Abstract
Biopharmaceuticals are currently becoming one of the fastest growing segments of the global pharmaceutical industry, being used in practically all branches of medicine from disease treatment to prevention. Virus-like particles (VLP) hold tremendous potential as a vaccine candidate due to their anticipated immunogenicity and safety profile when compared to inactivated or live attenuated viral vaccines. Nevertheless, there are several challenges yet to be solved in the development and manufacturing of these products, which ultimately can increase time to market. Suchlike virus-based products, the development of a platform approach is often hindered due to diversity and inherent variability of physicochemical properties of the product. In the present work, a flow-through chromatographic purification strategy for hepatitis C VLP expressed using the baculovirus-insect cell expression system was developed. The impact of operational parameters, such as residence time and ionic strength were studied using scaled-down models and their influence on the purification performance was described. The flow-through strategy herein reported made use of radial-flow chromatography columns packed with an anion exchanger and was compared with a bind and elute approach using the same chromatography media. Overall, by selecting the optimal operational setpoints, we were able to achieve higher VLP recoveries in the flow-through process (66% versus 37%) with higher removal of DNA, baculovirus and host-cell protein (92%, 99% and 50% respectively).
1. Introduction
Hepatitis C virus (HCV) is a global health issue that leads to approximately 200 million people infected worldwide. This virus causes an estimated 476,000 deaths per year due to the high rate of chronic infection, which can progress eventually to liver cirrhosis and hepatocellular carcinoma [,]. The treatments for chronic hepatitis C infection are based on antiviral therapies that are expensive, limiting their access in the low and middle-income countries. Furthermore, reinfection even after successful treatments has been reported []. Thus, the need for developing a vaccine is still very high. There are different types of vaccine platforms varying from live attenuated, inactivated, DNA vaccines, subunit vaccines and recombinant viral vectors []. Virus-like particles (VLP) appear as an appealing model of subunit vaccines due to their efficacy and safety profiles. Their self-assembled capsids or envelopes display a conformation similar to native viruses, being capable of triggering both humoral and cell-mediated immune responses []. Furthermore, due to their lack of viral genome, VLP are non-infectious thus avoiding the drawbacks seen with live attenuated viral vaccines.
Production of VLP can be performed using different production systems, although, for more complex VLPs, the baculovirus-insect cells system is preferable since it can induce more post-translational modifications and express multiple-component VLP []. Immunogenicity of HCV-VLP was already evaluated in animal models with encouraging results for an HCV vaccine design [,,]. Besides the production system, one of the most critical parts in vaccine developments is the purification process, as downstream processing of biopharmaceuticals, such as VLP, can count for up to 80% of manufacturing costs []. Thus, efficient, robust and fast processes are needed.
Conventional purification methods for this type of biologics still make use of sucrose or caesium chloride gradient ultracentrifugation. Although this technique often results in concentrated and pure samples [], the associated recovery yields are rather low. Furthermore, ultracentrifugation techniques are labor intensive processes with high cost of goods related to equipment and maintenance and are often associated with limited scalability [,,]. More recently, and due to the stringent purity guidelines, several examples of VLP purification making use of a combination of different unit operations are found in the literature [,]. These strategies commonly rely on the combination of filtration [,] and chromatography operations [,]. Chromatography is often used at two distinct steps: intermediate purification, aiming at bulk impurity removal and polishing, targeted to the removal of residual impurities. Ion exchange chromatography (IEC) in the bind-and-elute mode is one of the most common methods employed for intermediate purification []. The latest developments in chromatographic materials have been providing improved capacities and scalability from laboratory to commercial scale. Nonetheless, unique characteristics of virions, including their biophysical properties, stability and variability must be considered alongside the choice of the purification strategy for maximum efficiency. For IEC one important parameter to be considered upon process development is the VLP charge. Konz and coworkers [] reported a serotype dependence on chromatographic retention for adenovirus. A similar behavior was illustrated for enveloped viruses such as influenza by Michen and coworkers [], where a strain dependence of the virus isoelectric points was reported, suggesting that retention in ion exchange adsorbers will also vary with different virus strains. In addition to this, although there is a wide diversity of adsorptive surfaces and chemistries, the relatively large dimensions of viruses or VLP, between 100 and 200 nm, makes adsorption of these species restricted to the bead surface []. For positive chromatographic purification, where the product of interest is adsorbed in the chromatographic matrix, this might represent a capacity limitation. On the contrary, impurities such as host cell DNA and protein will access the porous surface area within the beads. Negative chromatographic purification, also known as flow-through (FT) chromatography, rather aims at adsorbing product impurities, overcoming the previous product capacity limitations.
The present work reports the development of a flow-through chromatographic strategy aimed at the purification of Hepatitis C VLP produced using insect cell-based expression with recombinant baculovirus. A negative chromatographic strategy using an anion exchange resin was selected for its simplicity of operation and design. The proposed strategy aims to deliver product with sufficient purity and quantity for preclinical studies. Optimization of operational parameters such as residence time and buffer ionic strength and their impact on the separation performance was performed. Additionally, the use of radial-flow chromatography was evaluated due to its potential to overcome pressure-related limitations in conventional axial-bed columns [,], with proven benefits in scale-up, performance [], and potential productivity increase.
