Influence of Carbon Nanosheets on the Behavior of 1,2-Dipalmitoyl-sn-glycerol-3-phosphocholine Langmuir Monolayers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization of Carbon Nanosheets (CN)
2.3. Study of Monolayers at the Water/Vapor Interface
3. Results and Discussion
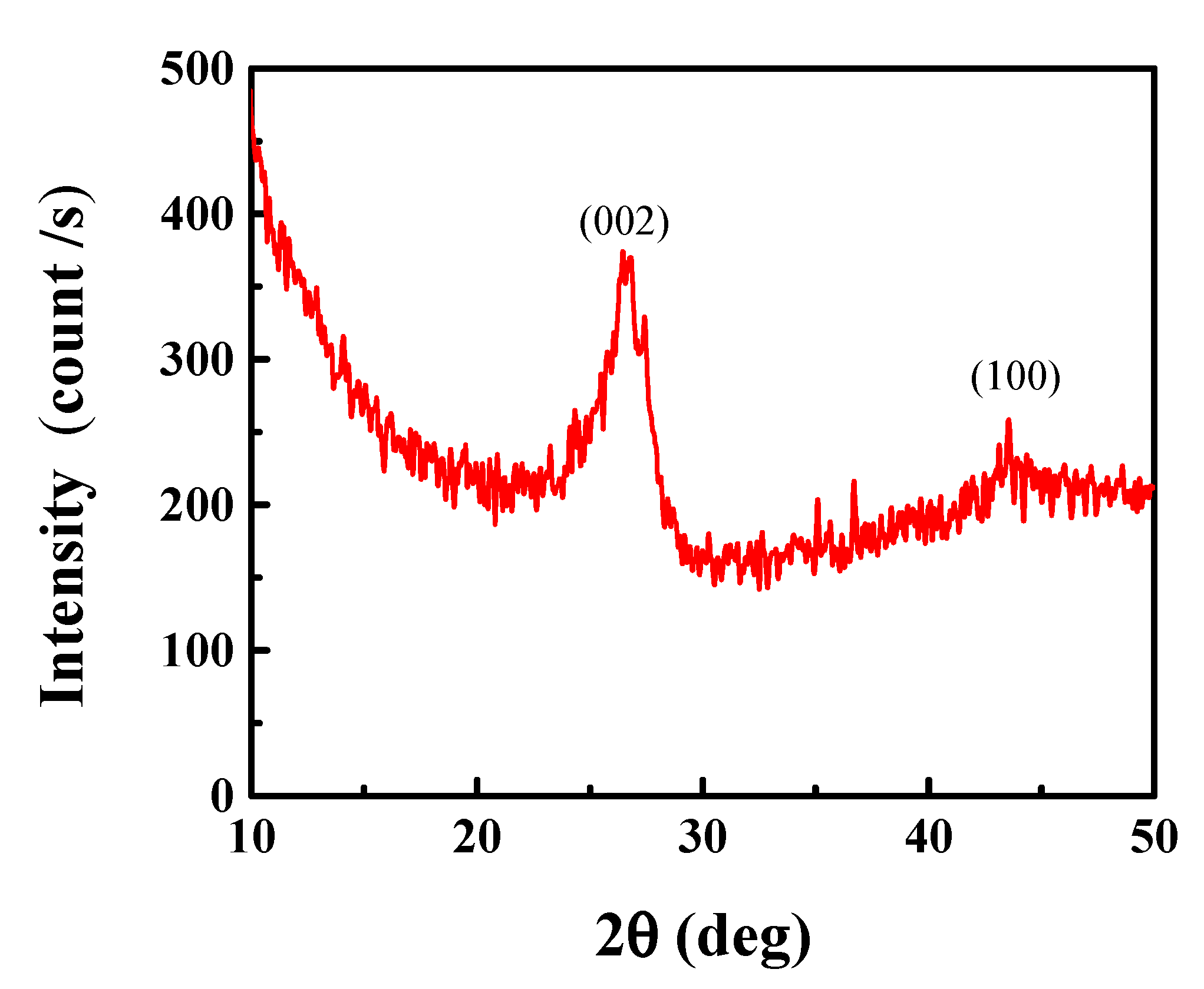
3.1. CN Characterization
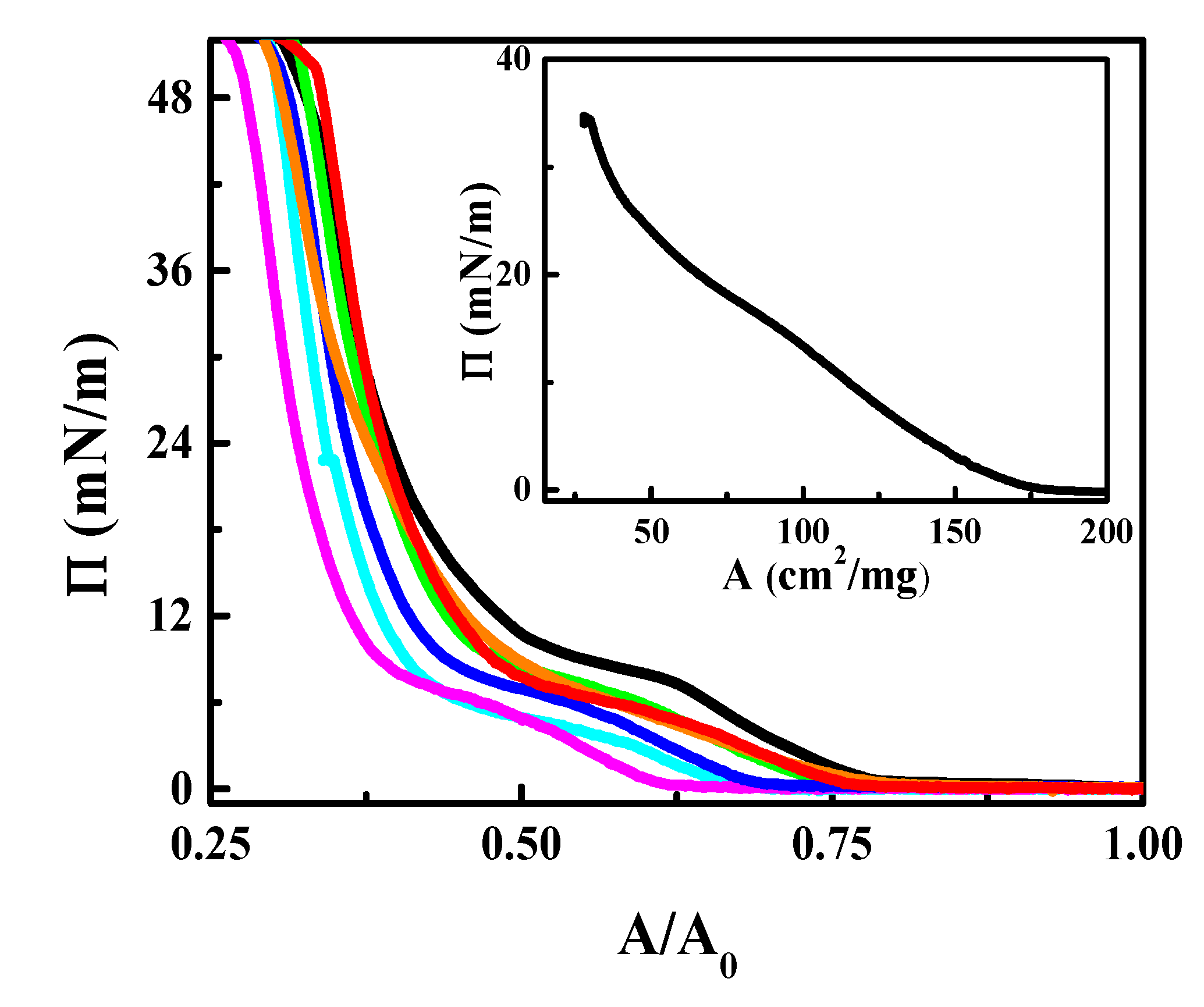
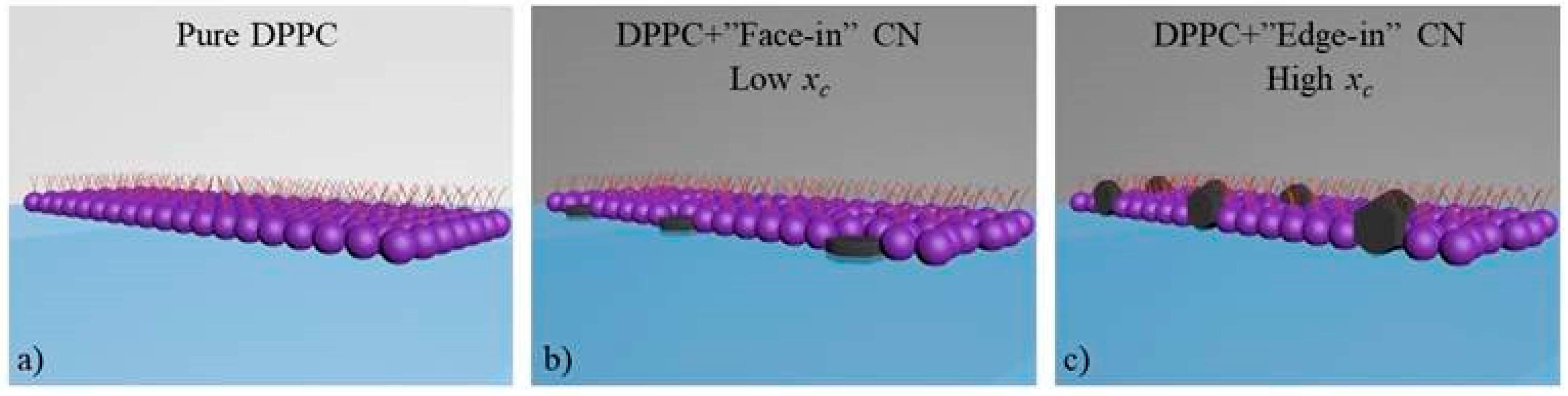
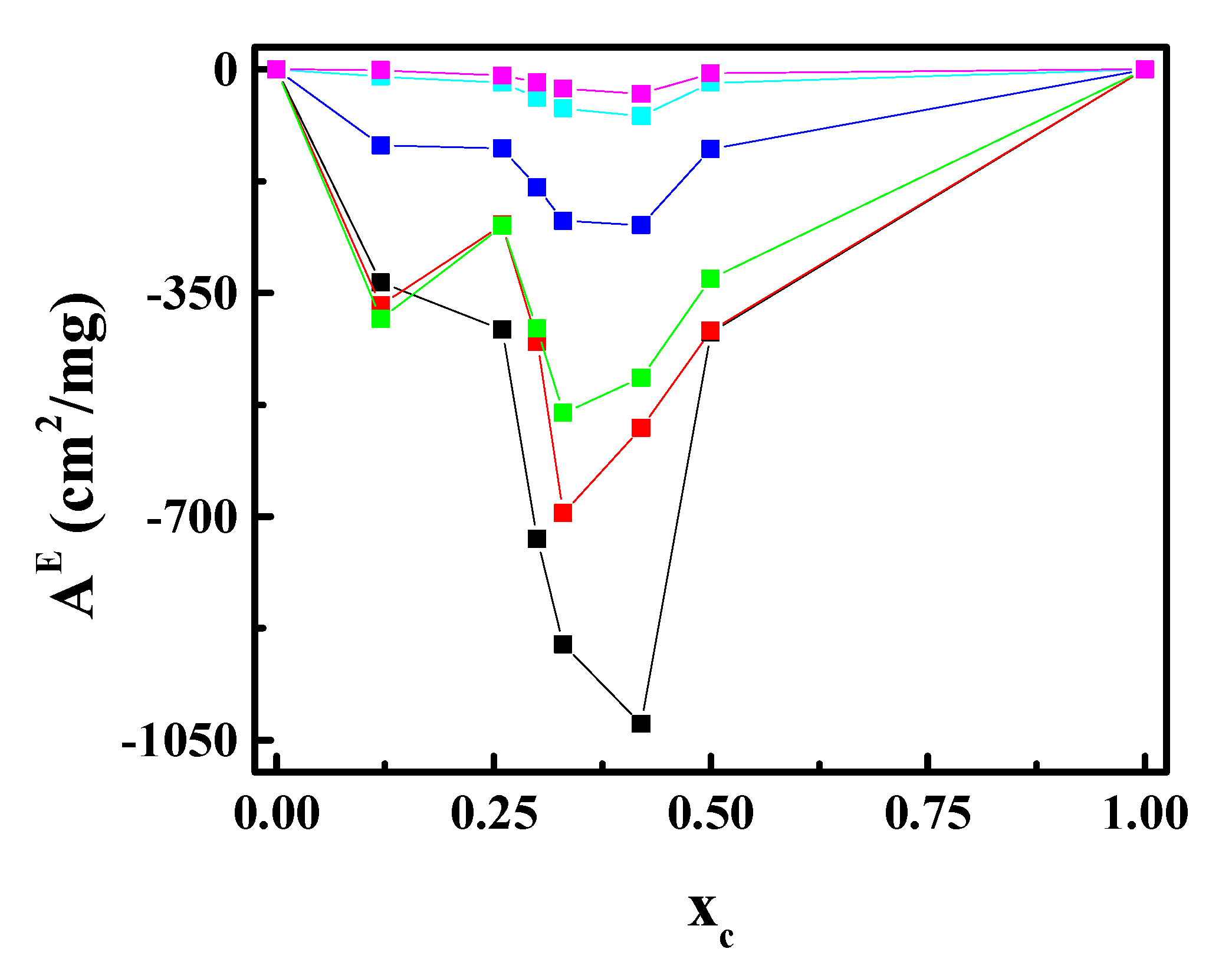
3.2. CN Incorporation into 1,2-Dipalmitoyl-sn-glycerol-3-phosphocholine (DPPC) Langmuir Monolayers
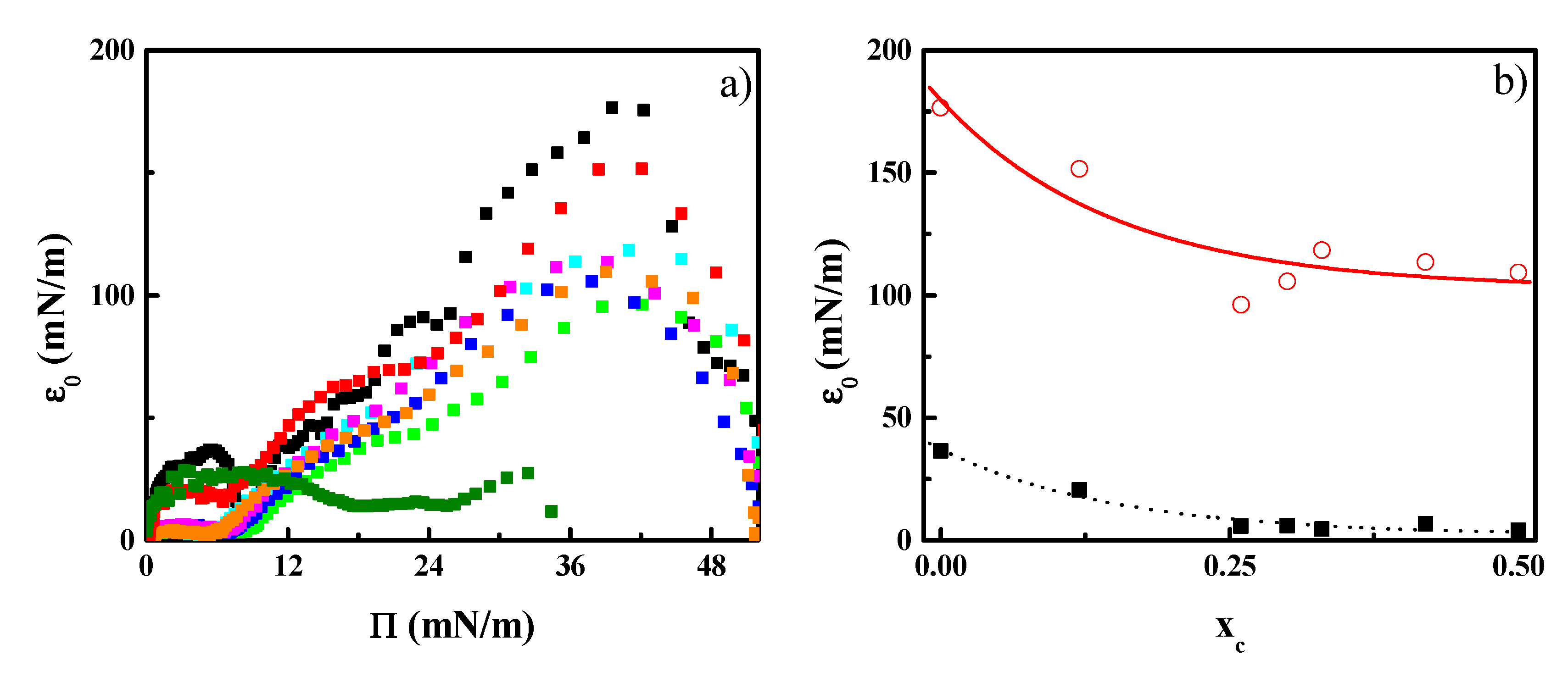
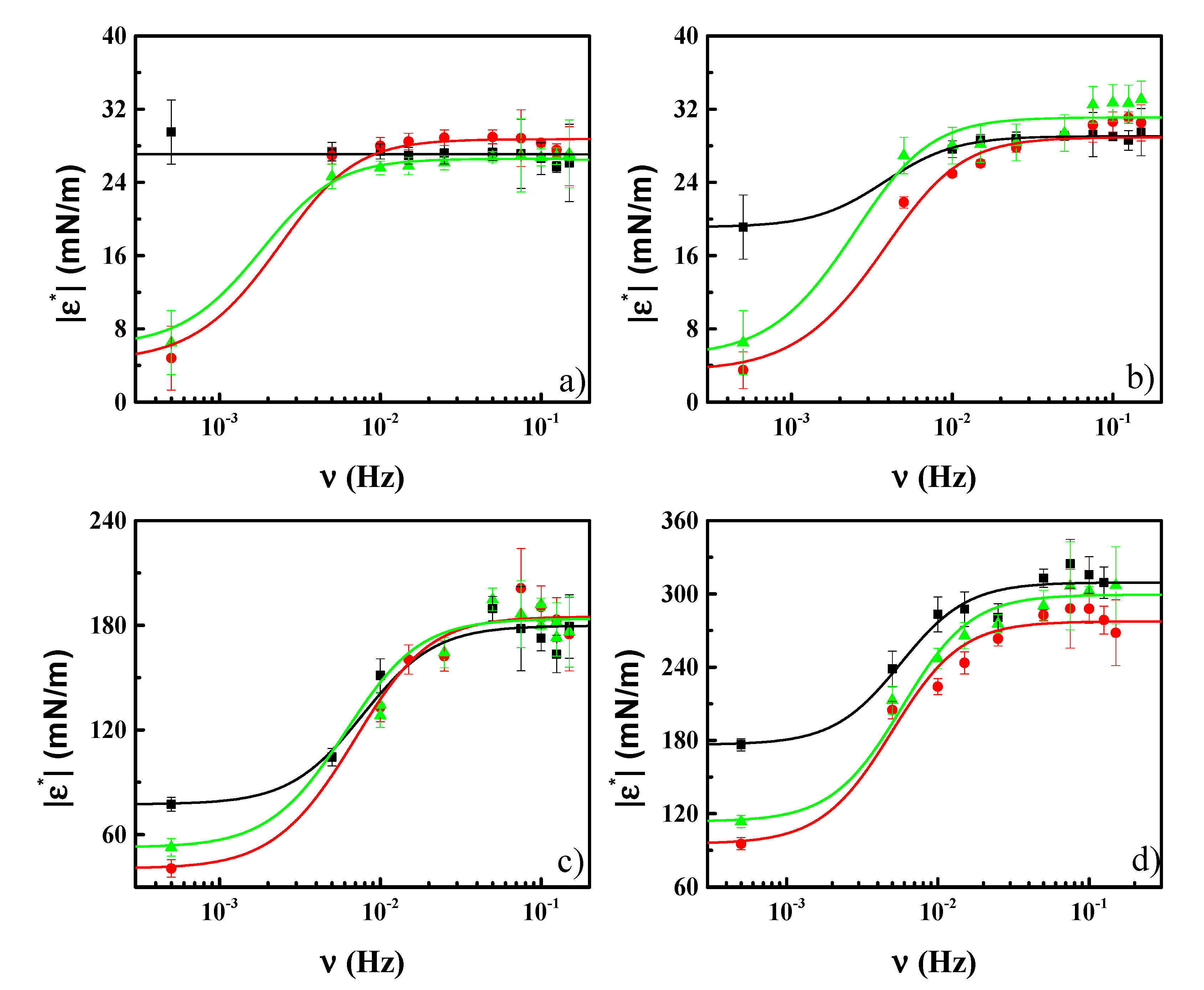
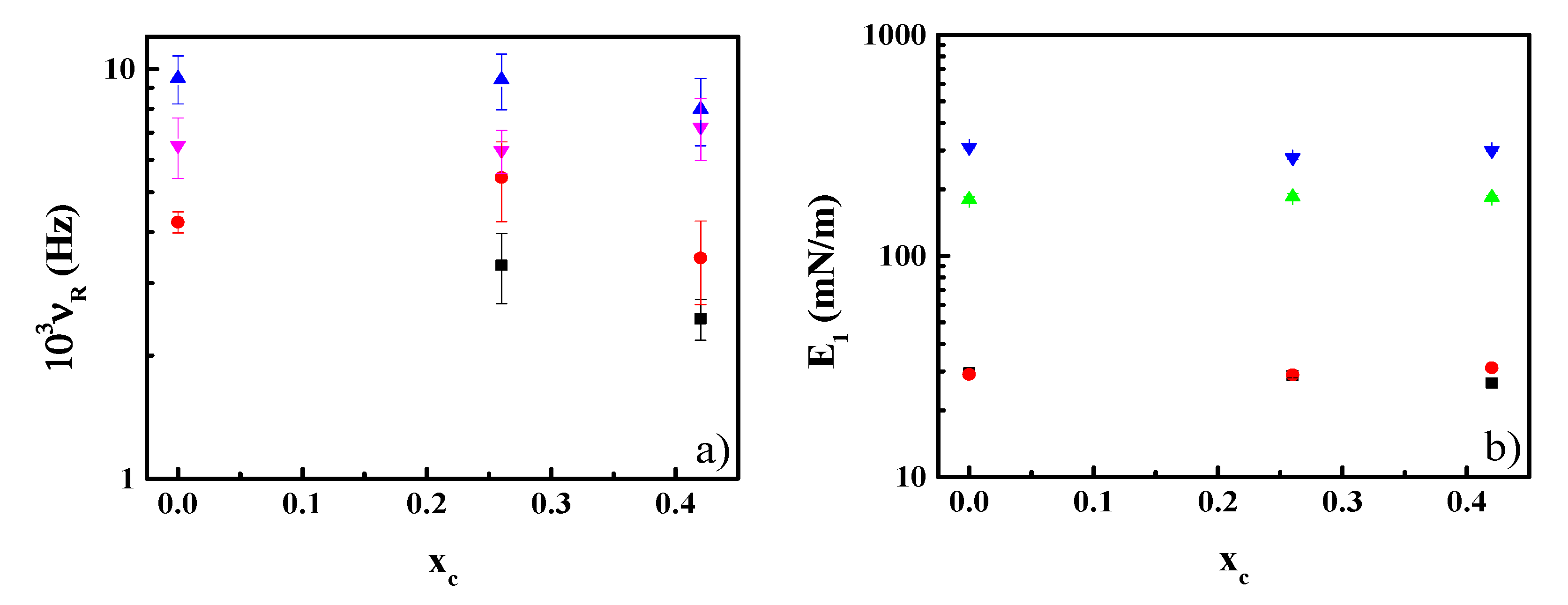
3.3. Effect of CN Incorporation in the Dilational Response of DPPC Langmuir Monolayers
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wu, C.-Y.; Ge, W. (Eds.) Particulate Materials: Synthesis, Characterisation, Processing and Modelling; Royal Society of Chemistry: Cambridge, UK, 2012. [Google Scholar]
- Pavese, G.; Alados-Arboledas, L.; Cao, J.; Satheesh, S.K. Carbonaceous particles in the atmosphere: Experimental and modelling issues. Adv. Meteorol. 2014, 2014, 529850. [Google Scholar] [CrossRef]
- Park, M.; Joo, H.S.; Lee, K.; Jang, M.; Kim, S.D.; Kim, I.; Borlaza, L.J.S.; Lim, H.; Shin, H.; Chung, K.H.; et al. Differential toxicities of fine particulate matters from various sources. Sci. Rep. 2018, 8, 17007. [Google Scholar] [CrossRef] [PubMed]
- De Volder, M.F.; Tawfick, S.H.; Baughman, R.H.; Hart, A.J. Carbon Nanotubes: Present and Future Commercial Applications. Science 2013, 339, 535–539. [Google Scholar] [CrossRef]
- Rauti, R.; Musto, M.; Bosi, S.; Prato, M.; Ballerini, L. Properties and behavior of carbon nanomaterials when interfacing neuronal cells: How far have we come? Carbon 2019, 143, 430–446. [Google Scholar] [CrossRef]
- Zaytseva, O.; Neumann, G. Carbon nanomaterials: Production, impact on plant development, agricultural and environmental applications. Chem. Biol. Technol. Agric. 2016, 3, 17. [Google Scholar] [CrossRef]
