Thermal Decontamination of Spent Activated Carbon Contaminated with Radiocarbon and Tritium
Abstract
1. Introduction
2. Materials and Methods
2.1. Spent Activated Carbon
2.2. Analysis of Textural Properties
2.3. TGA-FTIR Analysis
2.4. Trial Thermal Desorption
2.5. Kinetic Analysis and Prediction of the Thermal Desorption Reaction
3. Results and Discussion
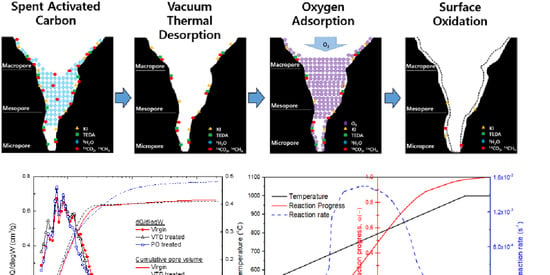
3.1. Changes in the Porous Texture
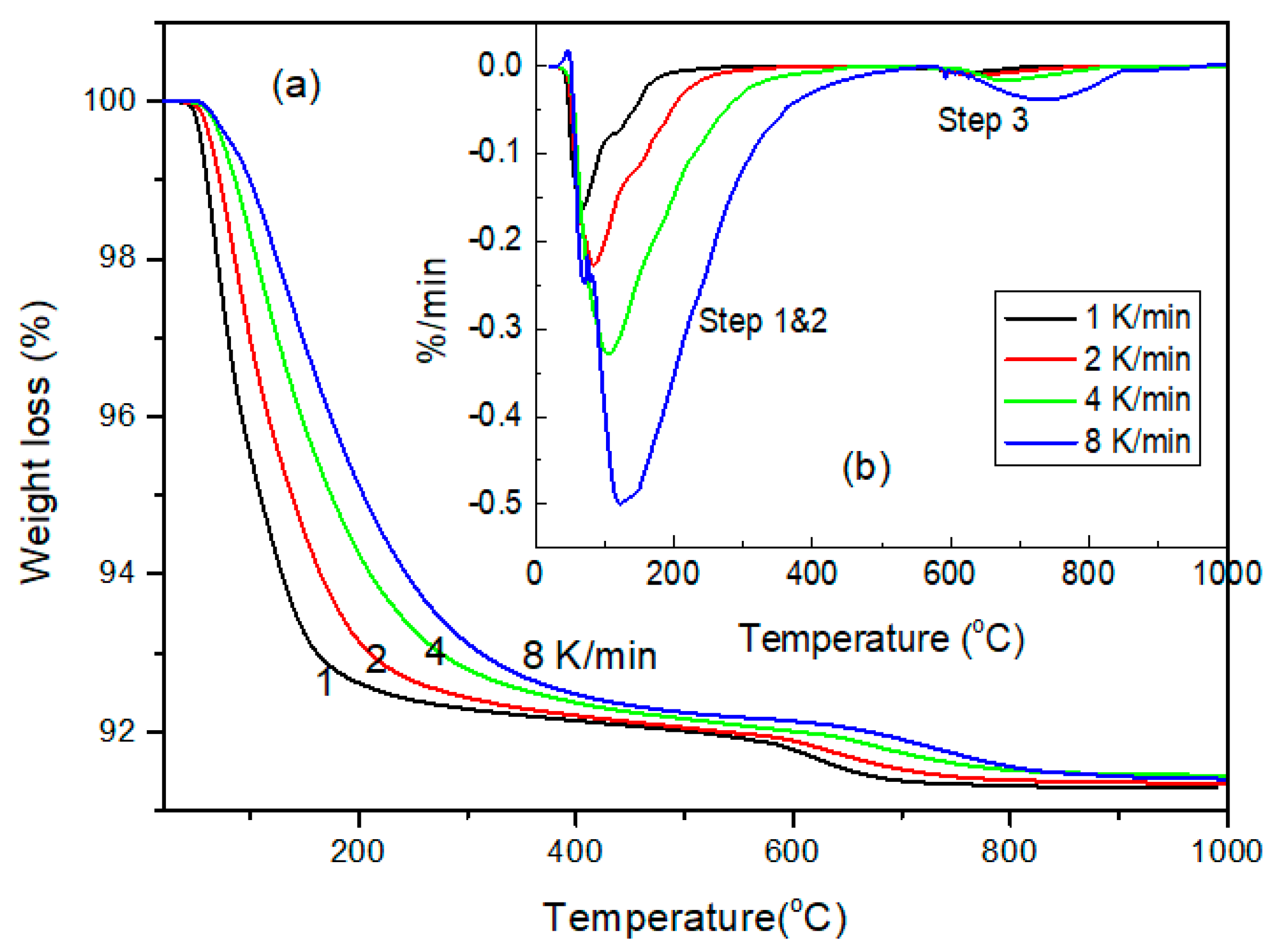
3.2. Thermal Desorption Characteristics
3.2.1. Thermal Desorption Steps
3.2.2. Thermal Desorption Mechanism
3.2.3. Thermal Desorption Characteristics of 3H and 14C species
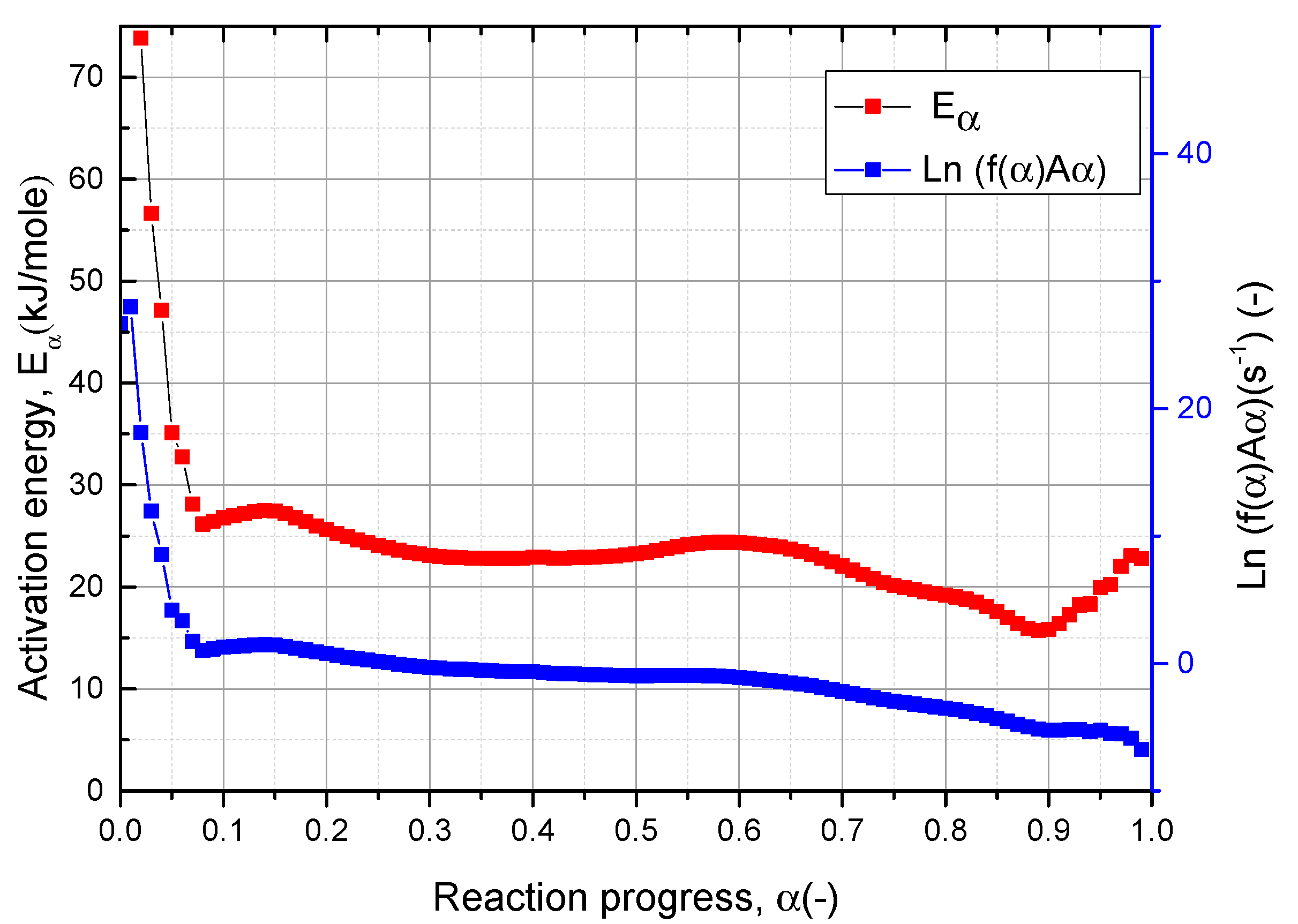
3.3. Kinetic Analysis and Prediction
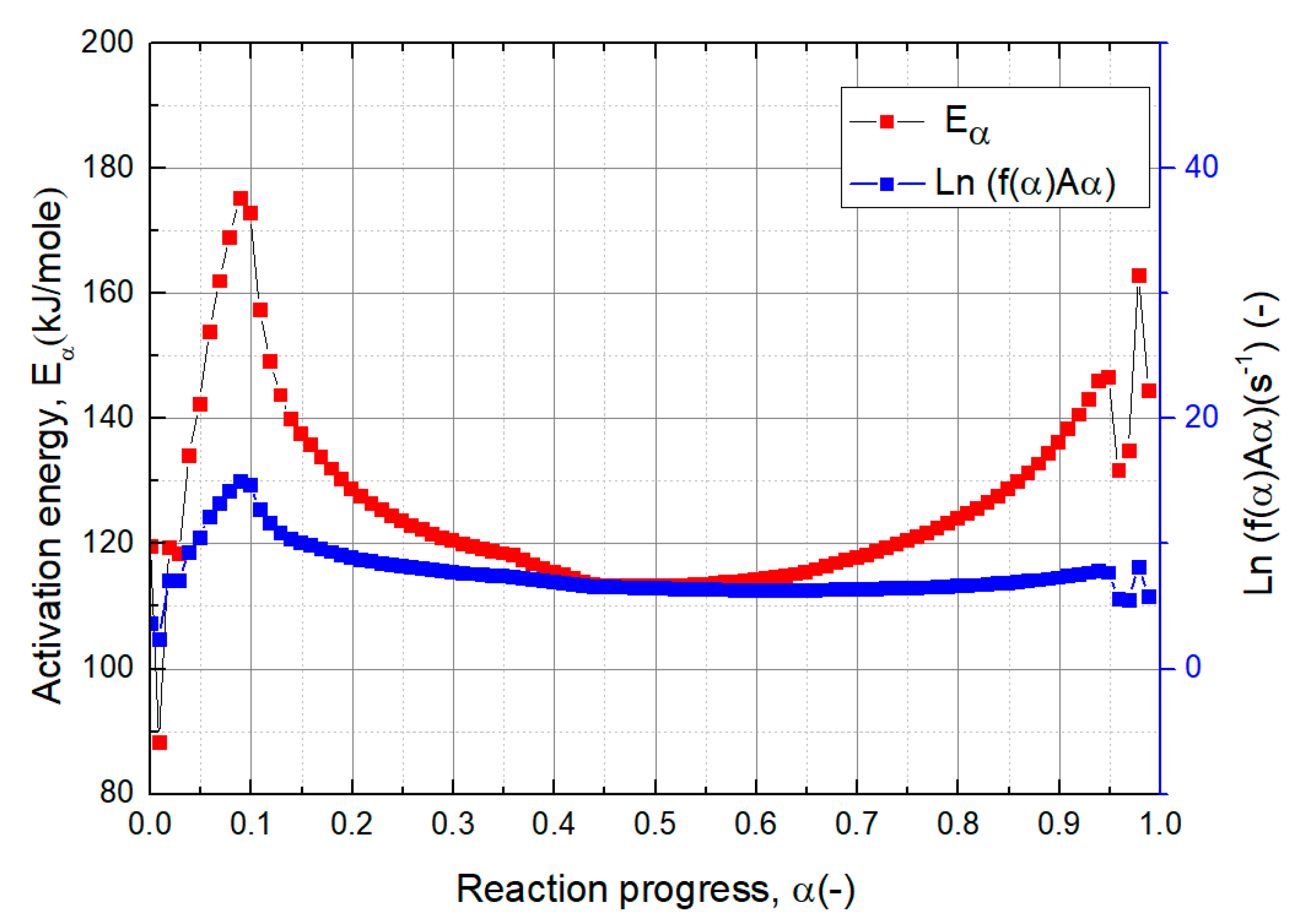
3.3.1. Kinetic Analysis of Vacuum Thermal Desorption Reaction
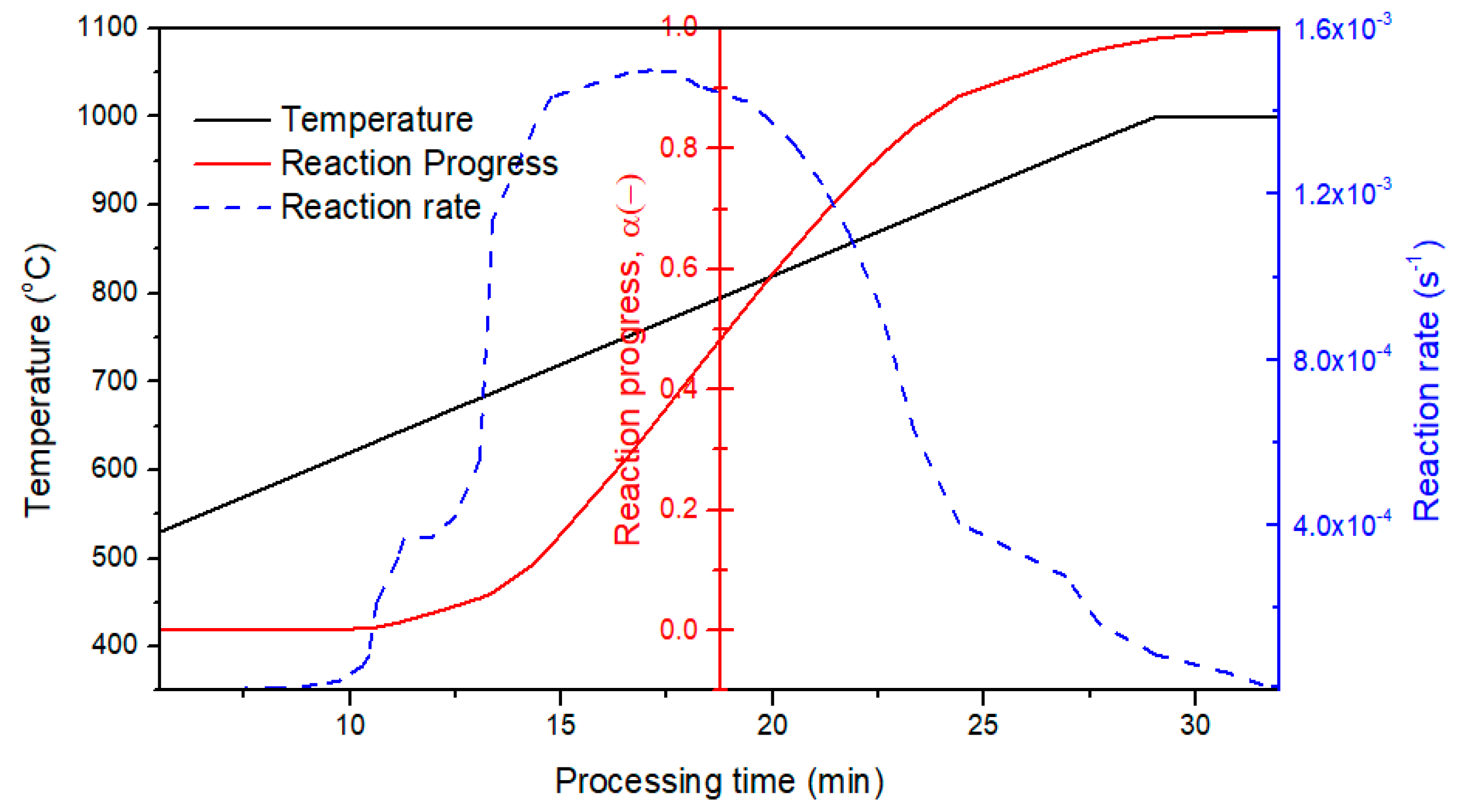
3.3.2. Kinetic Prediction for Vacuum Thermal Desorption Process
3.4. Trial Treatment of Spent Activated Carbon
3.4.1. Vacuum Thermal Desorption
3.4.2. Vacuum Thermal Desorption Followed by Surface Oxidation
