Abstract
The traditional two-column reactive distillation (RD) process is used for the production of butyric anhydride, which is synthesized with butyric acid and acetic anhydride via a reversible reaction. In this work, a novel process with a single RD column (SRDC) is designed for the production of butyric anhydride, where the second distillation column for separating excess reactant is removed based on the boiling point profile of the reaction system. Two applications of the proposed SRDC process, namely SRDC with excess butyric acid or acetic anhydride circulating internally, are economically optimized, and the results show that both SRDC processes have a lower total annual cost (TAC) than the traditional two-column process. Furthermore, from the perspective of TAC, the application with an excess feed of butyric acid is better than the application with excess acetic anhydride. The developed technique may also be applied to retrofit other traditional two-column RD processes, where the overhead and bottom products are the lightest and heaviest components of the reaction system, respectively, and no azeotrope is involved in the RD column.
1. Introduction
Butyric anhydride is an important intermediate product, which is mainly used in the production of cellulose acetate butyrate, butyl butyryl lactate, and spices. The existing production of butyric anhydride is a reversible anhydride exchange reaction of butyric acid and acetic anhydride. The boiling point profile of the reaction systems shows that reactive distillation (RD) can be used to produce butyric anhydride. RD is an intensified process which couples the chemical reaction with distillation separation in one piece of equipment, thus equipment costs are reduced. RD can also improve the conversion and yield of the reversible reaction by removing the product from the reaction system in time; in addition, the concentration of reactants increases and the reaction rate also increases as the products are moved out. In the past decades, the batch RD process has been used to produce butyric anhydride [1], with the purity of butyric anhydride being high. The main disadvantages of the batch process include difficulty of operation, long production period, high labor costs due to the difficulty of automation, and high energy consumption due to the need to steam out the transition components [1].
In recent years, continuous manufacturing processes have been introduced to produce fine chemicals, such as butyric anhydride, due to the numerous advantages over batch-wise manufacturing; these include lower capital and operating costs, improved controllability and product consistency, and lower environmental footprint [2,3,4].
Gao [1] introduced continuous RD technology for producing butyric anhydride based on the analysis of the physical properties of the reaction system; results revealed that the continuous RD process is feasible for the production of butyric anhydride.
The operation of the continuous RD process can be classified into two types, namely, neat and excess. For neat operation, reactants are fed into the RD column at the same ratio as the stoichiometric of reaction, while the composition of the column overhead and bottom products are not determined in this situation, since strict flow control cannot be guaranteed in practical production; for example, Xu et al. [5] compared the neat and excess operations for synthesizing N-propyl propionate, the dynamic simulation results show that the product purity requirements cannot be ensured during neat operation; Daniel et al. [6] showed the same result with a process combining a continuous stirred tank reactor and an RD column for production of isoamyl acetate on a pilot scale.
Therefore, the excess operation is often adopted in practice; this requires one feed for the reactants according to the reaction stoichiometric, which helps avoid the disadvantage of the neat operation, while an additional distillation column is needed to separate the excess reactant from the product. Chung et al. [7] studied the influence of the excess ratio on the reactant conversion and energy consumption, and the results show that there is an inherent trade-off between the number of reactive trays and energy consumption; Chua et al. [8] compared different choices of excess reactant for producing isopropanol with the RD process.
As a result of the reliability of the excess operation, Li et al. [9] proposed a two-column RD process to produce butyric anhydride with an excess feed of butyric acid, thus the reliability of the process was validated by simulation and experiments.
In this paper, a novel continuous process with a single RD column (SRDC) is proposed to produce butyric anhydride using the excess operation, where the second distillation column in the traditional RD process for separating excess reactant is removed based on the analysis of the boiling point profile of the reaction system. In order to ensure the stable composition of the RD column, an excess reactant is usually needed in the traditional RD process, and for this reason a second distillation column is needed to separate the product from the excess reactant. SRDC completes the separation of the excess reactant and the product in the stripping section or the rectifying section of the RD column. Compared with the traditional RD process, product purification is removed and the total annual cost (TAC) of butyric anhydride production is greatly reduced.