- Donaldson, K.; Seaton, A. A short history of the toxicology of inhaled particles. Part. Fibre Toxicol. 2012, 9, 13. [Google Scholar] [CrossRef]
- Beddoes, C.M.; Case, C.P.; Briscoe, W.H. Understanding Nanoparticle Cellular Entry: A Physicochemical Perspective. Adv. Colloid Interface Sci. 2015, 218, 48–68. [Google Scholar] [CrossRef]
- Morawska, L.; Ayoko, G.A.; Bae, G.N.; Buonanno, G.; Chao, C.Y.H.; Clifford, S.; Fu, S.C.; Hänninen, O.; He, C.; Isaxon, C.; et al. Airborne particles in indoor environment of homes, schools, offices and aged care facilities: The main routes of exposure. Environ. Int. 2017, 108, 75–83. [Google Scholar] [CrossRef]
- Sosnowski, T.R. Particles on the lung surface—Physicochemical and hydrodynamic effects. Curr. Opin. Colloid Interface Sci. 2018, 36, 1–9. [Google Scholar] [CrossRef]
- Rissler, J.; Gudmundsson, A.; Nicklasson, H.; Swietlicki, E.; Wollmer, P.; Löndahl, J. Deposition efficiency of inhaled particles (15–5000 nm) related to breathing pattern and lung function: An experimental study in healthy children and adults. Part. Fibre Toxicol. 2017, 14, 10. [Google Scholar] [CrossRef]
- Dobrowolska, K.; Jablczynska, K.; Kondej, D.; Sosnowski, T.R. Interactions of insoluble micro- and nanoparticles with the air-liquid interface of the model pulmonary fluids. Physicochem. Probl. Miner. Process. 2018, 54, 151–162. [Google Scholar] [CrossRef]
- Lopez-Rodriguez, E.; Pérez-Gil, J. Structure-function relationships in pulmonary surfactant membranes: From biophysics to therapy. Biochim. Biophys. Acta Biomembr. 2014, 1838, 1568–1585. [Google Scholar] [CrossRef] [PubMed]
- Bernhard, W. Lung surfactant: Function and composition in the context of development and respiratory physiology. Ann. Anat. Anat. Anz. 2016, 208, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Maestro, A.; Guzman, E.; Ortega, F.; Rubio, R.G. Contact angle of micro- and nanoparticles at fluid interfaces. Curr. Opin. Colloid Interface Sci. 2014, 19, 355–367. [Google Scholar] [CrossRef]
