Latest Developments in Membrane (Bio)Reactors
Abstract
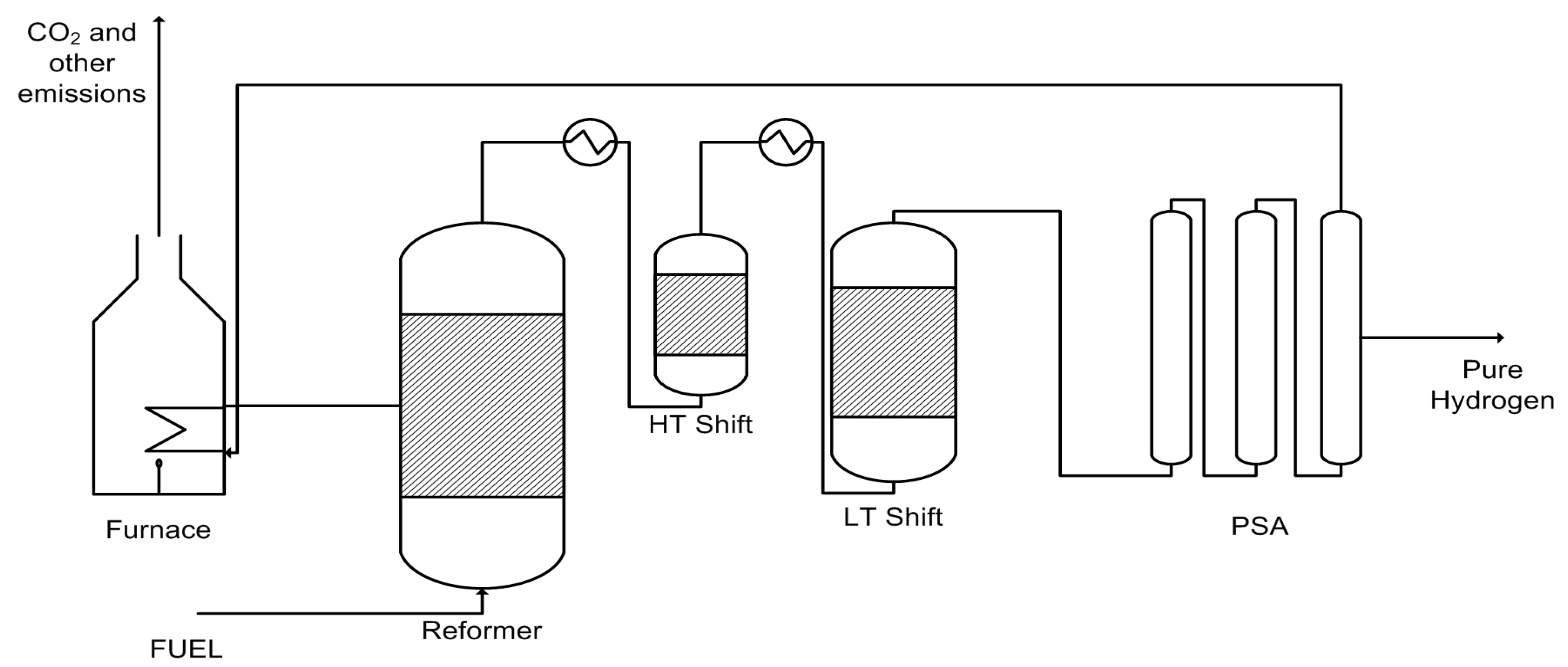
1. Introduction
2. Inorganic Membrane Reactors
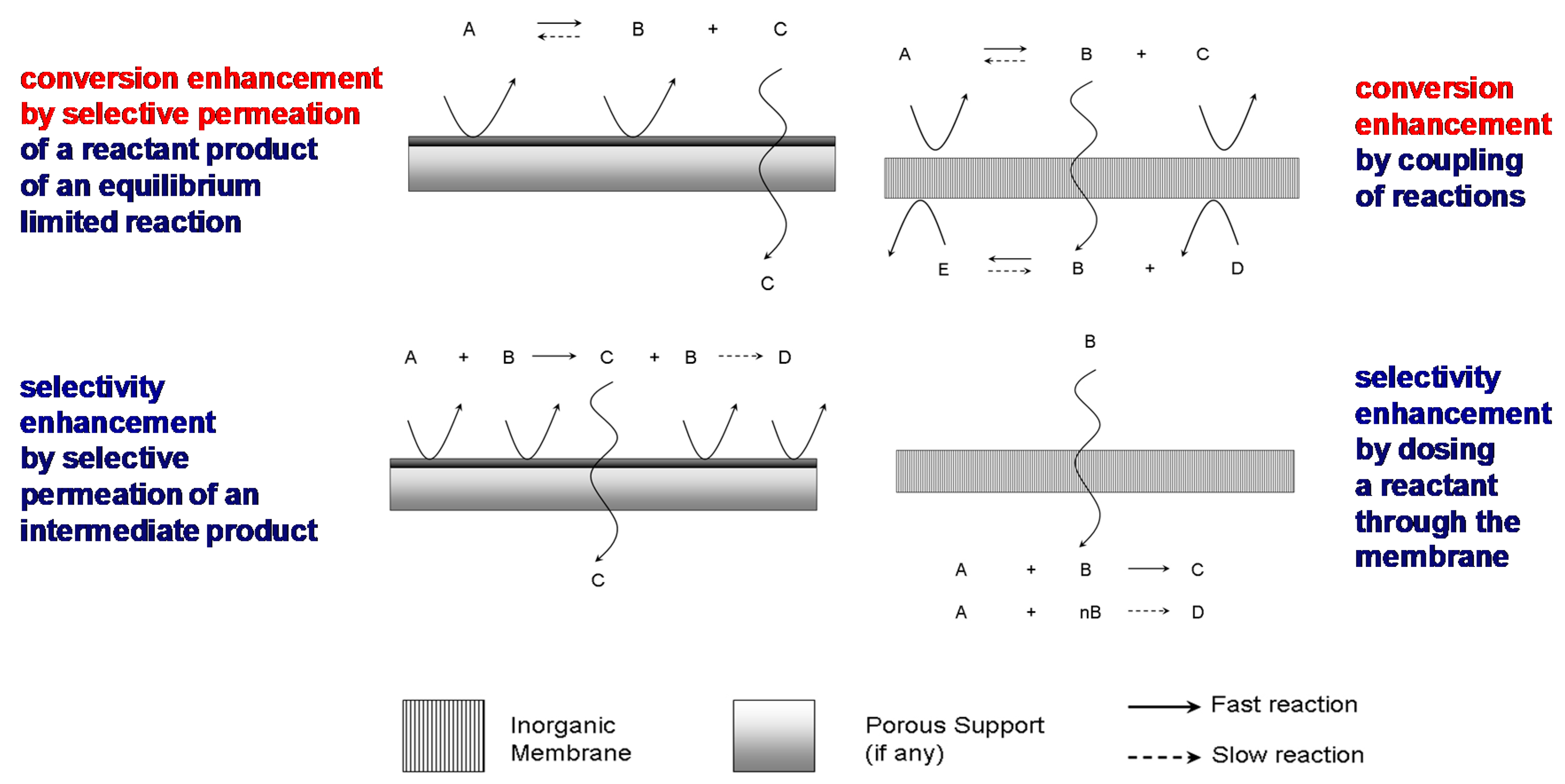
2.1. Applications of Inorganic Membrane Reactors
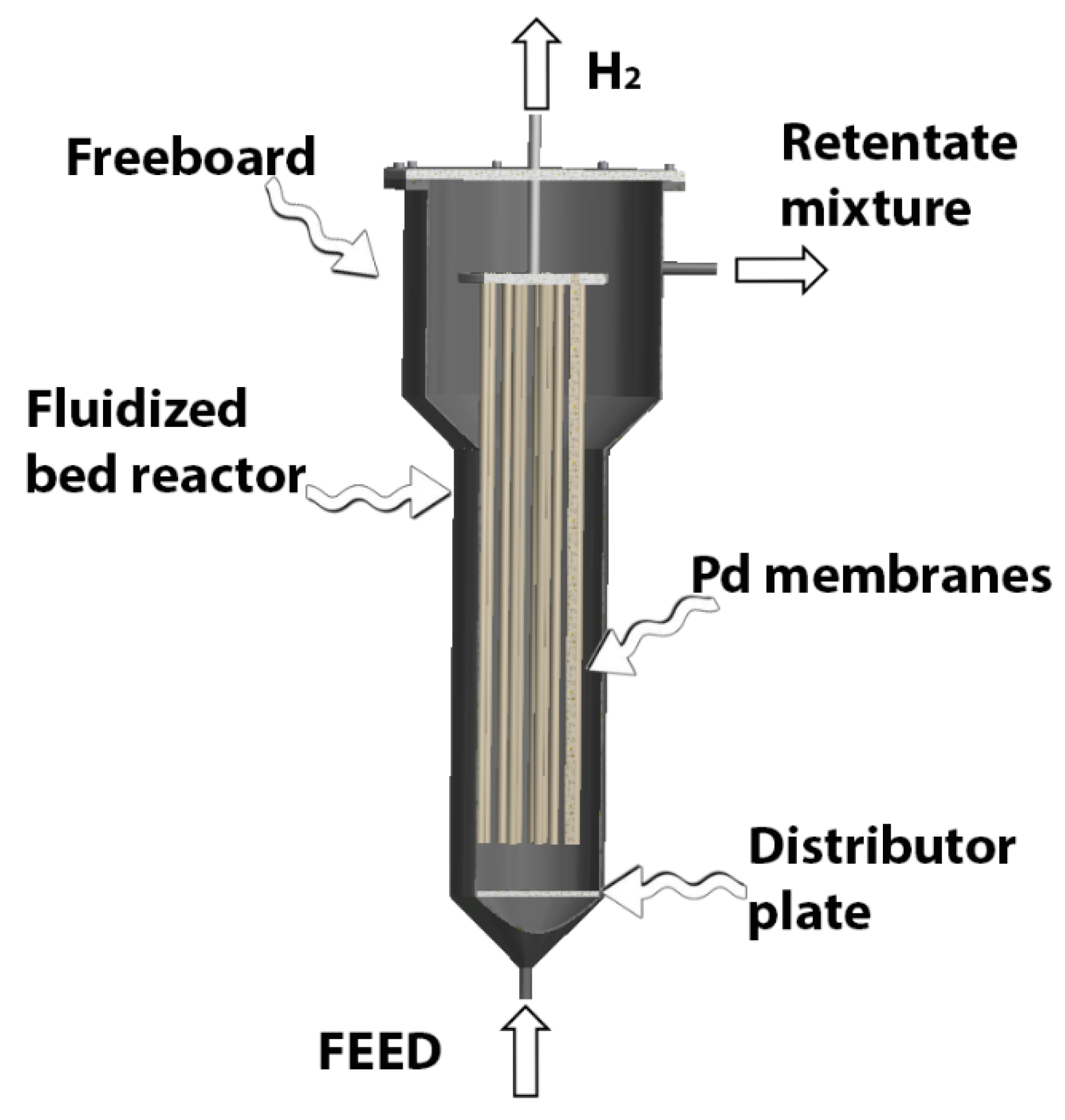
2.2. Inorganic Membrane Reactors Configurations
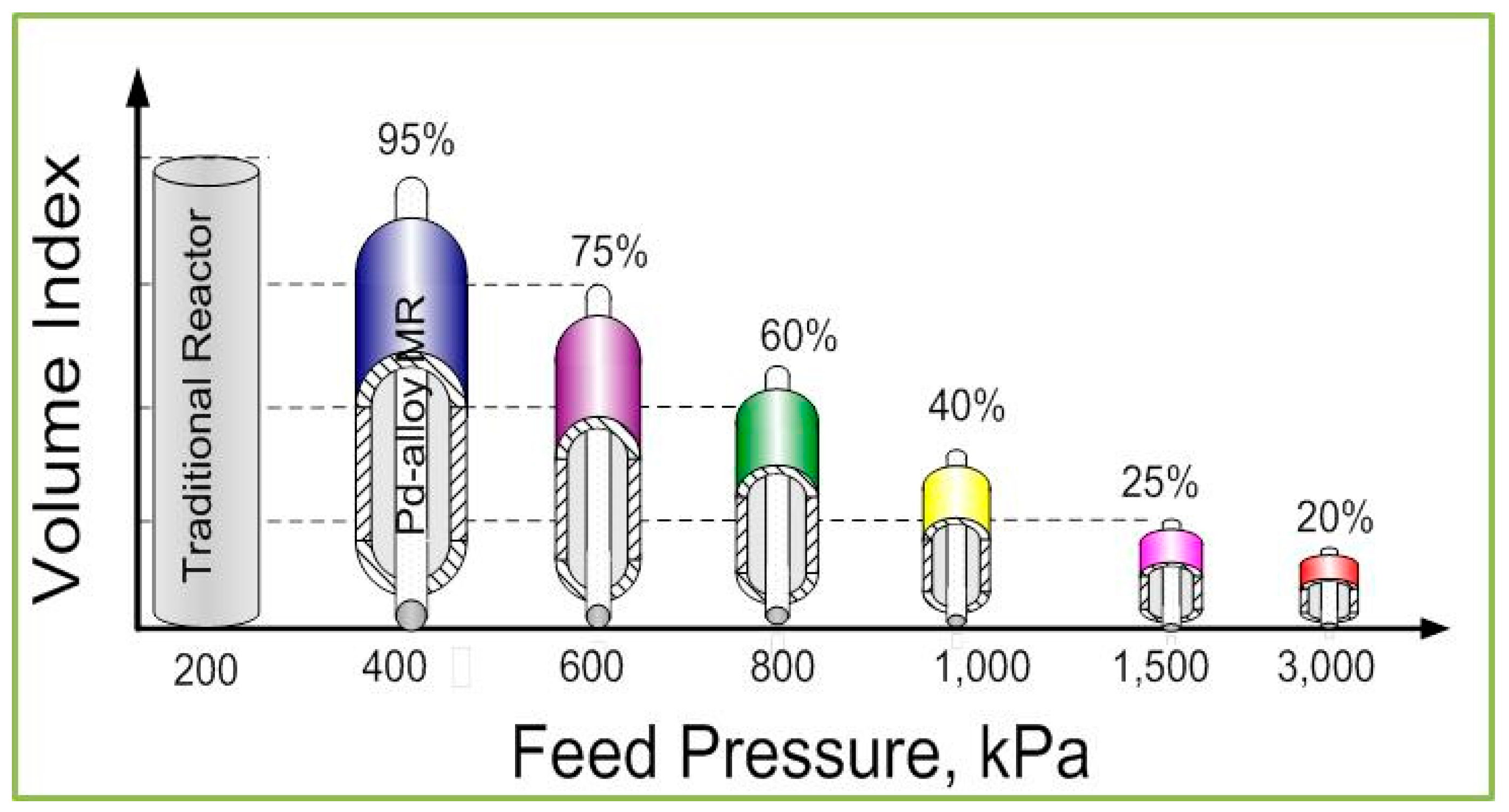
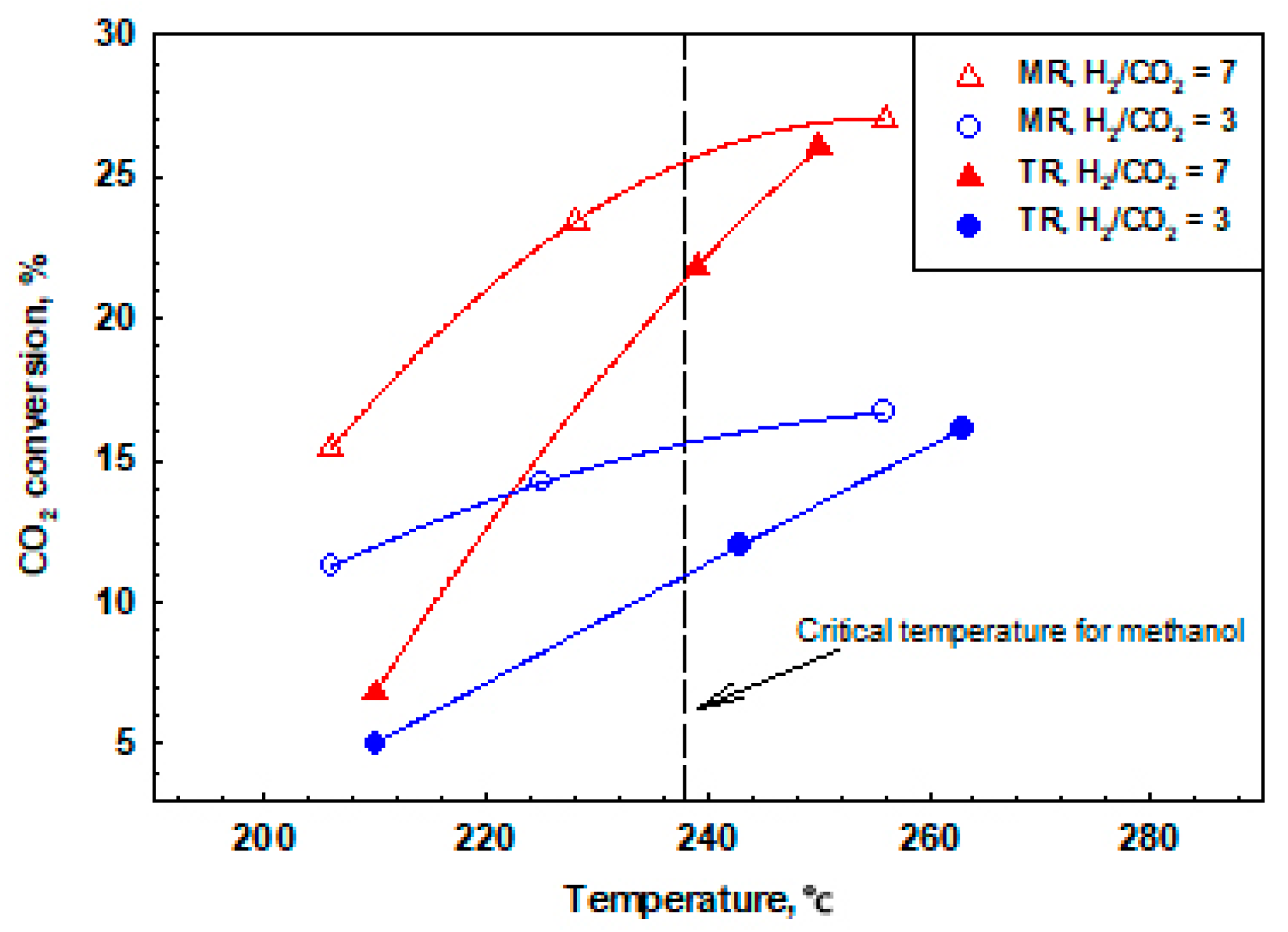
2.3. New Trends and Applications of Inorganic Membrane Reactors
3. Membrane Bioreactors
3.1. Applications of Membrane Bioreactors
3.1.1. Wastewater Treatment
3.1.2. Water Recycling
3.1.3. Bioconversion and Manufacturing of Bio-Products
3.1.4. Food Production
