Remediation of Lead and Nickel Contaminated Soil Using Nanoscale Zero-Valent Iron (nZVI) Particles Synthesized Using Green Leaves: First Results
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of nZVI Particles
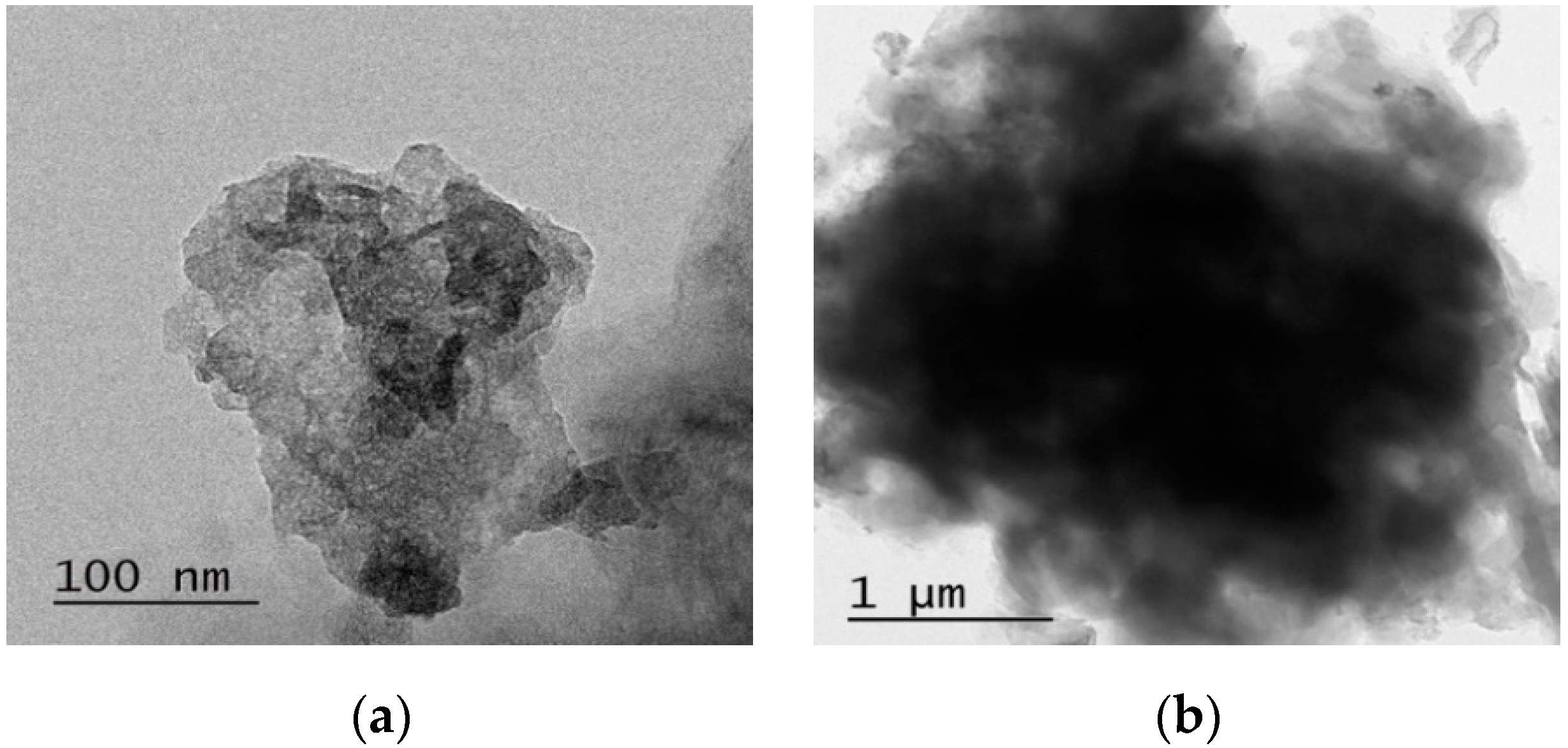
2.2. Characterization of nZVI Particles
2.3. Soil and its Original Characteristics