The rest of this paper is organized as follows. Section 2 summarizes the reaction kinetics and thermodynamic data needed for the simulation of the RD process. The traditional two-column RD process for the production of butyric anhydride is simulated in Section 3. Section 4 analyzes the boiling point profile of the reaction system and presents a novel SRDC process with internal material circulation for butyric anhydride production, where two different applications are simulated and compared. Finally the paper is concluded in Section 5.
2. Reaction System
2.1. Synthesis of Butyric Anhydride
The reaction of acetic acid with butyric anhydride is homogeneous; it is also a series of reversible reactions without a catalyst, which is carried out in two steps as shown by Equations (1) and (2). The reaction rates of the two steps are quite different, and the rate of Reaction (1) is much greater than that of the second step. According to Li et al. [10], the most suitable reaction temperature is 343.15 K.
2.2. Reaction Kinetics
Li et al. [10] have studied and estimated the kinetics of Reactions (1) and (2) in the temperature range of 323.15 K to 353.15 K, as shown by Equations (3) and (4):
In Equations (3) and (4), n is the reaction step; + and − represent forward and backward reaction respectively; kn+, kn− are the reaction rate constants for reaction n with unit dm3·mol−1·min−1, which are calculated by Equations (5)–(8):
2.3. Thermodynamics Data
Table 1 shows the information on reactants, products, and the boiling point at a reactive pressure of 20 kPa. There is no binary or ternary azeotropic phenomenon in the system [10].

Table 1.
Information of pure components at a reactive pressure on 20 kPa.
As can be seen from Table 1, the reactants of the reaction, namely, acetic anhydride and butyric acid, have boiling points higher than the lighter product acetic acid, and lower than the heavier product butyric anhydride, which is key to the proposed SRDC process with the internal circulation of excess reactant.
Table 2 shows binary interaction parameters estimated using Aspen Plus (Aspen Plus 8.4, Aspen Tech, Bedford, CO, USA, 2014), VLE-HOC is a physical property method. The non-random two liquid (NRTL) activity coefficient model was used to calculate phase equilibrium in this work [11], which is calculated by Equations (9)–(12):

Table 2.
Binary interaction parameters.
3. Simulation of Two-Column RD Process for the Production of Butyric Anhydride
According to the above reaction kinetic and thermodynamic data, and the process introduced in the literature [9], the traditional two-column RD process is operated with a 10% excess feed of butyric acid as simulated with Aspen Plus, where the pressure of both columns is 20 kPa, and the residence time of reactive tray is 6 min. Figure 1 shows the process parameters and simulation results of the process streams, where NF means feed position; Nr, Nrxn and Ns represent the number of rectifying, reactive, stripping trays, respectively; and NT means the total number of column trays. The reactants are fed into the RD column C1; the overhead discharge of C1 is acetic acid, while the bottom discharge is the mixture of excess butyric acid and butyric anhydride, which is fed into distillation column C2, whose overhead discharge is the excess butyric acid to be recycled back to C1, and the bottom discharge is the product butyric anhydride, whose purity is more than 94 mol %, as seen from Figure 1.
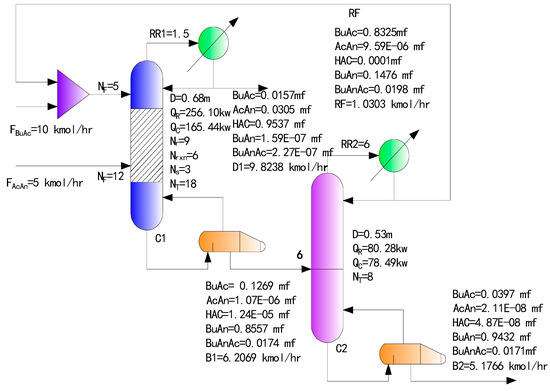
Figure 1.
Traditional two-column reactive distillation (RD) process.
4. Single RD Column with Internal Material Circulation
4.1. Principle and Two Applications
Before the reactants are fed, it is supposed that the excess operation has been used to prepare the initial bottom materials of the RD column before start-up, e.g., the mole ratio of acetic anhydride to butyric acid is 1.1:2 for the bottom materials. The neat operation is used for the RD column after the process is running in a steady state. As a result of the different boiling points of the pre-excessed acetic anhydride reactant and the acetic acid, they can be separated easily from in the rectifying section of the column. As shown by Figure 2a, the acetic anhydride is always excessive and circulates in the column, thus the conversion of butyric acid is very promising, similar to the case of the traditional two-column RD process with the excess feed of acetic anhydride. Of course, the condenser duty of the RD column in Figure 2a must be tuned carefully to implement the internal circulation of the excessed acetic anhydride.