3.1.5. Biofuel Production
3.1.6. Pharmaceuticals and Biotechnology
3.1.7. Other Applications
3.2. Membrane Bioreactor Configurations and Types
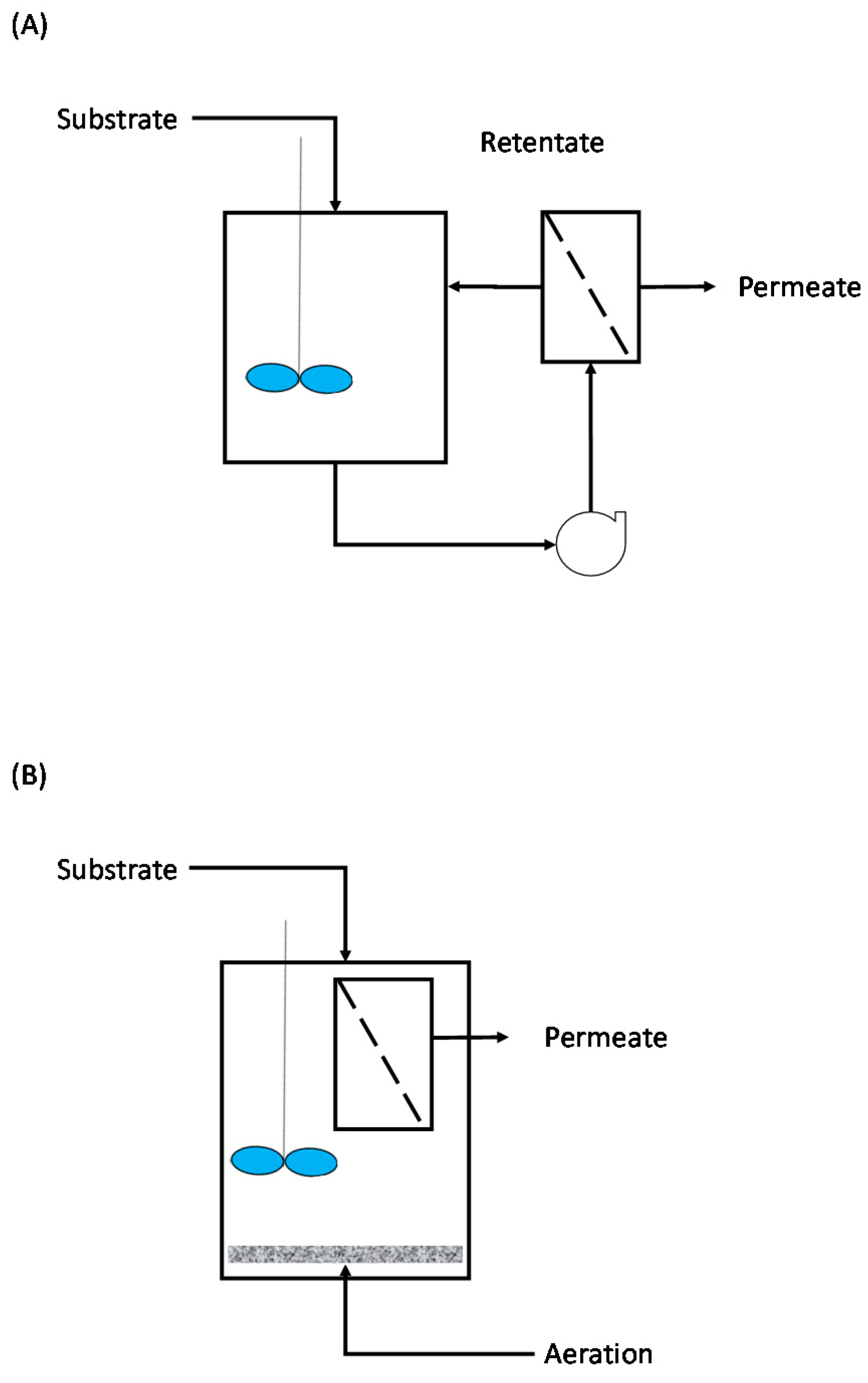
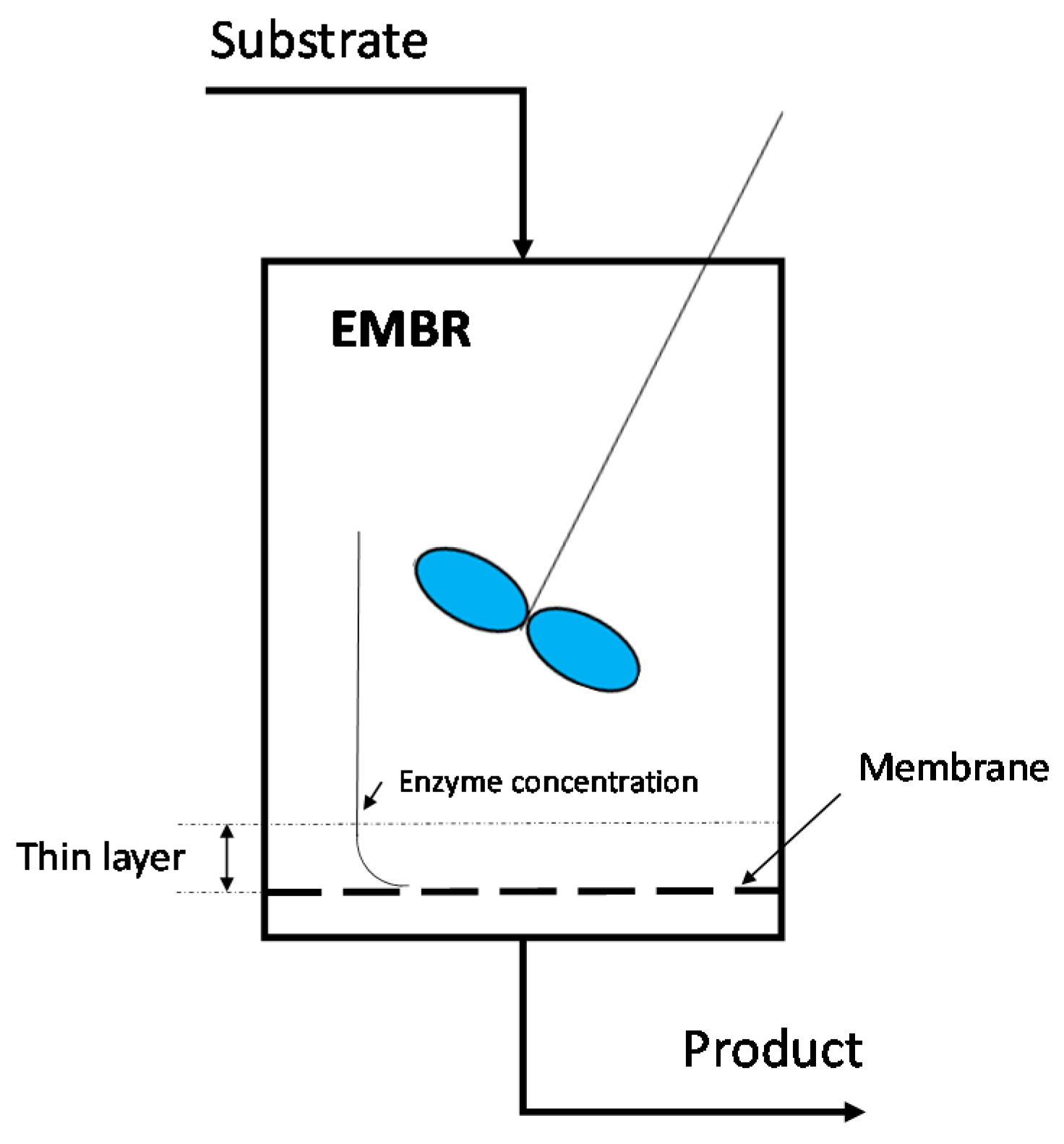
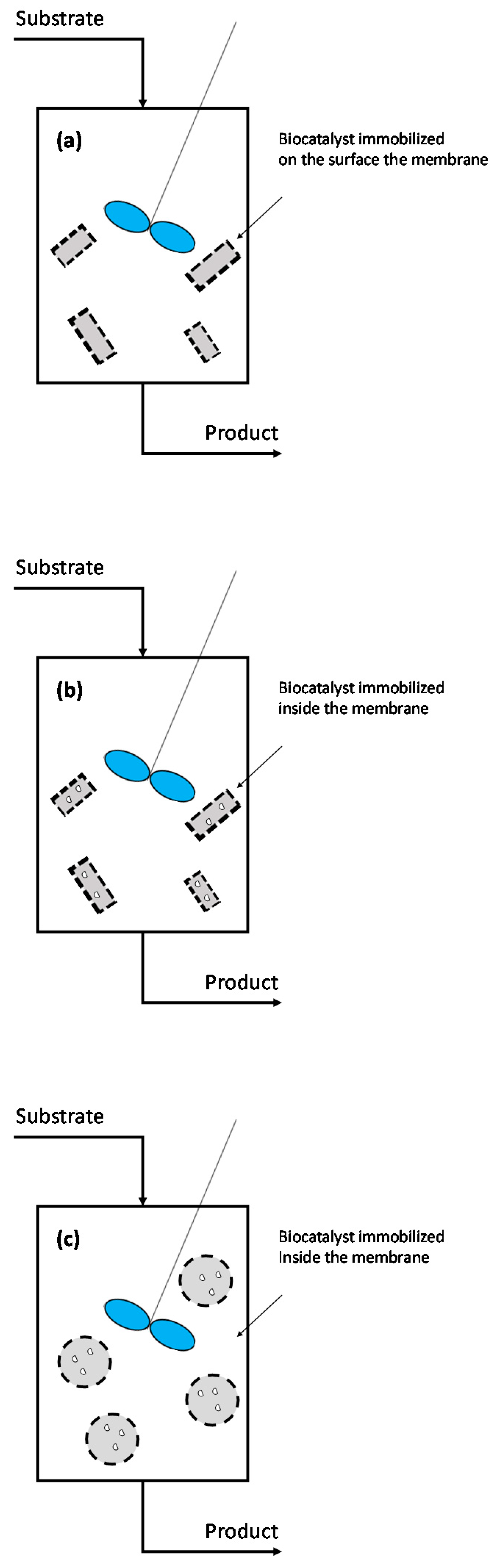
3.2.1. Enzymatic MBRs
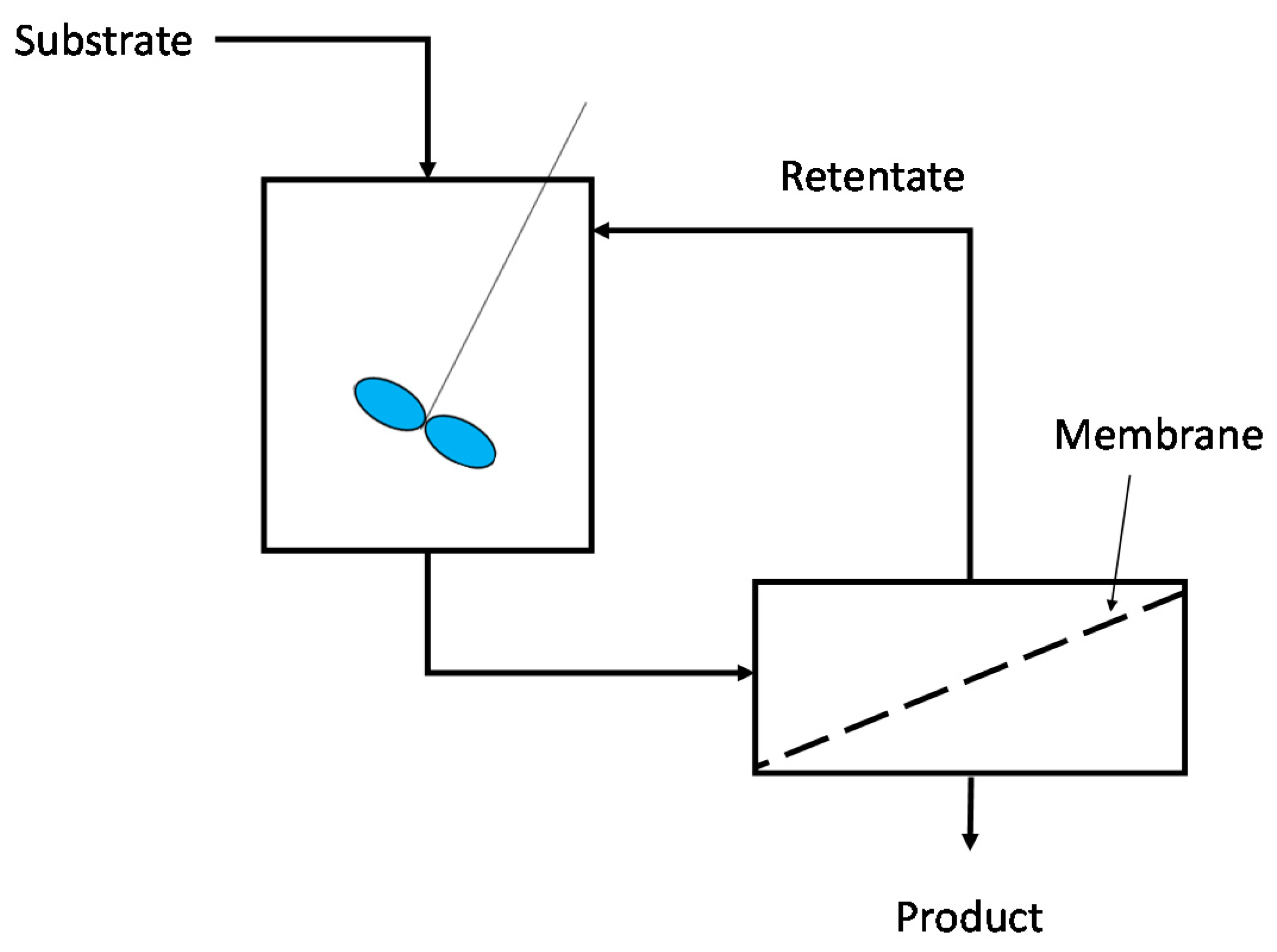
3.2.2. Free-Enzymatic MBRs
3.2.3. Continuous Stirred MBRs
3.2.4. Electrodialysis MBRs
3.2.5. Forward Osmosis MBRs
3.2.6. Membrane Photo-Bioreactor
3.3. Membrane Materials for Membrane Bioreactors
3.4. Challenges and Prospects
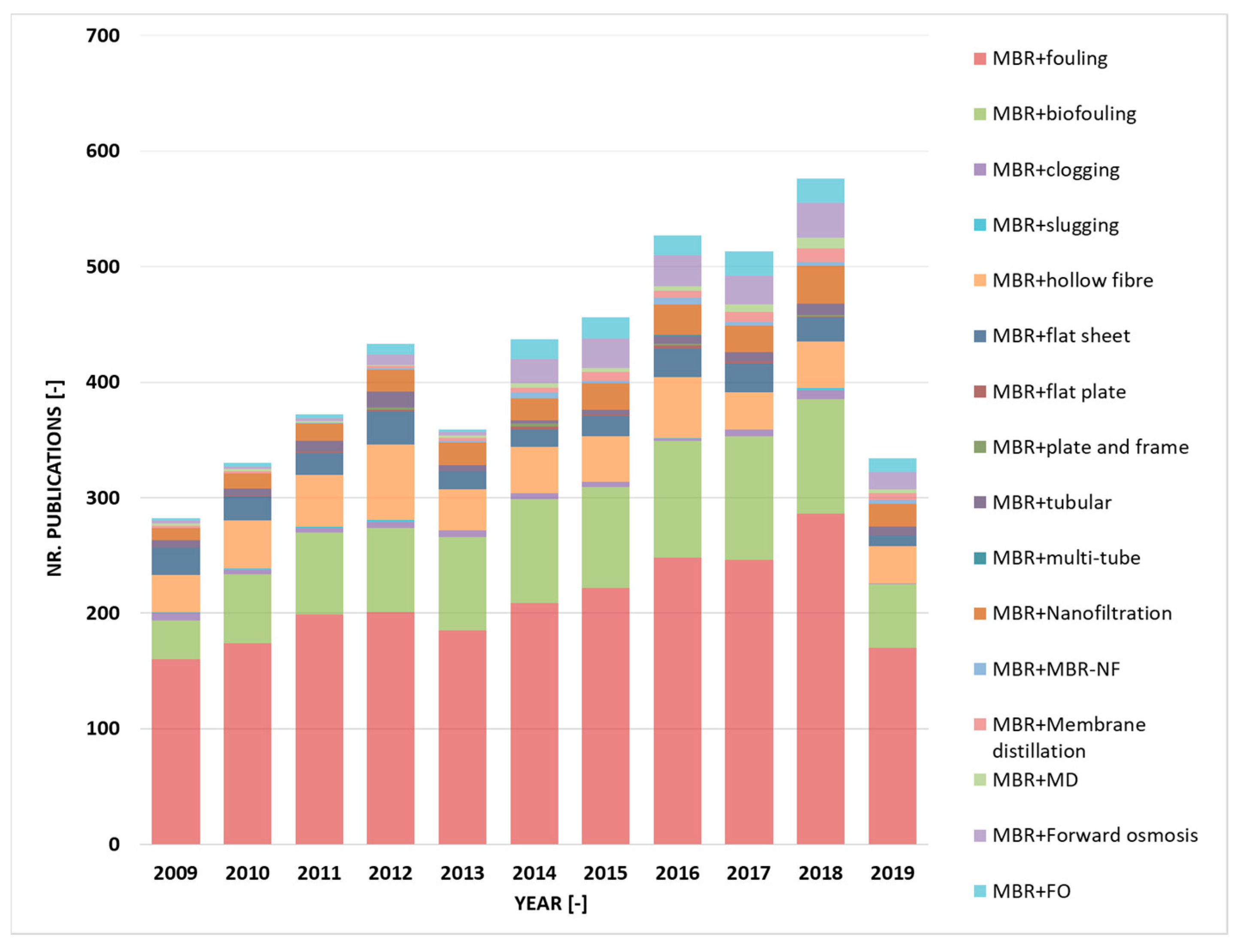
3.4.1. Fouling
3.4.2. Energy Consumption
3.5. Market Status and Economics
- -
- capital expenditure (CAPEX),
- -
- operating expenditure (OPEX),
- -
- material cost,
- -
- product cost.
3.6. New Trends and Future Perspectives
4. Conclusions
- -
- More efficient fouling control to guarantee the long-term performance of the system;
- -
- Reduced capital and operational cost associated with the MBR process (e.g., by lowering the membrane production cost, extending the membrane lifetime, and a reduction in the energy consumption);
- -
- Improved nutrient removal;
- -
- Positioning of MBRs in a more efficient application. Generally, MBRs are best suited for applications with a high effluent quality and cases with limited land use (e.g., densly populated areas).
