Abstract
In recent years, a strong interest has emerged in polysaccharide-hybrid composites and their potential applications, which have interesting functional and technological properties. This review summarizes and discusses the reported advantages and limitations of the functionalization of conventional and nonconventional polysaccharides by adding TiO2 nanoparticles as a reinforcement agent. Their effects on the mechanical, thermal, and UV-barrier properties as well as their water-resistance are discussed. In general, the polysaccharide–TiO2 hybrid materials showed improved physicochemical properties in a TiO2 content-dependent response. It showed antimicrobial activity against bacteria (gram-negative and gram-positive), yeasts, and molds with enhanced UV-protective effects for food and non-food packaging purposes. The reported applications of functionalized polysaccharide–TiO2 composites include photocatalysts (dye removal from aqueous media and water purification), biomedical (wound-healing material, drug delivery systems, biosensor, and tissue engineering), food preservation (fruits and meat), cosmetics (sunscreen and bleaching tooth treatment), textile (cotton fabric self-cleaning), and dye-sensitized solar cells. Furthermore, the polysaccharide–TiO2 showed high biocompatibility without adverse effects on different cell lines, indicating that their use in food, pharmaceutical, and biomedical applications is safe. However, it is necessary to evaluate the structural changes promoted by the storage conditions (time and temperature) on the physicochemical properties of polysaccharide–TiO2 hybrid composites to guarantee their stability during a determined time.
1. Introduction
In recent years, the development of functional and eco-friendly materials with advanced properties as an alternative to replacing conventional nondegradable polymers has gained attention, particularly, for the polysaccharide-hybrid materials which exhibit diversified technological applications (antimicrobial agent, packaging material, water pollutant degradation, energy storage, and biosensors) [1,2]. Hybrid materials refer to combining two or more organic or inorganic components such as organic–organic (starch–cellulose), inorganic–inorganic (TiO2–Ag), and organic–inorganic (starch–TiO2) compounds, and they are synthesized by different routes such as covalent immobilization, electrostatic binding, and polymerization methods among others [3]. These synthetic routes are effective to fabricate polysaccharide-hybrid structures with enhanced technological properties and applications [3]. Currently, there is a special interest in combining organic compounds such as polysaccharides with inorganic compounds like titanium dioxide (TiO2) to obtain hybrid materials with enhanced stability and new functionalities [4,5,6,7].
Titanium dioxide (TiO2) is a versatile material with interesting characteristics (biocompatible, chemical stability, high reactivity, electrochemical properties, low cost, and safe production) [2,8,9,10] used as a photocatalyst for water-dye degradation [11]. It is employed as a white colorant in the food and pharmaceutical industries [11]. Furthermore, TiO2 exhibits UV-protection and antimicrobial properties [12,13]. Moreover, TiO2 is widely used to develop energy storage systems like lithium-ion batteries and dye-sensitized solar cells [2,14,15,16]. Recently, TiO2 has been used as a physical cross-linking agent to improve the technological and functional properties of polysaccharide-based materials [17,18,19].
In the last decade, the use of TiO2 as a reinforcement agent of polysaccharide-based materials has been explored [5,11,20]. Teymourpour et al. [6] reported that the soybean polysaccharide biocomposite reinforced with TiO2 showed good antimicrobial activity against Escherichia coli and Staphylococcus aureus. Ahmadi et al. [21] developed a carboxymethyl cellulose film cross-linked with TiO2 with improved UV-protective effects. Urruela-Barrios et al. [5] fabricated alginate-gelatin hydrogels combined with TiO2 for tissue regeneration. Tunma [22] fabricated an active packaging with the cassava starch–TiO2 composite for banana and tomato preservation. Khodadadi et al. [23] reported that the pectin–TiO2:Cu composite is effective for methyl orange dye removal. Nonetheless, Khanmirzaei and Ramesh [24] fabricated a rice starch/ionic liquid/TiO2 nanocomposite for solar cell applications. Furthermore, the incorporation of TiO2 in different polysaccharide matrixes (carboxymethyl cellulose; starch; sodium alginate; agar; and a combination of k-Carrageenan, xanthan gum, and gellan gum) had a positive impact on their mechanical, thermal, and physicochemical properties, which exhibit potential industrial applications [17,20,25,26,27].
This review summarizes the advantages and limitations that the use of TiO2 nanoparticles as a reinforcement agent offers during the development of polysaccharide-based hybrid materials and provides an overview of the effect of diverse polysaccharide compounds reinforced with TiO2 on the antimicrobial activity and water treatment, potential food packaging material, pharmaceutical, and biomedical applications.
2. Hybrid Materials
The functionalization of organic materials through introduction of inorganic (metallic or metal oxide) nanoparticles is a strategy widely used to fabricate hybrid materials with enhanced properties, which has increased considerably in the last five years [3]. Hybrid materials, in particular, organic–inorganic materials, are commonly prepared using polysaccharides in combination with inorganic nanoparticles like TiO2 [8,28], where the most common method for their preparation is the evaporative casting-plate via mechanical stirring (Figure 1a) and chemical or physical surface deposition (Figure 1b) [29]. Furthermore, the hybrid materials are classified as class I (organic and inorganic presenting noncovalent interactions) or class II (organic and inorganic exhibiting covalent interactions) depending on the intra- and intermolecular interactions among the organic matrix and cross-linking agent [3].
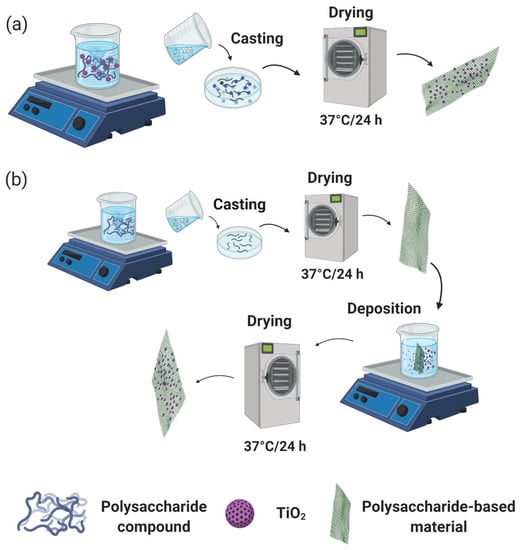
Figure 1.
Mechanical stirring (a) and chemical deposition (b) methods for polysaccharide-hybrid material preparation (adapted from Anaya-Esparza et al. [1], Miyazaki et al. [29], and Tang et al. [31]) (Figure created in Biorender.com).
In general, cross-linking agents (glutaraldehyde, polyethylene glycol, calcium carbonate, and TiO2) tend to limit interaction of the polymer chains with water molecules and provide structural integrity during exposure to a defined pressure and moisture conditions [3]. It could react with the available functional groups (–OH and –NH2) present in the organic matrix. Nonetheless, the type and concentration of the reinforcement agent determine the effects (positively or negatively) in the technological and functional properties of the organic compound [30]. TiO2 has the potential to enhance the properties of polysaccharide-based films such as starch (from potato, corn, and rice), sodium alginate, carboxymethyl cellulose, pullulan, agar, gums (k-Carrageenan, xanthan gum, and gellan), and chitosan, as is discussed below.
3. Polysaccharide–TiO2 Hybrid Materials
Polysaccharides are biopolymers composed of monosaccharides connected by glycosidic bonds [3]. They are obtained from different sources (plants, animals, algae, and microbial) and exhibit a wide range of applications because they are low cost, abundant, biodegradable, edible, and biocompatible with numerous organic and inorganic compounds [3]. However, most of their potential applications are limited by their poor physical properties (high solubility, low water barrier ability, low thermal stability, poor gas permeability, and mechanical resistance). Thus, their functionalization is necessary [32]. Moreover, nano-TiO2 can improve the physicochemical (Figure 2) and functional properties of polysaccharide-based materials like starch, sodium alginate, carboxymethyl cellulose, chitosan, pullulan, agar, k-Carrageenan, xanthan gum, and gellan gum-based materials [1,5,17,20,21,22,33]. Figure 3 summarizes the main applications of polysaccharide-based materials functionalized with TiO2 nanoparticles.
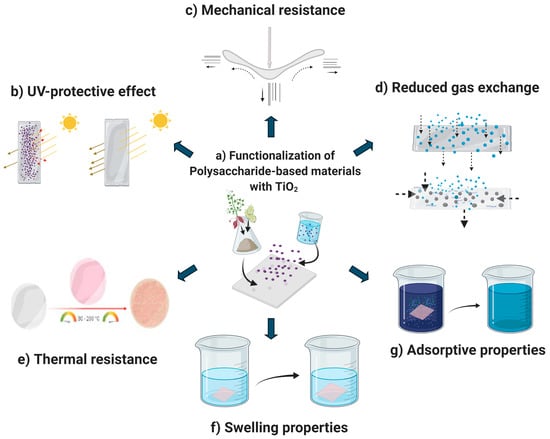
Figure 2.
Schematic representation of the functionalization of polysaccharide-based materials with TiO2 (a) and their effects on the UV-blocking (b), mechanical (c), gas exchange (d), thermal (e), swelling (f), and adsorptive (g) properties (adapted from Hou et al. [34], Goudarzi et al. [35], de Moura et al. [36], and Dai et al. [37]) (Figure created in Biorender.com).
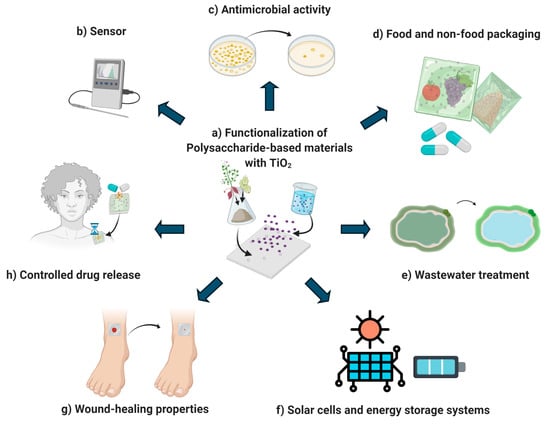
Figure 3.
Schematic representation of the functionalization of polysaccharide-based materials with TiO2 (a) and their main applications: sensor development (b), antimicrobial activity (c), food and non-food packaging (d), wastewater treatment (e), solar cells and energy storage systems (f), wound-healing (g), and controlled drug release (h) (adapted from de Moura et al. [36], Dai et al. [37], Ismail et al. [38], and Al-Morakam [39]) (Figure created in Biorender.com).
3.1. Starch–TiO2 Hybrid Material
Starch is one of the most versatile polysaccharides with relevant applications. However, their hydrophilic nature limits their uses [26]. Therefore, the addition of inorganic compounds like TiO2 into the starch matrix is a feasible strategy for development of functional starch-based materials with improved physicochemical properties for food and non-food packaging, photocatalyst, biomedical, and solar cell applications [3,40], as listed in Table 1.

Table 1.
Effect of TiO2 incorporation on starch matrix properties.
