Effect of Pasteurisation on Methane Yield from Food Waste and Other Substrates in Anaerobic Digestion
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Pasteurisation Procedure
2.3. Experimental Set-Up
2.4. Determination of Biogas Production
2.5. Laboratory Analyses
3. Results
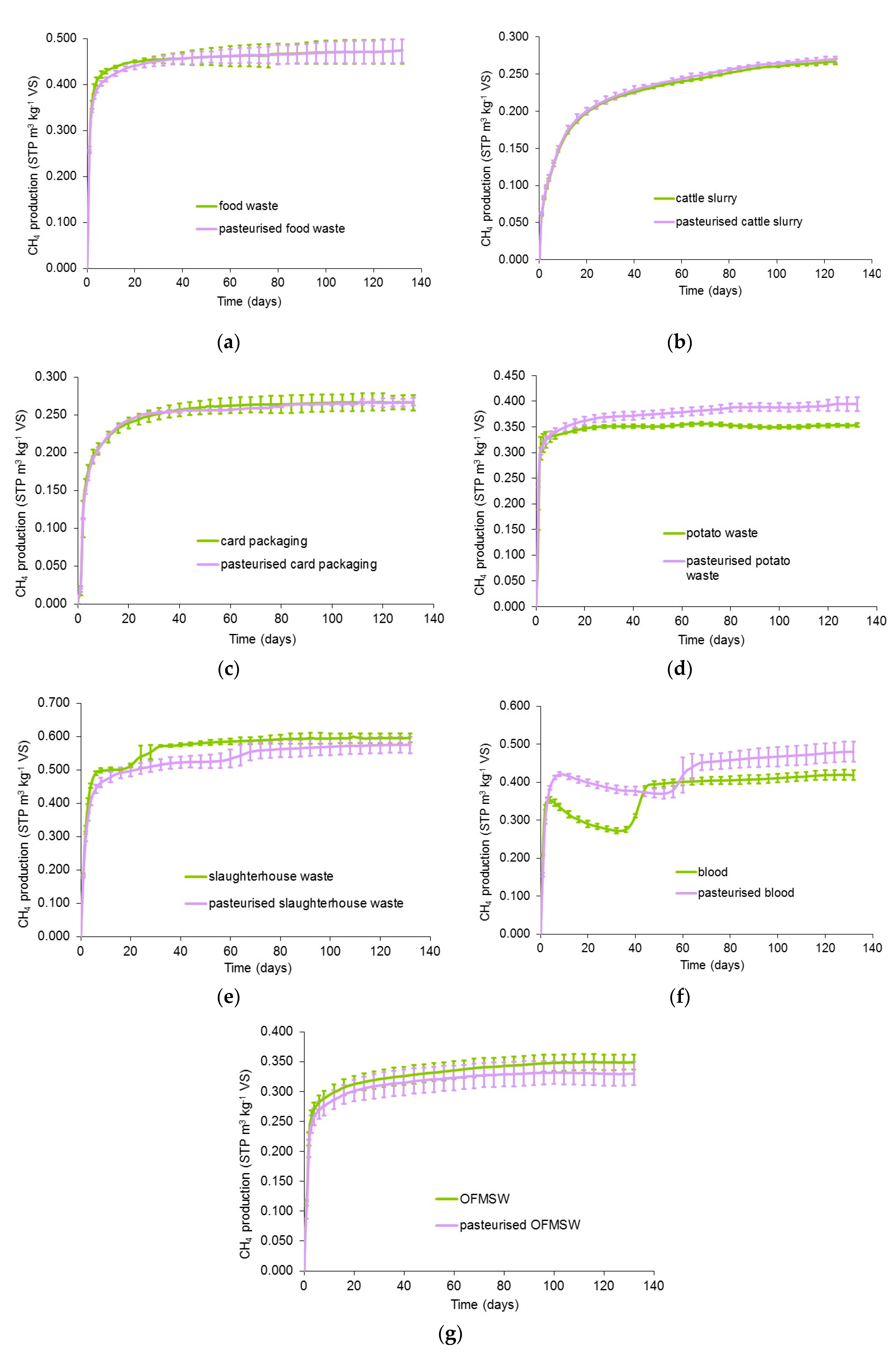
3.1. Methane Yields of Pasteurised and Unpasteurised Substrates
3.2. Comparison of Experimental and Theoretical Methane Yields
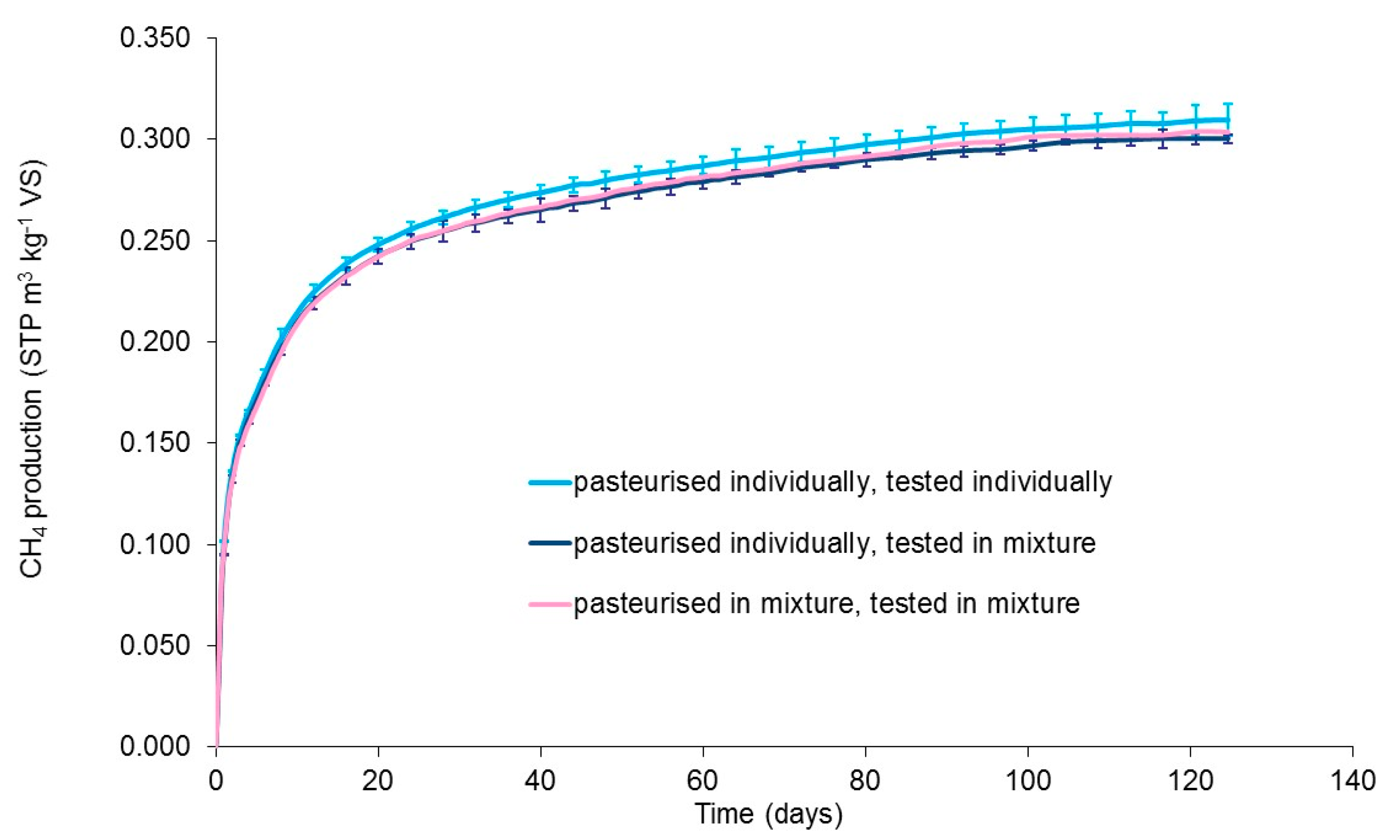
3.3. Effect of Co-Pasteurisation of Food Waste and Cattle Slurry
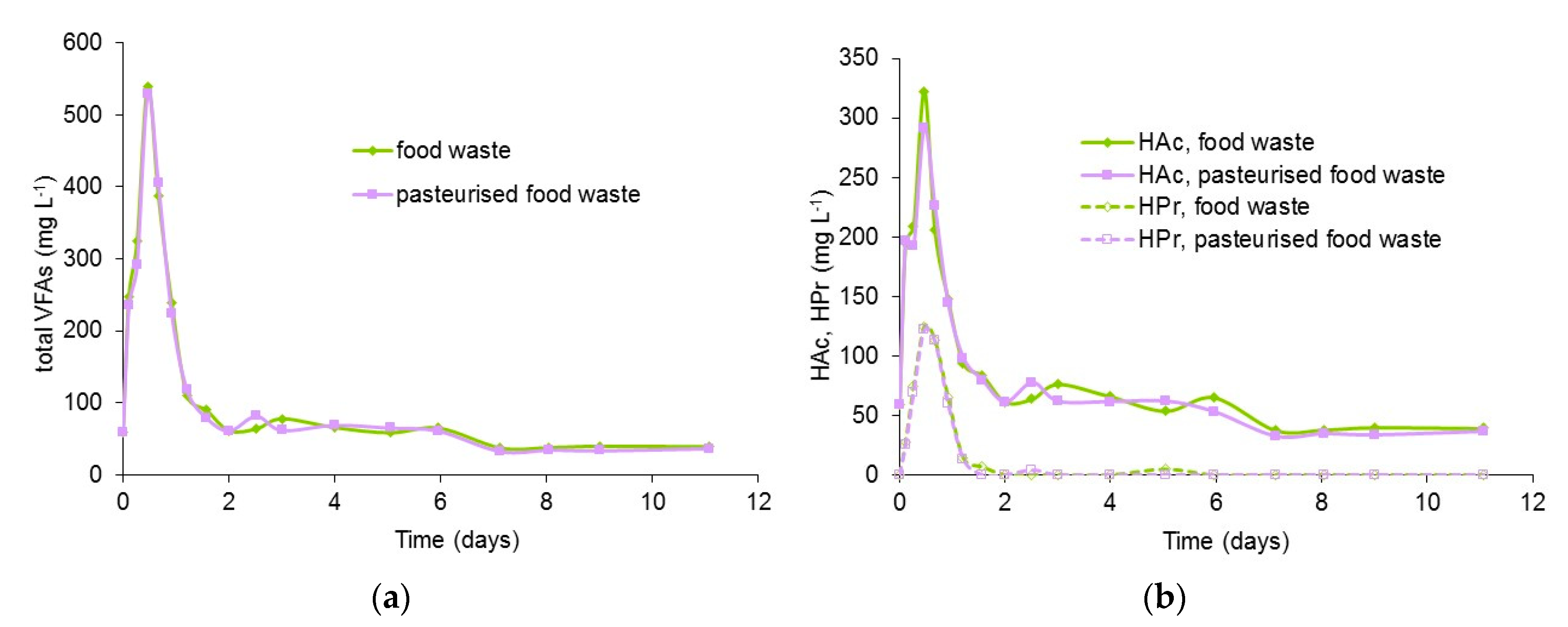
3.4. Profiles of VFA and Ammonia in Digestion of Pasteurised and Unpasteurised Food Waste
4. Discussion
4.1. Discussion of the Results from the Digestion Experiments with Pasteurised and Unpasteurised Substrates
4.2. Discussion of the Testing Procedure
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Appendix A. BMP Test on Cellulose Standard (Quality Control)

References