Funding
Conflicts of Interest
Abbreviations
| Acronym | Definition |
| MBR | Membrane Bioreactor |
| AMBR | Aerobic Membrane Bioreactor |
| AnMBR | Anaerobic Membrane Bioreactor |
| HRT | Hydraulic Residence Time |
| SRT | Solids Residence Time |
| MLD | Mega liters per day |
| FOMBR | Forward Osmosis Membrane Bioreactor |
| sMBR | Submerged Membrane Bioreactor |
| iMBR | Immersed Membrane Bioreactor |
| UASB | Up-flow Anaerobic Sludge Blank |
| GSB | Granular Sludge Bed |
| EMBR | Enzymatic Membrane Bioreactors |
| CSMBR | Continuous Stirred Membrane Bioreactor |
| MPBR | Membrane Photo Bioreactor |
| PVDF | Polyvinylidene difluoride |
| PE | Polyethylene |
| PP | Polypropelene |
| PES | Polyethersulfone |
| PTFE | Polytetrafluoroethylene |
References
- Gallucci, F.; Basile, A.; Hai, F.I. Introduction—A Review of Membrane Reactors. In Membranes for Membrane Reactors: Preparation, Optimization and Selection; John Wiley & Sons: Chichester, UK, 2011; pp. 1–61. [Google Scholar] [CrossRef]
- Scholes, C.A.; Smith, K.H.; Kentish, S.; Stevens, G.W. CO2 capture from pre-combustion processes—Strategies for membrane gas separation. Int. J. Greenh. Gas Control. 2010, 4, 739–755. [Google Scholar] [CrossRef]
- Voldsund, M.; Jordal, K.; Anantharaman, R. Hydrogen production with CO2 capture. Int. J. Hydrogen Energy 2016, 41, 4969–4992. [Google Scholar] [CrossRef]
- Zhang, G.; Jin, W.; Xu, N. Design and Fabrication of Ceramic Catalytic Membrane Reactors for Green Chemical Engineering Applications. Engineering 2018, 4, 848–860. [Google Scholar] [CrossRef]
- Buonomenna, M.; Bae, J. Membrane processes and renewable energies. Renew. Sustain. Energy Rev. 2015, 43, 1343–1398. [Google Scholar] [CrossRef]
- Basile, A.; De Falco, M.; Centi, G.; Iaquaniello, G. Membrane Reactor Engineering; John Wiley & Sons: Hoboken, NJ, USA, 2016; ISBN 9781118906804. [Google Scholar]
- Wei, Y.; Yang, W.; Caro, J.; Wang, H. Dense ceramic oxygen permeable membranes and catalytic membrane reactors. Chem. Eng. J. 2013, 220, 185–203. [Google Scholar] [CrossRef]
- Zornoza, B.; Casado, C.; Navajas, A.; Casado-Coterillo, C. Advances in Hydrogen Separation and Purification with Membrane Technology; Elsevier: Amsterdam, The Netherlands, 2013; ISBN 9780444563521. [Google Scholar]
- Sirkar, K.K.; Fane, A.; Wang, R.; Wickramasinghe, S.R. Process intensification with selected membrane processes. Chem. Eng. Process. Process. Intensif. 2015, 87, 16–25. [Google Scholar] [CrossRef]
- Chaubey, R.; Sahu, S.; James, O.O.; Maity, S. A review on development of industrial processes and emerging techniques for production of hydrogen from renewable and sustainable sources. Renew. Sustain. Energy Rev. 2013, 23, 443–462. [Google Scholar] [CrossRef]
- Saeidi, S.; Amin, N.A.S.; Rahimpour, M.R. Hydrogenation of CO2 to value-added products—A review and potential future developments. J. CO2 Util. 2014, 5, 66–81. [Google Scholar] [CrossRef]
- Meng, L.; Tsuru, T. Microporous membrane reactors for hydrogen production. Curr. Opin. Chem. Eng. 2015, 8, 83–88. [Google Scholar] [CrossRef]
- Sunarso, J.; Hashim, S.S.; Zhu, N.; Zhou, W. Perovskite oxides applications in high temperature oxygen separation, solid oxide fuel cell and membrane reactor: A review. Prog. Energy Combust. Sci. 2017, 61, 57–77. [Google Scholar] [CrossRef]
- Barbieri, G.; Brunetti, A.; Caravella, A.; Drioli, E. Pd-based membrane reactors for one-stage process of water gas shift. RSC Adv. 2011, 1, 651–661. [Google Scholar] [CrossRef]
- Saeidi, S.; Fazlollahi, F.; Najari, S.; Iranshahi, D.; Klemeš, J.J.; Baxter, L.L. Hydrogen production: Perspectives, separation with special emphasis on kinetics of WGS reaction: A state-of-the-art review. J. Ind. Eng. Chem. 2017, 49, 1–25. [Google Scholar] [CrossRef]
- Brunetti, A.; Caravella, A.; Barbieri, G.; Drioli, E. Simulation study of water gas shift reaction in a membrane reactor. J. Membr. Sci. 2007, 306, 329–340. [Google Scholar] [CrossRef]
- Chiuta, S.; Everson, R.C.; Neomagus, H.W.; van Der Gryp, P.; Bessarabov, D. Reactor technology options for distributed hydrogen generation via ammonia decomposition: A review. Int. J. Hydrogen Energy 2013, 38, 14968–14991. [Google Scholar] [CrossRef]
- Gao, Y.; Jiang, J.; Meng, Y.; Yan, F.; Aihemaiti, A. A review of recent developments in hydrogen production via biogas dry reforming. Energy Convers. Manag. 2018, 171, 133–155. [Google Scholar] [CrossRef]
- Iulianelli, A.; Ribeirinha, P.; Mendes, A.; Basile, A. Methanol steam reforming for hydrogen generation via conventional and membrane reactors: A review. Renew. Sustain. Energy Rev. 2014, 29, 355–368. [Google Scholar] [CrossRef]
- Mendes, D.; Sá, S.; Tosti, S.; Sousa, J.; Madeira, L.M.; Mendes, A. Experimental and modeling studies on the low-temperature water-gas shift reaction in a dense Pd–Ag packed-bed membrane reactor. Chem. Eng. Sci. 2011, 66, 2356–2367. [Google Scholar] [CrossRef]
- Matsuka, M.; Higashi, M.; Ishihara, T. Hydrogen production from methane using vanadium-based catalytic membrane reactors. Int. J. Hydrogen Energy 2013, 38, 6673–6680. [Google Scholar] [CrossRef]
- Silva, F.S.A.; Benachour, M.; Abreu, C.A.M. Evaluating Hydrogen Production in Biogas Reforming in a Membrane Reactor. Braz. J. Chem. Eng. 2015, 32, 201–210. [Google Scholar] [CrossRef]
- Gil, A.G.; Wu, Z.; Chadwick, D.; Li, K. Ni/SBA-15 Catalysts for combined steam methane reforming and water gas shift—Prepared for use in catalytic membrane reactors. Appl. Catal. A Gen. 2015, 506, 188–196. [Google Scholar] [CrossRef]
- Gallucci, F.; Paturzo, L.; Fama, A.; Basile, A. Experimental Study of the Methane Steam Reforming Reaction in a Dense Pd/Ag Membrane Reactor. Ind. Eng. Chem. Res. 2004, 43, 928–933. [Google Scholar] [CrossRef]
- Gallucci, F.F.; Fernandez, E.E.; Corengia, P.; Annaland, M.M.V.S. Recent advances on membranes and membrane reactors for hydrogen production. Chem. Eng. Sci. 2013, 92, 40–66. [Google Scholar] [CrossRef]
- Caravella, A.; Barbieri, G.; Drioli, E. Concentration polarization analysis in self-supported Pd-based membranes. Sep. Purif. Technol. 2009, 66, 613–624. [Google Scholar] [CrossRef]
- Nordio, M.; Soresi, S.; Manzolini, G.; Melendez, J.; van Sint Annaland, M.; Tanaka, D.P.; Gallucci, F. Effect of sweep gas on hydrogen permeation of supported Pd membranes: Experimental and modeling. Int. J. Hydrogen Energy 2019, 44, 4228–4239. [Google Scholar] [CrossRef]
- Caravella, A.; Melone, L.; Sun, Y.; Brunetti, A.; Drioli, E.; Barbieri, G. Concentration polarization distribution along Pd-based membrane reactors: A modelling approach applied to Water-Gas Shift. Int. J. Hydrogen Energy 2016, 41, 2660–2670. [Google Scholar] [CrossRef]
- Dittmeyer, R.; Boeltken, T.; Piermartini, P.; Selinsek, M.; Loewert, M.; Dallmann, F.; Kreuder, H.; Cholewa, M.; Wunsch, A.; Belimov, M.; et al. Micro and micro membrane reactors for advanced applications in chemical energy conversion. Curr. Opin. Chem. Eng. 2017, 17, 108–125. [Google Scholar] [CrossRef]
- Wunsch, A.; Kant, P.; Mohr, M.; Haas-Santo, K.; Pfeifer, P.; Dittmeyer, R. Recent Developments in Compact Membrane Reactors with Hydrogen Separation. Membranes 2018, 8, 107. [Google Scholar] [CrossRef]
- Wilhite, B.A. Unconventional microreactor designs for process intensification in the distributed reforming of hydrocarbons: A review of recent developments at Texas A&M University. Curr. Opin. Chem. Eng. 2017, 17, 100–107. [Google Scholar] [CrossRef]
- Gallucci, F.; van Sint Annaland, M.; Kuipers, J.A.M. Autothermal Reforming of Methane with Integrated CO2 Capture in a Novel Fluidized Bed Membrane Reactor. Part 1: Experimental Demonstration. Top. Catal. 2008, 51, 133–145. [Google Scholar] [CrossRef]
- Marra, L.; Wolbers, P.; Gallucci, F.; van Sint Annaland, M. Development of a RhZrO2 catalyst for low temperature autothermal reforming of methane in membrane reactors. Catal. Today 2014, 236, 23–33. [Google Scholar] [CrossRef]
- Peters, T.; Stange, M.; Sunding, M.F.; Bredesen, R. Stability investigation of micro-configured Pd–Ag membrane modules – Effect of operating temperature and pressure. Int. J. Hydrogen Energy 2015, 40, 3497–3505. [Google Scholar] [CrossRef]
- Helmi, A.; Voncken, R.; Raijmakers, A.; Roghair, I.; Gallucci, F.; van Sint Annaland, M. On concentration polarization in fluidized bed membrane reactors. Chem. Eng. J. 2018, 332, 464–478. [Google Scholar] [CrossRef]
- De Nooijer, N.; Gallucci, F.; Pellizzari, E.; Meléndez, J.; Tanaka, D.P.; Manzolini, G.; van Sint Annaland, M. On concentration polarisation in a fluidized bed membrane reactor for biogas steam reforming: Modelling and experimental validation. Chem. Eng. J. 2018, 348, 232–243. [Google Scholar] [CrossRef]
- Helmi, A.; Fernandez, E.; Rey, J.M.; Tanaka, D.P.; Gallucci, F.; van Sint Annaland, M. Fluidized Bed Membrane Reactors for Ultra Pure H2 Production—A Step forward towards Commercialization. Molecules 2016, 21, 376. [Google Scholar] [CrossRef]
- Medrano, J.A.; Spallina, V.; van Sint Annaland, M.; Gallucci, F. Thermodynamic analysis of a membrane-assisted chemical looping reforming reactor concept for combined H2 production and CO2 capture. Int. J. Hydrogen Energy 2014, 39, 4725–4738. [Google Scholar] [CrossRef]
- Medrano, J.; Potdar, I.; Melendez, J.; Spallina, V.; Tanaka, D.P.; van Sint Annaland, M.; Gallucci, F. The membrane-assisted chemical looping reforming concept for efficient H2 production with inherent CO2 capture: Experimental demonstration and model validation. Appl. Energy 2018, 215, 75–86. [Google Scholar] [CrossRef]
- Spallina, V.; Pandolfo, D.; Battistella, A.; Romano, M.C.; van Sint Annaland, M.; Gallucci, F. Techno-economic assessment of membrane assisted fluidized bed reactors for pure H 2 production with CO2 capture. Energy Convers. Manag. 2016, 120, 257–273. [Google Scholar] [CrossRef]
- Wassie, S.A.; Gallucci, F.; Zaabout, A.; Cloete, S.; Amini, S.; van Sint Annaland, M. Hydrogen production with integrated CO2capture in a novel gas switching reforming reactor: Proof-of-concept. Int. J. Hydrogen Energy 2017, 42, 14367–14379. [Google Scholar] [CrossRef]
- Wassie, S.A.; Cloete, S.; Spallina, V.; Gallucci, F.; Amini, S.; van Sint Annaland, M. Techno-economic assessment of membrane-assisted gas switching reforming for pure H2 production with CO2 capture. Int. J. Greenh. Gas Control. 2018, 72, 163–174. [Google Scholar] [CrossRef]
- Silva, J.M.; Ribeiro, L.S.; Órfão, J.; Tosti, S.; Soria, M.; Madeira, L.M. From sorption-enhanced reactor to sorption-enhanced membrane reactor: A step towards H2 production optimization through glycerol steam reforming. Chem. Eng. J. 2019, 368, 795–811. [Google Scholar] [CrossRef]
- Wang, M.; Zhou, Y.; Tan, X.; Gao, J.; Liu, S. Nickel hollow fiber membranes for hydrogen separation from reformate gases and water gas shift reactions operated at high temperatures. J. Membr. Sci. 2019, 575, 89–97. [Google Scholar] [CrossRef]
- Løvvik, O.M.; Peters, T.; Bredesen, R. First-principles calculations on sulfur interacting with ternary Pd–Ag-transition metal alloy membrane alloys. J. Membr. Sci. 2014, 453, 525–531. [Google Scholar] [CrossRef]
- Bhushan, B.; Goswami, N.; Parida, S.; Singha, A.; Rath, B.; Sodaye, H.; Bindal, R.; Kar, S. Tantalum membrane reactor for enhanced HI decomposition in Iodine–Sulphur (IS) thermochemical process of hydrogen production. Int. J. Hydrogen Energy 2017, 42, 5719–5732. [Google Scholar] [CrossRef]
- Jo, Y.S.; Cha, J.; Lee, C.H.; Jeong, H.; Yoon, C.W.; Nam, S.W.; Han, J. A viable membrane reactor option for sustainable hydrogen production from ammonia. J. Power Sources 2018, 400, 518–526. [Google Scholar] [CrossRef]
- Lamb, K.E.; Viano, D.M.; Langley, M.J.; Hla, S.S.; Dolan, M.D. High-Purity H2 Produced from NH3 via a Ruthenium-Based Decomposition Catalyst and Vanadium-Based Membrane. Ind. Eng. Chem. Res. 2018, 57, 7811–7816. [Google Scholar] [CrossRef]
- Peters, T.; Liron, O.; Tschentscher, R.; Sheintuch, M.; Bredesen, R. Investigation of Pd-based membranes in propane dehydrogenation (PDH) processes. Chem. Eng. J. 2016, 305, 191–200. [Google Scholar] [CrossRef]
- Ricca, A.; Montella, F.; Iaquaniello, G.; Palo, E.; Salladini, A.; Palma, V. Membrane assisted propane dehydrogenation: Experimental investigation and mathematical modelling of catalytic reactions. Catal. Today 2019, 331, 43–52. [Google Scholar] [CrossRef]
- Ricca, A.; Palma, V.; Iaquaniello, G.; Palo, E.; Salladini, A.A. Highly selective propylene production in a membrane assisted catalytic propane dehydrogenation. Chem. Eng. J. 2017, 330, 1119–1127. [Google Scholar] [CrossRef]
- Arratibel, A.; Medrano, J.A.; Meléndez, J.; Tanaka, D.P.; van Sint Annaland, M.; Gallucci, F. Attrition-resistant membranes for fluidized-bed membrane reactors: Double-skin membranes. J. Membr. Sci. 2018, 563, 419–426. [Google Scholar] [CrossRef]
- Lee, M.R.; Park, M.-J.; Jeon, W.; Choi, J.-W.; Suh, Y.-W.; Suh, D.J. A kinetic model for the oxidative coupling of methane over Na2WO4/Mn/SiO2. Fuel Process. Technol. 2012, 96, 175–182. [Google Scholar] [CrossRef]