3.1.1. Food and Non-Food Packaging Applications of Starch–TiO2 Hybrid Material
Most of the investigations reported in the literature for the corn starch–TiO2 hybrid composite are focused on developing eco-friendly food and non-food packaging alternatives to existing conventional plastic packaging [26]. The reinforcement of starch film with TiO2 promotes an increase in mechanical and thermal properties with higher degradation temperature (344 °C) compared with starch-based film (230 °C) in a dose-dependent response [7], and it exhibited high-pressure resistance (8.39 N/mm2) compared with the starch film (3.63 N/mm2) [25]. Wang et al. [41] reported that the mechanical properties of the hybrid film could be influenced (negatively or positively) depending on the TiO2 content. Moreover, the increasing hydrogen bonds and electrostatic interactions (between the –OH groups of the starch and the Ti2+ atoms) in the hybrid film are responsible for the improved properties. Furthermore, the starch–TiO2 hybrid film exhibited low–moderate antibacterial activity against S. aureus, but not against E. coli [54].
Additionally, corn starch may be combined with polyvinyl alcohol (PVA) and reinforced through TiO2 addition to fabricate ternary-hybrid films (starch–PVA–TiO2) with enhanced functional and technological properties [58]. The addition of TiO2 in the starch/PVA matrix improved its thermal (increased) and water resistance (increased), and moisture and oxygen (reduced) permeability through hydrogen, Ti=C bonds, and attractive electrostatic forces, where nano-TiO2 may promote the formation of a compact and dense structure, acting as an obstacle to the diffusion of water and oxygen molecules through a hybrid film without altering the starch/PVA structure [58,59].
Kochkina and Butikova [42] mentioned that the incorporation of TiO2 into a starch/PVA film improves their physicochemical properties and extends their functionalities. The addition of TiO2 in small quantities (0.1% w/w) into a biodegradable corn starch/PVA film did not alter its structure. However, it promotes intermolecular interaction through hydrogen-bond formation, improving its thermal stability (from 190 to 230 °C) and water vapor permeability (decrease from 12.6 to 5.39 × 10−7 g/m h Pa) as well as improving its mechanical (tensile strength from 16.22 to 21.11 MPa) and UV-barrier properties. Similar findings were reported during evaluation of the mechanical (tensile strength increased) and physicochemical (swelling degree increased) properties in a corn starch/PVA film reinforced with TiO2 nanoparticles [43].
Liu et al. [44] informed that incorporation of TiO2 (0.6%) in a high-amylose starch/PVA-based membrane promoted a decrease in the optical transmittance with an increase in the mechanical (tensile strength to 9.53 MPa and elongation at break to 49.5%) properties of the film. The compatibility of high-amylose starch and TiO2 were due to the formation of hydrogen and C–O–Ti bonds. They argued that TiO2 could be adsorbed in the starch granule surface by the TiO2–amylose (changes in the intensity and profile peaks in the range of 900–1000 cm−1 of the FTIR spectra) interactions that improve the miscibility of the starch/PVA structure. Furthermore, the compatibility of the TiO2 with a starch matrix is OH availability-dependent; however, an excessive concentration of TiO2 (>1% w/w) in the polymeric matrix could negatively affect the physicochemical properties of the starch/PVA films. Furthermore, the hybrid membrane showed that moderate antimicrobial activity performed by an agar test diffusion assay against E. coli (inhibition zone of 12.26 mm) and S. aureus (inhibition zone of 10.22 mm), which was attributable to the ability of TiO2 to restrain the growth of bacteria due to its photocatalytic properties. Similar trends were reported by Lin et al. [58], who informed that a starch/PVA/TiO2 (0.03% w/w) hybrid composite exhibited bacteriostatic activity against E. coli and Listeria monocytogenes, but the effect was dependent on the strain and TiO2 content. Likewise, Ahmed et al. [56] found that the addition of TiO2 nanoparticles in a PVA–starch–g–C3–N4–Ag hydrogel membrane enhanced the antibacterial activity against E. coli (inhibition zone of 37.33 mm) and S. aureus (inhibition zone of 33.25 mm).
Additionally, starch obtained from nonconventional sources like wheat, rice, tapioca, cassava, potato, and sago has been functionalized with TiO2 nanoparticles to fabricate hybrid packaging materials. However, although nano-TiO2 can act as a physical cross-linking agent of starch-based film, the type of starch matrix will have a significant impact on the properties of hybrid films [60].
Furthermore, in a wheat starch-based film, TiO2 acts as a physical cross-linker agent improving its thermal stability (mass loss of 50% at 289 °C), UV-barrier (blocking >99% of UV-light), mechanical (an increase of tensile strength and Young’s modulus), and water-related properties with a decrease in water vapor permeability and an increase in the hydrophobicity of the hybrid material [27,45]. However, a high amount of TiO2 negatively affects the mechanical properties of the film, and the preparation method of the wheat starch–TiO2 hybrid material strongly influences their physicochemical properties and potential applications [46].
The functionalization of potato starch-based films adding TiO2 nanoparticles promotes an increase in its hydrophobicity, enhancing its water-related, mechanical, thermal, and UV-barrier properties. These facts were attributable to the stable formation of hydrogen bonds between oxygen in TiO2 and hydrogen in C–O–H of starch, which decreases the possible active sites for water molecule retention and thermal decomposition of the starch composite [19,47]. Another root vegetable starch source for making hybrid materials is the cassava [22]. Tunma [22] developed an active packaging with the cassava starch–TiO2 composite aimed to enlarge the shelf life of bananas and tomato fruits, finding that banana (14 days) and tomatoes (21 days) packaged in the hybrid films extended their shelf life beyond that of those packaged with petroleum-based films (5 and 10 days, respectively). Furthermore, the active material showed antibacterial activity against Bacillus cereus and E. coli through bacterial membrane alteration.
Malathi and Singh [4] informed that a rice starch-based film reinforced with TiO2 exhibited antimicrobial activity against E. coli (a reduction >90% of viable cells) under UV-light (4 h), but the effect was dose-dependent, attributed to the photocatalytic activity of TiO2 that may promote a membrane cell alteration, affecting cell viability and cell growth. Moreover, the presence of TiO2 in the organic matrix improved the water vapor permeability, solubility, tensile strength, and elongation. According to the authors, TiO2 can act as a physical cross-linking agent restringing segment rotation and molecular mobility between starch chains and water molecules through hydrogen bonding or O–Ti–O and C–O–Ti bonding, enhancing the physicochemical properties of the hybrid film.
Additionally, starch has been combined with other organic matrices (pectin, kefiran, cinnamon essential oil, and poly(ε-caprolactone)) and reinforced with TiO2. Dash et al. [48] developed a biodegradable starch–pectin–TiO2 film with enhanced UV-barrier properties for UV-sensible food and non-food compounds preservation (i.e., ascorbic acid). The addition of TiO2 promotes a decrease in the moisture content, solubility, and moisture uptake with an increase in the thermal properties of the hybrid film in a dose-pendent manner. On the other hand, they reported that the mechanical properties of the hybrid film were negatively affected by the presence of nano-TiO2 and mentioned that, at high concentrations, TiO2 acts as an anti-plasticizer agent reducing the water molecule movement into the polymeric matrix, affecting the flexibility of the film. Goudarzi and Shahabi-Ghahfarrokhi [49] developed a photo-producible starch/kefiran–TiO2 composite as a potential alternative for food packaging applications. They reported that enhanced physicochemical, water-related, and thermal properties of starch/kefiran by the presence of TiO2 could be improved by the UV-radiation (345 nm) during a defined time (1 h).
Arezoo et al. [50] informed that sago starch film combined with cinnamon essential oil and TiO2 showed antimicrobial activity against E. coli, Salmonella Typhimurium, and S. aureus. Also, their incorporation of sago starch-based film improved its mechanical, optical, and water-related properties. On the other hand, Fei et al. [32] evaluated the effects on the physicochemical properties and structure of a starch–poly(ε-caprolactone (PCL)) composite by nano-TiO2 incorporation and reported that mechanical properties and water resistance were improved by TiO2 addition compared with non-reinforced material, but these effects were dose-dependent with an optimum TiO2 concentration of 6 wt%. According to the authors, the formation of intramolecular hydrogen bonds and covalent interaction (C–O–Ti bond) between starch and nano-TiO2 promotes a decrease in the rigidity of the starch–PCL structure.
Furthermore, the functionality of starch–TiO2 hybrid films could be enhanced by the surface modification of TiO2 with the presence of other inorganic compounds in its network. Chueangchayaphan et al. [51] evaluated the influence of Al2O3 on the properties of the starch–TiO2–Al2O3 hybrid composite and found that physicochemical (water contact angle, hardness, and thermal stability) properties of the ternary hybrid film improved in a Al2O3-dependent concentration. Similarly, Hajizadeh et al. [52] informed that the incorporation of TiO2:Ag-doped nanoparticles into the starch matrix increased the water resistance of the hybrid film and significantly inhibited the growth of E. coli and S. aureus, associated with the antibacterial effect of TiO2.
According to the results, the incorporation of TiO2 into starch-based materials can improve the physicochemical, thermal, mechanical, optical, and water-related properties through covalent and noncovalent interactions with the potential to develop food and non-food packaging.
3.1.2. Other Applications of Starch–TiO2 Hybrid Material
Other potential applications of starch–TiO2, such as environmental remediation, biomedical, and dye-sensitized solar cells, have been investigated (Table 1). Yun et al. [53] prepared a starch/PVA film reinforced with TiO2 and found that tensile strength, degree of swelling, solubility, and water vapor absorption were enhanced up to 1.14–1.52 times compared with films without nano-TiO2, whereas the elongation at break was negatively affected (decreased 1.60 times). Moreover, the authors reported that the starch/PVA/TiO2 film exhibited photocatalytic activity (under UV and visible light) against bisphenol A (degree of decomposition of 0.825 and 0.534, respectively) and 2,4-dichlorophenoxyacetic acid (decomposition degree of 0.597 and 0.396, respectively) in aqueous solution (10 ppm) after 4 h of exposure. Furthermore, starch/PVA/TiO2 has a photocatalytic degradation efficiency of 37% against methylene blue dye (10 mg/L) after 90 min of UV-light irradiation [54]. Similarly, Yun et al. [55] developed a starch/PVA film reinforced with TiO2 and poly(methyl methacrylate-co-acrylamide) for photocatalytic purposes. They reported that hybrid films showed photocatalytic properties against methylene blue and acetaldehyde under UVA and visible light irradiation. The photocatalytic efficiency of starch–TiO2 hybrid materials is directly proportional to the TiO2 concentration [61]. However, it should consider that the enhanced functional properties of starch by the addition of TiO2 could be negatively affected by a long UVA exposure time because a rupture of polysaccharide structure may occur [35].
Additionally, Ahmed et al. [56] evaluated in vivo the wound-dressing properties of a PVA–starch–g–C3–N4–Ag–TiO2 hydrogel membrane in an open excision-type wound-healing study in adult female Albino mice. They found that hybrid membranes had better-wound healing (reduction wound area of 97%) efficiency than cotton gauze (reduction wound area of 19.5%) or untreated (reduction wound area of 7.4%) groups after seven days of evolution. Nonetheless, animals treated with the hybrid hydrogel membrane showed better re-epithelization with good anti-inflammatory response than control groups, which are parameters related to the wound-healing activity. Ujcic et al. [57] developed a biodegradable wheat starch–TiO2 composite with a controlled drug (vancomycin) release profile and bacteriostatic properties against S. aureus without TiO2 release from the hybrid matrix to the medium.