2.4. Pollution of Soil Samples and Nanoparticle Addition
2.5. Remediation of Soil Samples
3. Results
3.1. Characteristics of nZVI Particles
3.2. Soil Characteristics
3.3. Remediation of Contaminated Soil
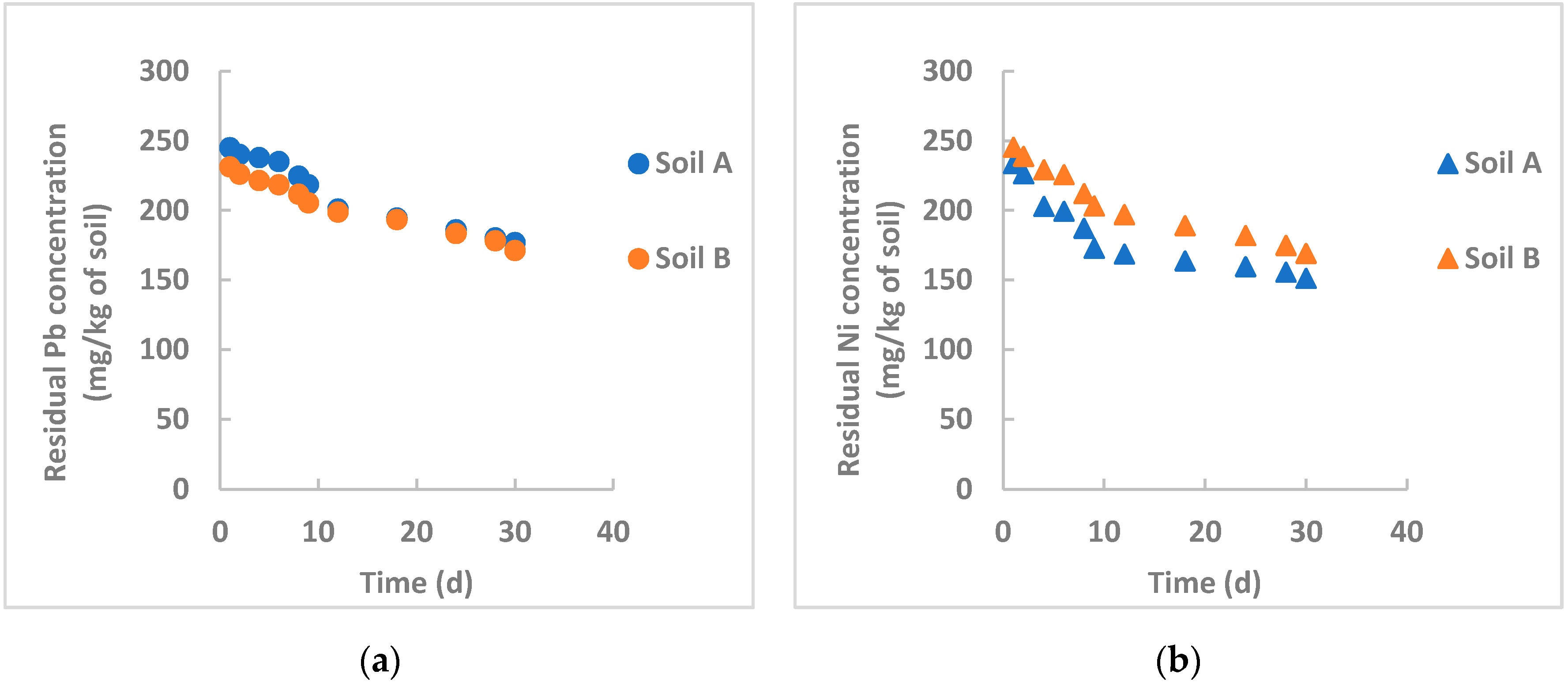
3.3.1. Remediation by Chemically Synthesized nZVI Particles
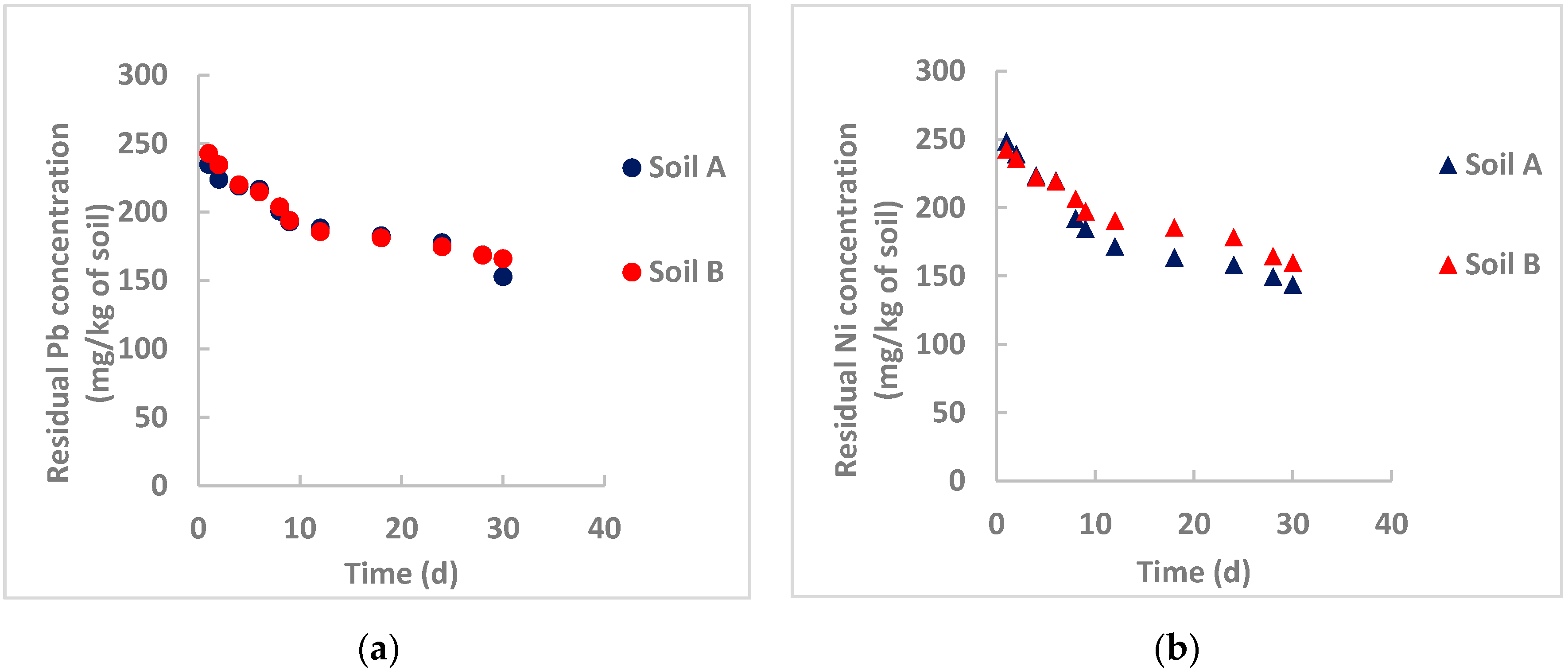
