. [Google Scholar] [CrossRef]
- Wang, Q.; Lu, Y.; Xin, Y.; Wei, L.; Huang, S.; Xu, J. Genome editing of model oleaginous microalgae Nannochloropsis spp. by CRISPR/Cas9. Plant J. 2016, 88, 1071–1081. [Google Scholar] [CrossRef]
- Choong, Y.J.; Yokoyama, H.; Matsumura, Y.; Lam, M.K.; Uemura, Y.; Dasan, Y.K.; Kadir, W.N.A.; Lim, J.W. The potential of using microalgae for simultaneous oil removal in wastewater and lipid production. Int. J. Environ. Sci. Technol. 2020, 17, 2755–2766. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Cheng, J.J.; Mos, M.; Daroch, M. Biological potential of microalgae in China for biorefinery-based production of biofuels and high value compounds. New Biotechnol. 2015, 32, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Li, Z.H.; Hiltunen, E. Strategies for Lipid Production Improvement in Microalgae as a Biodiesel Feedstock. BioMed Res. Int. 2016, 2016, 8792548. [Google Scholar] [CrossRef] [PubMed]
- Aratboni, H.A.; Rafiei, N.; Garcia-Granados, R.; Alemzadeh, A.; Morones-Ramirez, J.R. Biomass and lipid induction strategies in microalgae for biofuel production and other applications. Microb. Cell Factories 2019, 18, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Komolafe, O.; Orta, S.B.V.; Monje-Ramirez, I.; Noguez, I.Y.; Harvey, A.P.; Ledesma, M.T.O. Biodiesel production from indigenous microalgae grown in wastewater. Biores. Technol. 2014, 154, 297–304. [Google Scholar] [CrossRef]
- Lackner, M. 3rd-Generation Biofuels: Bacteria and Algae as Sustainable Producers and Converters. In Handbook of Climate Change Mitigation and Adaptation, 2nd ed.; Chen, W.Y., Suzuki, T., Lackner, M., Eds.; Springer: Berlin, Germany, 2016; pp. 3173–3210. [Google Scholar]
- Álvarez, X.; Otero, A. Nutrient removal from the centrate of anaerobic digestion of high ammonium industrial wastewater by a semi-continuous culture of Arthrospira sp. and Nostoc sp. PCC 7413. Environ. Biol. Fishes 2020, 7413, 1–10. [Google Scholar] [CrossRef]
- Benemann, J.; Vanolst, J.; Massingill, M.; Carlberg, J.; Weissman, J.; Brune, D. The controlled eutrophication process: Using Microalgae for CO2 utilization and agricultural fertilizer recycling. In Proceedings of the 7th International Conference on Greenhouse Gas Control Technologies, Vancouver, BC, Canada, 5–9 September 2004; pp. 1433–1438. [Google Scholar] [CrossRef]
- Molazadeh, M.; Ahmadzadeh, H.; Pourianfar, H.R.; Lyon, S.; Rampelotto, P.H. The Use of Microalgae for Coupling Wastewater Treatment with CO2 Biofixation. Front. Bioeng. Biotechnol. 2019, 7, 42. [Google Scholar] [CrossRef]
- Allen, M.M. Simple Conditions for Growth of Unicellular Blue-Green Algae on Plates. J. Phycol. 1968, 4, 1–4. [Google Scholar] [CrossRef]
- Álvarez, X. Modulación de la Producción y Caracterización Estructural de los Exopolisacáridos en Cianobacterias Diazotróficas, y Estudio de su Utilización para el Tratamiento del Digestato Líquido de la Digestión Anaeróbica de Efluente Efluentes de una Procesadora de Pescado. Ph.D. Thesis, Universidade de Santiago de Compostela, Santiago de Compostela, Spain, 2016. [Google Scholar]
- International Council for Standardization in Haematology. Recommended Methods for the Visual Determination of White Blood Cell Count and Platelet Counts, 1st ed.; World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- Arredondo, B.; Voltolina, D.; Zenteno, T.; Arce, M.; Gómez, G. Métodos y Herramientas Analíticas en la Evaluación de la Biomasa Microalgal, 2nd ed.; Centro de Investigaciones Biológicas del Noroeste: La Paz, Mexico, 2017. [Google Scholar]