- Liu, X.; Lendormi, T.; Lanoisellé, J.-L. Overview of hygienization pretreatment for pasteurization and methane potential enhancement of biowaste: Challenges, state of the art and alternative technologies. J. Clean. Prod. 2019, 236, 117525. [Google Scholar] [CrossRef]
- Nag, R.; Whyte, P.; Markey, B.K.; O’Flaherty, V.; Bolton, D.; Fenton, O.; Richards, K.G.; Cummins, E. Ranking hazards pertaining to human health concerns from land application of anaerobic digestate. Sci. Total Environ. 2020, 710, 136297. [Google Scholar] [CrossRef] [PubMed]
- Nag, R.; Auer, A.; Markey, B.K.; Whyte, P.; Nolan, S.; O’Flaherty, V.; Russell, L.; Bolton, D.; Fenton, O.; Richards, K.; et al. Anaerobic digestion of agricultural manure and biomass—Critical indicators of risk and knowledge gaps. Sci. Total Environ. 2019, 690, 460–479. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Liu, Y. Is anaerobic digestion a reliable barrier for deactivation of pathogens in biosludge? Sci. Total Environ. 2019, 668, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Tampio, E. Utilization of Food Waste via Anaerobic Digestion: From Feedstock to Biogas and Fertilizers; Tampere University of Technology Publications: Tampere, Finland, 2016; Volume 1405. [Google Scholar]
- EU ABP Regulation 1774/2002. Regulation (EC) No 1774/2002 of the European Parliament and of the Council of 3 October 2002 Laying Down Health Rules Concerning Animal by-Products not Intended for Human Consumption; European Commission: Brussels, Belgium, 2002; Available online: https://op.europa.eu/en/publication-detail/-/publication/28ab554e-8e93-4976-89a9-8b6c9d17dfb4 (accessed on 9 March 2020).
- EU ABP Regulation 1069/2009. Regulation (EC) No 1069/2009 of the European Parliament and of the Council of 21 October 2009 Laying down Health Rules as Regards Animal by-Products and Derived Products not Intended for Human Consumption and Repealing Regulation (EC) No 1774/2002 (Animal by-Products Regulation); European Commission: Brussels, Belgium, 2009; Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32009R1069 (accessed on 9 March 2020).