- Mendoza, A.J.; Guzman, E.; Martinez-Pedrero, F.; Ritacco, H.; Rubio, R.G.; Ortega, F.; Starov, V.M.; Miller, R. Particle laden fluid interfaces: Dynamics and interfacial rheology. Adv. Colloid Interface Sci. 2014, 206, 303–319. [Google Scholar] [CrossRef] [PubMed]
- Maestro, A.; Santini, E.; Zabiegaj, D.; Llamas, S.; Ravera, F.; Liggieri, L.; Ortega, F.; Rubio, R.G.; Guzman, E. Particle and Particle-Surfactant Mixtures at Fluid Interfaces: Assembly, Morphology, and Rheological Description. Adv. Condens. Matter Phys. 2015, 2015, 917516. [Google Scholar] [CrossRef]
- Maestro, A.; Santini, E.; Guzman, E. Physico-chemical foundations of particle-laden fluid interfaces. Eur. Phys. J. E 2018, 41, 97. [Google Scholar] [CrossRef]
- Maestro, A.; Guzmán, E. Colloids at Fluid Interfaces. Processes 2019, 7, 942. [Google Scholar] [CrossRef]
- Guzmán, E.; Santini, E. Lung surfactant-particles at fluid interfaces for toxicity assessments. Curr. Opin. Colloid Interface Sci. 2019, 39, 24–39. [Google Scholar] [CrossRef]
- Arick, D.Q.; Choi, Y.H.; Kim, H.C.; Won, Y.-Y. Effects of nanoparticles on the mechanical functioning of the lung. Adv. Colloid Interface Sci. 2015, 225, 218–228. [Google Scholar] [CrossRef]
- Kondej, D.; Sosnowski, T.R. Alteration of biophysical activity of pulmonary surfactant by aluminosilicate nanoparticles. Inhal. Toxicol. 2013, 25, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wu, Y.K.; Ren, Q.Z.; Zhang, L.G.; Liu, S.J.; Zuo, Y.Y. Biophysical Assessment of Pulmonary Surfactant Predicts the Lung Toxicity of Nanomaterials. Small Methods 2018, 2, 8. [Google Scholar] [CrossRef]
- Sosnowski, T.R.; Kubski, P.; Wojciechowski, K. New experimental model of pulmonary surfactant for biophysical studies. Colloid Surf. A 2017, 519, 27–33. [Google Scholar] [CrossRef]
- Guzman, E.; Liggieri, L.; Santini, E.; Ferrari, M.; Ravera, F. Influence of silica nanoparticles on phase behavior and structural properties of DPPC-Palmitic acid Langmuir monolayers. Colloids Surf. A 2012, 413, 280–287. [Google Scholar] [CrossRef]
- Guzman, E.; Liggieri, L.; Santini, E.; Ferrari, M.; Ravera, F. DPPC-DOPC Langmuir monolayers modified by hydrophilic silica nanoparticles: Phase behaviour, structure and rheology. Colloids Surf. A 2012, 413, 174–183. [Google Scholar] [CrossRef]