- Tiemersma, T.P.; Tuinier, M.J.; Gallucci, F.; Kuipers, J.A.M.; van Sint Annaland, M. A kinetics study for the oxidative coupling of methane on a Mn/Na2WO4/SiO2 catalyst. Appl. Catal. A Gen. 2012, 433–434, 96–108. [Google Scholar] [CrossRef]
- Spallina, V.; Velarde, I.C.; Jimenez, J.A.M.; Godini, H.R.; Gallucci, F.; van Sint Annaland, M. Techno-economic assessment of different routes for olefins production through the oxidative coupling of methane (OCM): Advances in benchmark technologies. Energy Convers. Manag. 2017, 154, 244–261. [Google Scholar] [CrossRef]
- Cruellas, A.; Bakker, J.; van Sint Annaland, M.; Medrano, J.; Gallucci, F. Techno-economic analysis of oxidative coupling of methane: Current state of the art and future perspectives. Energy Convers. Manag. 2019, 198, 111789. [Google Scholar] [CrossRef]
- Keller, G.E.; Bhasin, M.M. Synthesis of ethylene via oxidative coupling of methane. I. Determination of active catalysts. J. Catal. 1982, 73, 9–19. [Google Scholar] [CrossRef]
- Sofranko, J.A.; Leonard, J.J.; Jones, C.A. The oxidative conversion of methane to higher hydrocarbons. J. Catal. 1987, 103, 302–310. [Google Scholar] [CrossRef]
- Pak, S.; Qiu, P.; Lunsford, J.H. Elementary reactions in the oxidative coupling of methane over Mn/Na2WO4/SiO2 and Mn/Na2WO4/MgO catalysts. J. Catal. 1998, 179, 222–230. [Google Scholar] [CrossRef]
- Makri, M.; Vayenas, C.G. Successful scale up of gas recycle reactor separators for the production of C2H4 from CH4. Appl. Catal. A Gen. 2003, 244, 301–310. [Google Scholar] [CrossRef]
- Baerns, M.; Buyevskaya, O. Simple chemical processes based on low molecular-mass alkanes as chemical feedstocks. Catal. Today 1998, 45, 13–22. [Google Scholar] [CrossRef]
- Cruellas, A.; Melchiori, T.; Gallucci, F.; van Sint Annaland, M. Advanced reactor concepts for oxidative coupling of methane. Catal. Rev. 2017, 59, 234–294. [Google Scholar] [CrossRef]
- Tiemersma, T.; Chaudhari, A.; Gallucci, F.; Kuipers, H.; van Sint Annaland, M. Integrated autothermal oxidative coupling and steam reforming of methane. Part 1: Design of a dual-function catalyst particle. Chem. Eng. Sci. 2012, 82, 200–214. [Google Scholar] [CrossRef]
- Godini, H.R.; Trivedi, H.; De Villasante, A.G.; Görke, O.; Jašo, S.; Simon, U.; Berthold, A.; Witt, W.; Wozny, G. Design and demonstration of an experimental membrane reactor set-up for oxidative coupling of methane. Chem. Eng. Res. Des. 2013, 91, 2671–2681. [Google Scholar] [CrossRef]
- Godini, H.R.; Azadi, M.; Khadivi, M.; Gharibi, A.; Jazayeri, S.M.; Salerno, D.; Penteado, A.; Mokhtarani, B.; Orjuela, A.; Karsten, T.; et al. Conceptual Process Design and Economic Analysis of Oxidative Coupling of Methane. Comput. Aided Chem. Eng. 2018, 44, 361–366. [Google Scholar] [CrossRef]
- Vamvakeros, A.; Egan, C.K.; Ismagilov, I.Z.; Shearing, P.; Cernik, R.; Di Michiel, M.; Vaughan, G.B.M.; van Sint Annaland, M.; Jacques, S.D.; Middelkoop, V.; et al. Real time chemical imaging of a working catalytic membrane reactor during oxidative coupling of methane. Chem. Commun. 2015, 51, 12752–12755. [Google Scholar] [CrossRef] [PubMed]
- Di Felice, L.; Middelkoop, V.; Anzoletti, V.; Snijkers, F.; van Sint Annaland, M.; Gallucci, F. New high temperature sealing technique and permeability data for hollow fiber BSCF perovskite membranes. Chem. Eng. Process. Process. Intensif. 2016, 107, 206–219. [Google Scholar] [CrossRef]
- Gorbe, J.; Lasobras, J.; Francés, E.; Herguido, J.; Menéndez, M.; Kumakiri, I.; Kita, H. Preliminary study on the feasibility of using a zeolite A membrane in a membrane reactor for methanol production. Sep. Purif. Technol. 2018, 200, 164–168. [Google Scholar] [CrossRef]
- Masuda, T.; Asanuma, T.; Shouji, M.; Mukai, S.R.; Kawase, M.; Hashimoto, K. Methanol to olefins using ZSM-5 zeolite catalyst membrane reactor. Chem. Eng. Sci. 2003, 58, 649–656. [Google Scholar] [CrossRef]
- Tago, T.; Iwakai, K.; Morita, K.; Tanaka, K.; Masuda, T. Control of acid-site location of ZSM-5 zeolite membrane and its application to the MTO reaction. Catal. Today 2005, 105, 662–666. [Google Scholar] [CrossRef]
- Li, Y.; Chen, H.; Liu, J.; Li, H.; Yang, W. Pervaporation and vapor permeation dehydration of Fischer–Tropsch mixed-alcohols by LTA zeolite membranes. Sep. Purif. Technol. 2007, 57, 140–146. [Google Scholar] [CrossRef]
- Espinoza, R.; Du Toit, E.; Santamaria, J.; Menéndez, M.; Coronas, J.; Irusta, S. Use of membranes in fischer-tropsch reactors. Stud. Surf. Sci. Catal. 2000, 130, 389–394. [Google Scholar] [CrossRef]
- De Falco, M.; Capocelli, M.; Basile, A. Selective membrane application for the industrial one-step DME production process fed by CO2 rich streams: Modeling and simulation. Int. J. Hydrogen Energy 2017, 42, 6771–6786. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, N.; Qian, Y.; Liu, X.; Caro, J.; Huang, A. Efficient Synthesis of Dimethyl Ether from Methanol in a Bifunctional Zeolite Membrane Reactor. Angew. Chem. Int. Ed. 2016, 55, 12678–12682. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, G.; Marigliano, G.; Golemme, G.; Drioli, E. Simulation of CO2 hydrogenation with CH3OH removal in a zeolite membrane reactor. Chem. Eng. J. 2002, 85, 53–59. [Google Scholar] [CrossRef]
- Sawamura, K.-I.; Shirai, T.; Takada, M.; Sekine, Y.; Kikuchi, E.; Matsukata, M. Selective permeation and separation of steam from water–methanol–hydrogen gas mixtures through mordenite membrane. Catal. Today 2008, 132, 182–187. [Google Scholar] [CrossRef]
- Gallucci, F.; Paturzo, L.; Basile, A. An experimental study of CO2 hydrogenation into methanol involving a zeolite membrane reactor. Chem. Eng. Process. Process. Intensif. 2004, 43, 1029–1036. [Google Scholar] [CrossRef]
- Calabro, V. Engineering aspects of membrane bioreactors. In Membrane Reactor Engineering: Applications for a Greener Process Industry; John Wiley & Sons: Hoboken, NJ, USA, 2013; Volume 2, ISBN 9780857097347. [Google Scholar]
- Coutte, F.; Lecouturier, D.; Firdaous, L.; Kapel, R.; Bazinet, L.; Cabassud, C.; Dhulster, P. Recent Trends in Membrane Bioreactors; Elsevier, B.V.: Amsterdam, The Netherlands, 2017; ISBN 9780444636744. [Google Scholar]
- Belleville, M.-P.; Paolucci-Jeanjean, D.; Rios, G. Membrane Bioreactors and the Production of Food Ingredients; Woodhead Publishing Limited: Cambridge, UK, 2013; ISBN 9781845696450. [Google Scholar]
- Xiao, K.; Liang, S.; Wang, X.; Chen, C.; Huang, X. Current state and challenges of full-scale membrane bioreactor applications: A critical review. Bioresour. Technol. 2019, 271, 473–481. [Google Scholar] [CrossRef]
- Ladewig, B.; Al-Shaeli, M.N.Z. Fundamentals of Membrane Reactors; Springer: Singapore, 2017; ISBN 9789811020131. [Google Scholar]
- Nagy, E. Membrane Bioreactor. In Basic Equations of Mass Transport through a Membrane Layer, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 381–415. ISBN 9780128137222. [Google Scholar]
- Rios, G.; Belleville, M.-P.; Paolucci, D.; Sanchez-Marcano, J. Progress in enzymatic membrane reactors—A review. J. Membr. Sci. 2004, 242, 189–196. [Google Scholar] [CrossRef]
- Cassano, A.; Figoli, A.; Galiano, F.; Argurio, P.; Molinari, R. Membrane operations in wastewater treatment: Complexation reactions coupled with membranes, pervaporation and membrane bioreactors. In Handbook of Membrane Reactors; Woodhead Publishing Limited: Cambridge, UK, 2013; Volume 2, pp. 731–762. ISBN 9780857097347. [Google Scholar]
- Galinha, C.F.; Sanches, S.; Crespo, J.G. Membrane Bioreactors. In Fundamental Modeling of Membrane Systems; Luis, P., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; Volume 2, pp. 263–288. ISBN 9780080885049. [Google Scholar]
- Ozgun, H.; Dereli, R.K.; Ersahin, M.E.; Kinaci, C.; Spanjers, H.; van Lier, J.B. A review of anaerobic membrane bioreactors for municipal wastewater treatment: Integration options, limitations and expectations. Sep. Purif. Technol. 2013, 118, 89–104. [Google Scholar] [CrossRef]
- Deowan, S.; Bouhadjar, S.; Hoinkis, J. Membrane bioreactors for water treatment. In Handbook of Membrane Separations: Chemical, Pharmaceutical, Food, and Biotechnological Applications, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 741–758. ISBN 9781466555587. [Google Scholar]
- Asif, M.B.; Hai, F.I.; Jegatheesan, V.; Price, W.E.; Nghiem, L.D.; Yamamoto, K. Applications of Membrane Bioreactors in Biotechnology Processes; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128136065. [Google Scholar]
- Giorno, L.; Mazzei, R.; Drioli, E. Biochemical Membrane Reactors in Industrial Processes. In Membrane Operations, Innovative Separations and Transformations; Drioli, E., Giorno, L., Eds.; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2009; ISBN 9783527320387. [Google Scholar]
- He, Y.; Bagley, D.M.; Leung, K.T.; Liss, S.N.; Liao, B.Q. Recent advances in membrane technologies for biorefining and bioenergy production. Biotechnol. Adv. 2012, 30, 817–858. [Google Scholar] [CrossRef]
- Aslam, M.; Ahmad, R.; Yasin, M.; Khan, A.L.; Shahid, M.K.; Hossain, S.; Khan, Z.; Jamil, F.; Rafiq, S.; Bilad, M.R.; et al. Anaerobic membrane bioreactors for biohydrogen production: Recent developments, challenges and perspectives. Bioresour. Technol. 2018, 269, 452–464. [Google Scholar] [CrossRef]
- Shuit, S.H.; Ong, Y.T.; Lee, K.T.; Subhash, B.; Tan, S.H. Membrane technology as a promising alternative in biodiesel production: A review. Biotechnol. Adv. 2012, 30, 1364–1380. [Google Scholar] [CrossRef]
- Pellegrin, M.-L.; Min, K.; Diamond, J.; Sadler, M.E.; Greiner, A.D.; Zhang, K.; Aguinaldo, J.; Arabi, S.; Liu, M.; Burbano, M.S.; et al. Membrane Processes. Water Environ. Res. 2017, 89, 1092–1175. [Google Scholar] [CrossRef] [PubMed]
- De Cazes, M.; Abejón, R.; Belleville, M.-P.; Sanchez-Marcano, J. Membrane Bioprocesses for Pharmaceutical Micropollutant Removal from Waters. Membranes 2014, 4, 692–729. [Google Scholar] [CrossRef] [PubMed]
- Mazzei, R.; Piacentini, E.; Gebreyohannes, A.Y.; Giorno, L. Membrane Bioreactors in Food, Pharmaceutical and Biofuel Applications: State of the Art, Progresses and Perspectives. Curr. Org. Chem. 2017, 21, 1671–1701. [Google Scholar] [CrossRef]
- Krzeminski, P.; Leverette, L.; Malamis, S.; Katsou, E. Membrane bioreactors—A review on recent developments in energy reduction, fouling control, novel configurations, LCA and market prospects. J. Memb. Sci. 2017, 527, 207–227. [Google Scholar] [CrossRef]
- Kanai, M.; Ferre, V.; Wakahara, S.; Yamamoto, T.; Moro, M. A novel combination of methane fermentation and MBR—Kubota Submerged Anaerobic Membrane Bioreactor process. Desalination 2010, 250, 964–967. [Google Scholar] [CrossRef]
- Stuckey, D.C. Recent developments in anaerobic membrane reactors. Bioresour. Technol. 2012, 122, 137–148. [Google Scholar] [CrossRef]
- Bakonyi, P.; Nemestóthy, N.; Simon, V.; Bélafi-Bakó, K. Fermentative hydrogen production in anaerobic membrane bioreactors: A review. Bioresour. Technol. 2014, 156, 357–363. [Google Scholar] [CrossRef]
- Dvořák, L.; Gómez, M.; Dolina, J.; Černín, A. Anaerobic membrane bioreactors—A mini review with emphasis on industrial wastewater treatment: Applications, limitations and perspectives. Desalin. Water Treat. 2016, 57, 19062–19076. [Google Scholar] [CrossRef]
- Chang, S. Anaerobic Membrane Bioreactors (AnMBR) for Wastewater Treatment. Adv. Chem. Eng. Sci. 2014, 4, 56–61. [Google Scholar] [CrossRef]
- Lin, H.; Peng, W.; Zhang, M.; Chen, J.; Hong, H.; Zhang, Y. A review on anaerobic membrane bioreactors: Applications, membrane fouling and future perspectives. Desalination 2013, 314, 169–188. [Google Scholar] [CrossRef]
- Dereli, R.K.; Ersahin, M.E.; Ozgun, H.; Ozturk, I.; Jeison, D.; van Der Zee, F.; van Lier, J.B. Potentials of anaerobic membrane bioreactors to overcome treatment limitations induced by industrial wastewaters. Bioresour. Technol. 2012, 122, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Shin, C.; Bae, J. Current status of the pilot-scale anaerobic membrane bioreactor treatments of domestic wastewaters: A critical review. Bioresour. Technol. 2018, 247, 1038–1046. [Google Scholar] [CrossRef]
- Skouteris, G.; Hermosilla, D.; Expósito, P.L.; Negro, C.; Blanco, A. Anaerobic membrane bioreactors for wastewater treatment: A review. Chem. Eng. J. 2012, 198–199, 138–148. [Google Scholar] [CrossRef]
- Satyawali, Y.; Vanbroekhoven, K.; Dejonghe, W. Process intensification: The future for enzymatic processes? Biochem. Eng. J. 2017, 121, 196–223. [Google Scholar] [CrossRef]
- Cath, T.Y.; Childress, A.; Elimelech, M. Forward osmosis: Principles, applications, and recent developments. J. Membr. Sci. 2006, 281, 70–87. [Google Scholar] [CrossRef]
- Judd, S.; Judd, C. Fundamentals. In The MBR Book—Principles and Applications of Membrane Bioreactors for Water and Wastewater Treatment, 2nd ed.; Butterworth-Heinemann: Oxford, UK, 2011; ISBN 978-0-08-096682-3. [Google Scholar]
- Casey, E. Membrane Bioreactors for Wastewater Treatment; Elsevier Ltd.: Amsterdam, The Netherlands, 2015; ISBN 9781466555587. [Google Scholar]
- The MBR Site, (n.d.). Available online: https://www.thembrsite.com (accessed on 1 May 2020).