- Ware, A.; Power, N. What is the effect of mandatory pasteurisation on the biogas transformation of solid slaughterhouse wastes? Waste Manage. 2016, 48, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Luste, S.; Luostarinen, S. Anaerobic co-digestion of meat-processing by-products and sewage sludge—Effect of hygienization and organic loading rate. Bioresour. Technol. 2010, 101, 2657–2664. [Google Scholar] [CrossRef] [PubMed]
- Grim, J.; Malmros, P.; Schnürer, A.; Nordberg, A. Comparison of pasteurization and integrated thermophilic sanitation at a full-scale biogas plant – heat demand and biogas production. Energy 2015, 79, 419–427. [Google Scholar] [CrossRef]
- Nazari, L.; Yuan, Z.; Santoro, D.; Sarathy, S.; Ho, D.; Batstone, D.; Xu, C.; Ray, M.B. Low-temperature thermal pre-treatment of municipal wastewater sludge: Process optimization and effects on solubilization and anaerobic degradation. Water Res. 2017, 113, 111–123. [Google Scholar] [CrossRef]
- Liu, X.; Souli, I.; Chamaa, M.-A.; Lendormi, T.; Sabourin, C.; Lemée, Y.; Boy, V.; Chaira, N.; Ferchichi, A.; Morançais, P.; et al. Effect of thermal pretreatment at 70 °C for one hour (EU hygienization conditions) of various organic wastes on methane production under mesophilic anaerobic digestion. AIMS Environ. Sci. 2018, 5, 117–129. [Google Scholar] [CrossRef]
- Anukam, A.; Mohammadi, A.; Naqvi, M.; Granström, K. A review of the chemistry of anaerobic digestion: Methods of accelerating and optimizing process efficiency. Processes 2019, 7, 504. [Google Scholar] [CrossRef]
- Rafique, R.; Poulsen, T.G.; Nizami, A.S.; Asam, Z.; Murphy, J.D.; Kiely, G. Effect of thermal, chemical and thermo-chemical pre-treatments to enhance methane production. Energy 2010, 35, 4556–4561. [Google Scholar] [CrossRef]
- Luste, S.; Heinonen-Tanski, H.; Luostarinen, S. Co-digestion of dairy cattle slurry and industrial meat-processing by-products—effect of ultrasound and hygienization pre-treatments. Bioresour. Technol. 2012, 104, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Luste, S.; Luostarinen, S. Enhanced methane production from ultrasound pre-treated and hygienized dairy cattle slurry. Waste Manage. 2011, 31, 2174–2179. [Google Scholar] [CrossRef]
- Climent, M.; Ferrer, I.; del Baeza, M.M.; Artola, A.; Vazquez, F.; Font, X. Effects of thermal and mechanical pretreatments of secondary sludge on biogas production under thermophilic conditions. Chem. Eng. J. 2007, 133, 335–342. [Google Scholar] [CrossRef]
- Edstrom, M.; Nordberg, A.; Thyselius, L. Anaerobic treatment of animal byproducts from slaughterhouses at laboratory and pilot scale. Appl. Biochem. Biotechnol. 2003, 109, 127–138. [Google Scholar] [CrossRef]
- Hejnfelt, A.; Angelidaki, I. Anaerobic digestion of slaughterhouse by-products. Biomass Bioenergy 2009, 33, 1046–1054. [Google Scholar] [CrossRef]
- Luste, S.; Luostarinen, S.; Sillanpää, M. Effect of pre-treatments on hydrolysis and methane production potentials of by-products from meat-processing industry. J. Hazard. Mater. 2009, 164, 247–255. [Google Scholar] [CrossRef]
- Rodriguez-Abalde, A.; Fernandez, B.; Silvestre, G.; Flotats, X. Effects of thermal pre-treatments on solid slaughterhouse waste methane potential. Waste Manage. 2011, 31, 1488–1493. [Google Scholar] [CrossRef]
- Ajandouz, E.H.; Desseaux, V.; Tazi, S.; Puigserver, A. Effect of temperature and pH on the kinetics of caramelisation, protein cross-linking and Maillard reactions in aqueous model systems. Food Chem. 2008, 107, 1244–1252. [Google Scholar] [CrossRef]
- Mersad, A.; Lewandowski, R.; Heyd, B.; Decloux, M. Colorants in the sugar industry: Laboratory preparation and spectrometric analysis. Int. Sugar J. 2003, 105, 269–281. [Google Scholar]
- Martins, S.I.F.S.; Boekel, M.A.J.S. A kinetic model for the glucose/glycine Maillard reaction pathways. Food Chem. 2005, 90, 257–269. [Google Scholar] [CrossRef]
- Michalska, A.; Honke, J.; Lysiak, G.; Andlauer, W. Effect of drying parameters on the formation of early and intermediate stage products of the Maillard reaction in different plum (Prunus domestica L.) cultivars. LWT Food Sci. Technol. 2016, 65, 932–938. [Google Scholar] [CrossRef]
- Kung, L., Jr.; Shaver, R.D.; Grant, R.J.; Schmidt, R.J. Silage review: Interpretation of chemical, microbial, and organoleptic components of silages. J. Dairy Sci. 2018, 101, 4020–4033. [Google Scholar] [CrossRef] [PubMed]
- Banks, C.; Heaven, S.; Zhang, Y.; Baier, U. Food Waste Digestion: Anaerobic Digestion of Food Waste for a Circular Economy; IEA Bioenergy, Task 37; International Energy Agency (IEA) and MaREI Centre University College Cork: Cork, Ireland, 2018; Available online: https://www.ieabioenergy.com/publications/food-waste-digestion-anaerobic-digestion-of-food-waste-for-a-circular-economy/ (accessed on 16 July 2020).