2. Materials and Methods
2.1. Preparation of Hepatitis C VLP Feedstock
Production of hepatitis C VLP was carried out in a disposable stirred-tank bioreactor. Spodoptera frugiperda insect ovary cells (Sf9) in suspension culture (cat# 11496015, Thermo Fisher Scientific, MA, USA) were cultured in Sf-900TM II serum-free medium (cat# 10902179, Thermo Fisher Scientific, MA, USA) using a 2 L working volume disposable Mobius® 3 L Bioreactors (EMD Millipore, Billerica, MA, USA). Cultivations were carried out at 27 °C, dpO2 of 30% and an aeration rate of 0.01 vvm. Twenty-four hours after inoculation (1 × 106 cells mL−1) cells were co-infected at a multiplicity of infection of 1 for each recombinant baculovirus expressing Gag-MLV and HCV-E1/E2. Bioreactor bulk harvesting was performed at 96 hours post infection.
Upon harvesting, bulk clarification was performed using disposable Optiscale depth filtration devices with Polygard® CN membrane material (EMD Millipore, Bedford, MA, USA) The filtration flux was set to 98 L m−2 h−1 using a Tandem 1082 Pump (Sartorius Stedim Biotech, Gottingen, Germany). Clarified bulk concentration was carried out using UF cassettes (Pellicon® XL, EMD Millipore, Bedford, MA, USA) with a nominal pore size of 300 kDa, composed of composite regenerated cellulose. The concentration was performed until a volume reduction factor of 5 was achieved.
2.2. Anion Exchange Chromatography
Anion exchange (AEX) chromatographic media, Fractogel® TMAE (Merck Millipore, Darmstadt, Germany) was used for VLP purification. The resin was used either in slurry format for batch adsorption studies or packed in different chromatographic column formats.
2.2.1. Column Experiments
For packed bed formats column equilibration was performed with five column volumes (CV) of equilibration buffer (50 mM HEPES, 150 mM NaCl, pH = 7.4) and regenerated using 0.5 M of sodium hydroxide after each experiment. An Äkta Explorer 10s (GE Healthcare, Uppsala, Sweden) equipped with UV, conductivity, and pH detectors, and a fraction collector FRAC-950 (GE Healthcare, Uppsala, Sweden) was used for all column chromatography experiments. Sample injection was performed using a 50 mL superloop (GE Healthcare, Uppsala, Sweden).
Scouting experiments for bind and elute purification were carried out with a 1 mL pre-packed axial column (Merck Millipore, Darmstadt, Germany). After column equilibration, 10 CV of the VLP feedstock were injected. After sample application, the column was washed with five CV of the equilibration buffer and a linear gradient was performed by modulating NaCl concentration in the range of 150–2000 mM. A second set of experiments using step elution was also performed. Similarly, after column equilibration, 10 CVs of the VLP feedstock were injected, followed by a washing step of five CV with an equilibration buffer. Step elution was performed using 400, 550 and 700 mM of NaCl in 50 mM Hepes, pH = 7.4.
Radial-flow chromatographic experiments were carried out using a pre-packed 5 mL column (Procxys, Amsterdam, The Netherlands). After equilibration buffer injection (50 mM HEPES, 300 mM NaCl, pH = 7.4, five CV) sample injection was performed using a 50 mL superloop, followed by a wash step with the equilibration buffer and a final elution step using 50 mM HEPES, 1000 mM NaCl, pH = 7.4)
2.2.2. Static Adsorption Experiments
Small-scale batch adsorption studies were conducted using a fluid/solid ratio of 4. To different 250 μL aliquots of settled adsorbent previously equilibrated at different NaCl concentrations was added 1.0 mL of fluid with a determined and equal concentration of VLP. Concentration of NaCl in each tube was adjusted to 150, 300, 450 and 575 mM by using a concentrated NaCl buffered with 50 mM HEPES, pH = 7.4. The samples were incubated at 25 °C for 80 min under agitation. After incubation, the supernatant was recovered and analyzed for VLP, baculovirus, DNA and host-cell protein composition.
2.2.3. Batch Uptake Experiments
Adsorption of VLP and baculovirus (BV) was studied as a function of time in 15 mL flasks under agitation at room temperature (25 °C). Each flask contained 2 mL of settled resin previously equilibrated with 50 mM HEPES, 300 mM of NaCl, pH = 7.4. At time 0, 6 mL of VLP feedstock at different concentrations were added to each flask. Fixed volumes samples of 100 μL were withdrawn at different time points during an 80 min experiment. Samples were analyzed to obtain transient unabsorbed VLP and BV concentrations. A rate model based on the kinetic form of the Langmuir adsorption isotherm was used to simultaneously fit the transient data and estimate the adsorption isotherm parameters.