Khanmirzaei and Ramesh [24] fabricated a dye-sensitized solar cell using a nanocomposite polymer electrolyte formed with a rice starch–TiO2 hybrid material. They reported that, during solar cell development, the ionic conductivity of rice starch/ionic liquid composite was enhanced by incorporating TiO2 with an efficiency of 0.17 at 1000 W/m2 light intensity.
In summary, the starch–TiO2 hybrid material exhibited photocatalytic and antimicrobial activities and good ionic conductivity for potential environmental, biomedical, and dye-sensitized solar cell applications.
3.2. Sodium Alginate–TiO2 Hybrid Material
Sodium alginate (SA) is a degradable, nontoxic, functional, and compatible biopolymer with a wide range of applications [31,62]. Recently, several authors have combined SA with TiO2 nanoparticles aimed to enhance the technological and functional properties of alginate-based materials (Table 2).

Table 2.
Effect of TiO2 incorporation on sodium alginate matrix properties.
3.2.1. Environmental Applications of Sodium Alginate–TiO2 Hybrid Material
The use of SA–TiO2 hybrid material as a photocatalyst as an alternative to conventional catalysts has been explored [63] (Table 2). Thakur and Arobita [63], using a cross-linked SA–TiO2 hydrogel, reported a maximum adsorption capacity of methyl violet of 1156.1 mg g−1 with an adsorption efficiency of 99.6% in comparison with SA-based film (85%), attributed to the presence of TiO2 in the hybrid hydrogel, which acts as an anionic center that participates in the electrostatic attraction with methyl violet dye. Similarly, Reveendran and Ong [64] informed that an SA–TiO2 hybrid film was effective for the degradation of Congo red (5 mg L−1 at pH 8) under UV-radiation (6 h) without significant losses of catalytic activity after two cycles of reuse. In general, TiO2 favored the surface adsorption and photocatalytic degradation of dyes; however, a high dye concentration could inhibit the photocatalytic properties of TiO2 (surface saturation) because dye molecules tend to absorb energy (light and photons), thereby reducing the generation of reactive oxygen species and hydroxyl radicals.
Dai et al. [37] developed an SA–TiO2 hybrid aerosol as a novel oil/water separation and wastewater treatment. The hybrid aerogel showed oil/water separation efficiency of 99.7% after 60 cycles of reuse compared with sodium alginate aerogel. Furthermore, the hybrid aerogel exhibited excellent photocatalytic degradation after six repeated uses against methyl orange (>85% in 2.5 h) dye under simulated sunlight irradiation (150 min). Furthermore, Thomas et al. [65] synthesized a cross-linked SA/carboxymethyl cellulose (CMC) with nano-TiO2 and graphene oxide (GO) composite for Congo red (30 mg L−1) dye degradation under direct sunlight irradiation (240 min). They reported that SA–CMC–TiO2–GO (1.2 g L−1) showed higher dye degradation (98%) compared to SA–CMC–TiO2 (70%) or SA–CMC–GO (60%); nonetheless, the SA–CMC–TiO2–GO retained its degradation efficiency up to seven consecutive cycles. The enhanced photocatalytic properties of the hybrid material were attributed to the reduction of electro-hole pair recombination by the presence of TiO2 and GO in the polymeric matrix, generating a higher concentration of hydroxyl radical.
The addition of TiO2 into the polymeric matrix-like sodium alginate provides a major stabilization of TiO2, enhancing its photocatalytic and dye-removal properties.
3.2.2. Biomedical Applications of Sodium Alginate–TiO2 Hybrid Material
The potential biomedical applications of the SA–TiO2 hybrid material as a scaffold for tissue engineering, as a drug delivery system, and as a wound-healing material have been investigated [66]. Naik et al. [66] evaluated the application of TiO2–Hap–SA composite scaffolds as a bone implant material. In general, the hybrid material showed controlled swelling, acceptable degradation rate, excellent bio-mineralization, and biocompatibility with high cell viability in the human MG-63 cell line. Moreover, the hybrid material exhibited a controlled drug release profile of the methotrexate drug, which are suitable characteristics for biomedical applications. Furthermore, Selvi et al. [63] fabricated sodium SA–PVA–TiO2–curcumin patches as a wound-healing material. They found that the hybrid material reinforced with 100 µg mL−1 of TiO2 promoted antibacterial activity (by agar test diffusion assay) against Bacillus subtilis (inhibition of 11 mm) and Klebsiella pneumonia (inhibition of 8 mm), attributed to the ability of TiO2 to interact with the cell membrane, to increase its permeability, and to lead to cell death. Urruela-Barrios et al. [5] mentioned that an SA/gelatin hydrogel 3D printing reinforced with TiO2 and β-tricalcium phosphate exhibited a potential use for tissue engineering application. The fabricated hybrid material by the micro-extrusion process exhibits adequate porosity and mechanical resistance. However, the authors mentioned that further studies are needed to prove the material’s safety and biocompatibility. According to the evidence, incorporation of TiO2 into sodium alginate is a viable strategy to enhance its biological properties.
3.2.3. Food and Non-Food Packaging Applications of Sodium Alginate–TiO2 Hybrid Material
Table 2 lists reports on the use of the sodium alginate–TiO2 hybrid film for food and non-food applications. Tang et al. [31] developed a degradable SA film reinforced with TiO2:Au for food packaging applications with advanced UV- and water-barrier properties and antimicrobial capacity. The incorporation of TiO2:Au nanoparticles into the SA matrix improved its water resistance, mainly attributed to the increase in the surface hydrophobicity of the hybrid film. Furthermore, it showed higher antibacterial activity against S. aureus and E. coli (95 and 90%, respectively) in UV-light presence compared with the SA–TiO2 film (90 and 80%, respectively), which was associated with an enhanced TiO2:Au electron-hole recombination, improving the photocatalytic ability of the SA–TiO2:Au hybrid film. Additionally, the zein/sodium alginate (90:10) film functionalized with TiO2 (0.5%) and betanin (1%) showed interesting antioxidant properties 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH, radical scavenging of 64%) without toxicity effects on endothelial cells and high antimicrobial activity (by agar test diffusion assay) against E. coli (inhibition zone of 15.4 mm) and S. aureus (inhibition zone of 16.9 mm), which exhibited potential to be used for the preservation of fresh foods [68].
In general, the SA–TiO2 hybrid material can overcome the disadvantages of conventional nano-photocatalysts, which are hard to recycle. Furthermore, the obtained hybrid composite could be a low-cost and eco-friendly alternative for the removal and degradation of dyes from aqueous solutions.
3.2.4. Other Applications of Sodium Alginate–TiO2 Hybrid Material
Other investigated applications of the sodium alginate–TiO2 hybrid composite include the separation process, dye-sensitized solar cell, and molecular electronics [30,69,70]. Premakshi et al. [30] used a cross-linked SA-based membrane with TiO2 for dehydration of isopropanol by pervaporation. They reported that the hybrid membrane showed improved separation ability for the water–isopropanol system and suggested that the membrane exhibited high selectivity toward water molecules even with higher water concentrations in the feed (flux of 25 × 102 kg/m2 h), associated with the ability of TiO2 to form hydrogen-bonding interactions with the sodium alginate and water molecules. Furthermore, the SA–TiO2 membrane showed enhanced mechanical and thermal properties in a dose-dependent response with an optimum concentration of TiO2 of 10% w/w.
Uddin et al. [69] developed an SA–TiO2 film for dye-sensitized solar cell applications to increase the ionic conductivity and to reduce the fragility of electrodes. They reported that the highest conductivity was achieved with 8% of TiO2 (0.0472 S/m) at room temperature, which increased by 38% with an increase of the temperature (25 to 64 °C) due to nano-TiO2 creating overlapping paths in the hybrid network which allowed the charge carriers to pass through the less resistant routes. However, at high TiO2 concentration (>8%), electrodes were fragile and unstable. According to Peining et al. [16], the conductivity efficiency of the hybrid electrodes was improved because TiO2 may act as a scattering center, reflecting the photons into the electrode. Furthermore, the hybrid composite showed improved mechanical and optical properties compared to a pure sodium alginate film in a dose-dependent manner, which was associated with the hydrogen-bond formation between both components and the increase in the π-conjugated system. Likewise, Padma et al. [70] prepared an SA-based membrane reinforced with TiO2 nanoparticles for molecular electronics applications and found that the membrane-forming solution showed improved dielectric properties and ac-electrical conductivity with better thermal stability (250 °C) than the sodium alginate membrane (180 °C).
According to these data, the SA–TiO2 hybrid material showed potential separation processes, sensitized solar cells, and molecular electronics applications.
3.3. Cellulose–TiO2 Hybrid Material
Cellulose is an organic compound that can form intra- and intermolecular interactions with inorganic compounds [71,72]. For this reason, it is an excellent candidate for supporting nano-TiO2 (Table 3).

Table 3.
Effect of TiO2 incorporation on cellulose-based materials properties.
3.3.1. Food and Non-Food Applications of Cellulose–TiO2 Hybrid Material
Table 3 lists the work on cellulose-based materials functionalized with TiO2 for food and non-food packaging development with beneficial properties. The sol-gel method is a viable strategy to developed cellulose–TiO2 hybrid materials [11]. In general, the incorporation of TiO2 into the cellulose matrix improved mechanical, thermal, UV-light blocking, and water-related properties in a dose-dependent manner, attributed to the strong interactions between TiO2 and –OH functional groups of carboxymethyl cellulose (CMM) structure. However, a high amount of TiO2 and an inhomogeneous dispersion through films negatively affects its physicochemical (thermal, mechanical, and water-barrier) properties. Furthermore, the CMC–TiO2 showed antimicrobial activity against E. coli and S. aureus, associated with the reactive oxygen species (ROS) generation ability of TiO2 with cell growth inhibition properties [21,36,79].
El-Wakil et al. [73] informed that the wheat gluten–nanocellulose–TiO2 hybrid film could be a viable alternative for the development of food active packaging materials. The hybrid film showed antimicrobial activity (reduction of viable cells of 98.5%) against Saccharomyces cerevisiae, E. coli, and S. aureus through oxidation of bacteria cell membranes. Fathi-Achacholouei and Zahedi [74] reported that a carboxymethyl cellulose–sodium montmorillonite (5%)–TiO2 (1%) hybrid composite showed enhanced UV-light blocking (removing more than 98% of visible light), mechanical (an increase of tensile strength and elongation at break), thermal (glass transition increased from 72 to 80.3 °C), and water-related (moisture uptake reduction in 40%) properties compared with a carboxymethyl cellulose-based film.
Alizadeh-Sani et al. [75] evaluated the efficacy of a whey protein isolate–cellulose nanofiber functionalized with TiO2 (1% w/w) and rosemary essential oil (REO, 2% w/w) in the preserving quality (microbial deterioration and sensory attributes) parameters of refrigerated meat during storage (4 °C). They reported that the lamb meat treated with the hybrid film showed acceptable microbial quality (total viable count of 4.1 log colony-forming unit (CFU) g−1) after six days of storage without changes in color, odor, texture, and overall acceptability. Moreover, the treated meat showed reduced lipid oxidation during storage, associated with the presence of antioxidant compounds (80% of radical scavenging by DPPH assay) into the film [76]. Additionally, the TiO2 and REO incorporation in a whey protein isolate/cellulose nanofiber film improve mechanical and water-related properties with a decrease in its transparency. Also, the hybrid film showed remarkable antimicrobial activity against pathogenic bacteria (L. monocytogenes, E. coli O157:H7, S. enteritidis, and P. fluorescens) in a dose-dependent response, attributed to the presence of TiO2 and bioactive compounds (polyphenol) in the REO, altering the cell membrane and finally cell death [74]. Furthermore, the authors informed that a low content of TiO2 migrated from the polymeric matrix to the meat product but was under Food Drug Administration (FDA) limit recommendations [76].