- Morales-Polo, C.; Cledera-Castro, M.D.M.; Moratilla Soria, B.Y. Reviewing the anaerobic digestion of food waste: From waste generation and anaerobic process to its perspectives. Appl. Sci. 2018, 8, 1804. [Google Scholar] [CrossRef]
- Mirmohamadsadeghi, S.; Karimi, K.; Tabatabaei, M.; Aghbashlo, M. Biogas production from food wastes: A review on recent developments and future perspectives. Bioresour. Technol. Rep. 2019, 7, 100202. [Google Scholar] [CrossRef]
- Baek, G.; Kim, D.; Kim, J.; Kim, H.; Lee, C. Treatment of cattle manure by anaerobic co-digestion with food waste and pig manure: Methane yield and synergistic effect. Int. J. Environ. Res. Public Health 2020, 17, 4737. [Google Scholar] [CrossRef]
- Hegde, S.; Trabold, T.A. Anaerobic digestion of food waste with unconventional co-substrates for stable biogas production at high organic loading rates. Sustainability 2019, 11, 3875. [Google Scholar] [CrossRef]
- Xu, F.Q.; Li, Y.Y.; Ge, X.M.; Yang, L.C.; Li, Y. Anaerobic digestion of food waste—Challenges and opportunities. Bioresour. Technol. 2018, 247, 1047–1058. [Google Scholar] [CrossRef]
- Chow, W.L.; Chong, S.; Lim, J.W.; Chan, Y.J.; Chong, M.F.; Tiong, T.J.; Chin, J.K.; Pan, G.-T. Anaerobic co-digestion of wastewater sludge: A review of potential co-substrates and operating factors for improved methane yield. Processes 2020, 8, 39. [Google Scholar] [CrossRef]
- Atandi, E.; Rahman, S. Prospect of anaerobic co-digestion of dairy manure: A review. Environ. Technol. Rev. 2012, 1, 127–135. [Google Scholar] [CrossRef]
- Zarkadas, I.S.; Sofikiti, A.S.; Voudrias, E.A.; Pilidis, G.A. Thermophilic anaerobic digestion of pasteurised food wastes and dairy cattle manure in batch and large volume laboratory digesters: Focussing on mixing ratios. Renew. Energy 2015, 80, 432–440. [Google Scholar] [CrossRef]
- Pagliaccia, P.; Gallipoli, A.; Gianico, A.; Gironi, F.; Montecchio, D.; Pastore, C.; di Bitonto, L.; Braguglia, C.M. Variability of food waste chemical composition: Impact of thermal pre-treatment on lignocellulosic matrix and anaerobic biodegradability. J. Environ. Manage. 2019, 236, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Banks, C.J.; Zhang, Y. Technical Report: Optimising Inputs and Outputs from Anaerobic Digestion Processes; Defra Project Code WR0212 (Department for Environment, Food and Rural Affairs, London, UK); University of Southampton: Southampton, UK, 2010. Available online: http://randd.defra.gov.uk/Document.aspx?Document=WR0212_8889_TRP.pdf (accessed on 10 September 2020).
- Zhang, Y.; Kusch-Brandt, S.; Gu, S.; Heaven, S. Particle size distribution in municipal solid waste pre-treated for bioprocessing. Resources 2019, 8, 166. [Google Scholar] [CrossRef]
- Gonzalez-Estrella, J.; Asato, C.M.; Jerke, A.C.; Stone, J.J.; Gilcrease, P.C. Effect of structural carbohydrates and lignin content on the anaerobic digestion of paper and paper board materials by anaerobic granular sludge. Biotechnol. Bioeng. 2017, 114, 951–960. [Google Scholar] [CrossRef]
- Yuan, X.; Wen, B.; Ma, X.; Zhu, W.; Wang, X.; Chen, S.; Cui, Z. Enhancing the anaerobic digestion of lignocellulose of municipal solid waste using a microbial pretreatment method. Bioresour. Technol. 2014, 154, 1–9. [Google Scholar] [CrossRef]
- Zhang, Y.; Banks, C.J.; Heaven, S. Anaerobic digestion of two biodegradable municipal waste streams. J. Environ. Manage. 2012, 104, 166–174. [Google Scholar] [CrossRef]