- Zhu, C.J.; Lee, Y.K. Determination of biomass dry weight of marine microalgae. Environ. Biol. Fishes 1997, 9, 189–194. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Herbert, D.; Phipps, P.; Strange, R. Chapter III Chemical Analysis of Microbial Cells. Methods Microbiol. 1971, 5, 209–344. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A Rapid Method of Total Lipid Extraction and Purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Marsh, J.B.; Weinstein, D.B. Simple charring method for determination of lipids. J. Lipid Res. 1966, 7, 574–576. [Google Scholar]
- AOAC. Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemists: Rockville, MD, USA, 2005. [Google Scholar]
- AOAC. Official Methods of Analysis, 17th ed.; Association of Official Analytical Chemists: Rockville, MD, USA, 2000. [Google Scholar]
- AOAC. Official Methods of Analysis, 16th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1995. [Google Scholar]
- D’Elia, C.F.; Steudler, P.A.; Corwin, N. Determination of total nitrogen in aqueous samples using persulfate digestion1. Limnol. Oceanogr. 1977, 22, 760–764. [Google Scholar] [CrossRef]
- Tandon, H.L.S.; Cescas, M.P.; Tyner, E.H. An Acid-Free Vanadate-Molybdate Reagent for the Determination of Total Phosphorus in Soils. Soil Sci. Soc. Am. J. 1968, 32, 48–51. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington, DC, USA, 2017. [Google Scholar]
- Evans, S.J.; Johnson, M.S.; Leah, R.T. Determination of mercury in fish tissue: A rapid automated technique for routine analysis. Varian Instrum. Work 1986, 60, 1–6. [Google Scholar]
- AOAC. Official Methods of Analysis, 21st ed.; Association of Official Analytical Chemists: Rockville, MD, USA, 2019. [Google Scholar]
- Deaker, M.; Maher, W. Determination of arsenic in arsenic compounds and marine biological tissues using low volume microwave digestion and electrothermal atomic absorption spectrometry. J. Anal. At. Spectrom. 1999, 14, 1193–1207. [Google Scholar] [CrossRef]
- Chen, M.; Li, J.; Dai, X.; Sun, Y.; Chen, F. Effect of phosphorus and temperature on chlorophyll a contents and cell sizes of Scenedesmus obliquus and Microcystis aeruginosa. Limnology 2010, 12, 187–192. [Google Scholar] [CrossRef]
- Cheirsilp, B.; Srinuanpan, S.; Mandik, Y. Efficient harvesting of microalgal biomass and direct conversion of microalgal lipids into biodiesel. In Microalgae Cultivation for Biofuels Production, 1st ed.; Yousuf, A., Ed.; Academic Press: Cambridge, UK, 2010; Volume 1, pp. 83–96. [Google Scholar] [CrossRef]
- Osundeko, O.; Dean, A.P.; Davies, H.; Pittman, J.K. Acclimation of Microalgae to Wastewater Environments Involves Increased Oxidative Stress Tolerance Activity. Plant Cell Physiol. 2014, 55, 1848–1857. [Google Scholar] [CrossRef]
- Wollmann, F.; Dietze, S.; Ackermann, J.; Bley, T.; Walther, T.; Steingroewer, J.; Krujatz, F. Microalgae wastewater treatment: Biological and technological approaches. Eng. Life Sci. 2019, 19, 860–871. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.S.; Dahms, H.-U.; Won, E.-J.; Lee, J.-S.; Shin, K.-H. Microalgae—A promising tool for heavy metal remediation. Ecotoxicol. Environ. Saf. 2015, 113, 329–352. [Google Scholar] [CrossRef]