2.3. Analytical Methods
2.3.1. DNA Quantification
Total double-stranded DNA of process samples was measured using the fluorescent-based Quant-iTTM PicoGreen® dsDNA Assay kit (Life Technologies, Carlsbad, CA, USA) according to manufacturer’s instructions. The assay was performed in black 96-well microplates, flat transparent (Corning, NY, USA) and the fluorescence was measured on Infinite® 200 PRO NanoQuant (Tecan, Männedorf, Switzerland).
2.3.2. Host Cell Protein
The concentration of host cell proteins (HCP) from SF9 insect cells was determined using a two-site immunoenzymetric assay (Cygnus Technologies, Inc., Southport, NC, USA) without changes to the manufacturer’s protocol. The absorbance was measured at 450 nm with a reference at 650 nm using an Infinite® 200 PRO NanoQuant (Tecan, Männedorf, Switzerland).
2.3.3. VLP Quantification
VLP were quantified following Gag p30 protein content in process samples using a commercially available QuickTiter MuLV core antigen ELISA kit (Cell Biolabs, San Diego, CA, USA) according to the manufacturer’s instructions. The absorbance was measured at 450 nm with a reference at 620 nm using an Infinite® 200 PRO NanoQuant (Tecan, Männedorf, Switzerland).
2.3.4. Baculovirus Particle Quantification
Quantification of genome-containing particles of process samples was performed under a two-step procedure. Firstly, the viral DNA was extracted and purified with the High Pure Viral Nucleic Acid Kit (Roche Diagnostics, Mannheim, Germany) as described in the manufacturer’s instructions. All samples were previously diluted 10 times, before genome extraction, with D-PBS (Thermo Fisher Scientific, MA, USA). Secondly, the number of viral DNA copies was determined by real-time PCR using the LightCycler system (Roche Diagnostics, Rotkreuz, Switzerland). Purified recombinant baculovirus viral DNA stocks previously quantified by another validated qPCR method [] were used as external standards.
2.3.5. Nanoparticles Tracking Analysis
Presence and size distribution of virus-like particles and other remaining bulk particles was measured using a NanoSight NS500 (Malvern Instruments Ltd., Malvern, UK). In order to be in the range of 108–109 particles mL−1, the instrument’s linear range, the process samples were diluted in D-PBS (Thermo Fisher Scientific, MA, USA). The processing of video frames was done with Nanoparticle Tracking Analysis (NTA) 3.2 Analytical software. The camera level and the screen gain were set to 16 and 1 respectively. For each sample 60 s videos were acquired and particles between 70 and 150 nm were considered.
3. Results and Discussion
The objective of the present work was to develop a scalable purification process, based on flow-through chromatography, using radial-flow columns, for hepatitis C VLP that maximizes productivity and is capable of supply sufficient purity and quantity required for preclinical studies. There are no specific guidelines with regard to the purity of preclinical material. It is however expected an increase in the drug purity when moving from discovery, throughout preclinical and clinical stages []. Focus in the preclinical stage should not be on achieving maximum purity but rather on obtaining a drug substance with an impurity profile that ideally will be similar and consistent with that of the later clinical material [,]. During a preclinical scenario the drug’s toxic and pharmacological effects are evaluated through in vitro and in vivo laboratory animal testing. The quantity requirements for preclinical studies are several orders of magnitude above the drug discovery stage. Additionally, the production process should be, at this stage, stable or similar to the substance production process for clinical testing. This will enable consistent results and safety in later clinical testing []. In Figure 1 are proposed two different routes for VLP purification. The first approach was supported by chromatography while the second relied on density gradient centrifugation. Although this last approach is often used to prepare virus stocks and is capable of providing materials with high purity and concentration, it presents several drawbacks related to process recovery and scalability. Chromatography-based purification is widely used in the biopharmaceutical industry, providing not only scalability but also the possibility of exploiting the difference in properties between the product of interest and associated impurities. The inclusion of tangential filtration steps, in the purification workflow provides the process with the capability of conditioning the VLP sample with the appropriate buffer composition before chromatography and, later on, formulates the purified sample.
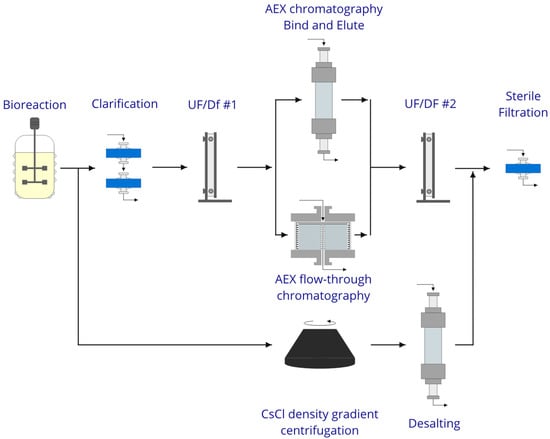
Figure 1.
Possible processing routes for hepatitis C virus-like particles (VLP); clarification performed using a train of depth filters; concentration and buffer exchange using tangential flow filtration with membrane cassettes devices.