- Calabro, V.; Iorio, G. Economic aspects of membrane bioreactors. In Handbook of Membrane Reactors; Elsevier BV: Amsterdam, The Netherlands, 2013; Volume 2, pp. 888–911. ISBN 9780857097347. [Google Scholar]
- Deng, L.; Guo, W.S.; Ngo, H.H.; Zhang, H.; Wang, J.; Li, J.; Xia, S.; Wu, Y. Biofouling and control approaches in membrane bioreactors. Bioresour. Technol. 2016, 221, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Shi, Y.; Jegatheesan, V.; Haq, I.U. A Review on the Mechanism, Impacts and Control Methods of Membrane Fouling in MBR System. Membranes 2020, 10, 24. [Google Scholar] [CrossRef]
- Aslam, M.; Charfi, A.; Lesage, G.; Heran, M.; Kim, J. Membrane bioreactors for wastewater treatment: A review of mechanical cleaning by scouring agents to control membrane fouling. Chem. Eng. J. 2017, 307, 897–913. [Google Scholar] [CrossRef]
- Ho, K.; Teow, Y.; Ang, W.; Mohammad, A.W. An Overview of Electrically-enhanced Membrane Bioreactor (EMBR) for Fouling Suppression. J. Eng. Sci. Technol. Rev. 2017, 10, 128–138. [Google Scholar] [CrossRef]
- Iorhemen, O.T.; Hamza, R.A.; Tay, J.H. Membrane Bioreactor (MBR) Technology for Wastewater Treatment and Reclamation: Membrane Fouling. Membranes 2016, 6, 33. [Google Scholar] [CrossRef]
- Judd, S. The status of membrane bioreactor technology. Trends Biotechnol. 2008, 26, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Zhang, S.; Oh, Y.; Zhou, Z.; Shin, H.-S.; Chae, S.-R. Fouling in membrane bioreactors: An updated review. Water Res. 2017, 114, 151–180. [Google Scholar] [CrossRef] [PubMed]
- Neoh, C.H.; Noor, Z.Z.; Mutamim, N.S.A.; Lim, C.K. Green technology in wastewater treatment technologies: Integration of membrane bioreactor with various wastewater treatment systems. Chem. Eng. J. 2016, 283, 582–594. [Google Scholar] [CrossRef]
- Pellegrin, M.; Burbano, M.S.; Sadler, M.E.; Diamond, J.; Baker, S.; Greiner, A.D.; Arabi, S.; Wong, J.; Doody, A.; Padhye, L.P.; et al. Membrane Processes. Water Environ. Res. 2016, 88, 1050–1124. [Google Scholar] [CrossRef]