3.5. Recyclability of Treated Spent Activated Carbon
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lüth, H. Solid Surfaces, Interfaces, and Thin Films, 5th ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 518–520. [Google Scholar]
- Zhou, Z.; Hao, S.; Gao, L.; Zhang, Y. Study on adsorption performance of coal based activated carbon to radioactive iodine and stable iodine. Ann. Nucl. Energy 2014, 72, 237–241. [Google Scholar] [CrossRef]
- Gourani1, M.; Sadighzadeh, A.; Mizani, F. Effect of impregnating materials in activated carbon on Iodine -131 (131I) removal efficiency. Radiat. Prot. Environ. 2012, 37, 179–183. [Google Scholar]
- Kim, S.-H.; Chuang, J.C.; Kelly, P.B.; Clifford, A.J. Carbon isotopes profiles of human whole blood, plasma, red blood cells, urine and feces for biological/biomedical 14C-accelerator mass spectrometry applications. Anal. Chem. 2011, 83, 3312–3318. [Google Scholar] [PubMed]
- IAEA. Management of Waste Containing Tritium and Carbon-14; Technical Report Series, No. 421; International Atomic Energy Agency: Vienna, Austria, 2004; pp. 3–4. [Google Scholar]
- Kurk, M.; Jaronic, M.; Choma, J. Comparative analysis of simple and advance sorption methods for assessment of microporosity of activated carbons. Carbon 1998, 36, 1447–1458. [Google Scholar]
- Silvestre-Albero, A.; Silvestre-Albero, J.; Martı’nez-Escandell, M.; Futamura, R.; Itoh, T.; Kaneko, K.; Rodrı’guez-Reinoso, F. Non-porous reference carbon for N2 (77.4 K) and Ar (87.3 K) adsorption. Carbon 2014, 66, 699–704. [Google Scholar] [CrossRef]
- Nakai, K.; Nakada, Y.; Hakuman, M.; Yoshida, M.; Senda, Y.; Tateishi, Y.; Sonoda, J.; Naono, H. High resolution N2 adsorption isotherms at 77.4 K and 87.3 K by carbon blacks and activated carbon fibers—Analysis of porous texture of activated carbon fibers by αs-method. J. Colloid. Interface Sci. 2012, 367, 383–393. [Google Scholar]
- Tarazona, P. Free-energy density functional for hard spheres. Phys. Rev. A 1985, 31, 2672–2679. [Google Scholar]
- Tarazona, P.; Marconi, P.M.B.; Evans, R. Phase equilibria of fluid interfaces and confined fluids—Non-local versus local density functionals. Mol. Phys. 1987, 60, 573–595. [Google Scholar]