3.1. Bind and Elute Purification
Chromatographic purification of virus and VLP is usually performed with anion exchangers in a bind and elute mode [,]. In order to assess this route of purification 10 mL of a VLP sample obtained after concentration and buffer exchange were applied to a 1 mL column. Elution of the adsorbed VLP was then performed with a two-step linear gradient followed by a column stripping step and depicted in Figure 2. The flow-through and wash steps pools returned 39% of VLP recovery. In addition to this, the observed VLP recovery across the elution gradient was in the range of 3–10%. A step-wise elution with 400, 550 and 700 mM of NaCl followed by a column stripping step with 1 M of NaCl was also performed. The obtained VLP recovery in the elution step varied between 33% and 37%. The obtained recovery value was in line with the reported values in the literature for the used resin (35%) []. The column stripping with 1 M of NaCl yielded an additional recovery of 17–23%. Although VLP recovery was mildly affected by the composition of the elution buffer, the same behavior was not observed for the monitored impurities.
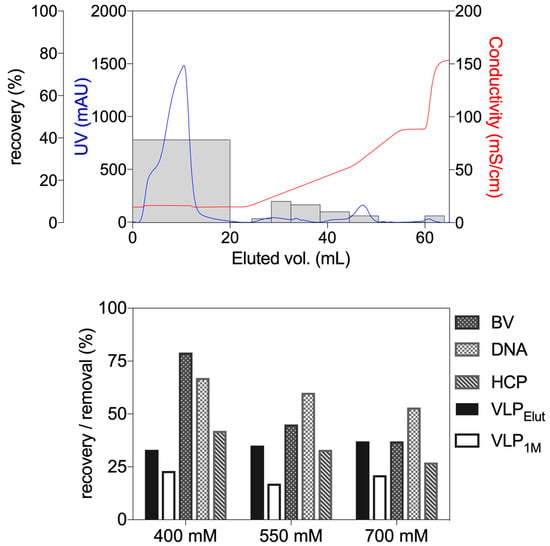
Figure 2.
Bind and elute chromatography. Top—gradient elution profile obtained after loading a Fractogel® TMAE anion exchange 1 mL column with 10 mL of hepatitis C feedstock, recovered after concentration and buffer exchange. Loading was carried at a linear velocity of 200 cm h−1. Columns correspond to VLP recovery of each pool recovered. Elution performed using linear gradients from 0–25% and 25–50% of elution buffer, followed by a column stripping step gradient at 100% of elution buffer (2 M NaCl). Bottom—step elution at 400, 550 and 700 mM of NaCl (VLP recovery—black columns) followed by a column stripping step at 1 M of NaCl (VLP recovery—empty columns; n = 1). Removal of baculovirus (BV), DNA and host-cell protein (HCP) was also reported for each elution step.
A possible explanation for the linear gradient elution results might be associated with different VLP adsorption mechanisms or to particle charge heterogeneity within the VLP population leading to different binding strengths. On the other hand, step elution allowed us to simultaneously displace the different variants of VLP, thus returning a single elution pool. Another constraint to the proposed strategy is related to the adsorption capacity of IEC resins. Previous studies evaluating virus and VLP purification using IEC resins suggest that due to the relative size of these species adsorption is carried mainly at the outer surface of the IEC beads []. A targeted capture of VLP was compromised due to the limitation of adsorption capacity.
3.2. Flow-Through Purification
Flow-through chromatography is perhaps one of the most common used tools to polish monoclonal antibodies. Operating close to neutral pH and at low conductivity, many viruses and trace impurities such as DNA, endotoxin and a large percentage of host cell proteins display a negative charge, thus binding to the AEX matrix, whereas the typically basic antibody species will not []. This type of operation can circumvent the aforementioned capacity limitation for VLP purification by selecting operating conditions that promote the adsorption of impurities while the VLP product flows through. On top of that, this type of operation also avoids elution steps with high salts concentrations that can compromise the immunogenic effect of the VLP []. Optimal depletion of impurities and VLP recovery is dependent upon certain process parameters. Residence time and ionic strength of the loaded sample are the most common parameters used to modulate product binding [,]. The route chosen to select the optimal operation points is described next.
3.2.1. Ionic Strength Optimization
The results of the experiment reported in Figure 3 found clear support for the dependence of VLP binding on ionic strength. The graph shows that for higher NaCl concentration the recovery of VLP in the supernatant gradually increases. An opposite behavior can be found for DNA, host cell protein and baculovirus. The decrease observed in HCP, DNA and BV removal, limits the range of NaCl concentrations that can be used for a flow-through purification of VLP. Concentrations bellow 200 mM enable a higher impurity clearance, however, VLP recovery will be negatively affected. Salt concentration above 200 mM of NaCl allow higher VLP recovery but at the expense of lower purity.
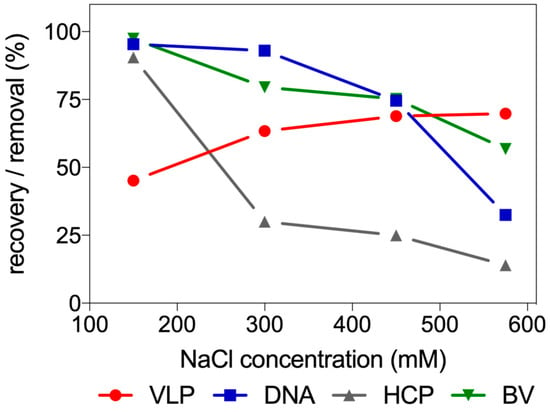
Figure 3.