3.3.2. Remediation by nZVI Particles Synthesized Using Neem Leaves
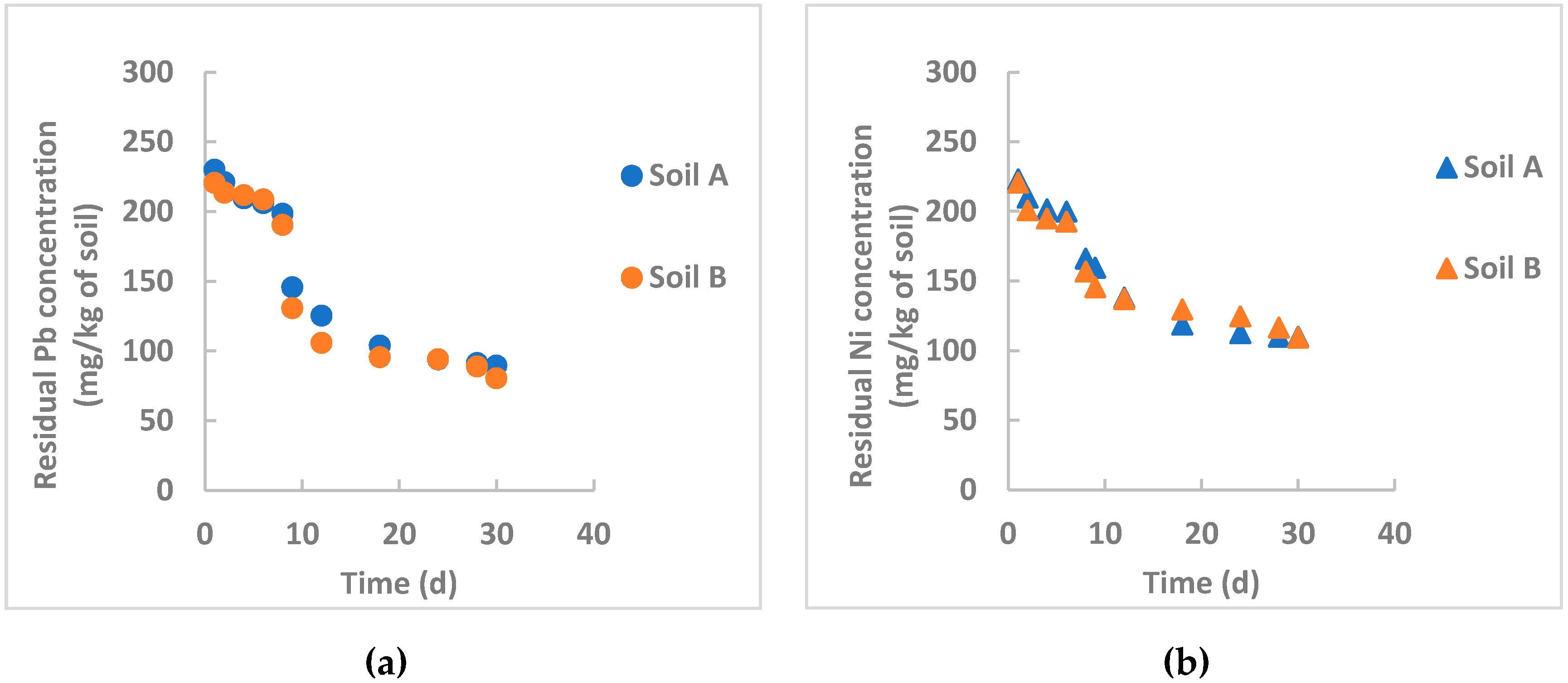
3.3.3. Remediation by nZVI Particles Synthesized Using Mint Leaves
4. Discussion
- The chemically synthesized particles provided the lowest efficiencies.
- The particles achieved by the processing of mint leaves showed the best results for the removal of both the metals.
- With identical nanoparticles dosage, the removal of lead by all the tested particles was constantly higher than nickel, suggesting a higher affinity of the particle for the metal.
- A double dosage improved the removal, albeit to a small extent (the maximum improvement was 26% for lead removal by neem-derived particles).