- El-Sayed, S.A.; Mostafa, M.E. Pyrolysis characteristics and kinetic parameters determination of biomass fuel powders by differential thermal gravimetric analysis (TGA/DTG). Energ. Convers. Manag. 2014, 85, 165–172. [Google Scholar] [CrossRef]
- Cvetanovic, R.J.; Amenomiya, Y. A temperature programmed desorption technique for investigation of practical catalysts. Catal. Rev. Sci. Eng. 1972, 6, 21. [Google Scholar] [CrossRef]
- Friedman, H.L. Kinetics of thermal degradation of char-forming plastics from thermogravimetry application to a phenolic plastic. J. Polym. Sci. 1964, 6, 183–195. [Google Scholar] [CrossRef]
- Berger, A.H.; Bhown, A.S. Comparing physisorption and chemisorption solid sorbents for use separating CO2 from flue gas using temperature swing adsorption. Energy Procedia 2011, 4, 562–567. [Google Scholar] [CrossRef]
- González-García, C.M.; González, J.F.; Román, S. Removal efficiency of radioactive methyl iodide on TEDA-impregnated activated carbons. Fuel Process. Technol. 2011, 92, 247–252. [Google Scholar] [CrossRef]
- Mahle, J.J.; Peterson, G.W.; Schindler, B.J.; Smith, P.B.; Rossin, J.A.; Wagner, G.W. Role of TEDA as an Activated Carbon Impregnant for the Removal of Cyanogen Chloride from Air Streams: Synergistic Effect with Cu(II). J. Phys. Chem. C 2010, 114, 20083–20090. [Google Scholar] [CrossRef]
- Barkauskas, J.; Dervinyte, M. An investigation of the functional groups on the surface of activated carbons. J. Serb. Chem. Soc. 2004, 69, 363–375. [Google Scholar] [CrossRef]
- Liang, X.; Chi, J.; Yang, Z. The influence of the functional group on activated carbon for acetone adsorption property by molecular simulation study. Microporous Mesoporous Mater. 2018, 262, 77–88. [Google Scholar] [CrossRef]
- Ryczkowski, J.; Pasieczna, S.; Figueiredo, J.L.; Pereira, M.F.R.; Borowiecki, T. Characterization of activated carbon by FT-IR/PAS and TPD. J. Phys. IV Fr. 2004, 117, 57–63. [Google Scholar] [CrossRef]
- NIST Chemistry WebBook. Available online: https://webbook.nist.gov/cgi/cbook.cgi?ID=C124389&Units=SI&Type=IR-SPEC (accessed on 20 February 2020).