Batch adsorption study using Fractogel® TMAE at different load conditions. Evaluation of the impact of different NaCl concentrations in VLP recovery and removal of DNA, BV and HCP.
The insect cell-baculovirus system (IC-BEVS) is broadly used for the production of recombinant viral proteins for vaccine applications, and particularly appropriate for the production of virus-like particles that often require the simultaneous expression of several recombinant proteins. Nevertheless, alongside with VLP, baculovirus are also expressed. This species can be expressed in different proportions in comparison to VLP, depending on the cell line used and bioreaction parameters []. In addition to this, due to the similar size and charge of baculovirus, VLP might compete for the adsorber binding sites, leading to a reduced adsorption capacity for baculovirus. Batch uptake studies are frequently performed to determine adsorption conditions as part of method development for packed bed applications. The transient and equilibrium data gathered could be correlated with theoretical or empirical equations that allow us to further characterize the adsorptive system. Batch uptake studies were performed for different initial concentration of VLP and BV. The resulting transient adsorption kinetics from the batch adsorption experiments are plotted in Figure 4.
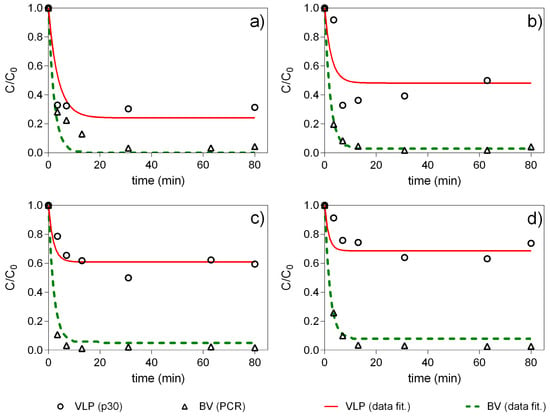
Figure 4.
Batch uptake experiments at different feedstock concentrations. (a) C0 = ¼ Cfeedstock; (b) C0 = ½ Cfeedstock; (c) C0 = ¾ Cfeedstock; (d) C0 = Cfeedstock. All experiments were carried at constant solid/fluid ratio. The concentration of NaCl is all samples is 300 mM. = 51.5 ng mL−1; = 2.3 × 1010 copies mL−1.
The equilibrium concentration of the considered species, VLP and baculovirus were estimated by simultaneously fitting the transient experimental data of the four experiments to a kinetic form of a Langmuir adsorption isotherm as described elsewhere []. This figure illustrates that there is a rapid drop in liquid phase concentration within the first 20 min, leading to a local minimum in the concentration of VLP and baculovirus in the liquid phase in each batch sorption system, which remained constant for the rest of the equilibration period. A closer analysis of this figure reveals that the equilibrium liquid phase concentration of VLP was strongly dependent on the initial loading concentration. On the other hand, independently of the concentration of the initial sample the baculovirus liquid phase equilibrium concentration fell abruptly to less than 10% of the initial concentration in the four experiments. This fact suggests that at the selected NaCl concentration (300 mM) there was a highly favorable adsorption of baculovirus contrarily to what was observed for the VLP. The analysis of the adsorption isotherms in Figure 5 revealed two favorable adsorption equilibrium isotherms. The nearly rectangular shape of the BV isotherm indicates that the adsorption of these species to the chromatography media was nearly independent of the bulk concentration and would probably be adsorbed more preferentially with comparison to VLP.
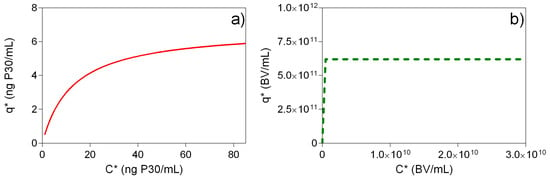
Figure 5.
Equilibrium adsorption isotherm of VLP (a) and baculovirus (b) obtained with the estimated parameters in Table 1. A Langmuir adsorption isotherm () was considered. The equilibrium adsorbed concentration in the stationary phase is denoted by , whereas depicts the associated liquid phase equilibrium concentration.

Table 1.
Best-fit values of the kinetic form of the Langmuir adsorption isotherm. The saturation capacity of the anion-exchanger is denoted by qsat (P30 ng mL−1 or copies mL−1), where Kd and k1 are the dissociation constant (ng mL−1 or copies mL−1) and the forward interaction rate constant (mL ng−1 min−1 or mL copies−1 min−1) of the adsorption model considered respectively.
3.2.2. Throughput and Residence Time Optimization in Radial-Flow Columns
In a flow-through process, impurities are retained in the stationary phase while the product of interest is collected in the flow-through pool []. Product recovery and impurity clearance are therefore dependent on the amount of feed processed. Figure 6a reports the throughput scouting experiments carried out. From the analysis of this figure, it is possible to see that VLP recovery was low for smaller injected volumes, improving with the amount of feed processed through the column. Conversely, impurity clearance reached the highest level for smaller injected volumes and decreased with an increase in throughput.