- Kinuthia, G.K.; Ngure, V.; Beti, D.; Lugalia, R.; Wangila, A.; Kamau, L. Levels of heavy metals in wastewater and soil samples from open drainage channels in Nairobi, Kenya: Community health implication. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Shakoor, M.B.; Bibi, I.; Niazi, N.K.; Shahid, M.; Nawaz, M.F.; Farooqi, A.; Naidu, R.; Rahman, M.M.; Murtaza, G.; Lüttge, A. The evaluation of arsenic contamination potential, speciation and hydrogeochemical behaviour in aquifers of Punjab, Pakistan. Chemosphere 2018, 199, 737–746. [Google Scholar] [CrossRef]
- Nagi, M.; He, M.; Li, D.; Gebreluel, T.; Cheng, B.; Wang, C. Utilization of tannery wastewater for biofuel production: New insights on microalgae growth and biomass production. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Koreivienė, J.; Valčiukas, R.; Karosienė, J.; Baltrėnas, P. Testing of Chlorella/Scenedesmus Microalgae Consortia for Remediation of Wastewater, CO2 Mitigation and Algae Biomass Feasibility for Lipid Production. J. Environ. Eng. Landsc. Manag. 2014, 22, 105–114. [Google Scholar] [CrossRef]
- Lu, W.; Wang, Z.; Wang, X.; Yuan, Z. Cultivation of Chlorella sp. using raw dairy wastewater for nutrient removal and biodiesel production: Characteristics comparison of indoor bench-scale and outdoor pilot-scale cultures. Biores. Technol. 2015, 192, 382–388. [Google Scholar] [CrossRef]
- Marazzi, F.; Bellucci, M.; Fantasia, T.; Ficara, E.; Mezzanotte, V. Interactions between Microalgae and Bacteria in the Treatment of Wastewater from Milk Whey Processing. Water 2020, 12, 297. [Google Scholar] [CrossRef]
- Whitton, R.; Le Mével, A.; Pidou, M.; Ometto, F.; Villa, R.; Jefferson, B. Influence of microalgal N and P composition on wastewater nutrient remediation. Water Res. 2016, 91, 371–378. [Google Scholar] [CrossRef]
- Jebali, A.; Acién, F.; Gómez, C.; Fernández-Sevilla, J.; Mhiri, N.; Karray, F.; Dhouib, A.; Molina-Grima, E.; Sayadi, S. Selection of native Tunisian microalgae for simultaneous wastewater treatment and biofuel production. Biores. Technol. 2015, 198, 424–430. [Google Scholar] [CrossRef]
- Sweiss, M. Microalgas para Tratamiento de Aguas Residuales y Producción de Biomasa Desde Bioprospección Hasta Biotecnología. Ph.D. Thesis, Universidad de Bath, Inglaterra, UK, 2017. [Google Scholar]
- Hena, S.A.; Abida, N.; Tabassum, S. Screening of facultative strains of high lipid producing microalgae for treating surfactant mediated municipal wastewater. RSC Adv. 2015, 5, 98805–98813. [Google Scholar] [CrossRef]
- Ling, Y.; Sun, L.-P.; Wang, S.-Y.; Lin, C.S.K.; Sun, Z.; Zhou, Z.-G. Cultivation of oleaginous microalgae Scenedesmus obliquus coupled with wastewater treatment for enhanced biomass and lipid production. Biochem. Eng. J. 2019, 148, 162–169. [Google Scholar] [CrossRef]
- Wu, Y.-H.; Li, X.; Yu, Y.; Hu, H.-Y.; Zhang, T.-Y.; Li, F.-M. An integrated microalgal growth model and its application to optimize the biomass production of Scenedesmus sp. LX1 in open pond under the nutrient level of domestic secondary effluent. Biores. Technol. 2013, 144, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Hena, S.; Fatimah, S.; Tabassum, S. Cultivation of algae consortium in a dairy farm wastewater for biodiesel production. Water Res. Ind. 2015, 10, 1–14. [Google Scholar] [CrossRef]
- Kuo, C.-M.; Chen, T.-Y.; Lin, T.-H.; Kao, C.-Y.; Lai, J.-T.; Chang, J.-S.; Lin, C.-S. Cultivation of Chlorella sp. GD using piggery wastewater for biomass and lipid production. Biores. Technol. 2015, 194, 326–333. [Google Scholar] [CrossRef]