- Walker, M.; Zhang, Y.; Heaven, S.; Banks, C.J. Potential errors in the quantitative evaluation of biogas production in anaerobic digestion processes. Bioresour. Technol. 2009, 100, 6339–6346. [Google Scholar] [CrossRef]
- Symons, G.E.; Buswell, A.M. The methane fermentation of carbohydrates. J. Am. Chem. Soc. 1933, 55, 2028–2036. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Hansen, K.H.; Angelidaki, I.; Ahring, B.K. Anaerobic digestion of swine manure: Inhibition by ammonia. Water Res. 1998, 32, 5–12. [Google Scholar] [CrossRef]
- US EPA. Method 9071B: N-Hexane Extractable Material (HEM) for Sludge, Sediment, and Solid Samples. Test Methods for Evaluating Solid Waste, Physical/chemical Methods; US EPA SW-846 Compendium; US Environmental Protection Agency: Washington, DC, USA, 1998. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral-detergent fiber and non-starch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Kitcherside, M.A.; Glen, E.F.; Webster, A.J.F. FibreCap: An improved method for the rapid analysis of fibre in feeding stuffs. Anim. Feed Sci. Tech. 2000, 86, 125–132. [Google Scholar] [CrossRef]
- Mousavioun, P.; Doherty, W.O.S. Chemical and thermal properties of fractionated bagasse soda lignin. Ind. Crops Prod. 2010, 31, 52–58. [Google Scholar] [CrossRef]
- Achinas, S.; Li, Y.; Achinas, V.; Euverink, G.J.W. Biogas potential from the anaerobic digestion of potato peels: Process performance and kinetics evaluation. Energies 2019, 12, 2311. [Google Scholar] [CrossRef]
- Cuetos, M.J.; Gómez, X.; Martínez, E.J.; Fierro, J.; Otero, M. Feasibility of anaerobic co-digestion of poultry blood with maize residues. Bioresour. Technol. 2013, 144, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Kramer, S.L.; Waibel, P.E.; Behrends, B.R.; El Kandelgy, S.M. Amino acids in commercially produced blood meals. J. Agr. Food Chem. 1978, 26, 979–981. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, I.R.; Pullammanappallil, P.C. Protein degradation during anaerobic wastewater treatment: Derivation of stoichiometry. Biodegradation 2001, 12, 247–256. [Google Scholar] [CrossRef]
- Atelge, M.; Atabani, A.; Banu, J.R.; Krisa, D.; Kaya, M.; Eskicioglu, C.; Kumar, G.; Lee, C.; Yildiz, Y.S.; Unalan, S.; et al. A critical review of pretreatment technologies to enhance anaerobic digestion and energy recovery. Fuel 2020, 270, 117494. [Google Scholar] [CrossRef]
- Schirmer, M.; Jekle, M.; Becker, T. Starch gelatinization and its complexity for analysis. Starch 2015, 67, 30–41. [Google Scholar] [CrossRef]
- Liu, Q.; Tarn, R.; Lynch, D.; Skjodt, N.M. Physicochemical properties of dry matter and starch from potatoes grown in Canada. Food Chem. 2007, 105, 897–907. [Google Scholar] [CrossRef]
- Sveinbjornsson, J.; Murphy, M.; Uden, P. In vitro evaluation of starch degradation from feeds with or without various heat treatments. Anim. Feed Sci. Tech. 2007, 132, 171–185. [Google Scholar] [CrossRef]
- Eriksson, T.; Murphy, M. Ruminal digestion of leguminous forage, potatoes and fodder beets in batch culture: I. Fermentation pattern. Anim. Feed Sci. Tech. 2004, 111, 73–88. [Google Scholar] [CrossRef]
- Zhu, F.; Hua, Y.; Li, G. Physicochemical properties of potato, sweet potato and quinoa starch blends. Food Hydrocoll. 2020, 100, 105278. [Google Scholar] [CrossRef]