- Guzman, E.; Liggieri, L.; Santini, E.; Ferrari, M.; Ravera, F. Influence of silica nanoparticles on dilational rheology of DPPC-palmitic acid Langmuir monolayers. Soft Matter 2012, 8, 3938–3948. [Google Scholar] [CrossRef]
- Guzman, E.; Liggieri, L.; Santini, E.; Ferrari, M.; Ravera, F. Mixed DPPC-cholesterol Langmuir monolayers in presence of hydrophilic silica nanoparticles. Colloids Surf. B 2013, 105, 284–293. [Google Scholar] [CrossRef]
- Guzman, E.; Orsi, D.; Cristofolini, L.; Liggieri, L.; Ravera, F. Two-Dimensional DPPC Based Emulsion-like Structures Stabilized by Silica Nanoparticles. Langmuir 2014, 30, 11504–11512. [Google Scholar] [CrossRef]
- Orsi, D.; Guzman, E.; Liggieri, L.; Ravera, F.; Ruta, B.; Chushkin, Y.; Rimoldi, T.; Cristofolini, L. 2D dynamical arrest transition in a mixed nanoparticle-phospholipid layer studied in real and momentum spaces. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef]
- Guzman, E.; Ferrari, M.; Santini, E.; Liggieri, L.; Ravera, F. Effect of silica nanoparticles on the interfacial properties of a canonical lipid mixture. Colloids Surf. B 2015, 136, 971–980. [Google Scholar] [CrossRef]
- Guzman, E.; Santini, E.; Ferrari, M.; Liggieri, L.; Ravera, F. Interfacial Properties of Mixed DPPC-Hydrophobic Fumed Silica Nanoparticle Layers. J. Phys. Chem. C 2015, 119, 21024–21034. [Google Scholar] [CrossRef]
- Orsi, D.; Rimoldi, T.; Guzman, E.; Liggieri, L.; Ravera, F.; Ruta, B.; Cristofolini, L. Hydrophobic Silica Nanoparticles Induce Gel Phases in Phospholipid Monolayers. Langmuir 2016, 32, 4868–4876. [Google Scholar] [CrossRef] [PubMed]
- Guzman, E.; Santini, E.; Ferrari, M.; Liggieri, L.; Ravera, F. Effect of the Incorporation of Nanosized Titanium Dioxide on the Interfacial Properties of 1,2-Dipalmitoyl-sn-glycerol-3-phosphocholine Langmuir Monolayers. Langmuir 2017, 33, 10715–10725. [Google Scholar] [CrossRef] [PubMed]