Yu et al. [78] used a cellulose nanofibril–TiO2 hybrid composite as a reinforcement agent for the development of PVA-based packaging. The mechanical and UV-barrier properties improved in the presence of the cellulose–TiO2 hybrid composite. Furthermore, the PVA–cellulose–TiO2 composite did not show toxicity against cancerous (Caco-2 cell) and normal colon cells in a concentration of one mg mL-1, whereas the cells showed epithelial integrity and viability (membranes, mitochondria, and nuclei were normal and without the presence of TiO2 nanoparticles within the cells), associated with the strong interaction between cellulose and TiO2 avoiding the nanoparticle release from the hybrid material to the medium. Furthermore, the hybrid material did not affect the typical intestinal bacteria (E. coli, Lactobacillus acidophilus, and Bifidobacterium animalis Bif-g cells). On the other hand, the addition of the carboxymethyl cellulose–TiO2 hybrid composite into the PVA system could negatively affect the mechanical and barrier properties as a result of a saturation of the polymeric matrix [90].
To summarize, research-based reports support that incorporation of TiO2 into the cellulosic matrix improved the technological (mechanical, thermal, UV-protective, and water-related) properties of cellulose-based materials with potential food and non-food packaging applications.
3.3.2. Environment Applications of Cellulose–TiO2 Hybrid Material
Usage of cellulose as a supporting material of TiO2 for removal and degradation of diverse organic and inorganic pollutants from aqueous media have been explored [70]. Miao et al. [74] fabricated a cellulose–TiO2 mesoporous hybrid material capable of reducing Ag(I) into Ag and Au(III) to Au through photocatalytic reactions. Uddin et al. [81] prepared photoactive fibers combining cellulose and TiO2 nanoparticles for photocatalytic purposes. They found that hybrid materials showed adsorptive and photocatalytic activities against methylene blue (0.05% w/v) and heptane-extracted bitumen fraction (0.2 w/v% of a mixture of heavy aromatic hydrocarbons) under simulated sunlight with catalytic properties even after 20 reaction cycles and without cellulose-support degradation.
Zeng et al. [82] reported that TiO2 immobilization in a cellulose matrix is a viable alternative for removal and photocatalytic degradation of water-dye pullulans, which is low-cost and easy to be applied. They found that a macroporous hybrid structure formed among cellulose and TiO2 (through electrostatic and hydrogen-bonding interactions) showed efficient adsorptive and photocatalytic properties against phenol dye. Furthermore, immobilized TiO2 nanoparticles on cellulose supports have been used for Rhodamine B degradation under UV-radiation (120 min) through the de-ethylation process promoted by •OH radicals adsorbed onto the surface of hybrid material and associated with the hydrophobicity/hydrophilicity of TiO2-coated samples, which is pH-dependent (decreased while increased pH from 1.5 to 6) [83].
Additionally, ternary cellulose-based hybrid materials have been developed to improve the photocatalytic properties of cellulose–TiO2. Jo et al. [84] fabricated a cellulose/carrageenan–TiO2 hybrid hydrogel with enhanced adsorptive and photocatalytic (removal of 115.3 mg g−1 with 85% of degradation) properties against methylene blue (60 mg L−1) compared with cellulose–TiO2 (removal of 0.8 mg g−1 with 33% of degradation) and cellulose (without effect) materials under UV-radiation (254 nm) after 3 h of exposure in a carrageenan dose-dependent and its adsorptive properties.
Wang et al. [85] investigated the photocatalytic and antimicrobial activity of a cellulose fiber-based paper filled with TiO2:Ag (40% w/w) nanoparticles. They reported that the cellulose–TiO2:Ag hybrid film exhibited stable degradation rates (0.95) against methylene orange (0.02 g L−1) in an aqueous solution under UV-radiation (254 nm) after four hours of exposure in comparison with a cellulose–TiO2 (0.90) film with good efficiency after three photocatalytic cycles (degradation rate of 0.95 and 0.85, respectively), but the effects were in a TiO2:Ag nanoparticles dose-dependent response. Furthermore, the hybrid material showed antibacterial activity against E. coli, where photocatalytic and antimicrobial activities were attributable to silver ion release.
Mohamed et al. [71] informed that a cellulose–TiO2:N-doped hybrid film showed remarkable photocatalytic activity under UVA (30 W at 312 nm) and visible (30 W at >420 nm) light for methylene blue (40 mg L−1) degradation (96 and 78%, respectively) after 360 min of exposure. They mentioned that the presence of nitrogen atoms into the TiO2 network through hydrogen-bonding interactions (Ti–O–C) enhances the absorption energy of the hybrid film, promoting high catalytic activity.
In general, immobilization of TiO2 onto a cellulose matrix is a feasible way to improve the adsorptive, photocatalytic, and antimicrobial properties of cellulose-based materials, which are suitable for removal of pollutants from aqueous media.
3.3.3. Other Applications of Cellulose–TiO2 Hybrid Material
Other investigated applications of the cellulose–TiO2 hybrid composite include textile (UV-protective and self-cleaning properties) and ammonia gas sensors. The introduction of TiO2 nanoparticles in a cellulose matrix improved the UV-protective properties, associated with the optical and light scattering capability of TiO2 [86]. Kale et al. [87] developed cotton fabric self-cleaning by coating cellulose–TiO2 on its surface with stable properties after ten washing cycles in a dose-dependent manner. On the other hand, the self-cleaning properties of a cellulose–TiO2 coating could be negatively affected by the surface modification of TiO2 with SiO2 (cellulose–TiO2:SiO2) [88].
Pang et al. [89] developed a cellulose/TiO2–polyaniline (PANI) hybrid composite through P–N heterojunctions to improve ammonia-sending properties in a homemade test system at room temperature and reported that cellulose–TiO2–PANI showed higher gas sensitivity performance than the cellulose–PANI sensor, associated with the P–N heterojunction at the interface of PANI and TiO2 nanoparticles.
According to the evidence, the cellulose–TiO2 hybrid material could be used for the development of fabric cotton and biosensors with advanced properties.
3.4. Chitosan–TiO2 Hybrid Material
Chitosan is a deacetylated form of chitin with a poly-cationic character and is nontoxic, biodegradable, and biocompatible with organic and inorganic compounds [91]. Recently, a detailed review of the chitosan–TiO2 hybrid composite has been published [1]. It is a versatile hybrid material with enhanced physicochemical, mechanical, and barrier properties with diversified applications that include antimicrobial, environmental, biomedical, and food and non-food packaging applications [1]. Nonetheless, further studies on the chitosan–TiO2 applications have emerged (Table 4).

Table 4.
Effect of TiO2 incorporation on chitosan-based materials properties.
3.4.1. Environmental Applications of Chitosan–TiO2 Hybrid Material
The Chitosan–TiO2 hybrid composite has been used for the removal and degradation of diverse organic compounds from aqueous media under UV-light irradiation. Mahmoud et al. [92] prepared a chitosan–acrylic acid (CS–AA) hydrogel reinforced with nano-TiO2 with enhanced swelling and adsorptive properties for methylene blue dye removal from aqueous solution (20 mg L−1). They informed of a remarkable change in the adsorption rate of MB (90%) using the CS–AA–TiO2 hybrid hydrogel (0.20 g) compared with CS-AA (60%) in pH-dependent response with an optimum pH value of 10, mainly associated with the porosity and large surface area of the hybrid hydrogel. El-Ella et al. [93] investigated the carcinogenic ethidium bromide (EtBr) efficiency degradation (in aqueous media at pH 12 and aeration condition) using a chitosan–polyvinylidene–TiO2:Au hybrid composite under sunlight conditions (400–600 W/m2). They found that the hybrid film removes 70 to 90% of the EtBr dye, where 60% of it was photodegraded in the first 60 min. According to the authors, the pH of the medium and aeration promotes •OH formation, facilitating dye adsorption and photodegradation. Additionally, Ikhlef-Taguelmimt et al. [94] immobilized TiO2 nanoparticles into chitosan support for tetracycline (TC) degradation under UV-irradiation (360 nm at 30 W). The efficiency removal (87%) was dependent on agitation speed in the batch system and TiO2 concentration; moreover, the efficiency was TC concentration-dependent with a decrease when increased from 30 to 40 mg L−1 after 60 min of reaction, associated with the saturation of active sites by agglomeration on the catalyst surface, decreasing light absorption capacity, and leading photocatalytic properties.
Xu et al. [95] functionalized a ceramic disk filter (CDF) with the chitosan–TiO2 hybrid composite for bacterial removal from drinking water. They reported that CDF–chitosan–TiO2 showed enhanced E. coli removal (99%) from contaminated water (1 × 106 CFU mL−1) compared with CDF (93%), mainly attributed to the direct interaction of bacteria (negative charge) with the chitosan–TiO2 (positive charge) composite and the oxidative stress in cell membrane from ROS generated by TiO2. Moreover, they explain that the ROS generation of TiO2 is enhanced by the presence of chitosan due to the prevention of radical recombination, inhibiting oxygen reduction and water oxidation. Furthermore, Marey [96] reported that the chitosan–TiO2 composite is a viable strategy for removing turbidity (total solid soluble (TSS)) from wastewater because the hybrid composite showed adsorption (efficiency TSS removal of 18% nephelometric turbidity unit (NTU)) and photocatalytic (under visible light) properties in pH-dependent response, associated with the poly-cationic properties of chitosan.
In summary, chitosan-based materials functionalized with TiO2 nanoparticles could be a viable, low-cost, and efficient alternative for water treatment.
3.4.2. Food and Non-Food Applications of Chitosan–TiO2 Hybrid Material
In general, the physicochemical properties of chitosan have been improved by the incorporation of TiO2 nanoparticles. Hussein et al. [97] reported that chitosan–TiO2 composites via the chemical route (291 °C) showed enhanced thermal stability compared to the physically prepared (273 °C) and chitosan-based (240 °C) material. Nugraheni et al. [98] informed that the PVA–chitosan–TiO2 hybrid membrane showed enhanced swelling properties in alkaline conditions (pH 10) compared with the acidic conditions (pH 4), associated with the protonation of an amine group from chitosan in acidic conditions. On the other hand, the physical interaction of TiO2:Ag with chitosan influences the properties of the hybrid film, and high amounts of TiO2:Ag nanoparticles could affect the plasticizing or elasticity of chitosan-based films due to the agglomeration of particles into the polymeric matrix [99]. Additionally, the chitosan–TiO2 composite has been used for food preservation. Hosseinzadeh et al. [100] evaluated the chitosan-based film reinforced with TiO2 (1% w/v) and Cymbopogon citratus essential oil (1.5% w/v) for preserving quality (microbial, physicochemical, and sensory) parameters of minced meat at cold storage (4 °C). They reported that minced meat treated with the hybrid film showed acceptable microbial quality (total viable count of <7 log CFU g−1) after 10 days of storage without significant changes in pH values (5.94 to 6.83) and organoleptic (color, odor, and taste) properties.