- Ai, Y.; Jan, J.-I. Gelatinization and rheological properties of starch. Starch 2014, 67, 213–224. [Google Scholar] [CrossRef]
- Wilkins, K. Volatile organic compounds from household waste. Chemosphere 1994, 29, 47–53. [Google Scholar] [CrossRef]
- Agapiou, A.; Vamvakari, J.P.; Andrianopoulos, A.; Pappa, A. Volatile emissions during storing of green food waste under different aeration conditions. Environ. Sci. Pollut. Res. 2016, 23, 8890–8901. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Zhang, Y.; Kusch-Brandt, S.; Banks, C.J. Comparison of variable and constant loading for mesophilic food waste digestion in a long-term experiment. Energies 2020, 13, 1279. [Google Scholar] [CrossRef]
- Holliger, C.; Alves, M.; Andrade, D.; Angelidaki, I.; Astals, S.; Baier, U.; Bougrier, C.; Buffière, P.; Carballa, M.; De Wilde, V.; et al. Towards a standardization of biomethane potential tests. Water Sci. Technol. 2016, 74, 2515–2522. [Google Scholar] [CrossRef] [PubMed]
- Koch, K.; Hefner, S.D.; Weinrich, S.; Astals, S.; Holliger, C. Power and limitations of biochemical methane potential (BMP) tests. Front. Energy Res. 2020, 8, 63. [Google Scholar] [CrossRef]
| Food Waste | Slaughter-House Waste 1 | Animal Blood 2 | Cattle Slurry | Potato Waste | Card Packaging | OFMSW | |
|---|---|---|---|---|---|---|---|
| Basic characteristics relevant for anaerobic digestion including nutrients | |||||||
| pH | 4.71 ± 0.01 (1:5) 3 | 5.96 ± 0.04 (1:5) 3 | 7.23 ± 0.06 | 7.83 ± 0.07 (1:5) 3 | 8.12 ± 0.01 (1:5) 3 | 7.21± 0.03 (1:30) 3 | 6.39 ± 0.01 (1:5) 3 |
| TS (% WW) | 23.7 ± 0.1 | 20.8 ± 0.3 | 19.7 ± 0.3 | 9.31 ± 0.14 | 24.7 ± 0.0 | 93.9 ± 0.1 | 52.8 ± 0.6 |
| VS (% WW) | 21.7 ± 0.1 | 19.4 ± 0.3 | 18.9 ± 0.3 | 6.52 ± 0.04 | 23.1 ± 0.0 | 78.5 ± 0.4 | 33.6 ± 0.6 |
| VS (% TS) | 91.4 ± 0.4 | 93.2 ± 0.1 | 95.6 ± 0.1 | 70.0 ± 0.6 | 93.2 ± 0.0 | 83.6 ± 0.5 | 63.5 ± 1.9 |
| TOC (% TS) | 47.6 ± 0.5 | 45.5 ± 1.7 | 41.9 ± 0.7 | 38.9 ± 1.0 | 42.7 ± 1.1 | 41.6 ± 0.7 | 35.0 ± 0.4 |
| TAN (% TS) | - | - | - | 1.15 ± 0.01 | - | - | - |
| TKN (% TS) | 3.42 ± 0.04 | 7.95 ± 0.12 | 14.7 ± 0.0 | 3.50 ± 0.05 | 1.53 ± 0.01 | 0.144 ± 0.001 | 1.39 ± 0.08 |
| TP (g kg−1 TS) | 5.41 ± 0.32 | 8.10 ± 0.13 | 0.835 ± 0.036 | 8.58 ± 0.63 | 3.59 ± 0.48 | 0.134 ± 0.003 | 2.17 ± 0.25 |
| TK (g kg−1 TS) | 14.3 ± 0.8 | 10.9 ± 0.1 | 3.71 ± 0.11 | 16.7 ± 0.2 | 23.8 ± 0.8 | 0.221 ± 0.011 | 4.26 ± 0.37 |
| TOC/TKN | 13.9 ± 0.2 | 5.73 ± 0.23 | 2.85 ± 0.05 | 11.1 ± 0.3 | 27.9 ± 0.7 | 288 ± 5 | 25.2 ± 1.5 |
| Biodegradable C/TKN | 13.6 ± 0.3 | 5.58 ± 0.25 | 2.85 ± 0.05 | 8.12 ± 2.00 | 27.5 ± 0.8 | 207 ± 54 | 19.6 ± 3.9 |
| CV (kJ g−1 TS) | 20.66 ± 0.18 | 26.21±0.01 | 22.91 ± 0.25 | 16.75 ± 0.10 | 16.50 ± 0.10 | 17.18 ± 0.36 | 13.90 ± 0.23 |
| Biochemical composition of substrates, expressed on a VS basis (in g kg−1 VS) | |||||||
| Non-structural carbohydrates 4 | 508.9 ± 4.9 | <10 | 25.1 ± 2.2 | 144.5 ± 12.0 | 832.0 ± 3.7 | 14.6 | 313.2 ± 47.1 |
| Lipids 5 | 151.2 ± 0.9 | 348.9 ± 7.6 | <10 | 93.6 ± 0.8 | <10 | <10 | 68.6 ± 5.4 |
| Crude proteins | 235.0 ± 2.6 | 537.6 ± 7.8 | 964.9 ± 2.2 | 213.5 ± 3.7 | 102.7 ± 0.3 | 10.8 ± 0.0 | 130.0 ± 7.4 |
| Hemi-cellulose | 38.1 ± 3.7 | 46.3 ± 2.9 | - | 225.6 ± 8.2 | 22.0 ± 0.4 | 127.8 6 | 52.2 ± 12.3 |
| Cellulose | 50.4 ± 1.6 | 46.0 ± 4.0 | - | 96.7 ± 3.0 | 22.1 ± 2.8 | 623.9 6 | 252.0 ± 36.2 |