- Tan, X.; Acquah, I.; Liu, H.; Li, W.; Tan, S. A critical review on saline wastewater treatment by membrane bioreactor (MBR) from a microbial perspective. Chemosphere 2019, 220, 1150–1162. [Google Scholar] [CrossRef]
- Meng, F.; Chae, S.-R.; Drews, A.; Kraume, M.; Shin, H.-S.; Yang, F. Recent advances in membrane bioreactors (MBRs): Membrane fouling and membrane material. Water Res. 2009, 43, 1489–1512. [Google Scholar] [CrossRef]
- Patentscope, World Intellectual Property Organization. 2019. Available online: https://patentscope.wipo.int/search/en/result.jsf?_vid=P20-JZKXVG-60911 (accessed on 21 August 2019).
- Judd, S.; Judd, C. Largest MBR Plants (Over 100 MLD)—Worldwide. Available online: https://www.thembrsite.com/largest-membrane-bioreactor-plants-worldwide/ (accessed on 14 August 2019).
- Deibert, W.; Ivanova, M.E.; Baumann, S.; Guillon, O.; Meulenberg, W.A. Ion-conducting ceramic membrane reactors for high-temperature applications. J. Membr. Sci. 2017, 543, 79–97. [Google Scholar] [CrossRef]
- Baker, J.; Dudley, L. Biofouling in membrane systems—A review. Desalination 1998, 118, 81–89. [Google Scholar] [CrossRef]
- Komlenic, R. Rethinking the causes of membrane biofouling. Filtr. Sep. 2010, 47, 26–28. [Google Scholar] [CrossRef]

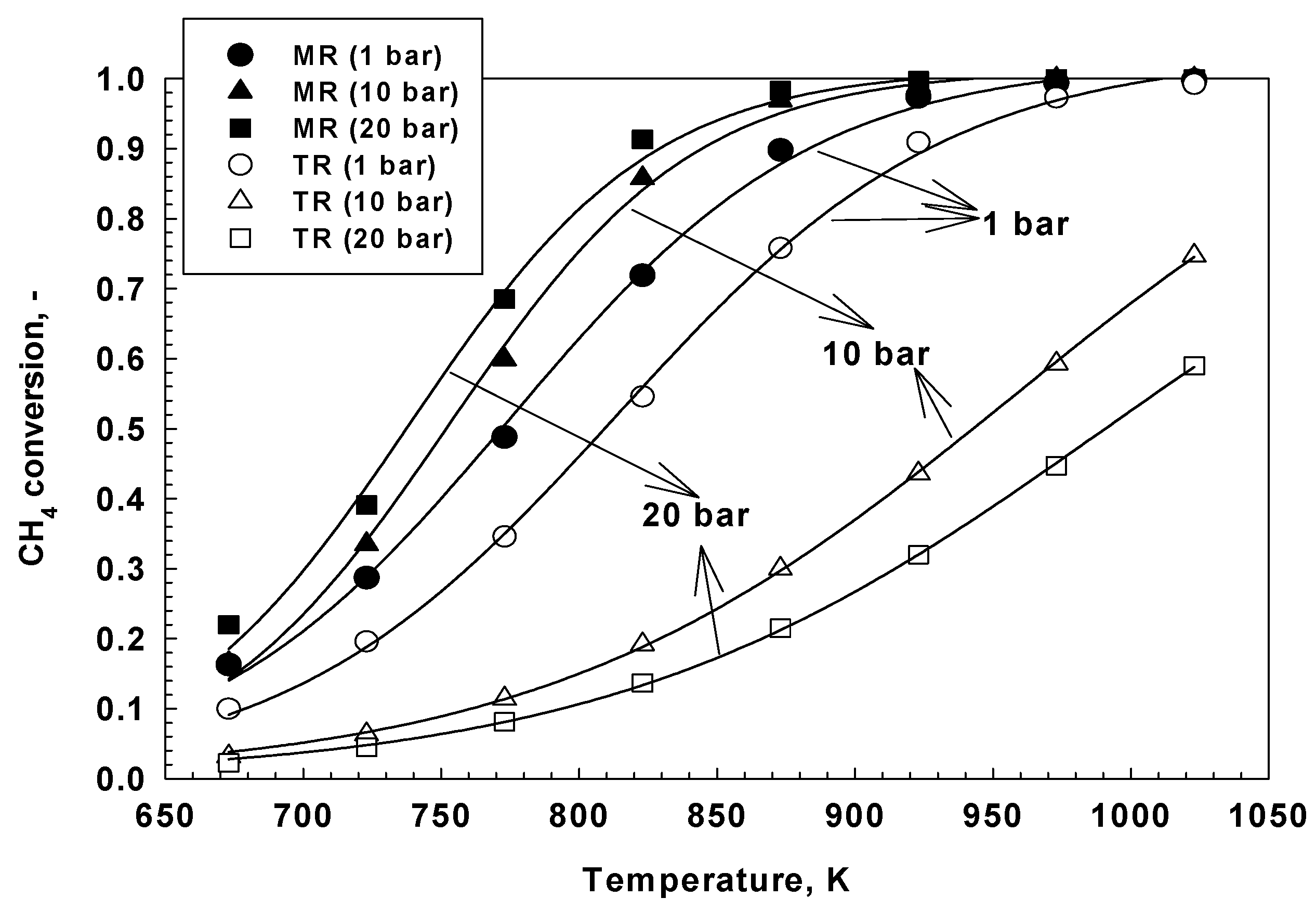
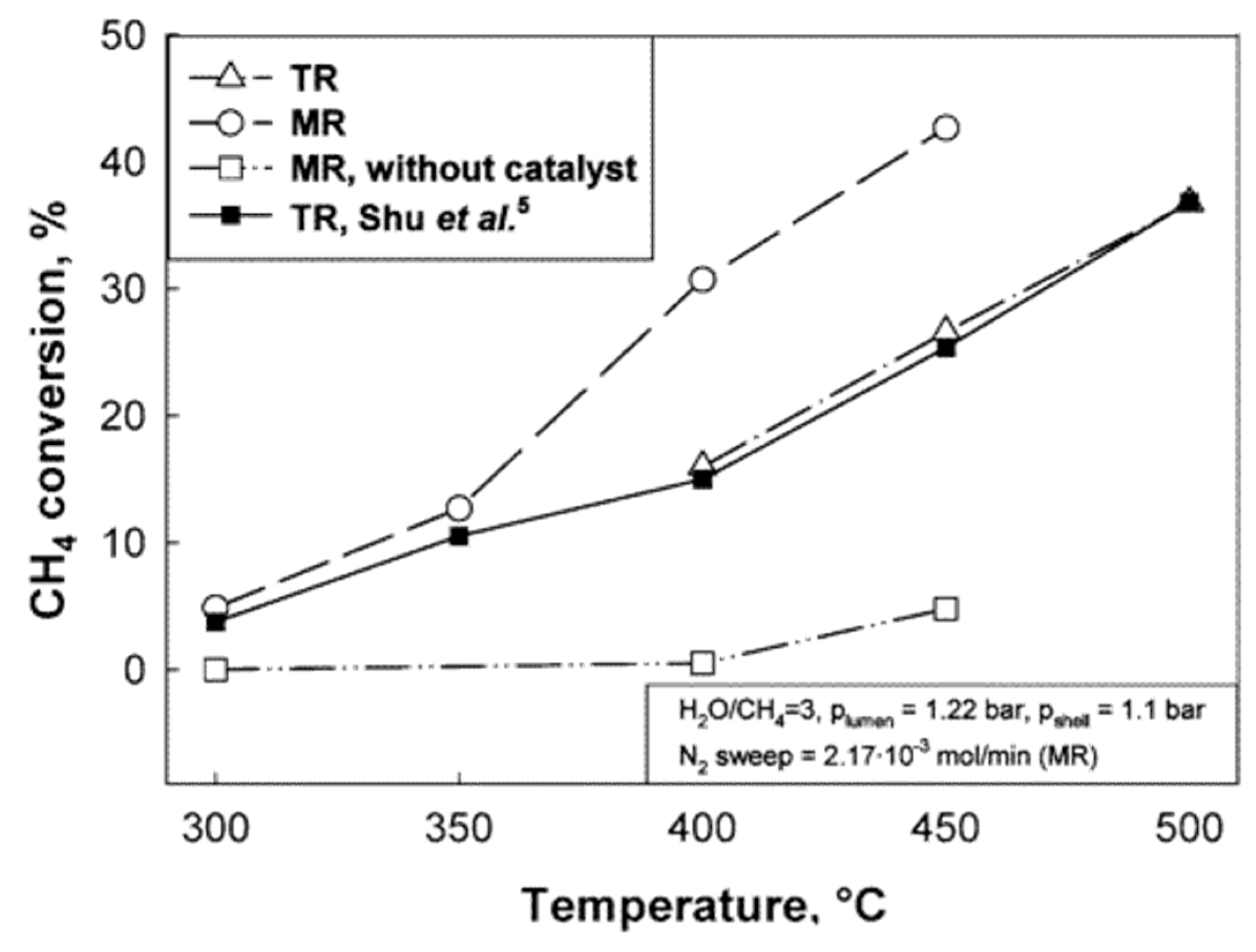

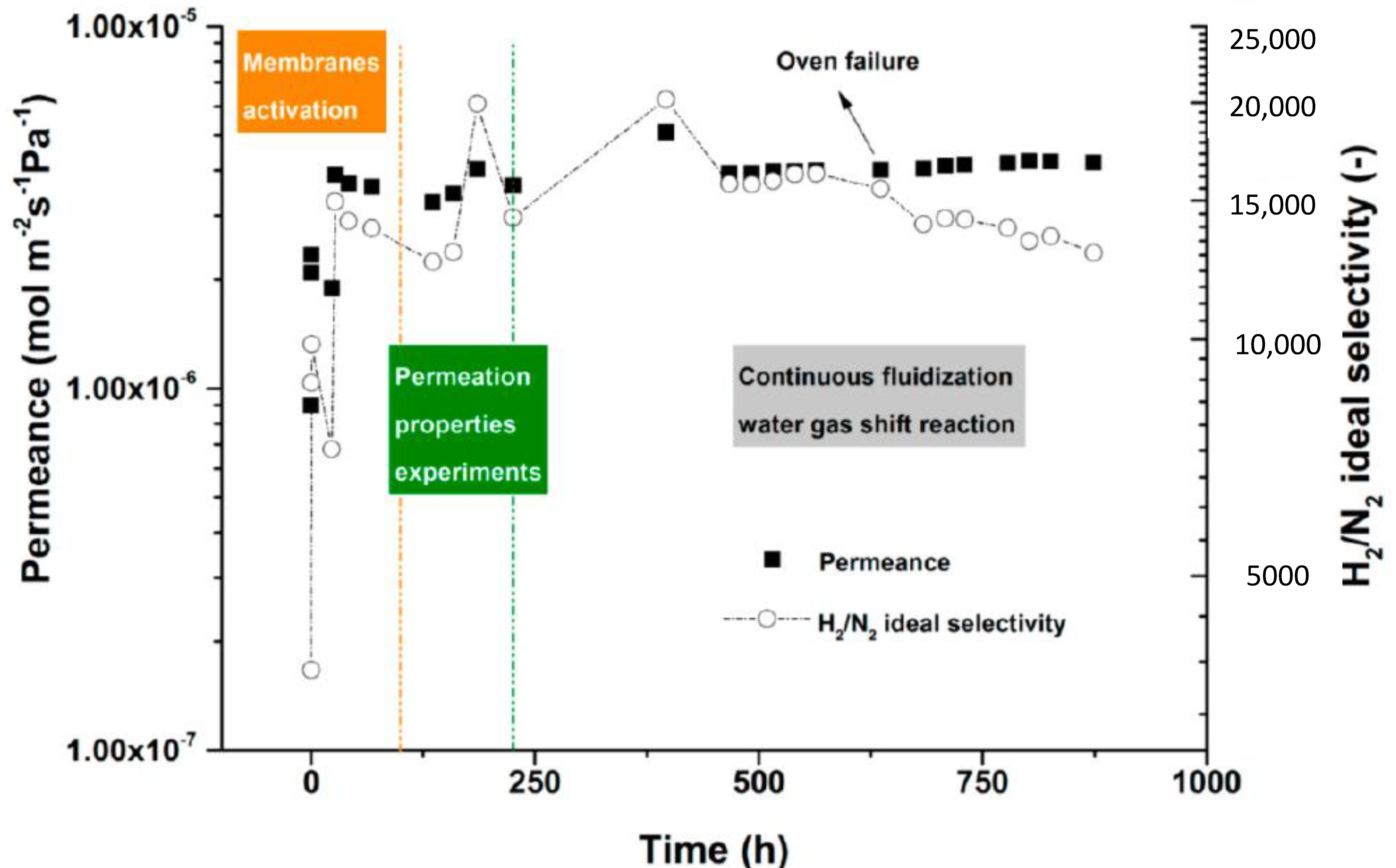
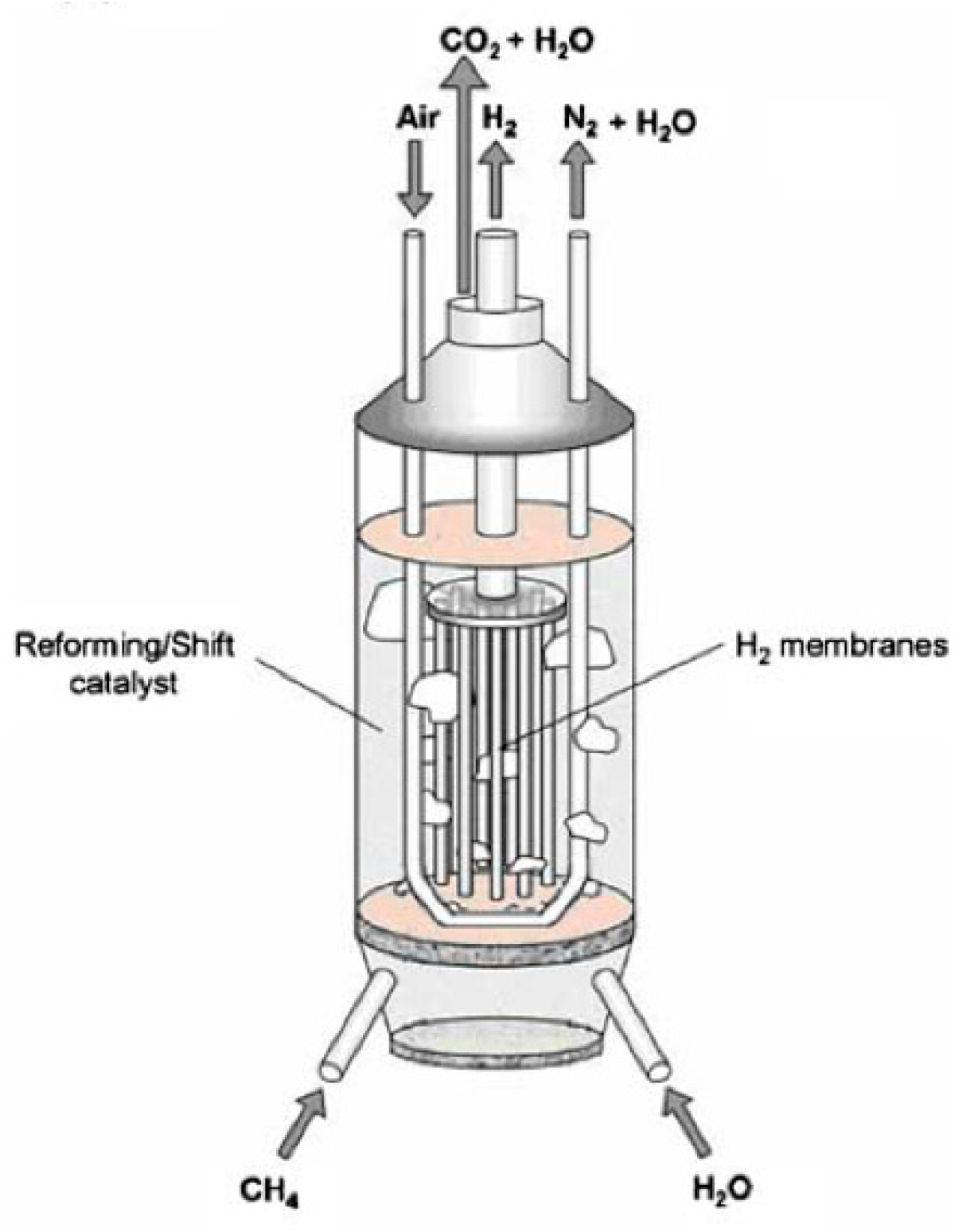

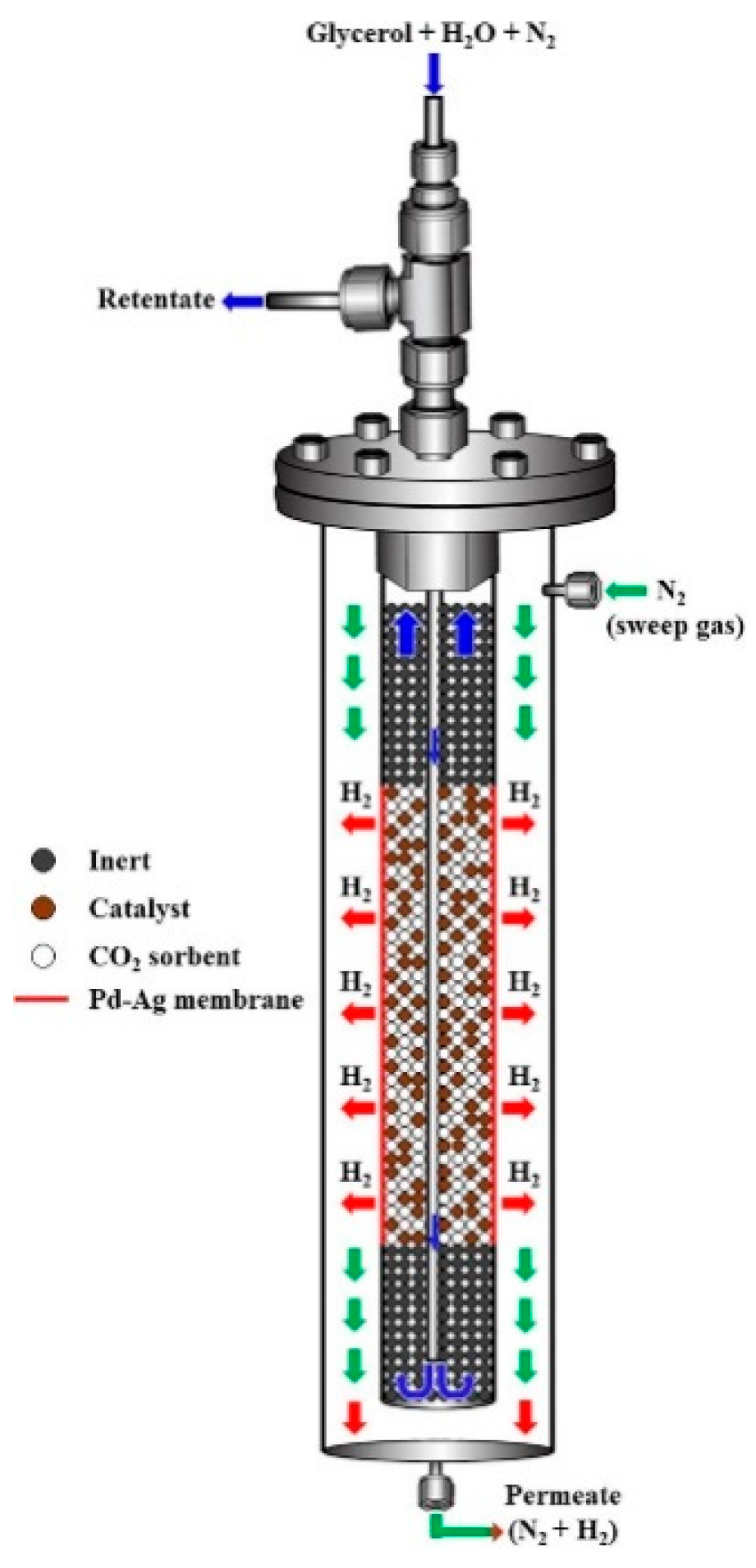
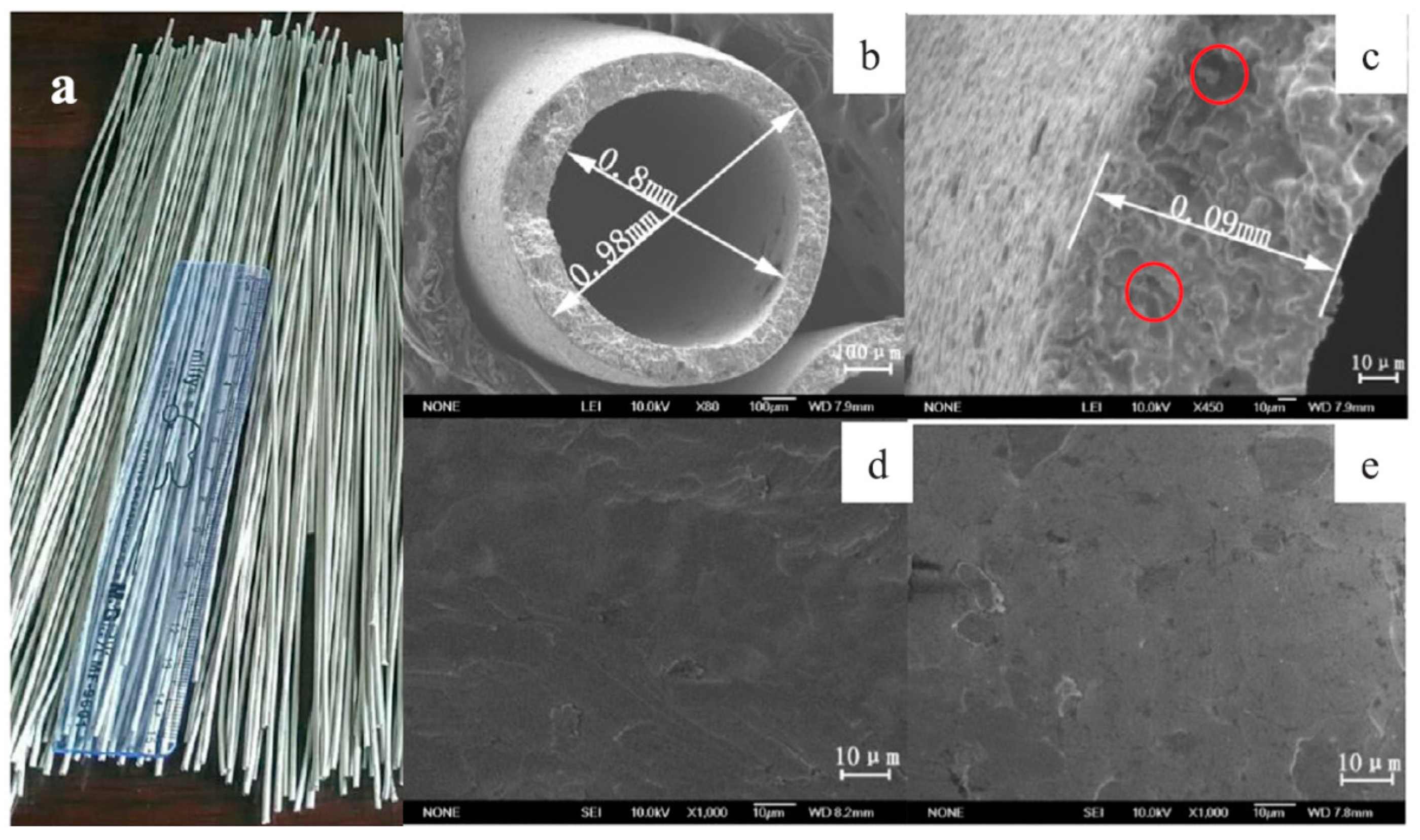
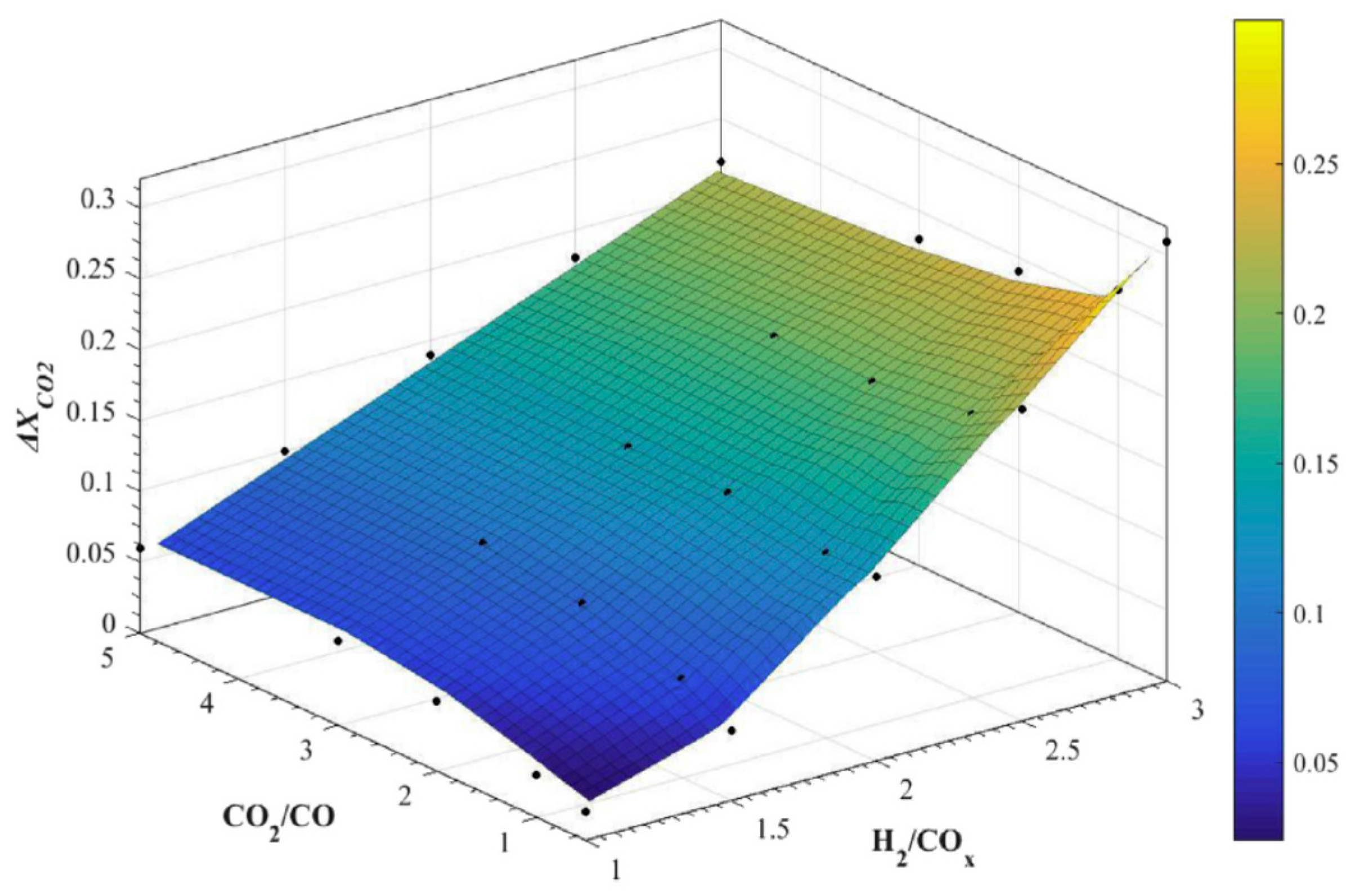
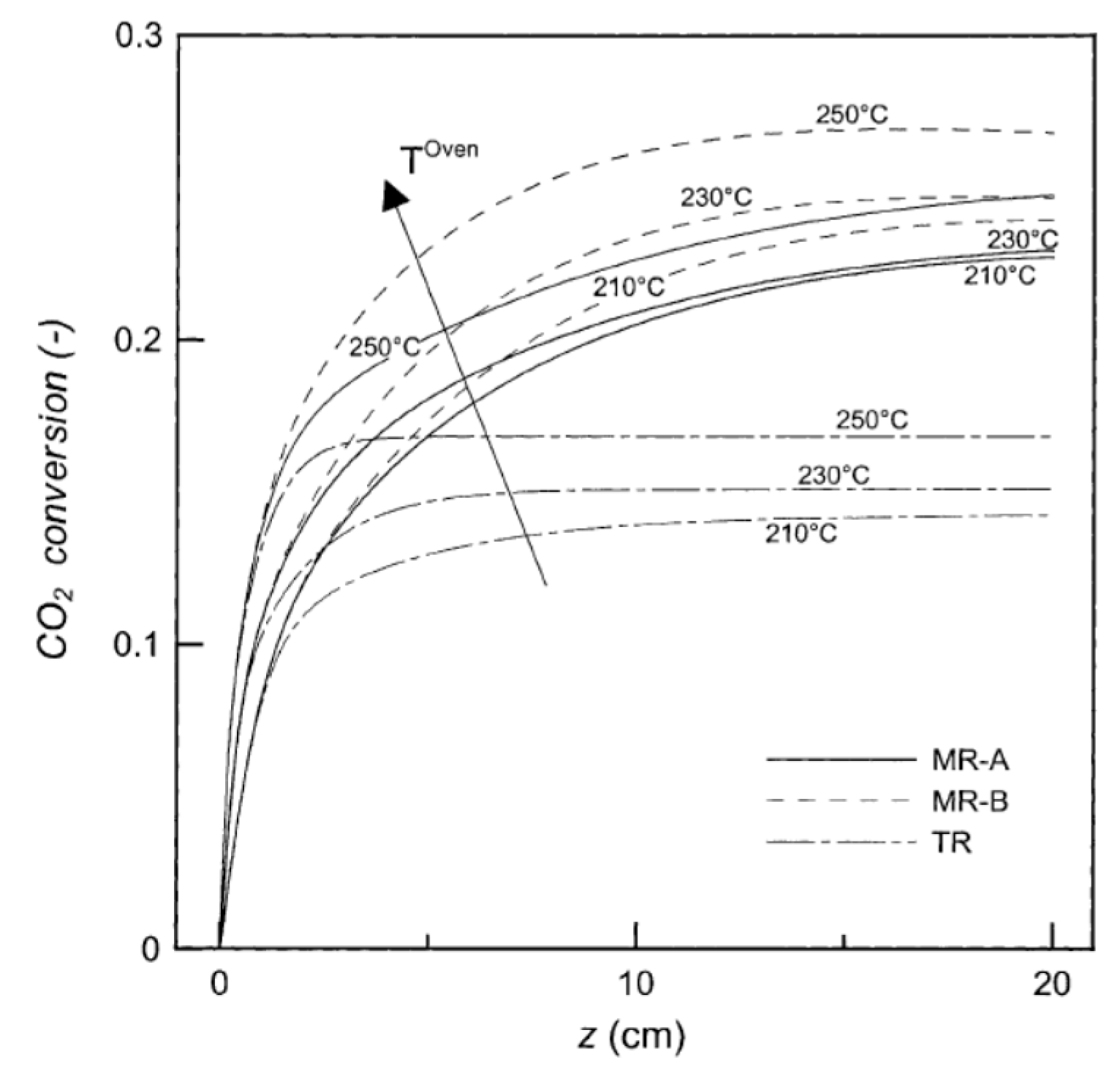
| Reaction | |
|---|---|
| Water gas shift (WGS) | |
| −41.1 | |
| Methane decomposition (Carbon production) | |
| 75 | |
| Steam reforming (SR) reactions | |
| 206.2 | |
| 164.9 | |
| 49 | |
| 239.5 | |
| 173 | |
| Partial and full oxidation reactions | |
| −802 | |
| −71 | |
| −35.6 | |
| −192.3 | |
| −14.4 | |
| Autothermal reforming (ATR) reactions | |
| 339 | |
| 0 | |
| −50 | |
| Dehydrogenation reaction | |
| 124 | |
| Ammonia decomposition | |
| 45.9 |
| Configuration | Turbulence Promotion * | Can Be Backflushed? | Membrane Processe(s) | Suitable for MBR? |