- Guzman, E.; Liggieri, L.; Santini, E.; Ferrari, M.; Ravera, F. Effect of Hydrophilic and Hydrophobic Nanoparticles on the Surface Pressure Response of DPPC Monolayers. J. Phys. Chem. C 2011, 115, 21715–21722. [Google Scholar] [CrossRef]
- Bykov, A.G.; Gochev, G.; Loglio, G.; Miller, R.; Panda, A.K.; Noskov, B.A. Dynamic surface properties of mixed monolayers of polystyrene micro- and nanoparticles with DPPC. Colloids Surf. A 2017, 521, 239–246. [Google Scholar] [CrossRef]
- Bykov, A.G.; Guzmán, E.; Rubio, R.G.; Krycki, M.M.; Milyaeva, O.Y.; Noskov, B.A. Influence of temperature on dynamic surface properties of spread DPPC monolayers in a broad range of surface pressures. Chem. Phys. Lipids 2019, 225, 104812. [Google Scholar] [CrossRef]
- Sheridan, A.J.; Slater, J.M.; Arnold, T.; Campbell, R.A.; Thompson, K.C. Changes to DPPC Domain Structure in the Presence of Carbon Nanoparticles. Langmuir 2017, 33, 10374–10384. [Google Scholar] [CrossRef]
- Bykov, A.G.; Noskov, B.A. Surface Dilatational Elasticity of Pulmonary Surfactant Solutions in a Wide Range of Surface Tensions. Colloid J. 2018, 80, 467–473. [Google Scholar] [CrossRef]
- Bykov, A.G.; Loglio, G.; Ravera, F.; Liggieri, L.; Miller, R.; Noskov, B.A. Dilational surface elasticity of spread monolayers of pulmonary lipids in a broad range of surface pressure. Colloids Surf. A 2018, 541, 137–144. [Google Scholar] [CrossRef]
- Bykov, A.G.; Loglio, G.; Miller, R.; Milyaeva, O.Y.; Michailov, A.V.; Noskov, B.A. Dynamic properties and relaxation processes in surface layer of pulmonary surfactant solutions. Colloids Surf. A 2019, 573, 14–21. [Google Scholar] [CrossRef]
- Keating, E.; Zuo, Y.Y.; Tadayyon, S.M.; Petersen, N.O.; Possmayer, F.; Veldhuizen, R.A.W. A modified squeeze-out mechanism for generating high surface pressures with pulmonary surfactant. Biochim. Biophys. Acta Biomembr. 2012, 1818, 1225–1234. [Google Scholar] [CrossRef] [PubMed]
- Valle, R.P.; Wu, T.; Zuo, Y.Y. Biophysical Influence of Airborne Carbon Nanomaterials on Natural Pulmonary Surfactant. ACS Nano 2015, 9, 5413–5421. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, E.; Santini, E.; Zabiegaj, D.; Ferrari, M.; Liggieri, L.; Ravera, F. Interaction of Carbon Black Particles and Dipalmitoylphosphatidylcholine at the Water/Air Interface: Thermodynamics and Rheology. J. Phys. Chem. C 2015, 119, 26937–26947. [Google Scholar] [CrossRef]