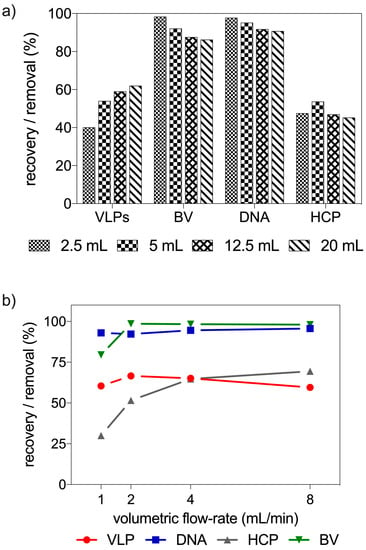
Figure 6.
Throughput and residence time optimization in radial-flow columns. (a) Effect of different loaded sample volumes (2.5, 5, 12.5 and 20 mL) in radial-flow columns (n = 1) and (b) impact of residence time in VLP recovery and impurity clearance in the radial-flow setup used.
Residence time of a chromatographic column is directly linked to volumetric flow rate. Shorter residence times can lead to higher productivities. On the other hand, lowering residence time can also lead to lower capacity utilization of the chromatographic resin. Figure 6b reports the dependence of VLP recovery and impurity clearance on the volumetric flow rate. With the exception of host-cell protein removal, the other species analyzed did not show a strong dependence on residence time. The dependence observed of host-cell protein removal with residence time was not expected and the data suggests that we must be in the presence of some competition for the adsorption sites. In addition, the particle size distribution of the FT purified samples reported in Figure 6b was also evaluated by nanoparticle tracking analysis. Total particle recovery of VLP with a size between 70 and 200 nm was in the range of 55–82%.
The developed FT strategy provided a VLP recovery yield in the range of 60–66%. This purification strategy enabled a removal of baculovirus and DNA of more than 80% and 92% respectively in a single step. Although the flow-velocities and observed recoveries were higher when comparing to the bind and elute chromatography (33–37%) described in Section 3.1 the chromatography media used for the flow-through purification was 2.5 times higher for the same processed volume of VLP feedstock. The developed approach should be seen as a first step in developing a purification process for the proposed hepatitis C vaccine candidate. Although the proposed method offered the possibility to avoid traditional CsCl gradients, providing products with a very low risk of toxicity, further improvements to final purity could be envisioned. One of these improvements could be the implementation of an endonuclease treatment step, especially if DNA clearance is not adequate [,]. However, the use of endonuclease could increase the cost of manufacturing.
Early-stage clinical studies are often associated with a high degree of uncertainty, therefore there is great benefit in producing material for phases-1 and -2 clinical trials using a standardized process and procedures. Virus serotype or differences in the epitopes displayed in a VLP in a classic bind-and-elute process can often result in different elution profiles, thus requiring a fine-tuning of the process. Approaches based on negative-mode purification such as the one herein reported are therefore particularly suited for a platform concept since the target product is not adsorbed. Flow-through chromatography is also often associated with high volumetric flow-rates. The range of available flow rates in a packed bed with an axial flow is limited by pressure drop constraints. Radial-flow chromatography can be used as an alternative to the axial flow chromatography. Due to the radial-flow of the mobile phase, a larger flow area is available with shorter path length, allowing higher volumetric flow rates with lower pressure.
Finally, the use of unit operations such as filtration and chromatography throughout the purification process introduces the potential for a simple, robust and scalable downstream process.
4. Conclusions
A rational design and implementation of a flow-through purification process for hepatitis C VLP using AEX chromatography with radial-flow columns was presented. Initial evaluation of the impact of process operating parameters and materials was performed using scaled-down tools—scout 1 mL columns and shake flasks experiments. Knowledge acquired was translated to a radial-flow setup. The selected operational parameters, such as residence time and the ionic strength loading conditions were studied and their impact on the purification performance was described.
Experimentally, the developed flow-through strategy implemented used 2.5 times more resin for the same volume of feedstock. However, this purification strategy provided a higher recovery yield (66%) when comparing to the bind and elute approach (37%). Furthermore, the developed flow-through approach enabled a baculovirus removal between 80% and 99%, a DNA removal from 92% to 96% and host-cell protein in the range of 30–70% in a single step. Moreover, by using a flow-through strategy, the risks associated with bind and elute mode regarding envelope VLP degradation are reduced, thus providing a robust chromatography step that could be efficiently integrated into a manufacturing platform of this vaccine candidate.
Author Contributions
Conceptualization, R.J.S.S.; methodology, R.J.S.S. and M.G.M.; software, R.J.S.S.; writing—original draft preparation, R.J.S.S. and M.G.M.; writing—review and editing, R.J.S.S., M.G.M., A.S.M., A.X., M.J.T.C. and C.P.; funding acquisition, M.J.T.C., P.M.A. and C.P. All authors have read and agreed to the published version of the manuscript.