| Lignin | 16.5 ± 0.2 | 18.5 ± 2.1 | - | 226.1 ± 7.3 | 11.2 ± 2.3 | 212.9 6 | 184.0 ± 25.9 |
| Elemental analysis (in % of TS) | |||||||
| N | 3.42 ± 0.04 | 7.95 ± 0.12 | 14.7 ± 0.0 | 3.50 ± 0.05 | 1.53 ± 0.01 | 0.14± 0.00 | 1.39 ± 0.08 |
| C | 47.9 ± 0.5 | 45.6 ± 1.7 | 42.1 ± 0.7 | 39.2 ± 1.0 | 43.7 ± 1.1 | 41.6 ± 0.7 | 35.1 ± 0.5 |
| H | 7.03 ± 0.26 | 8.04 ± 0.38 | 7.33 ± 0.37 | 5.18 ± 0.15 | 7.18 ± 0.20 | 4.76 ± 0.23 | 5.06 ± 0.32 |
| S | 0.15 ± 0.01 | 0.62 ± 0.03 | 1.00 ± 0.02 | 0.31 ± 0.02 | 0.06 ± 0.02 | 0.21 ± 0.00 | 0.27 ± 0.04 |
| O | 34.3 ± 2.5 | 23.3 ± 1.7 | 27.1 ± 0.9 | 23.1 ± 0.9 | 38.8 ± 1.3 | 36.9 ± 0.9 | 25.1 ± 1.2 |
| Potentially toxic elements (in mg kg−1 TS) | |||||||
| Cd | <1.0 | <1.0 | <1.0 | <1.0 | <1.0 | <0.05 | 1.50 ± 0.37 |
| Cr | 30.8 ± 0.6 | 14.6 ± 0.3 | <2.0 | 113 ± 2 | 6.9 ± 0.5 | 9.1 ± 0.9 | 263 ± 11 |
| Cu | 7.20 ± 0.81 | 37.9 ± 0.5 | 6.7 ± 0.3 | 58.4 ± 1.1 | 9.8 ± 0.7 | 20.3 ± 2.3 | 107 ± 10 |
| Hg | <0.010 | < 0.010 | <0.010 | <0.010 | <0.010 | <0.10 | 0.179 ± 0.018 |
| Ni | 7.0 ± 2.9 | 6.9 ± 0.3 | < 5.0 | 44.8 ± 0.6 | <5.0 | 4.5 ± 0.5 | 97.0 ± 2.9 |
| Pb | <10 | <10 | < 10 | <10 | <10 | 2.9 ± 0.4 | 162 ± 11 |
| Zn | 33 ± 11 | 250 ± 0 | 16.3 ± 0.2 | 231 ± 6 | 20.3 ± 0.5 | 16.2 ± 4.3 | 259 ± 4 |
| Substrate | Theoretical BMP Value (STP m3·kg−1 VS) | Unpasteurised | Pasteurised | ||
|---|---|---|---|---|---|
| Experimental BMP Value (STP m3 kg−1 VS) | Ratio of Experimental to Theoretical Value (%) | Experimental BMP Value (STP m3·kg−1 VS) | Ratio of Experimental to Theoretical Value (%) | ||
| Food waste | 0.507 | 0.475 ± 0.031 | 93.7 | 0.473 ± 0.026 | 93.3 |
| Cattle slurry | 0.393 | 0.267 ± 0.031 | 67.9 | 0.269 ± 0.019 | 68.4 |
| Card packaging | 0.327 | 0.266 ± 0.010 | 81.3 | 0.267 ± 0.005 | 81.7 |
| Potato waste | 0.407 | 0.353 ± 0.004 | 86.7 | 0.395 ± 0.014 | 97.1 |
| Slaughterhouse waste | 0.659 | 0.595 ± 0.014 | 90.3 | 0.575 ± 0.025 | 87.3 |
| Animal blood | 0.498 | 0.418 ± 0.013 | 83.9 | 0.479 ± 0.026 | 96.2 |
| OFMSW | 0.384 | 0.349 ± 0.013 | 90.9 | 0.330 ± 0.019 | 86.0 |
| Substrate | BMP Unpasteurised Material (STP m3·tonne−1 WW) | BMP Pasteurised Material (STP m3·tonne−1 WW) |
|---|---|---|
| Food waste | 102 | 102 |
| Cattle slurry | 17.4 | 17.5 |
| Card packaging | 210 | 211 |
| Potato waste | 81.5 | 91.2 |
| Slaughterhouse waste | 115 | 112 |
| Animal blood | 79.0 | 90.5 |
| OFMSW | 114 | 108 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Kusch-Brandt, S.; Heaven, S.; Banks, C.J. Effect of Pasteurisation on Methane Yield from Food Waste and Other Substrates in Anaerobic Digestion. Processes 2020, 8, 1351. https://doi.org/10.3390/pr8111351
Zhang Y, Kusch-Brandt S, Heaven S, Banks CJ. Effect of Pasteurisation on Methane Yield from Food Waste and Other Substrates in Anaerobic Digestion. Processes. 2020; 8(11):1351. https://doi.org/10.3390/pr8111351
Chicago/Turabian StyleZhang, Yue, Sigrid Kusch-Brandt, Sonia Heaven, and Charles J. Banks. 2020. "Effect of Pasteurisation on Methane Yield from Food Waste and Other Substrates in Anaerobic Digestion" Processes 8, no. 11: 1351. https://doi.org/10.3390/pr8111351
APA StyleZhang, Y., Kusch-Brandt, S., Heaven, S., & Banks, C. J. (2020). Effect of Pasteurisation on Methane Yield from Food Waste and Other Substrates in Anaerobic Digestion. Processes, 8(11), 1351. https://doi.org/10.3390/pr8111351