- Kondej, D.; Sosnowski, T.R. Carbon nanotubes influence surface properties of the phospholipid membrane. Toxicol. Lett. 2018, 295, S203–S204. [Google Scholar] [CrossRef]
- Sosnowski, T.R.; Kolinski, M.; Gradon, L. Interactions of Benzo a pyrene and Diesel Exhaust Particulate Matter with the Lung Surfactant System. Ann. Occup. Hyg. 2011, 55, 329–338. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, S. Effects of Fullerenes on Phospholipid Membranes: A Langmuir Monolayer Study. ChemPhysChem 2009, 10, 2284–2289. [Google Scholar] [CrossRef]
- Wang, Z.; Li, X.; Yang, S. Studies of Dipalmitoylphosphatidylcholine (DPPC) Monolayers Embedded with Endohedral Metallofullerene (Dy@C82). Langmuir 2009, 25, 12968–12973. [Google Scholar] [CrossRef]
- Li, S.; Stein, A.J.; Kruger, A.; Leblanc, R.M. Head Groups of Lipids Govern the Interaction and Orientation between Graphene Oxide and Lipids. J. Phys. Chem. C 2013, 117, 16150–16158. [Google Scholar] [CrossRef]
- Pavoski, G.; Maraschin, T.; de Carvalho-Fim, F.; Balzaretti, N.M.; Galland, G.B.; Moura, C.S.; de Sousa-Basso, N.R. Few Layer Reduced Graphene Oxide: Evaluation of the Best Experimental Conditions for Easy Production. Matter Res. 2017, 20, 53–61. [Google Scholar] [CrossRef]
- Hifeda, Y.F.; Rayfield, G.W. Evidence for First-Order Phase Transitions in Lipid and Fatty Acid Monolayers. Langmuir 1992, 8, 197–200. [Google Scholar] [CrossRef]
- Monroy, F.; Ortega, F.; Rubio, R.G.; Velarde, M.G. Surface rheology, equilibrium and dynamic features at interfaces, with emphasis on efficient tools for probing polymer dynamics at interfaces. Adv. Colloid Interface Sci. 2007, 134, 175–189. [Google Scholar] [CrossRef] [PubMed]
- Huh, S.H. Thermal Reduction of Graphene Oxide. In Physics and Applications of Graphene Experiment; Mikhailov, S., Ed.; InTech: London, UK, 2011. [Google Scholar]