- Jeong, D.; Jang, A. Exploration of microalgal species for simultaneous wastewater treatment and biofuel production. Environ. Res. 2020, 188, 109772. [Google Scholar] [CrossRef]
- Shen, Q.-H.; Jiang, J.-W.; Chen, L.-P.; Cheng, L.-H.; Xu, X.-H.; Chen, H.-L. Effect of carbon source on biomass growth and nutrients removal of Scenedesmus obliquus for wastewater advanced treatment and lipid production. Biores. Technol. 2015, 190, 257–263. [Google Scholar] [CrossRef]
- Ansari, F.A.; Ravindran, B.; Gupta, S.K.; Nasr, M.; Rawat, I.; Bux, F. Techno-economic estimation of wastewater phycoremediation and environmental benefits using Scenedesmus obliquus microalgae. J. Environ. Manag. 2019, 240, 293–302. [Google Scholar] [CrossRef]
- Ho, S.-H.; Kondo, A.; Hasunuma, T.; Chang, J.-S. Engineering strategies for improving the CO2 fixation and carbohydrate productivity of Scenedesmus obliquus CNW-N used for bioethanol fermentation. Biores. Technol. 2013, 143, 163–171. [Google Scholar] [CrossRef]
- Toledo-Cervantes, A.; Morales, M.; Novelo, E.; Revah, S. Carbon dioxide fixation and lipid storage by Scenedesmus obtusiusculus. Biores. Technol. 2013, 130, 652–658. [Google Scholar] [CrossRef]
- Choi, H.-J. Dairy wastewater treatment using microalgae for potential biodiesel application. Environ. Eng. Res. 2016, 21, 393–400. [Google Scholar] [CrossRef]
- Ambat, I.; Bec, S.; Peltomaa, E.; Srivastava, V.; Ojala, A.; Sillanpää, M. A synergic approach for nutrient recovery and biodiesel production by the cultivation of microalgae species in the fertilizer plant wastewater. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Wang, Z.; Yao, X.-D.; Miao, M.-S.; Chen, Q.-F.; Kong, Q.; Shang, D.-W.; Yu, J.-N.; Fu, X.-M. Effects of urban wastewater dilution on growth and biochemical properties of Scenedesmus sp. Desalin. Water Treat. 2016, 57, 29363–29370. [Google Scholar] [CrossRef]
- European Committee for Standardization. Liquid Petroleum Products—Fatty Acid Methyl Esters (FAME) for Use in Diesel Engines and Heating Applications—Requirements and Test Methods; European Standard 14214; European Committee for Standardization: Brussels, Belgium, 2012. [Google Scholar]
- Dijkstra, A.J. Revisiting the formation of-trans isomers during partial hydrogenation of triacylglycerol oils. Eur. J. Lipid Sci. Technol. 2006, 108, 249–264. [Google Scholar] [CrossRef]
- Hoekman, S.K.; Broch, A.; Robbins, C.; Ceniceros, E.; Natarajan, M. Review of biodiesel composition, properties, and specifications. Renew. Sustain. Energy Rev. 2012, 16, 143–169. [Google Scholar] [CrossRef]
- Castillo, O.S.; Torres-Badajoz, S.G.; Núñez-Colín, C.A.; Peña-Caballero, V.; Herrera, C.H.; Rodríguez-Núñez, J.R. Producción de biodiésel a partir de microalgas: Avances y perspectivas biotecnológicas. Hidrobiológica 2017, 27, 337–352. [Google Scholar]
- Quevedo, C.; Sonia, M.; Acosta, A. Crecimiento de Scenedesmus sp. en diferentes medios de cultivo para producción de proteína microalgal. Vitae-Columbia 2008, 15, 25–31. [Google Scholar]
- Verduga, M.E. Cultivo en Batch de Scenedesmus spp. en Aguas Residuales de Industrias Lácteas: Crecimiento, Productividad y Composición Bioquímica. Bachelor’s Thesis, Universidad de Guayaquil, Guayaquil, Ecuador, 2020. Available online: http://repositorio.ug.edu.ec/bitstream/redug/48682/1/CD_TESIS_ELOIZA.pdf (accessed on 15 May 2020).