- NIST Chemistry WebBook. Available online: https://webbook.nist.gov/cgi/cbook.cgi?Formula=CH4&NoIon=on&Units=SI&cIR=on (accessed on 20 February 2020).
- NIST Chemistry WebBook. Available online: https://webbook.nist.gov/cgi/cbook.cgi?Formula=H2O&NoIon=on&Units=SI&cIR=on (accessed on 20 February 2020).
- Roduit, B.; Borgeat, C.; Berger, B.; Folly, P.; Alonso, B.; Aebischer, J.N.; Stoessel, F. Advanced kinetic tools for the evaluation of decomposition reactions. J. Thermal. Anal. Calorim. 2005, 80, 229–636. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Saha, B.; Maiti, A.; Ghoshal, A.K. Model-free method for isothermal and non-isothermal decomposition kinetics analysis of PET sample. Thermochim. Acta 2006, 444, 46–52. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Chen, M. A comparison of isothermal with nonisothermal drying kinetics of municipal sewage sludge. J. Thermal. Anal. Calorim. 2015, 123, 665–673. [Google Scholar] [CrossRef]
- Cruz, G.; Crnkovic, P.M. Investigation into the kinetic behavior of biomass combustion under N2/O2 and CO2/O2 atmospheres. J. Thermal. Anal. Calorim. 2016, 123, 1003–1011. [Google Scholar] [CrossRef]
- Park, H.S.; Park, G.L.; Eun, H.C.; Choi, J.H.; Li, G.L.; Ahn, H.J.; Song, K.S. Treatment Method of Spent Air Cleaning Charcoal. Korean Patent 10-1545440, 11 August 2015. [Google Scholar]