Funding
R.J.S.S., M.G.M., and A.S.M., acknowledge FCT for fellowships SFRH/BPD/121558/2016, PD/BD/114034/2015 and PD/BD/135501/2018, respectively.
Acknowledgments
The authors also acknowledge Ana Sofia Coroadinha and Rute Castro for the support in VLP production. The authors would like to acknowledge Andreas Stein, Annika Aldinger and Achim Schwaemmle (Merck Millipore) for the AEX samples and for useful discussions.
Conflicts of Interest
The authors R.S., M.G.M., A.S.M., P.M.A., M.J.T.C. and C.P., declare no competing financial interests. A.X. is an employee of EMD Millipore, the manufacturer of Benzonase® endonuclease, bioreactors, clarification filters, and ultrafiltration cassettes used in the project.
References
- Torresi, J. The Rationale for a Preventative HCV Virus-Like Particle (VLP) Vaccine. Front. Microbiol. 2017, 8, 2163. [Google Scholar] [CrossRef] [PubMed]
- Torresi, J.; Johnson, D.; Wedemeyer, H. Progress in the development of preventive and therapeutic vaccines for hepatitis C virus. J. Hepatol. 2011, 54, 1273–1285. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.F.; Soares, H.R.; Guerreiro, M.R.; Alves, P.M.; Coroadinha, A.S. Viral vaccines and their manufacturing cell substrates: New trends and designs in modern vaccinology. Biotechnol. J. 2015, 10, 1329–1344. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.; Seong, B.L. Exploiting virus-like particles as innovative vaccines against emerging viral infections. J. Microbiol. 2017, 55, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Bellier, B.; Klatzmann, D. Virus-like particle-based vaccines against hepatitis C virus infection. Expert Rev. Vaccines 2013, 12, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Soares, H.R.; Castro, R.; Tomás, H.A.; Carrondo, M.J.T.; Alves, P.M.; Coroadinha, A.S. Pseudotyping retrovirus like particles vaccine candidates with Hepatitis C virus envelope protein E2 requires the cellular expression of CD81. AMB Express 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, R.N.; Azevedo, A.M.; Aires Barros, M.R.; Krajnc, N.L.; Kramberger, P.; Carbajal, M.L.; Grasselli, M.; Meyer, R.; Fernandez Lahore, M. Emerging technologies for the integration and intensification of downstream bioprocesses. Pharm. Bioprocess. 2013, 1, 423–440. [Google Scholar] [CrossRef]
- Croyle, M.A.; Anderson, D.J.; Roessler, B.J.; Amidon, G.L. Development of a Highly Efficient Purification Process for Recombinant Adenoviral Vectors for Oral Gene Delivery. Pharm. Dev. Technol. 1998, 3, 365–372. [Google Scholar] [CrossRef]
- Morenweiser, R. Downstream processing of viral vectors and vaccines. Gene Ther. 2005, 12, S103–S110. [Google Scholar] [CrossRef]
- Tseng, Y.-F.; Weng, T.-C.; Lai, C.-C.; Chen, P.-L.; Lee, M.-S.; Hu, A.Y.-C. A fast and efficient purification platform for cell-based influenza viruses by flow-through chromatography. Vaccine 2018, 36, 3146–3152. [Google Scholar] [CrossRef]
- Trilisky, E.I.; Lenhoff, A.M. Sorption processes in ion-exchange chromatography of viruses. J. Chromatogr. A 2007, 1142, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Lightfoot, E.N.; Moscariello, J.S. Bioseparations. Biotechnol. Bioeng. 2004, 87, 259–273. [Google Scholar] [CrossRef] [PubMed]
- Vicente, T.; Roldão, A.; Peixoto, C.; Carrondo, M.J.T.; Alves, P.M. Large-scale production and purification of VLP-based vaccines. J. Invertebr. Pathol. 2011, 107, S42–S48. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, S.B.; Silva, R.J.S.; Moreira, A.S.; Cunha, B.; Clemente, J.J.; Alves, P.M.; Carrondo, M.J.T.; Xenopoulos, A.; Peixoto, C. Efficient filtration strategies for the clarification of influenza virus-like particles derived from insect cells. Sep. Purif. Technol. 2019, 218, 81–88. [Google Scholar] [CrossRef]
- Carvalho, S.B.; Silva, R.J.S.; Moleirinho, M.G.; Cunha, B.; Moreira, A.S.; Xenopoulos, A.; Alves, P.M.; Carrondo, M.J.T.; Peixoto, C. Membrane-Based Approach for the Downstream Processing of Influenza Virus-Like Particles. Biotechnol. J. 2019, 14, 1800570. [Google Scholar] [CrossRef]
- Durous, L.; Rosa-Calatrava, M.; Petiot, E. Advances in Influenza Virus-Like Particles bioprocesses. Expert Rev. Vaccines 2019. [Google Scholar] [CrossRef]
- Moleirinho, M.G.; Silva, R.J.S.; Alves, P.M.; Carrondo, M.J.T.; Peixoto, C. Current challenges in biotherapeutic particles manufacturing. Expert Opin. Biol. Ther. 2019, 1–15. [Google Scholar] [CrossRef]