- López-Díaz, D.; Velázquez, M.M.; Blanco de La Torre, S.; Pérez-Pisonero, A.; Trujillano, R.; Fierro, J.L.G.; Claramunt, S.; Cirera, A. The Role of Oxidative Debris on Graphene Oxide Films. ChemPhysChem 2013, 14, 4002–4009. [Google Scholar] [CrossRef]
- Claramunt, S.; Varea, A.; López-Díaz, D.; Velázquez, M.M.; Cornet, A.; Cirera, A. The Importance of Interbands on the Interpretation of the Raman Spectrum of Graphene Oxide. J. Phys. Chem. C 2015, 115, 10123–10129. [Google Scholar] [CrossRef]
- Hontoria-Lucas, C.; López-Peinado, A.J.; López-González, J.D.D.; Rojas-Cervantes, M.L.; Martin-Aranda, R.M. Study of oxygen-containing groups in a series of graphite oxides: Physical and chemical characterization. Carbon 1995, 33, 1585–1592. [Google Scholar] [CrossRef]
- Yang, D.; Velamakanni, A.; Bozoklu, G.; Park, S.; Stoller, M.; Piner, R.D.; Stankovich, S.; Jung, I.; Field, D.A.; Ventrice, C.A.; et al. Chemical analysis of graphene oxide films after heat and chemical treatments by X-ray photoelectron and Micro-Raman spectroscopy. Carbon 2009, 47, 145–152. [Google Scholar] [CrossRef]
- Klopfer, K.J.; Vanderlick, T.K. Isotherms of Dipalmitoylphosphatidylcholine (DPPC) Monolayers: Features Revealed and Features Obscured. J. Colloid Interface Sci. 1996, 182, 220–229. [Google Scholar] [CrossRef]
- Arriaga, L.R.; Löpez-Montero, I.; Ignés-Mullol, J.; Monroy, F. Domain-growth kinetic origin of nonhorizontal phase coexistence plateaux in langmuir monolayers: Compression rigidity of a raft-like lipid distribution. J. Phys. Chem. B 2010, 114, 4509–4520. [Google Scholar] [CrossRef]
- Phillips, M.C.; Chapman, D. Monolayer characteristics of saturated 1,2-diacyl phosphatidylcholines (lecithins) and phosphatidylethanolamines at the air-water interface. Biochim. Biophys. Acta Biomembr. 1968, 163, 301–313. [Google Scholar] [CrossRef]
- Gaines, G.L. Insoluble Monolayers at Liquid–Gas Interfaces; Interscience Publishers: New York, NY, USA, 1966. [Google Scholar]
- Dynarowicz-Łątka, P.; Kita, K. Molecular interaction in mixed monolayers at the air/water interface. Adv. Colloid Interface Sci. 1999, 79, 1–17. [Google Scholar] [CrossRef]