Evidence indicates that chitosan functionalized with TiO2 showed enhanced physicochemical, antimicrobial, and barrier-protective properties for the development of diverse packaging materials.
3.4.3. Biomedical and Cosmetic Applications of Chitosan–TiO2 Hybrid Material
The potential biomedical and cosmetic applications of the chitosan–TiO2 hybrid composite have been explored. Hanafy et al. [101] informed that the hybrid composite formed by chitosan and TiO2 exhibited inhibition growth against Bacillus cereus (85%), S. aureus (79%), Candida albicans (46%), Aspergillus niger (81%), and E. coli (60%). Furthermore, in an open excision-type wound-healing study in adult female rats, the hybrid film recovered faster (98% of closure after 14 days of surgery) than chitosan-based films (86% of closure after 14 days of surgery) and promoted cell growth and higher re-epithelization processes without scar formation. On the other hand, Cheng et al. [102] coated chitosan–TiO2–Ag nano-powder on a bendable double mattress for antibacterial purposes. According to the authors, it showed an antibacterial effect against S. aureus (inhibition of 99%), which can reduce the incidence of bedsores in patients and can reduce the frequency of mattress disinfection, decreasing cleaning costs.
Petrick et al. [103] fabricated a chitosan–TiO2 composite as an active ingredient for the development of a multifunctional sunscreen composed of water and carboxymethyl cellulose as an emulsifier. They reported that hybrid cream (with 10% of TiO2) showed a moderate UV-protection effect (solar protection factor of 21.4); moreover, it exhibited excellent antimicrobial disinfection against E. coli (99.7%) within two hours, mainly by the photocatalytic properties of TiO2 in visible light. Moreover, Kolsuz-Ozcetin and Surmelioglu [101] informed that an experimental 6% hydrogen peroxide hydrogel combined with the chitosan–TiO2 composite provided effective bleaching without adverse effects on the tooth surface, which can be potentially employed for preventing dental-related problems. They argue that TiO2 accelerates the bleaching reaction under UV-light at 385 nm.
In summary, the chitosan–TiO2 hybrid composite exhibited enhanced antimicrobial, wound-healing, and UV-protective properties for biomedical and cosmetic applications.
3.5. Other Polysaccharides Functionalized with TiO2
Additionally, other nonconventional polysaccharides such as gellan gum, agar, gelatin, and pullulan among others have been functionalized with TiO2 to enhance their technological and functional properties (Table 5).

Table 5.
Effect of TiO2 incorporation on nonconventional polysaccharide-based materials properties.
3.5.1. Food and Non-Food Applications of Nonconventional Polysaccharides Functionalized with TiO2
Table 5 lists reports on the use of the chitosan–TiO2 hybrid material for developing food and non-food packaging applications. Li et al. [105] developed hybrid agar–TiO2 fibers through the wet spinning process with enhanced mechanical, and water- and UV-barrier properties, which were associated with the optical and hydrophilic properties of TiO2 nanoparticles. Moreover, the mechanical, UV-barrier, and water-related properties in an agar–carrageenan film were enhanced by the addition of 1% w/v of nano-TiO2 [34]. Similar trends were reported when TiO2 was added in a film composed of a mixture of K-Carrageenan, xanthan gum, and gellan gum (mechanical, thermal, and water- and UV-barrier properties improved); moreover, it exhibited partial inhibition of S. aureus [17].
Vejdan et al. [106] studied the effect of TiO2 incorporation in a fish gelatin/agar bilayer film. They found that the addition of TiO2 at low levels (<0.5 g/100 mL) positively influenced the water-related, mechanical, and UV-barrier properties of the hybrid film. However, higher concentrations of TiO2 (>0.5/100 mL) lead to a reduction of mechanical properties due to an inhomogeneous dispersion and agglomeration of inorganic particles in the polymeric matrix. Furthermore, the gelatin/agar–TiO2 could be a viable alternative for preventing fish oil oxidation, mainly by its low light transmission through the hybrid film capability [107].
Abdel-Baky et al. [108] reported that edible guar gum-based films incorporated with nano-TiO2 can maintain quality (soluble solids content, color, acidity, total phenol, and flavonoid compounds) attributes of some dates (Medjool and Barthy) during cold storage (for 8 weeks at 0 °C with 75% relative humidity) without microbial pollution (psychrophilic bacteria, mold, and yeast) growth. According to the authors, the hybrid film generates a low oxygen/high carbon dioxide microclimate, providing a physical barrier that decreases metabolic processes and prevents dehydration and microbial deterioration of the fruits.
Nasiri et al. [109] informed that bean pod shell gum combined with Mentha pulegium essential oil (4% w/w) and TiO2 (2% w/w) showed antimicrobial activity against S. aureus, B. cereus, E. coli, S. typhoid, and P. aureginosa. Gram-positive bacteria were more susceptible than gram-negative bacteria, which was related to the type of bacteria (cell physiology–morphology) and attributed by the variation on their cell wall.
Jin et al. [110] prepared a hybrid film composed of jackfruit filum polysaccharides and nano-TiO2 (JFPT) using the solvent casting method for food and non-food packaging purposes. The addition of TiO2 decreased the transparency, moisture uptake, and soluble matter of the hybrid film, and the mechanical and thermal properties were enhanced, which were ascribed to the formation of strong inter- and intramolecular interactions between the biopolymer and TiO2 nanoparticles. Furthermore, the JFPT composite showed higher antimicrobial activity against E. coli (79%) than S. aureus (60%), which was associated with the inherent differences of each bacteria cell wall structure and the ability of TiO2 to generate ROS to inactivate bacteria by causing cell lysis.
Salarbashi et al. [111] functionalized a soluble soybean polysaccharide with TiO2 and found that swelling degree, water vapor permeability, and thermal and mechanical properties considerably improve in a dose-dependent response. Furthermore, the hybrid film showed antimicrobial activity against Staphylococcus epidermis (4 mg mL−1) and Penicillium expansum (2.5 mg mL−1), attributed to the catalytic properties of TiO2, affecting the viability of the cells.
Liu et al. [20] reported that mechanical, and water- and UV-barrier characteristics of pullulan-based films were improved by the presence of TiO2 in a dose-dependent manner, associated with the formation of intermolecular hydrogen bonds during hybrid film preparation. On the other hand, the hybrid composite formed by pectin and TiO2:Cu-doped nanoparticles showed methyl orange dye photodegradation from aqueous media [23].
The incorporation of TiO2 into nonconventional polysaccharide-based materials significantly improved their physicochemical, mechanical, thermal, water-resistance, and UV-barrier properties, which are suitable characteristics for the development of food and non-food packaging materials.
3.5.2. Biomedical Applications of Nonconventional Polysaccharides Functionalized with TiO2
The potential biomedical applications of nonconventional polysaccharide-based materials functionalized with TiO2 have been investigated (Table 5). Razali et al. [33] informed that the addition of TiO2 in gellan gum films improved their thermal stability and antimicrobial activity (by agar test diffusion assay) against S. aureus (inhibition of 10 mm), Streptococcus sp. (inhibition of 12 mm), E. coli (inhibition of 11 mm), and Pseudomonas aeruginosa (inhibition of 10 mm) without significant changes in transparency (transmittance of 94%) compared with the gellan gum-based film (without antimicrobial activity and transmittance of 100%). The improved physicochemical properties were attributed to the hydrogen-bond formation between the biopolymer and nano-TiO2, while the antibacterial activity of the hybrid film was attributed to the presence of TiO2 and its ability to ROS generation, promoting a malfunction of bacteria membrane, leading to cell death. Furthermore, the biocompatibility of the gellan gum–TiO2 hybrid film has been tested in a mouse fibroblast cell line (3T3), indicating no cytotoxic effects. Moreover, in an open-excision-type wound-healing study in adult Sprague Dawley rat, the hybrid film promoted an accelerated re-epithelialization (more than 50% on day 3 of wound operation) without inflammatory phenomenon after 14 days of evaluation in comparison with a gellan gum-based film. This phenomenon could be related to the presence of nano-TiO2 in the polymeric matrix, which was able to promote cell growth and cell migration to accelerate open-excision wound healing, mainly by TiO2, which may affect protein interaction and subsequent cell adhesion and proliferation; nonetheless, the acceleration of the wound-healing process may be related to the antimicrobial properties of the hybrid film [38,112].
According to these data, nonconventional polysaccharides-based materials functionalized with TiO2 showed interesting properties for biomedical applications. However, further studies are required to validate their safety and efficacy use.
4. Conclusions
Evidence indicates that the use of TiO2 as a reinforcement agent in polysaccharide-based materials is a viable strategy that significantly enhanced their mechanical, thermal, and UV-barrier properties and water resistance. Biopolymer–TiO2 hybrid composite is an active research area for environmental remediation and biomedical applications. Moreover, it is a low-cost and eco-friendly alternative for the development of packaging materials for food and non-food purposes based on its antimicrobial and photocatalytic properties. However, it is necessary to evaluate the possible structural changes promoted by the storage time and work temperature on the physicochemical properties of polysaccharide–TiO2 hybrid composites to guarantee their stability and safe use during a determined time.