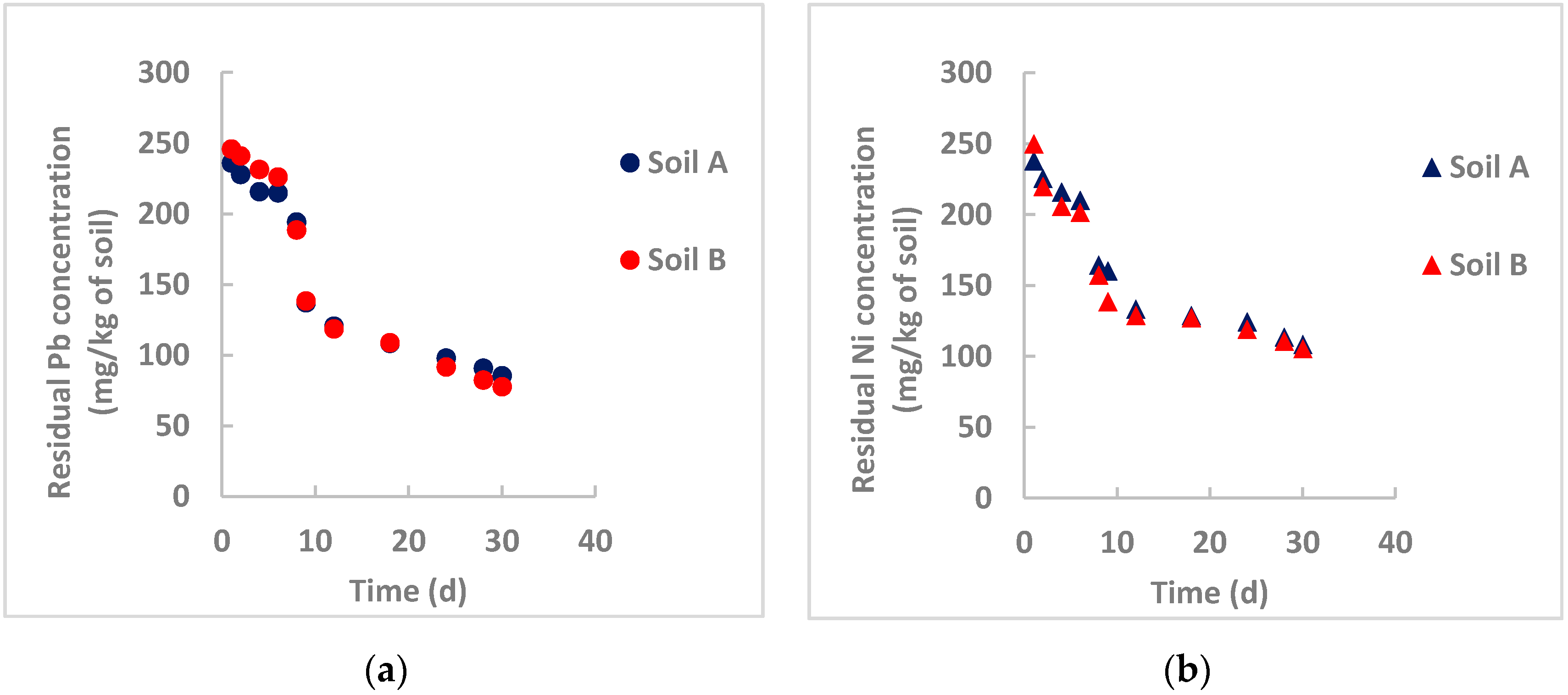
- Gil-Diaz et al. [32] studied in-field brownfield remediation polluted with arsenic and mercury using commercial nZVI particles for long times (32 months) and achieved an initial sharp reduction of the pollutants, followed by a rather constant residual concentration probably due to interferences of organic matter that can form stable complexes on the particle surface, especially at acidic pH and limiting the particle removal efficiency. Examining Figure 3, Figure 4 and Figure 5, this occurs for nickel removal in Soil B, where pH is moderately acidic (5.32) and the organic matter content is high (about 5%). This is evident for nickel, whereas the pH influence is not appreciable for lead. One hypothesis can be the different solubility product constant, KPS, for Ni(OH)2 and Pb(OH)2, which is equal to 6 × 10−16 and 1.4 × 10−20, respectively. A rough calculation can provide the saturation concentration of the heavy metals at the tested pH values. For lead, at saturation its concentration is lower than the initial concentration in both instances. Therefore, lead hydroxide also precipitates at an acidic pH. Heavy metal removal by nZVI particles is not only based on precipitation. For lead, this phenomenon could be more influential than the others (adsorption, coprecipitation, oxidation/reduction);
- Mystrioti et al. [33] studied the reduction of hexavalent chromium to Cr(III) by nZVI particles synthesized from several sources, namely Camellia sinensis (green tea), Syzygium aromaticum (clove), Mentha spicata (spearmint), Punica granatum juice (pomegranate), and red wine. The process was conducted on a liquid solution containing 50 mg/L of Cr(VI) in contact with different nanoparticle concentrations from the aforementioned sources. The reduction process, occurring on the particle surfaces, had better efficiency at a high particle concentration, and when a low concentration was used, the process kinetics clearly showed two different rates.