- Pereira Aguilar, P.; Schneider, T.A.; Wetter, V.; Maresch, D.; Ling, W.L.; Tover, A.; Steppert, P.; Jungbauer, A. Polymer-grafted chromatography media for the purification of enveloped virus-like particles, exemplified with HIV-1 gag VLP. Vaccine 2019, 37, 7070–7080. [Google Scholar] [CrossRef]
- Konz, J.O.; Livingood, R.C.; Bett, A.J.; Goerke, A.R.; Laska, M.E.; Sagar, S.L. Serotype Specificity of Adenovirus Purification Using Anion-Exchange Chromatography. Hum. Gene Ther. 2005, 16, 1346–1353. [Google Scholar] [CrossRef]
- Michen, B.; Graule, T. Isoelectric points of viruses. J. Appl. Microbiol. 2010, 109, 388–397. [Google Scholar] [CrossRef]
- Lee, M.F.X.; Chan, E.S.; Tan, W.S.; Tam, K.C.; Tey, B.T. Negative chromatography of hepatitis B virus-like particle: Comparative study of different adsorbent designs. J. Chromatogr. A 2016, 1445, 1–9. [Google Scholar] [CrossRef]
- Cabanne, C.; Raedts, M.; Zavadzky, E.; Santarelli, X. Evaluation of radial chromatography versus axial chromatography, practical approach. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2007, 845, 191–199. [Google Scholar] [CrossRef]
- Besselink, T.; van der Padt, A.; Janssen, A.E.M.; Boom, R.M. Are axial and radial flow chromatography different? J. Chromatogr. A 2013, 1271, 105–114. [Google Scholar] [CrossRef]
- Huang, S.H.; Roy, S.; Hou, K.C.; Tsao, G.T. Scaling-Up of Affinity Chromatography by Radial-Flow Cartridges. Biotechnol. Prog. 1988, 4, 159–165. [Google Scholar] [CrossRef]
- Vicente, T.; Peixoto, C.; Carrondo, M.J.T.; Alves, P.M. Purification of recombinant baculoviruses for gene therapy using membrane processes. Gene Ther. 2009, 16, 766–775. [Google Scholar] [CrossRef]
- Jagschies, G.; Lindskog, E.; Lacki, K.; Galliher, P. Biopharmaceutical Processing: Development, Design, and Implementation of Manufacturing Processes; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 9780128125526. [Google Scholar]
- Johnson, C.; Frantz, S. Role of Study Director and Study Monitor in Drug Development. In A Comprehensive Guide to Toxicology in Preclinical Drug Development; Academic Press: Cambridge, MA, USA, 2013; pp. 747–757. ISBN 9780123878151. [Google Scholar] [CrossRef]
- Segura, M.M.; Kamen, A.A.; Garnier, A. Overview of Current Scalable Methods for Purification of Viral Vectors. Methods Mol. Biol. 2011, 737, 89–116. [Google Scholar] [CrossRef] [PubMed]
- Nestola, P.; Peixoto, C.; Silva, R.R.J.S.; Alves, P.M.; Mota, J.P.B.; Carrondo, M.J.T. Improved virus purification processes for vaccines and gene therapy. Biotechnol. Bioeng. 2015, 112, 843–857. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.X.; Tressel, T. Basic Concepts in Q Membrane Chromatography for Large-Scale Antibody Production. Biotechnol. Prog. 2006, 22, 341–349. [Google Scholar] [CrossRef]
- Cruz, P.E.; Peixoto, C.C.; Moreira, J.L.; Carrondo, M.J.T. Production and quality analysis of Pr55gag particles produced in baculovirus-infected insect cells. J. Chem. Technol. Biotechnol. 1998, 72, 149–158. [Google Scholar] [CrossRef]
- Felberbaum, R.S. The baculovirus expression vector system: A commercial manufacturing platform for viral vaccines and gene therapy vectors. Biotechnol. J. 2015, 10, 702–714. [Google Scholar] [CrossRef]
- Chu, K.H.; Hashim, M.A. Protein adsorption on Ion exchange resin: Estimation of equilibrium isotherm parameters from batch kinetic data. Biotechnol. Bioprocess Eng. 2006, 11, 61–66. [Google Scholar] [CrossRef]
- Silva, R.J.S.; Mota, J.P.B.; Peixoto, C.; Alves, P.M.; Carrondo, M.J.T. Improving the downstream processing of vaccine and gene therapy vectors with continuous chromatography. Pharm. Bioprocess. 2015, 3, 489–505. [Google Scholar] [CrossRef]
- Bandeira, V.; Peixoto, C.; Rodrigues, A.F.; Cruz, P.E.; Alves, P.M.; Coroadinha, A.S.; Carrondo, M.J.T. Downstream processing of lentiviral vectors: Releasing bottlenecks. Hum. Gene Ther. Methods 2012, 23, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Cruz, P.E.; Silva, A.C.; Roldão, A.; Carmo, M.; Carrondo, M.J.T.; Alves, P.M. Screening of novel excipients for improving the stability of retroviral and adenoviral vectors. Biotechnol. Prog. 2006, 22, 568–576. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).