Author Contributions
Conceptualization, L.M.A.-E., Z.V.-d.l.M., A.P.-L. and E.M.-G.; methodology, L.M.A.-E., Z.V.-d.l.M., J.M.R.-G., R.R.-T., T.S.-C., S.A.-A., A.P.-L. and E.M.-G.; investigation, L.M.A.-E., Z.V.-d.l.M., J.M.R.-G., R.R.-T., T.S.-C., S.A.-A., A.P.-L. and E.M.-G.; writing—original draft preparation, L.M.A.-E., Z.V.-d.l.M., J.M.R.-G., R.R.-T., T.S.-C., S.A.-A., A.P.-L. and E.M.-G.; writing—review and editing, L.M.A.-E., Z.V.-d.l.M., A.P.-L. and E.M.-G.; supervision, L.M.A.-E., Z.V.-d.l.M., A.P.-L. and E.M.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors gratefully acknowledge the financial support from a scholarship (702634) from CONACYT-Mexico as well as Acoyani Garrido-Sandoval for his work on proofreading and edition.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Anaya-Esparza, L.M.; Ruvalcaba-Gómez, J.M.; Maytorena-Verdugo, C.I.; González-Silva, N.; Romero-Toledo, R.; Aguilera-Aguirre, S.; Pérez-Larios, A.; Montalvo-González, E. Chitosan-TiO2: A versatile hybrid composite. Materials 2020, 13. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Wu, Y.; Reddy, M.V.; Sreekumaran Nair, A.; Chowdari, B.V.R.; Ramakrishna, S. Long term cycling studies of electrospun TiO2 nanostructures and their composites with MWCNTs for rechargeable Lithium-ion batteries. RSC Adv. 2012, 2, 531–537. [Google Scholar] [CrossRef]
- Vilela, C.; Pinto, R.; Pinto, S.; Marques, P.; Silvestre, A.; Freire, C. Polysaccharide Based Hybrid. Materials Metals and Metal. Oxides, Graphene and Carbon Nanotubes; Springer: Berlin, Germany, 2014; Volume 53, ISBN 9783030003463. [Google Scholar]
- Malathi, A.N.; Singh, A.K. Antimicrobial activity of rice starch based film reinforced with titanium dioxide (TiO2) nanoparticles. Agric. Res. J. 2019, 56, 111. [Google Scholar] [CrossRef]
- Urruela-Barrios, R.; Ramírez-Cedillo, E.; de León, A.D.; Alvarez, A.J.; Ortega-Lara, W. Alginate/gelatin hydrogels reinforced with TiO2 and β-TCP fabricated by microextrusion-based printing for tissue regeneration. Polymers 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Teymourpour, S.; Abdorreza, M.N.; Nahidi, F. Functional, thermal, and antimicrobial properties of soluble soybean polysaccharide biocomposites reinforced by nano TiO2. Carbohydr. Polym. 2015, 134, 726–731. [Google Scholar] [CrossRef]
- Hejri, Z.; Seifkordi, A.A.; Ahmadpour, A.; Zebarjad, S.M.; Maskooki, A. Biodegradable starch/poly (vinyl alcohol) film reinforced with titanium dioxide nanoparticles. Int. J. Miner. Metall. Mater. 2013, 20, 1001–1011. [Google Scholar] [CrossRef]
- Suri, G.; Chhabra, P.; Gupta, R.; Saxena, S.; Tyagi, M.; Seshadri, G.; Verma, G.L.; Khandal, R.K. Challenges in preparation of metal-containing nanocomposites; Dispersion of titanium into plastics. E-Polymers 2010. [Google Scholar] [CrossRef][Green Version]
- Reddy, M.V.; Adams, S.; Liang, G.T.J.; Mingze, I.F.; Van Tu An, H.; Chowdari, B.V.R. Low temperature molten salt synthesis of anatase TiO2 and its electrochemical properties. Solid State Ionics 2014, 262, 120–123. [Google Scholar] [CrossRef]
- Zhou, J.J.; Wang, S.Y.; Gunasekaran, S. Preparation and characterization of whey protein film incorporated with TiO2 nanoparticles. J. Food Sci. 2009, 74. [Google Scholar] [CrossRef]
- Bardet, R.; Belgacem, M.N.; Bras, J. Different strategies for obtaining high opacity films of MFC with TiO2 pigments. Cellulose 2013, 20, 3025–3037. [Google Scholar] [CrossRef]
- Jesline, A.; John, N.P.; Narayanan, P.M.; Vani, C.; Murugan, S. Antimicrobial activity of zinc and titanium dioxide nanoparticles against biofilm-producing methicillin-resistant Staphylococcus aureus. Appl. Nanosci. 2015, 5, 157–162. [Google Scholar] [CrossRef]
- Popov, A.P.; Priezzhev, A.V.; Lademann, J.; Myllylä, R. TiO2 nanoparticles as an effective UV-B radiation skin-protective compound in sunscreens. J. Phys. D Appl. Phys. 2005, 38, 2564–2570. [Google Scholar] [CrossRef]
- Reddy, M.V.; José, R.; Teng, T.H.; Chowdari, B.V.R.; Ramakrishna, S. Preparation and electrochemical studies of electrospun TiO2 nanofibers and molten salt method nanoparticles. Electrochem. Acta 2010, 55, 3109–3117. [Google Scholar] [CrossRef]
- Reddy, M.V.; Valerie Teoh, X.W.; Nguyen, T.B.; Michelle Lim, Y.Y.; Chowdari, B.V.R. Effect of 0.5 M NaNO3: 0.5 M KNO3 and 0.88 M LiNO3: 0.12 M LiCl molten salts, and heat treatment on electrochemical properties of TiO2. J. Electrochem. Soc. 2012, 159, A762–A769. [Google Scholar] [CrossRef]
- Peining, Z.; Yongzhi, W.; Reddy, M.V.; Sreekumaran Nair, A.; Shengjie, P.; Sharma, N.; Peterson, V.K.; Chowdari, B.V.R.; Ramakrishna, S. TiO2 nanoparticles synthesized by the molten salt method as a dual functional material for dye-sensitized solar cells. RSC Adv. 2012, 2, 5123–5126. [Google Scholar] [CrossRef]
- Balasubramanian, R.; Kim, S.S.; Lee, J.; Lee, J. Effect of TiO2 on highly elastic, stretchable UV protective nanocomposite films formed by using a combination of k-Carrageenan, xanthan gum and gellan gum. Int. J. Biol. Macromol. 2019, 123, 1020–1027. [Google Scholar] [CrossRef]
- Afzal, S.; Samsudin, E.M.; Mun, L.K.; Julkapli, N.M.; Hamid, S.B.A. Room temperature synthesis of TiO2 supported chitosan photocatalyst: Study on physicochemical and adsorption photo-decolorization properties. Mater. Res. Bull. 2017, 86, 24–29. [Google Scholar] [CrossRef]
- Xiong, J.; Sheng, C.; Wang, Q.; Guo, W. Toughened and water-resistant starch/TiO2 bio-nanocomposites as an environment-friendly food packaging material. Mater. Res. Express 2019, 6. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Han, K.; Cai, Y.; Ma, M.; Tong, Q.; Sheng, L. Effect of nano-TiO2 on the physical, mechanical and optical properties of pullulan film. Carbohydr. Polym. 2019, 218, 95–102. [Google Scholar] [CrossRef]
- Ahmadi, R.; Tanomand, A.; Kazeminava, F.; Kamounah, F.S.; Ayaseh, A.; Ganbarov, K.; Yousefi, M.; Katourani, A.; Yousefi, B.; Kafil, H.S. Fabrication and characterization of a titanium dioxide (TiO2) nanoparticles reinforced bio-nanocomposite containing miswak (Salvadora persica L.) extract—The antimicrobial, thermo-physical and barrier properties. Int. J. Nanomed. 2019, 14, 3439–3454. [Google Scholar] [CrossRef]
- Tunma, S. Starch based nanocomposites in active packaging for extended shelf life of fresh fruits. Walailak J. Sci. Technol. 2018, 15, 273–281. [Google Scholar] [CrossRef]
- Khodadadi, B.; Sabeti, M.; Moradi, S.; Aberomand Azar, P.; Raeis Farshid, S. Synthesis of Cu-TiO2 nanocomposite and investigation of the effectiveness of PEG, Pectin, and CMCas Additives. Q. J. Appl. Chem. Res. 2012, 6, 33–41. [Google Scholar]
- Khanmirzaei, M.H.; Ramesh, S. Nanocomposite polymer electrolyte based on rice starch/ionic liquid/TiO2 nanoparticles for solar cell application. Meas. J. Int. Meas. Confed. 2014, 58, 68–72. [Google Scholar] [CrossRef]
- Kuz, P.; Ateş, M. Starch-based bioplastic materials for packaging industry. J. Sustain. Constr. Mater. Technol. 2020, 5, 399–406. [Google Scholar] [CrossRef]
- Amin, M.R.; Chowdhury, M.A.; Kowser, M.A. Characterization and performance analysis of composite bioplastics synthesized using titanium dioxide nanoparticles with corn starch. Heliyon 2019, 5. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, A.R.; Savadkoohi, B.; Zahedi, Y.; Hatami, M.; Ako, K. Fabrication and characterization of hybrid sodium montmorillonite/TiO2 reinforced cross-linked wheat starch-based nanocomposites. Int. J. Biol. Macromol. 2019, 131, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xiong, Z.; Xiong, H.; Wang, Z.; Din, Z.U.; Nawaz, A.; Wang, P.; Hu, C. Effects of nano-TiO2 on bonding performance, structure stability and film-forming properties of starch-g-VAc based wood adhesive. Carbohydr. Polym. 2018, 200, 477–486. [Google Scholar] [CrossRef]
- Miyazaki, T.; Ishikawa, K.; Shirosaki, Y.; Ohtsuki, C. Organic–inorganic composites designed for biomedical applications. Biol. Pharm. Bull. 2013, 36, 1670–1675. [Google Scholar] [CrossRef]
- Premakshi, H.G.; Kariduraganavar, M.Y.; Mitchell, G.R. Crosslinked nanocomposite sodium alginate-based membranes with titanium dioxide for the dehydration of isopropanol by pervaporation. Molecules 2020, 25. [Google Scholar] [CrossRef]
- Tang, S.; Wang, Z.; Li, P.; Li, W.; Li, C.; Wang, Y.; Chu, P.K. Degradable and photocatalytic antibacterial Au-TiO2/sodium alginate nanocomposite films for active food packaging. Nanomaterials 2018, 8. [Google Scholar] [CrossRef]
- Fei, P.; Shi, Y.; Zhou, M.; Cai, J.; Tang, S.; Xiong, H. Effects of nano-TiO2 on the properties and structures of starch/poly(ε-caprolactone) composites. J. Appl. Polym. Sci. 2013, 130, 4129–4136. [Google Scholar] [CrossRef]
- Razali, M.H.; Ismail, N.A.; Amin, K.A.M. Fabrication and characterization of antibacterial titanium dioxide nanorods incorporating gellan gum films. J. Pure Appl. Microbiol. 2019, 13, 1909–1916. [Google Scholar] [CrossRef]
- Hou, X.; Xue, Z.; Liu, J.; Yan, M.; Xia, Y.; Ma, Z. Characterization and property investigation of novel eco-friendly agar/carrageenan/TiO2 nanocomposite films. J. Appl. Polym. Sci. 2019, 136, 1–12. [Google Scholar] [CrossRef]
- Goudarzi, V.; Shahabi-Ghahfarrokhi, I. Development of photo-modified starch/kefiran/TiO2 bio-nanocomposite as an environmentally-friendly food packaging material. Int. J. Biol. Macromol. 2018, 116, 1082–1088. [Google Scholar] [CrossRef]
- De Moura, M.R.; Zucolotto, V.; Aouada, F.A.; Mattoso, L.H.C. Efficiency improvement of cellulose derivative nanocomposite using titanium dioxide nanoparticles. J. Nanosci. Nanotechnol. 2017, 17, 2206–2211. [Google Scholar] [CrossRef]