- Di Palma et al. [34] used chemically synthesized nZVI particles to reduce Cr(VI) to Cr(III) from neutral soil in the slurry mode. Their trials also showed that the reduction efficiency was influenced positively by the test duration and nZVI particle concentration, with a more evident two-step process at a low nZVI particle concentration.
- Wang et al. [35] synthesized nZVI particles from green tea and eucalyptus leaves to remove nitrate from wastewater and compared their performance to the results achieved with particles from chemical synthesis. The best results were achieved with the nanoparticles of chemical origin. However, after air contact for two months, the vegetal-origin nanoparticles did not change their performance and showed good stability, whereas for the others, the removal efficiency dropped by 50%.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kabata-Pendias, A.; Pendias, H. Trace Metals in Soils and Plants; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Usha, R.; Vasavi, A.; Thishya, K.; Janshi Rani, S.; Supraja, P. Phytoextraction of lead from industrial effluents by sunflower (Helianthus Annuus. L). Rasayan J. Chem. 2011, 4, 8–12. [Google Scholar]
- Burke, D.M.; Morris, M.A.; Holmes, J.D. Chemical oxidation of mesoporous carbon foams for lead ion adsorption. Sep. Purif. Technol. 2013, 104, 150–159. [Google Scholar] [CrossRef]
- National Safety Council. Lead Poisoning. Available online: https://www.nsc.org/new_resources/%20resources/document/lead_poisoning (accessed on 18 November 2015).
- Özcan, A.S.; Tunali, S.; Akar, T.; Özcan, A. Biosorption of Lead (II) ions onto waste biomass of Phaseolus vulgaris L.: Estimation of the equilibrium, kinetic and thermodynamic parameters. Desalination 2009, 244, 188–198. [Google Scholar] [CrossRef]
- Wuana, R.A.; Okieimen, F.E. Heavy metals in contaminated soils: A review of sources, chemistry, risk and best, available strategies for remediation. ISRN Ecol. 2011. ID 402647. [Google Scholar] [CrossRef]
- Dudka, S.; Miller, W.P. Permissible concentration of arsenic and lead in soils based on risk assessment. Water Air Soil Pollut. 1999, 113, 127–132. [Google Scholar] [CrossRef]
- Akhtar, N.; Iqbal, J.; Iqbal, M. Removal and recovery of nickel (II) from aqueous solution by loofa sponge-immobilized biomass of Chlorella sorakiniana: Characterisation study. J. Hazard. Mater. 2004, B108, 85–94. [Google Scholar] [CrossRef]
- Farooq, U.; Kozinski, J.A.; Khan, M.A.; Athar, M. Biosorption of heavy metal ions using wheat-based biosorbents: A review of the recent literature. Bioresour. Technol. 2010, 101, 5034–5043. [Google Scholar] [CrossRef]
- Senthil Kumar, P.S.; Ramalingam, S.; Dinesh Kirupha, S.; Murugesan, A.; Vidhyadevi, T.; Sivanesan, S. Adsorption behaviour of nickel (II) onto cashew nut shell: Equilibrium thermodynamics, kinetics, mechanism and process design. Chem. Eng. J. 2011, 167, 122–131. [Google Scholar] [CrossRef]
- Malamis, S.; Katsou, E. A review on zinc and nickel adsorption on natural and modified zeolite, bentonite and vermiculite: Examination of process parameters, kinetics and isotherms. J. Hazard. Mater. 2013, 252–253, 428–461. [Google Scholar] [CrossRef]
- Adriano, D.C. Trace Elements in Terrestrial Environment; Springer: New York, NY, USA, 2001. [Google Scholar]
- Iyaka, Y.A. Nickel in soils: A review of its distribution and impacts. Sci. Res. Essays 2011, 6, 6774–6777. [Google Scholar]