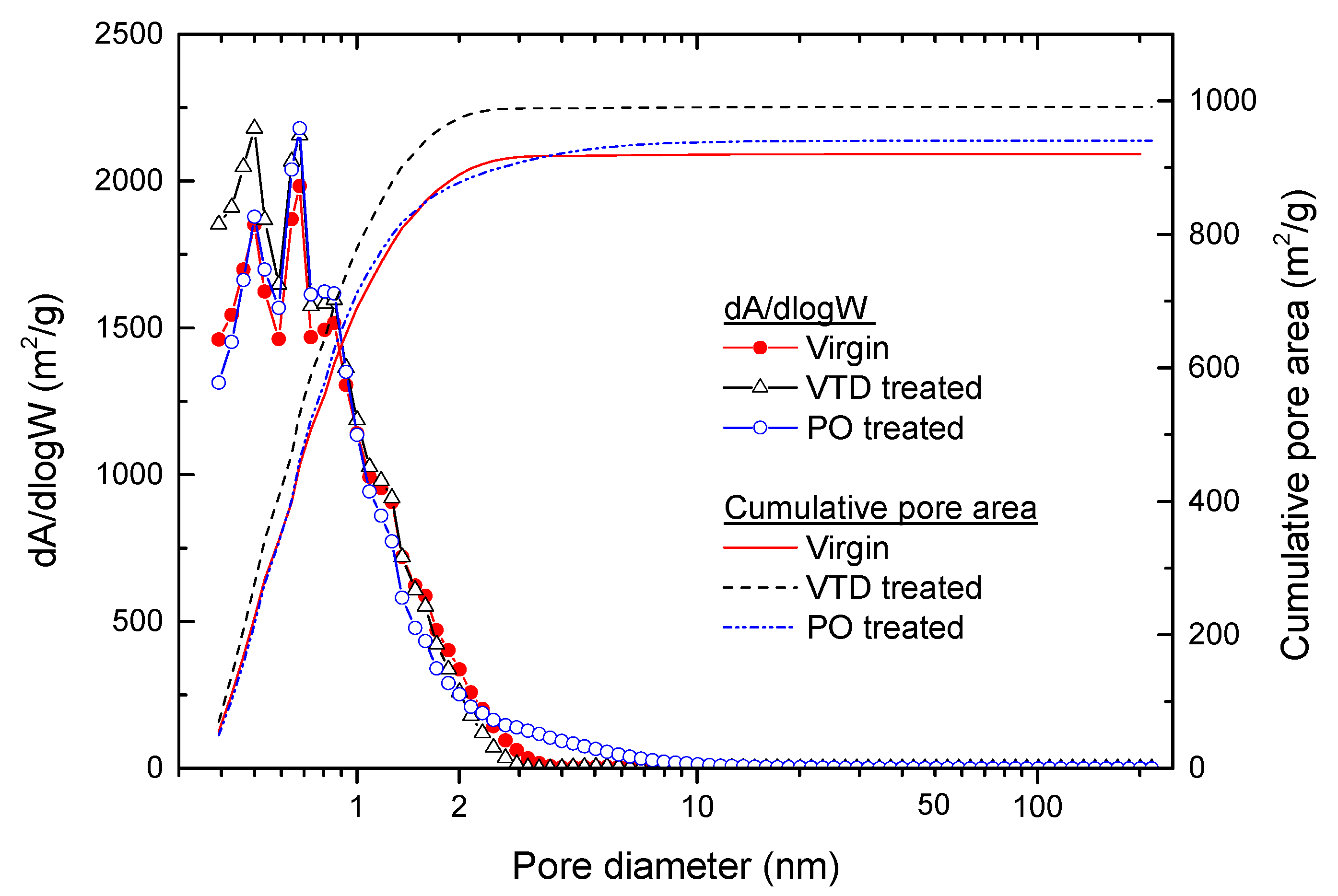

| GAC Sample | Pore Volume (cm3/g, STP) | Surface Area (m2/g) | |||
|---|---|---|---|---|---|
| Vmicro | Vmeso | Aext | Amicro | ABET | |
| Virgin | 0.483 | 0.015 | 6.1 | 1045.1 | 1108.4 |
| VTD-treated | 0.491 | 0.014 | 3.1 | 1077.2 | 1159.1 |
| PO-treated | 0.486 | 0.058 | 8.6 | 1040.0 | 1108.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.-C.; Lee, M.-W.; Eun, H.-C.; Kim, H.-J.; Lee, K.; Seo, B.-K. Thermal Decontamination of Spent Activated Carbon Contaminated with Radiocarbon and Tritium. Processes 2020, 8, 1359. https://doi.org/10.3390/pr8111359
Yang H-C, Lee M-W, Eun H-C, Kim H-J, Lee K, Seo B-K. Thermal Decontamination of Spent Activated Carbon Contaminated with Radiocarbon and Tritium. Processes. 2020; 8(11):1359. https://doi.org/10.3390/pr8111359
Chicago/Turabian StyleYang, Hee-Chul, Min-Woo Lee, Hee-Chul Eun, Hyung-Ju Kim, Keunyoung Lee, and Bum-Kyung Seo. 2020. "Thermal Decontamination of Spent Activated Carbon Contaminated with Radiocarbon and Tritium" Processes 8, no. 11: 1359. https://doi.org/10.3390/pr8111359
APA StyleYang, H.-C., Lee, M.-W., Eun, H.-C., Kim, H.-J., Lee, K., & Seo, B.-K. (2020). Thermal Decontamination of Spent Activated Carbon Contaminated with Radiocarbon and Tritium. Processes, 8(11), 1359. https://doi.org/10.3390/pr8111359