- Dai, J.; Tian, Q.; Sun, Q.; Wei, W.; Zhuang, J.; Liu, M.; Cao, Z.; Xie, W.; Fan, M. TiO2-alginate composite aerogels as novel oil/water separation and wastewater remediation filters. Compos. Part. B Eng. 2019, 160, 480–487. [Google Scholar] [CrossRef]
- Ismail, N.A.; Amin, K.A.M.; Majid, F.A.A.; Razali, M.H. Gellan gum incorporating titanium dioxide nanoparticles biofilm as wound dressing: Physicochemical, mechanical, antibacterial properties and wound healing studies. Mater. Sci. Eng. C 2019, 103, 109770. [Google Scholar] [CrossRef]
- AL-Mokaram, A.M.A.A.A.; Yahya, R.; Abdi, M.M.; Mahmud, H.N.M.E. The development of non-enzymatic glucose biosensors based on electrochemically prepared polypyrrole-chitosan-titanium dioxide nanocomposite films. Nanomaterials 2017, 7. [Google Scholar] [CrossRef]
- Ciesielski, W.; Krystyjan, M. Starch-metal complexes and their rheology. E-Polymers 2009, 1–13. [Google Scholar] [CrossRef][Green Version]
- Wang, C.R.; Yan, X.Z.; Yu, L.L.; Fang, R. Preparation and properties of glycerol plasticized-corn starch/titanium dioxide-Starch bionanocomposites. Adv. Mater. Res. 2014, 997, 480–483. [Google Scholar] [CrossRef]
- Kochkina, N.E.; Butikova, O.A. Effect of fibrous TiO2 filler on the structural, mechanical, barrier and optical characteristics of biodegradable maize starch/PVA composite films. Int. J. Biol. Macromol. 2019, 139, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Sreekumar, P.A.; Al-Harthi, M.A.; De, S.K. Reinforcement of starch/polyvinyl alcohol blend using nano¢ titanium dioxide. J. Compos. Mater. 2012, 46, 3181–3187. [Google Scholar] [CrossRef]
- Liu, C.; Xiong, H.; Chen, X.; Lin, S.; Tu, Y. Effects of nano-TiO2 on the performance of high-amylose starch based antibacterial films. J. Appl. Polym. Sci. 2015, 132, 2–8. [Google Scholar] [CrossRef]
- Ostafińska, A.; Mikešová, J.; Krejčíková, S.; Nevoralová, M.; Šturcová, A.; Zhigunov, A.; Michálková, D.; Šlouf, M. Thermoplastic starch composites with TiO2 particles: Preparation, morphology, rheology and mechanical properties. Int. J. Biol. Macromol. 2017, 101, 273–282. [Google Scholar] [CrossRef]
- Goudarzi, V.; Shahabi-Ghahfarrokhi, I.; Babaei-Ghazvini, A. Preparation of ecofriendly UV-protective food packaging material by starch/TiO2 bio-nanocomposite: Characterization. Int. J. Biol. Macromol. 2017, 95, 306–313. [Google Scholar] [CrossRef]
- Oleyaei, S.A.; Zahedi, Y.; Ghanbarzadeh, B.; Moayedi, A.A. Modification of physicochemical and thermal properties of starch films by incorporation of TiO2 nanoparticles. Int. J. Biol. Macromol. 2016, 89, 256–264. [Google Scholar] [CrossRef]
- Dash, K.K.; Ali, N.A.; Das, D.; Mohanta, D. Thorough evaluation of sweet potato starch and lemon-waste pectin based-edible films with nano-titania inclusions for food packaging applications. Int. J. Biol. Macromol. 2019, 139, 449–458. [Google Scholar] [CrossRef]
- Goudarzi, V.; Shahabi-Ghahfarrokhi, I. Photo-producible and photo-degradable starch/TiO2 bionanocomposite as a food packaging material: Development and characterization. Int. J. Biol. Macromol. 2018, 106, 661–669. [Google Scholar] [CrossRef]
- Arezoo, E.; Mohammadreza, E.; Maryam, M.; Abdorreza, M.N. The synergistic effects of cinnamon essential oil and nano TiO2 on antimicrobial and functional properties of sago starch films. Int. J. Biol. Macromol. 2020, 157, 743–751. [Google Scholar] [CrossRef]
- Chueangchayaphan, N.; Ting, K.A.; Yusoff, M.; Chueangchayaphan, W. Influence of Al2O3 particle size on properties of thermoplastic starch–TiO2–Al2O3 composites. Polym. Bull. 2019, 76, 5889–5902. [Google Scholar] [CrossRef]
- Hajizadeh, H.; Peighambardoust, S.J.; Peighambardoust, S.H.; Peressini, D. Physical, mechanical, and antibacterial characteristics of bio-nanocomposite films loaded with Ag-modified SiO2 and TiO2 nanoparticles. J. Food Sci. 2020, 85, 1193–1202. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.H.; Youn, Y.N.; Yoon, S.D.; Lee, J.U. Preparation and physical properties of starch-based nanocomposite films with the addition of titanium oxide nanoparticles. J. Ceram. Process. Res. 2012, 13, 59–64. [Google Scholar]
- Ghozali, M.; Restu, W.K.; Triwulandari, E.; Anwar, M. Effect of metal oxide as antibacterial agent on thermoplastic starch/metal oxide biocomposites properties. Polym. Technol. Mater. 2020, 59, 1317–1325. [Google Scholar] [CrossRef]
- Yun, Y.-H.; Hwang, K.-J.; Wee, Y.-J.; Yoon, S.-D. Synthesis, physical properties, and characterization of starch-based blend films by adding nano-sized TiO2/poly(methyl methacrylate-co-acrylamide). J. Appl. Polym. Sci. 2010, 116, 2658–2667. [Google Scholar] [CrossRef]
- Ahmed, A.; Niazi, M.B.K.; Jahan, Z.; Ahmad, T.; Hussain, A.; Pervaiz, E.; Janjua, H.A.; Hussain, Z. In-vitro and in-vivo study of superabsorbent PVA/Starch/g-C3N4/Ag@TiO2 NPs hydrogel membranes for wound dressing. Eur. Polym. J. 2020, 130, 109650. [Google Scholar] [CrossRef]
- Ujcic, A.; Krejcikova, S.; Nevoralova, M.; Zhigunov, A.; Dybal, J.; Krulis, Z.; Fulin, P.; Nyc, O.; Slouf, M. Thermoplastic starch composites with titanium dioxide and vancomycin antibiotic: Preparation, morphology, thermomechanical properties, and antimicrobial susceptibility testing. Front. Mater. 2020, 7, 1–13. [Google Scholar] [CrossRef]
- Lin, D.; Huang, Y.; Liu, Y.; Luo, T.; Xing, B.; Yang, Y.; Yang, Z.; Wu, Z.; Chen, H.; Zhang, Q.; et al. Physico-mechanical and structural characteristics of starch/polyvinyl alcohol/nano-titania photocatalytic antimicrobial composite films. Lwt 2018, 96, 704–712. [Google Scholar] [CrossRef]
- Mousazadeh, S.; Shakouri, A.; Hojjat, M.; Etemad, S.G.; Heris, S.Z. Rheological behavior of starch–poly(vinyl alcohol)–TiO2 nanofluids and their main and interactive effects. J. Appl. Polym. Sci. 2016, 133, 1–9. [Google Scholar] [CrossRef]
- Ujcic, A.; Nevoralova, M.; Dybal, J.; Zhigunov, A.; Kredatusova, J.; Krejcikova, S.; Fortelny, I.; Slouf, M. Thermoplastic starch composites filled with isometric and elongated TiO2-based nanoparticles. Front. Mater. 2019, 6, 1–13. [Google Scholar] [CrossRef]
- Longo, V.M.; Picon, F.C.; Zamperini, C.; Albuquerque, A.R.; Sambrano, J.R.; Vergani, C.E.; Machado, A.L.; Andrés, J.; Hernandes, A.C.; Varela, J.A.; et al. Experimental and theoretical approach of nanocrystalline TiO2 with antifungal activity. Chem. Phys. Lett. 2013, 577, 114–120. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, K.; Ma, S.; Liu, T.; Yao, M.; Li, J.; Wang, X.; Guan, F. Preparing an injectable hydrogel with sodium alginate and Type I collagen to create better MSCs growth microenvironment. E-Polymers 2019, 19, 87–91. [Google Scholar] [CrossRef]
- Thakur, S.; Arotiba, O. Synthesis, characterization and adsorption studies of an acrylic acid-grafted sodium alginate-based TiO2 hydrogel nanocomposite. Adsorpt. Sci. Technol. 2018, 36, 458–477. [Google Scholar] [CrossRef]
- Reveendran, G.; Ong, S.T. Application of experimental design for dyes removal in aqueous environment by using sodium alginate-TiO2 thin film. Chem. Data Collect. 2018, 15–16, 32–40. [Google Scholar] [CrossRef]
- Thomas, M.; Natarajan, T.S.; Sheikh, M.U.D.; Bano, M.; Khan, F. Self-organized graphene oxide and TiO2 nanoparticles incorporated alginate/carboxymethyl cellulose nanocomposites with efficient photocatalytic activity under direct sunlight. J. Photochem. Photobiol. A Chem. 2017, 346, 113–125. [Google Scholar] [CrossRef]
- Naik, K.; Chandran, V.G.; Rajashekaran, R.; Waigaonkar, S.; Kowshik, M. Mechanical properties, biological behaviour and drug release capability of nano TiO2-HAp-Alginate composite scaffolds for potential application as bone implant material. J. Biomater. Appl. 2016, 31, 387–399. [Google Scholar] [CrossRef]
- Selvi, R.T.; Prasanna, A.P.S.; Niranjan, R.; Kaushik, M.; Devasena, T.; Kumar, J.; Chelliah, R.; Oh, D.H.; Swaminathan, S.; Venkatasubbu, G.D. Metal oxide curcumin incorporated polymer patches for wound healing. Appl. Surf. Sci. 2018, 449, 603–609. [Google Scholar] [CrossRef]
- Amjadi, S.; Almasi, H.; Ghorbani, M.; Ramazani, S. Preparation and characterization of TiO2NPs and betanin loaded zein/sodium alginate nanofibers. Food Packag. Shelf Life 2020, 24, 100504. [Google Scholar] [CrossRef]
- Uddin, M.J.; Islam, J.M.M.; Rahman, M.A.; Khan, M.A. Development of photoactive titanium dioxide doped sodium alginate film for dye sensitized solar cell Application. Int. J. Thin Film. Sci. Technol. 2017, 6, 135–138. [Google Scholar] [CrossRef]
- Padma, G.T.; Rao, T.S.; Naidu, K.C.B. Preparation, characterization and dielectric properties of sodium alginate/titanium dioxide composite membranes. SN Appl. Sci. 2019, 1, 1–8. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Salleh, W.N.W.; Jaafar, J.; Ismail, A.F.; Abd Mutalib, M.; Jamil, S.M. Incorporation of N-doped TiO2 nanorods in regenerated cellulose thin films fabricated from recycled newspaper as a green portable photocatalyst. Carbohydr. Polym. 2015, 133, 429–437. [Google Scholar] [CrossRef]
- Alavi, M. Modifications of microcrystalline cellulose (MCC), nanofibrillated cellulose (NFC), and nanocrystalline cellulose (NCC) for antimicrobial and wound healing applications. E-Polymers 2019, 19, 103–119. [Google Scholar] [CrossRef]