- Basta, N.T.; Gradwohl, R. Remediation of heavy metals contaminated soil using rock phosphate. Better Crops 1998, 82, 29–31. [Google Scholar]
- Kuiken, T. Cleaning up contaminated waste site: Is nanotechnology the answer? Nano Today 2009, 5, 6–8. [Google Scholar] [CrossRef]
- Pasinszki, T.; Krebsz, M. Synthesis and Application of Zero-Valent Iron Nanoparticles in Water Treatment, Environmental Remediation, Catalysis, and Their Biological Effects. Nanomaterials 2020, 10, 917. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Saha, I.; Mukhopadhyay, A.; Chattopadhyay, D.; Gosh, U.; Chatterjee, D. Role of nanotechnology in water treatment and purification: Potential applications and implications. Int. J. Chem. Sci. Technol. 2013, 3, 59–64. [Google Scholar]
- Liao, C.J.; Chung, T.L.; Chen, W.L.; Kuo, S.L. Treatment of pentachlorophenol-contaminated soil using nano-scale zero-valent iron with hydrogen peroxide. J. Mol. Catal. A Chem. 2006, 265, 189–194. [Google Scholar] [CrossRef]
- Taghipour, M.; Jalali, M. Effect of clay minerals and nanoparticles on chromium fractionation in soil contaminated with leather factory waste. J. Hazard. Mater. 2015, 297, 127–133. [Google Scholar] [CrossRef]
- Allabaksh, M.B.; Mandal, B.K.; Kesarla, M.K.; Kumar, K.S.; Reddy, P.S. Preparation of stable Zero Valent Iron nanoparticles using different chelating agents. J. Chem. Pharm. Res. 2010, 2, 67–74. [Google Scholar]
- Valipour, M.; Shahbazi, K.; Khanmirzaei, A. Chemical immobilization of lead, cadmium, copper, and nickel in contaminated soils by phosphate amendments. CLEAN Soil Air Water 2016, 44, 572–578. [Google Scholar] [CrossRef]
- Yadegari, M. Performance of purslane (Portulaca oleracea) in nickel and cadmium contaminated soil as a heavy metals-removing crop. Iran. J. Plant Physiol. 2018, 8, 2447–2455. [Google Scholar]
- De Gisi, S.; Minetto, D.; Lofrano, G.; Libralato, G.; Conte, B.; Todaro, F.; Notarnicola, M. Nano-scale zero valent iron (nZVI) treatment of marine sediments slightly polluted by heavy metals. Chem. Eng. Trans. 2017, 60, 139–144. [Google Scholar]
- Vasarevičius, S.; Danila, V.; Paliulis, D. Application of stabilized nano Zero Valent Iron particles for immobilization of available Cd2+, Cu2+, Ni2+, and Pb2+ ions in soil. Int. J. Environ. Res. 2019, 13, 465–474. [Google Scholar] [CrossRef]
- Pattanayak, M.; Nayak, P.L. Green synthesis and characterisation of Zero Valent Iron nanoparticles from leaf extract of Azadirachta Indica (Neem). World J. Nano Sci. Technol. 2013, 2, 6–9. [Google Scholar]
- IS:3025-Part 11, Methods of Sampling and Test (Physical and Chemical) for Water and Wastewater, Part 11 pH Value; Bureau of Indian Standards: New Delhi, India, 2006.
- IS:3025-Part 14, Methods of Sampling and Test (Physical and Chemical) for Water and Wastewater, Part 14 Specific Conductance; Bureau of Indian Standards: New Delhi, India, 2002.
- ASTM D854, Standard Test Methods for Specific Gravity of Soil Solids by Water Pycnometer; ASTM International: West Conshohocken, PA, USA, 2014.
- ASTM D4959, Standard Test Method for Determination of Water Content of Soil by Direct Heating; ASTM International: West Conshohocken, PA, USA, 2016.
- ASTM D2974, Standard Test Methods for Moisture, Ash, and Organic Matter of Peat and Other Organic Soils; ASTM International: West Conshohocken, PA, USA, 2014.