- Tuantet, K.; Temmink, H.; Zeeman, G.; Janssen, M.; Wijffels, R.H.; Buisman, C.J. Nutrient removal and microalgal biomass production on urine in a short light-path photobioreactor. Water Res. 2014, 55, 162–174. [Google Scholar] [CrossRef]
- Kumar, D.; Singh, B. Ankit. Phycoremediation of nutrients and valorisation of microalgal biomass: An economic perspective. In Application of Microalgae in Wastewater Treatment, 1st ed.; Gupta, S., Bux, F., Eds.; Springer: Cham, Switzerland, 2019; Volume 2, pp. 1–15. [Google Scholar] [CrossRef]
- Choudhary, P.; Assemany, P.P.; Naaz, F.; Bhattacharya, A.; Castro, J.D.S.; Couto, E.D.A.D.C.; Calijuri, M.L.; Pant, K.K.; Malik, A. A review of biochemical and thermochemical energy conversion routes of wastewater grown algal biomass. Sci. Total. Environ. 2020, 726, 137961. [Google Scholar] [CrossRef]
- Shen, L.; Ndayambaje, J.D.; Murwanashyaka, T.; Cui, W.; Manirafasha, E.; Chen, C.; Wang, Y.; Lu, Y. Assessment upon heterotrophic microalgae screened from wastewater microbiota for concurrent pollutants removal and biofuel production. Biores. Technol. 2017, 245, 386–393. [Google Scholar] [CrossRef]
- Shen, Y.; Yang, T.; Zhu, W.; Zhao, Y. Wastewater treatment and biofuel production through attached culture of Chlorella vulgaris in a porous substratum biofilm reactor. Environ. Biol. Fishes 2016, 29, 833–841. [Google Scholar] [CrossRef]
- Abdel-Raouf, N.; Al-Homaidan, A.; Ibraheem, I. Microalgae and wastewater treatment. Saudi J. Biol. Sci. 2012, 19, 257–275. [Google Scholar] [CrossRef]
- Gupta, S.K.; Ansari, F.A.; Shriwastav, A.; Sahoo, N.K.; Rawat, I.; Bux, F. Dual role of Chlorella sorokiniana and Scenedesmus obliquus for comprehensive wastewater treatment and biomass production for bio-fuels. J. Clean. Prod. 2016, 115, 255–264. [Google Scholar] [CrossRef]
- Ding, J.; Zhao, F.; Cao, Y.; Xing, L.; Liu, W.; Mei, S.; Li, S. Cultivation of Microalgae in Dairy Farm Wastewater Without Sterilization. Int. J. Phytoremed. 2015, 17, 222–227. [Google Scholar] [CrossRef]
- Rani, A.S.; Rao, H.R.V.N.G.; Kumar, A.B.; Shruthi, M. Eco-Friendly Approach for Treating Dairy Effluent and Lipid Estimation Using Microalgae. Br. Biotechnol. J. 2015, 7, 33–39. [Google Scholar] [CrossRef]
- Brar, A.; Kumar, M.; Pareek, N. Comparative Appraisal of Biomass Production, Remediation, and Bioenergy Generation Potential of Microalgae in Dairy Wastewater. Front. Microbiol. 2019, 10, 678. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).