- El-Wakil, N.A.; Hassan, E.A.; Abou-Zeid, R.E.; Dufresne, A. Development of wheat gluten/nanocellulose/titanium dioxide nanocomposites for active food packaging. Carbohydr. Polym. 2015, 124, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Fathi-Achachlouei, B.; Zahedi, Y. Fabrication and characterization of CMC-based nanocomposites reinforced with sodium montmorillonite and TiO2 nanomaterials. Carbohydr. Polym. 2018, 199, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh Sani, M.; Ehsani, A.; Hashemi, M. Whey protein isolate/cellulose nanofibre/TiO2 nanoparticle/rosemary essential oil nanocomposite film: Its effect on microbial and sensory quality of lamb meat and growth of common foodborne pathogenic bacteria during refrigeration. Int. J. Food Microbiol. 2017, 251, 8–14. [Google Scholar] [CrossRef]
- Alizadeh-Sani, M.; Mohammadian, E.; McClements, D.J. Eco-friendly active packaging consisting of nanostructured biopolymer matrix reinforced with TiO2 and essential oil: Application for preservation of refrigerated meat. Food Chem. 2020, 322, 126782. [Google Scholar] [CrossRef]
- Alizadeh-Sani, M.; Khezerlou, A.; Ehsani, A. Fabrication and characterization of the bionanocomposite film based on whey protein biopolymer loaded with TiO2 nanoparticles, cellulose nanofibers and rosemary essential oil. Ind. Crops Prod. 2018, 124, 300–315. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, W.; Sun, L.; Kong, F.; Lin, M.; Mustapha, A. Preparation of cellulose nanofibril/titanium dioxide nanoparticle nanocomposites as fillers for PVA-based packaging and investigation into their intestinal toxicity. Int. J. Biol. Macromol. 2020, 156, 1174–1182. [Google Scholar] [CrossRef]
- de Matos Fonseca, J.; Valencia, G.A.; Soares, L.S.; Dotto, M.E.R.; Campos, C.E.M.; Moreira, R.F.P.M.; Fritz, A.R.M. Hydroxypropyl methylcellulose-TiO2 and gelatin-TiO2 nanocomposite films: Physicochemical and structural properties. Int. J. Biol. Macromol. 2020, 151, 944–956. [Google Scholar] [CrossRef]
- Miao, S.; Miao, Z.; Liu, Z.; Han, B.; Zhang, H.; Zhang, J. Synthesis of mesoporous TiO2 films in ionic liquid dissolving cellulose. Microporous Mesoporous Mater. 2006, 95, 26–30. [Google Scholar] [CrossRef]
- Uddin, M.J.; Cesano, F.; Bonino, F.; Bordiga, S.; Spoto, G.; Scarano, D.; Zecchina, A. Photoactive TiO2 films on cellulose fibres: Synthesis and characterization. J. Photochem. Photobiol. A Chem. 2007, 189, 286–294. [Google Scholar] [CrossRef]
- Zeng, J.; Liu, S.; Cai, J.; Zhang, L. TiO2 immobilized in cellulose matrix for photocatalytic degradation of phenol under weak UV light irradiation. J. Phys. Chem. C 2010, 114, 7806–7811. [Google Scholar] [CrossRef]
- Ortelli, S.; Blosi, M.; Albonetti, S.; Vaccari, A.; Dondi, M.; Costa, A.L. TiO2 based nano-photocatalysis immobilized on cellulose substrates. J. Photochem. Photobiol. A Chem. 2014, 276, 58–64. [Google Scholar] [CrossRef]
- Jo, S.; Oh, Y.; Park, S.; Kan, E.; Lee, S.H. Cellulose/carrageenan/TiO2 nanocomposite for adsorption and photodegradation of cationic dye. Biotechnol. Bioprocess. Eng. 2017, 22, 734–738. [Google Scholar] [CrossRef]
- Wang, J.; Liu, W.; Li, H.; Wang, H.; Wang, Z.; Zhou, W.; Liu, H. Preparation of cellulose fiber–TiO2 nanobelt–silver nanoparticle hierarchically structured hybrid paper and its photocatalytic and antibacterial properties. Chem. Eng. J. 2013, 228, 271–280. [Google Scholar] [CrossRef]
- Nelson, K.; Deng, Y. Enhanced light scattering from hollow polycrystalline TiO2 particles in a cellulose matrix. Langmuir 2008, 24, 975–982. [Google Scholar] [CrossRef]
- Kale, B.M.; Wiener, J.; Militky, J.; Rwawiire, S.; Mishra, R.; Jacob, K.I.; Wang, Y. Coating of cellulose-TiO2 nanoparticles on cotton fabric for durable photocatalytic self-cleaning and stiffness. Carbohydr. Polym. 2016, 150, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Veronovski, N.; Sfiligoj-Smole, M.; Viota, J.L. Characterization of TiO2/TiO2-SiO2 coated cellulose textiles. Text. Res. J. 2010, 80, 55–62. [Google Scholar] [CrossRef]
- Pang, Z.; Yang, Z.; Chen, Y.; Zhang, J.; Wang, Q.; Huang, F.; Wei, Q. A room temperature ammonia gas sensor based on cellulose/TiO2/PANI composite nanofibers. Coll. Surf. A Physicochem. Eng. Asp. 2016, 494, 248–255. [Google Scholar] [CrossRef]
- Behnezhad, M.; Goodarzi, M.; Baniasadi, H. Fabrication and characterization of polyvinyl alcohol/carboxymethyl cellulose/titanium dioxide degradable composite films: An RSM study. Mater. Res. Express 2019, 6. [Google Scholar] [CrossRef]
- Tripathi, S.; Mehrotra, G.K.; Dutta, P.K. Chitosan based antimicrobial films for food packaging applications. E-Polymers 2008, 1–7. [Google Scholar] [CrossRef]
- Mahmoud, G.A.; Sayed, A.; Thabit, M.; Safwat, G. Chitosan biopolymer based nanocomposite hydrogels for removal of methylene blue dye. SN Appl. Sci. 2020, 2. [Google Scholar] [CrossRef]
- El-Ella, A.A.; Youssef, A.M.; Ghannam, H.E.; Zedan, A.F.; Aboulthana, W.M.; Al-Sherbini, A.S.A. Synthesis of high efficient CS/PVDC/TiO2-Au nanocomposites for photocatalytic degradation of carcinogenic ethidium bromide in sunlight. Egypt. J. Chem. 2020, 63, 1619–1638. [Google Scholar] [CrossRef]
- Ikhlef-Taguelmimt, T.; Hamiche, A.; Yahiaoui, I.; Bendellali, T.; Lebik-Elhadi, H.; Ait-Amar, H.; Aissani-Benissad, F. Tetracycline hydrochloride degradation by heterogeneous photocatalysis using TiO2(P25) immobilized in biopolymer (Chitosan) under UV irradiation. Water Sci. Technol. 2020, 2, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Huang, G.; An, C.; Huang, J.; Chen, X.; Xin, X.; Song, P.; Feng, R.; Li, Y. Low-cost microbiological purification using a new ceramic disk filter functionalized by chitosan/TiO2 nanocomposites. Sep. Purif. Technol. 2020, 248, 116984. [Google Scholar] [CrossRef]
- Marey, A. Synthesis composite of TiO2/chitosan and TiO2/bentonite for removing turbidity from Ismailia canal as water treatment plant. Afr. J. Chem. Educ. 2020, 10, 124–133. [Google Scholar]
- Hussein, L.I.; Abdaleem, A.H.; Darwish, M.S.A.; Mostafa, M.H.; Elsawy, M.A. Chitosan/TiO2 nanocomposites: Effect of microwave heating and solution mixing techniques on physical properties. Egyp. J. Chem. 2020, 63, 449–460. [Google Scholar] [CrossRef]
- Nugraheni, A.D.; Purnawati, D.; Rohmatillah, A.; Mahardika, D.N.; Kusumaatmaja, A. Swelling of PVA/chitosan/TiO2 nanofibers membrane in different pH. Mater. Sci. Forum 2020, 990 MSF, 220–224. [Google Scholar] [CrossRef]
- Taspika, M.; Desiati, R.D.; Mahardika, M. Influence of TiO2/Ag particles on the properties of chitosan film. Adv. Nat. Sci. Nanosci. Nanotechnol. 2020, 11, 015017. [Google Scholar] [CrossRef]
- Hosseinzadeh, S.; Partovi, R.; Talebi, F.; Babaei, A. Chitosan/TiO2 nanoparticle/Cymbopogon citratus essential oil film as food packaging material: Physico-mechanical properties and its effects on microbial, chemical, and organoleptic quality of minced meat during refrigeration. J. Food Process. Preserv. 2020, 44, 1–12. [Google Scholar] [CrossRef]
- Hanafy, M.S.; Desoky, W.M.; Hussein, E.M.; El-Shaer, N.H.; Gomaa, M.; Gamal, A.A.; Esawy, M.A.; Guirguis, O.W. Biological applications study of bio-nanocomposites based on chitosan/TiO2 nanoparticles polymeric films modified by oleic acid. J. Biomed. Mater. Res. Part. A 2020. [Google Scholar] [CrossRef]
- Cheng, C.W.; Yang, C.I.; Ou, S.L.; Chi, H.W.; Lui, P.W.; Chen, W.Y.; Ao, W.; Lai, F.M. Bending mattress and antibacterial effect of TiO2/nAg/chitosan-nanoparticle-applied intelligent patient bed. Sensors Mater. 2020, 32, 1757–1766. [Google Scholar] [CrossRef]
- Petrick, J.; Ibadurrohman, M. Slamet Synthesis of chitosan/TiO2 nanocomposite for antibacterial sunscreen application. Int. Conf. Trends Mater. Sci. Inven. Mater. Ictmim 2020, 2259, 060020. [Google Scholar] [CrossRef]
- Kolsuz Ozcetin, H.; Surmelioglu, D. Effects of bleaching gel containing TiO2 and chitosan on tooth surface roughness, microhardness and colour. Aust. Dent. J. 2020. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Geng, C.; Xue, Z.; Xia, Y. Preparation and properties investigation of agar/TiO2 fibers. J. Appl. Sci. Eng. Inno. 2018, 5, 109–112. [Google Scholar]
- Vejdan, A.; Ojagh, S.M.; Adeli, A.; Abdollahi, M. Effect of TiO2 nanoparticles on the physico-mechanical and ultraviolet light barrier properties of fish gelatin/agar bilayer film. LWT Food Sci. Technol. 2016, 71, 88–95. [Google Scholar] [CrossRef]
- Vejdan, A.; Ojagh, S.M.; Abdollahi, M. Effect of gelatin/agar bilayer film incorporated with TiO2 nanoparticles as a UV absorbent on fish oil photooxidation. Int. J. Food Sci. Technol. 2017, 52, 1862–1868. [Google Scholar] [CrossRef]
- Abdel-Baky, E.; El-Duma Abdullah, Z.; El Din Aboul-Anean, H. Application of nano edible films to improve some sates in Saudi Arabia. Int. J. Pharm. Res. Allied Sci. 2020, 9, 69–84. [Google Scholar]
- Nasiri, M.; Sani, A.M.; Hakimzadeh, V.; Shahidi, M. Antimicrobial effects of edible nano-composite based on bean pod shell gum, nano-TiO2, and Mentha pulegium essential oil. J. Appl. Biol. Biotechnol. 2019, 7, 75–78. [Google Scholar] [CrossRef]
- Jin, B.; Li, X.; Zhou, X.; Xu, X.; Jian, H.; Li, M.; Guo, K.; Guan, J.; Yan, S. Fabrication and characterization of nanocomposite film made from a jackfruit filum polysaccharide incorporating TiO2 nanoparticles by photocatalysis. RSC Adv. 2017, 7, 16931–16937. [Google Scholar] [CrossRef]
- Salarbashi, D.; Tafaghodi, M.; Bazzaz, B.S.F.; Jafari, B. Characterization of soluble soybean (SSPS) polysaccharide and development of eco-friendly SSPS/TiO2 nanoparticle bionanocomposites. Int. J. Biol. Macromol. 2018, 112, 852–861. [Google Scholar] [CrossRef]
- Razali, M.H.; Ismail, N.A.; Mat Amin, K.A. Titanium dioxide nanotubes incorporated gellan gum bio-nanocomposite film for wound healing: Effect of TiO2 nanotubes concentration. Int. J. Biol. Macromol. 2020, 153, 1117–1135. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).