- Petrov, J.G.; Andreeva, T.D.; Moehwald, H. Dipolar Interactions and Miscibility in Binary Langmuir Monolayers with Opposite Dipole Moments of the Hydrophilic Heads. Langmuir 2009, 25, 3659–3666. [Google Scholar] [CrossRef]
- Ravera, F.; Ferrari, M.; Santini, E.; Liggieri, L. Influence of surface processes on the dilational visco-elasticity of surfactant solutions. Adv. Colloid Interface Sci. 2005, 117, 75–100. [Google Scholar] [CrossRef] [PubMed]
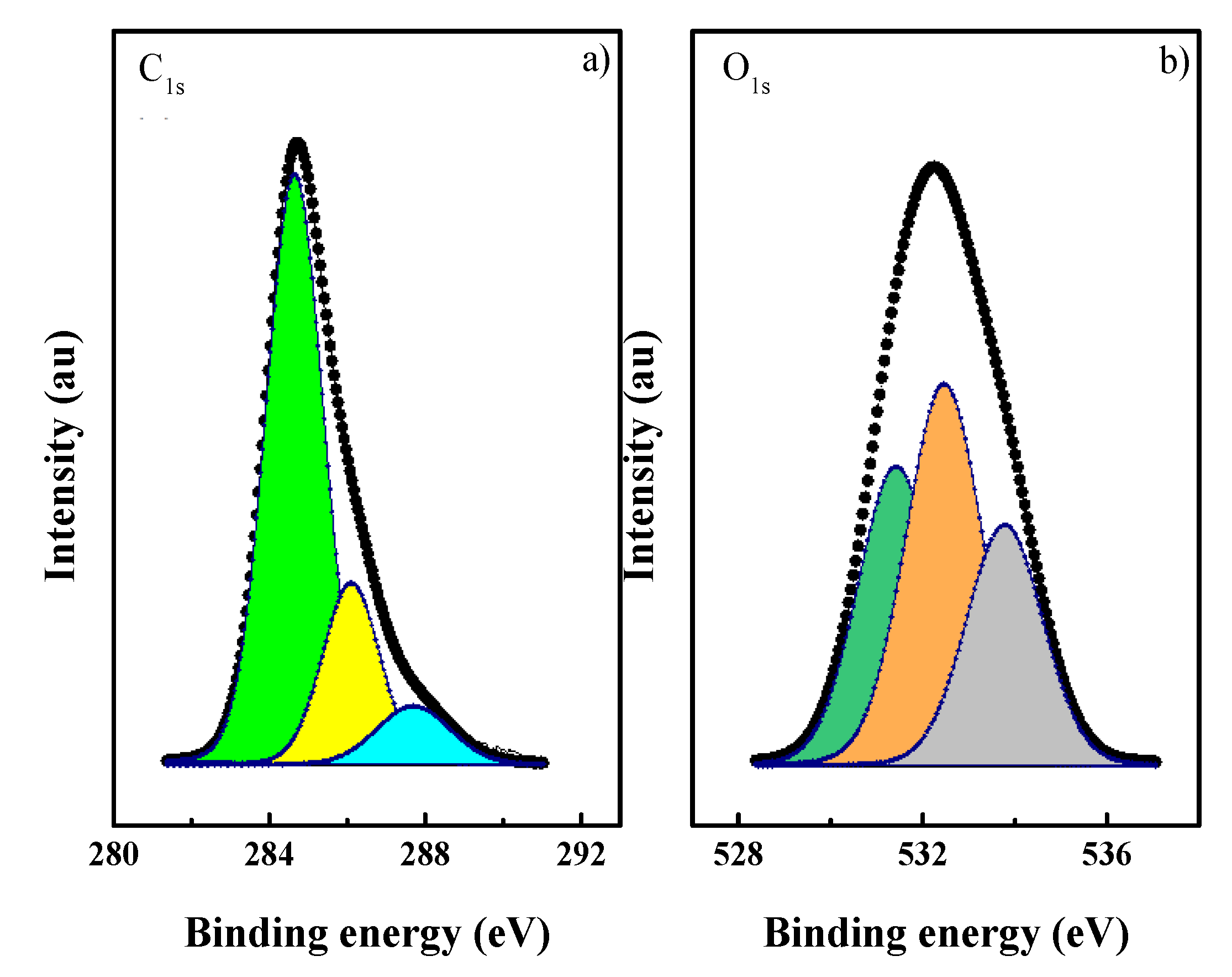
| C1s Emission | Max. Binding Energy/eV (Percentage) | O1s Emission | Max. Binding Energy/eV (Percentage) | O/C at |
|---|---|---|---|---|
| C=C | 284.8 (71) | C=O | 531.5 (33) | 0.154 |
| C–O | 286.2 (22) | C–O | 532.6 (41) | |
| C=O | 287.6 (7) | O–H | 533.8 (26) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz-López, R.; Guzmán, E.; Velázquez, M.M.; Fernández-Peña, L.; Merchán, M.D.; Maestro, A.; Ortega, F.; G. Rubio, R. Influence of Carbon Nanosheets on the Behavior of 1,2-Dipalmitoyl-sn-glycerol-3-phosphocholine Langmuir Monolayers. Processes 2020, 8, 94. https://doi.org/10.3390/pr8010094
Muñoz-López R, Guzmán E, Velázquez MM, Fernández-Peña L, Merchán MD, Maestro A, Ortega F, G. Rubio R. Influence of Carbon Nanosheets on the Behavior of 1,2-Dipalmitoyl-sn-glycerol-3-phosphocholine Langmuir Monolayers. Processes. 2020; 8(1):94. https://doi.org/10.3390/pr8010094
Chicago/Turabian StyleMuñoz-López, Ruth, Eduardo Guzmán, Maria Mercedes Velázquez, Laura Fernández-Peña, María Dolores Merchán, Armando Maestro, Francisco Ortega, and Ramón G. Rubio. 2020. "Influence of Carbon Nanosheets on the Behavior of 1,2-Dipalmitoyl-sn-glycerol-3-phosphocholine Langmuir Monolayers" Processes 8, no. 1: 94. https://doi.org/10.3390/pr8010094
APA StyleMuñoz-López, R., Guzmán, E., Velázquez, M. M., Fernández-Peña, L., Merchán, M. D., Maestro, A., Ortega, F., & G. Rubio, R. (2020). Influence of Carbon Nanosheets on the Behavior of 1,2-Dipalmitoyl-sn-glycerol-3-phosphocholine Langmuir Monolayers. Processes, 8(1), 94. https://doi.org/10.3390/pr8010094