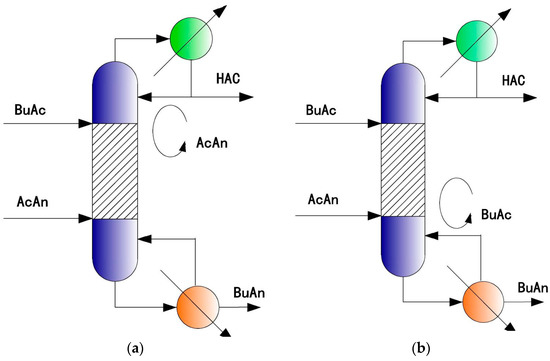
Figure 2.
A single RD column (SRDC) with internal circulating excess reactant. (a) SRDC with internal circulation of pre-excess AcAn; (b) SRDC with internal circulation of pre-excess BuAc.
Similarly, with an excess of butyric acid in the initial bottom material, the internal circulation of butyric acid can also be realized by tuning the reboiler duty of the RD column in Figure 2b carefully, since the difference in boiling points is also significant between butyric acid and butyric anhydride.
The above two applications of the single RD column with excess reactant circulating internally are the bases of the proposed SRDC process.
4.2. Simulation of the SRDC Process
The application of internal circulation of excess reactant depends on tuning the condenser duty or reboiler duty of the SRDC. Therefore, it is important to simulate the processes shown in Figure 2a,b whose corresponding flowsheets in ASPEN are shown with Figure 3a,b. The position of the reactive section is fixed, but the simulation strategy is different depending on the different excess feed. When acetic anhydride is in excess, the rectifying section and the reactive section are separated from the stripping section, the tear flow is supplemented with an excess of 10% acetic anhydride, and the rectifying section is used to separate the excess acetic anhydride and acetic acid. When butyric acid is in excess, the rectifying section is separated from the reactive section and the stripping section, the tear flow is supplemented with an excess of 10% butyric acid, and the stripping section is used for the separation of the excess butyric acid and butyric anhydride.
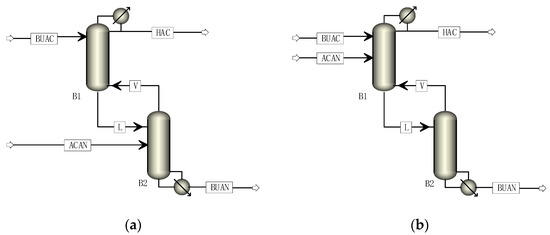
Figure 3.
Flowsheets of SRDC with internal circulation of excess reactant. (a) SRDC with internal circulation of pre-excess AcAn; (b) SRDC with internal circulation of pre-excess BuAc.
In Figure 3a, which represents the pre-excess of acetic anhydride, the upper column with the condenser is the only rectifying section of the SRDC, as shown in Figure 2a; the only material discharged from overhead is the acetic acid byproduct, the excess acetic anhydride circulates internally, while the lower column with the reboiler only includes the reactive and stripping sections of the SRDC, whose bottom discharge is the butyric anhydride product.
In Figure 3b, which represents the pre-excess of butyric acid, the upper column with the condenser only includes the reaction and rectifying sections of the SRDC, as shown in Figure 2b, whose overhead discharge is also the acetic acid byproduct; while the lower column with the reboiler only represents the stripping section of the SRDC, where the pre-excess butyric acid is separated from butyric anhydride and circulates internally; the bottom discharge is also the butyric anhydride product.
4.3. Simulation Results of the Optimized SRDC
On the basis of the flowsheets presented in Figure 3, sensitivity-based optimization was carried out to optimize the proposed two applications of the SRDC process, and the results are shown in the following.
Under the condition of a pre-excess of acetic anhydride, the simulation results of the optimized SRDC process are shown in Figure 4, the SRDC3 is the SRDC with excess acetic anhydride circulating internally.