- ASTM D422, Standard Test Method for Particle-Size Analysis of Soils; ASTM International: West Conshohocken, PA, USA, 2007.
- Gil-Díaz, M.; Rodríguez-Valdés, E.; Alonso, J.; Baragaño, D.; Gallego, J.R.; Lobo, M.C. Nanoremediation and long-term monitoring of brownfield soil highly polluted with As and Hg. Sci. Total Environ. 2019, 675, 165–175. [Google Scholar] [CrossRef]
- Mystrioti, C.; Xanthopoulou, T.D.; Tsakiridis, P.; Papassiopi, N.; Xenidis, A. Comparative evaluation of five plant extracts and juices for nanoiron synthesis and application for hexavalent chromium reduction. Sci. Total Environ. 2016, 539, 105–113. [Google Scholar] [CrossRef]
- Di Palma, L.; Gueye, M.T.; Petrucci, E. Hexavalent chromium reduction in contaminated soil: A comparison between ferrous sulphate and nanoscale zero-valent iron. J. Hazard. Mater. 2015, 281, 70–76. [Google Scholar] [CrossRef]
- Wang, T.; Lin, J.; Chen, Z.; Megharaj, M.; Naidu, R. Green synthesized iron nanoparticles by green tea and eucalyptus leaves extracts used for removal of nitrate in aqueous solution. J. Clean. Prod. 2014, 83, 413–419. [Google Scholar] [CrossRef]




| Characteristics | Soil A | Soil B |
|---|---|---|
| Soil type | Coarse-graded, sandy soil | Coarse-graded, sandy soil |
| pH | 6.65 | 5.32 |
| Conductivity (mS/cm) | 1.12 | 0.98 |
| Water content (%) | 13.7 | 12.9 |
| Specific gravity | 2.98 | 3.07 |
| Organic content (% by weight) | 2.78 | 4.94 |
| Lead concentration (mg/kg of soil) | 0.245 | 0.234 |
| Nickel concentration (mg/kg of soil) | 0.201 | 0.267 |
| Particle Origin | Pb Removal Efficiency at t = 30 days | Ni Removal Efficiency at t = 30 days | ||
|---|---|---|---|---|
| Particle dosage | ||||
| 0.1 g/kg of soil | 0.2 g/kg of soil | 0.1 g/kg of soil | 0.2 g/kg of soil | |
| Chemically synthesized | 21.6% | 18.5% | ||
| Neem leaves | 26.9% | 33.3% | 33.2% | 38.2% |
| Mint leaves | 62.3% | 66.1% | 50.6% | 56.1% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Francy, N.; Shanthakumar, S.; Chiampo, F.; Sekhar, Y.R. Remediation of Lead and Nickel Contaminated Soil Using Nanoscale Zero-Valent Iron (nZVI) Particles Synthesized Using Green Leaves: First Results. Processes 2020, 8, 1453. https://doi.org/10.3390/pr8111453
Francy N, Shanthakumar S, Chiampo F, Sekhar YR. Remediation of Lead and Nickel Contaminated Soil Using Nanoscale Zero-Valent Iron (nZVI) Particles Synthesized Using Green Leaves: First Results. Processes. 2020; 8(11):1453. https://doi.org/10.3390/pr8111453
Chicago/Turabian StyleFrancy, Nimita, Subramanian Shanthakumar, Fulvia Chiampo, and Yendaluru Raja Sekhar. 2020. "Remediation of Lead and Nickel Contaminated Soil Using Nanoscale Zero-Valent Iron (nZVI) Particles Synthesized Using Green Leaves: First Results" Processes 8, no. 11: 1453. https://doi.org/10.3390/pr8111453
APA StyleFrancy, N., Shanthakumar, S., Chiampo, F., & Sekhar, Y. R. (2020). Remediation of Lead and Nickel Contaminated Soil Using Nanoscale Zero-Valent Iron (nZVI) Particles Synthesized Using Green Leaves: First Results. Processes, 8(11), 1453. https://doi.org/10.3390/pr8111453