|---|---|---|---|---|
| Pleated filter cartridge | Very poor | No | DE-MF, low TSS waters | Yes |
| Flat sheet | Fair | No | ED, UF, RO | Yes |
| Spiral-wound | Poor | No | RO/NF, UF | Yes |
| Multi tubular | Very good | No | CF-MF/UF, high TSS waters, NF | No |
| Capillary tube | Fair | Yes | UF | No |
| Hollow fibre | Very poor—fair | Yes | MF/UF, RO | No |
| Installation | Location | Technology Suppliers | Capacity (PDF) | Commissoning Date |
|---|---|---|---|---|
| Beihu WWTP | Hubei, China | Beijing Origin Water Technology Co., Ltd. (BOW) | 1040 | 2019 |
| Hendriksdal Sweden | nr Stockholm, Sweden | SUEZ—Water Technologies & Solutions | 864 | 2019 (stage 2) |
| Al Ansab | Muscat, Oman | Kubota | 125 | 2018 |
| Water Affairs Integrative EPC | Xingi, Guizhou, China | Beijing Origin Water Technology Co., Ltd. (BOW) | 399 | 2016–2017 |
| Huaifang Water Recycling Project | Beijing China | Memstar | 780 | 2016 |
| Product | Supplier |
|---|---|
| Immersed flat sheet (FS) modules |
|
| Immersed hollow fiber (HF) modules |
|
| Side stream MBR products (MT) modules |
|
| Type | Product/Service | Companies |
|---|---|---|
| System integrators | Membranes, membrane modules, pumps, tanks, etc. |
|
| Component manufacturers | Membranes. |
|
| Membrane bioreactor systems | MBR system is built and membranes and membrane modules are provided by the membrane manufacturer. |
|
| Application-specific MBR and AnMBR | These companies focus on a specific area. |
|
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Helmi, A.; Gallucci, F. Latest Developments in Membrane (Bio)Reactors. Processes 2020, 8, 1239. https://doi.org/10.3390/pr8101239
Helmi A, Gallucci F. Latest Developments in Membrane (Bio)Reactors. Processes. 2020; 8(10):1239. https://doi.org/10.3390/pr8101239
Chicago/Turabian StyleHelmi, Arash, and Fausto Gallucci. 2020. "Latest Developments in Membrane (Bio)Reactors" Processes 8, no. 10: 1239. https://doi.org/10.3390/pr8101239
APA StyleHelmi, A., & Gallucci, F. (2020). Latest Developments in Membrane (Bio)Reactors. Processes, 8(10), 1239. https://doi.org/10.3390/pr8101239