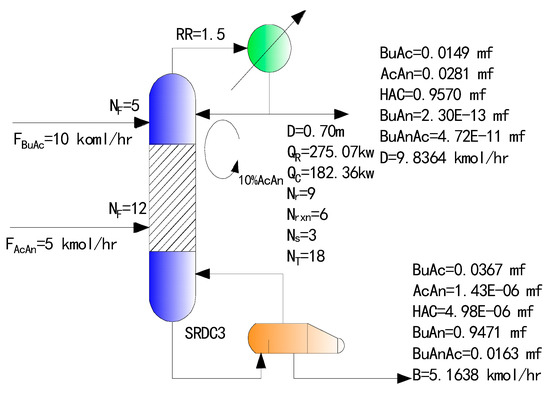
Figure 4.
SRDC3 (SRDC with excess acetic anhydride circulating internally) with excess acetic anhydride circulating internally.
As seen from Figure 4, the purity of the butyric anhydride product is higher than in the traditional two-column process. Figure 5 shows the temperature and liquid composition profiles between the SRDC trays.
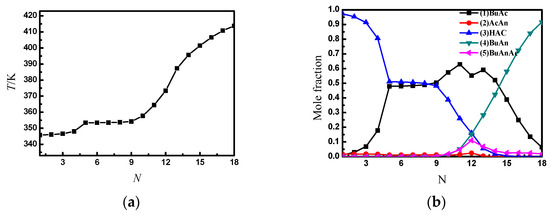
Figure 5.
The temperature and liquid composition profile between the SRDC3 trays. (a) The temperature profile between SRDC3 trays; (b) the liquid composition profile between SRDC3 trays.
Figure 6 shows the simulation results of the optimized SRDC under the condition of excess butyric acid, where the purity of the butyric anhydride product is also higher than that of the two-column process, the SRDC4 is the SRDC with excess butyric acid circulating internally.
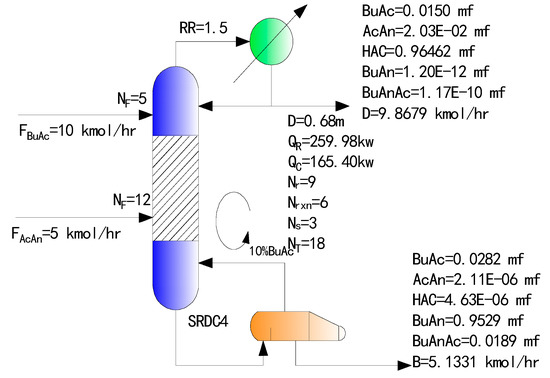
Figure 6.
SRDC4 (SRDC with excess butyric acid circulating internally) with excess butyric acid circulating internally.
Figure 7 shows the temperature and liquid composition profiles between the trays of the SRDC4, which are similar to those under the condition of an excess feed of acetic anhydride.
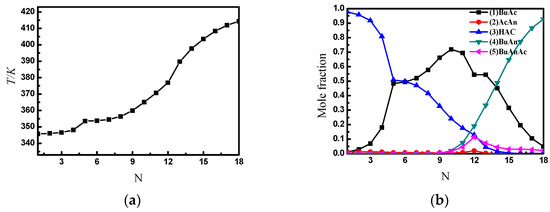
Figure 7.
The temperature and liquid composition profile between SRDC4 trays. (a) The temperature profile between SRDC4 trays; (b) the liquid composition profile between SRDC4 trays.
The results of the three processes are compared in Table 3.

Table 3.
Results of the three processes.
The conversion of reactants and the purity of the product are higher than in the two-column process.
4.4. Economic Comparison
In this section, the traditional two-column process with an excess of butyric acid and the proposed two applications of the SRDC process are compared from the perspective of TAC, the calculation of which is shown in Equation (13) [12], consisting of capital cost and energy cost. The capital cost includes the cost of the column, tray, and heat exchanger, and the energy cost includes the cost of steam and cooling water. As shown in Table 4, the TAC of the three processes are calculated and compared.

Table 4.
Economic comparison.
As seen in Table 4, both SRDC processes are more economical than the traditional two-column process. In addition, the excess of butyric acid is more economical than the excess of acetic anhydride for the SRDC process; this condition is the result of the removal of the second distillation column,
5. Conclusions
In this paper, a novel process for a single reactive distillation column with excess reactant circulating internally is proposed for the production of butyric anhydride. Two applications with different reactant pre-excesses are proposed, and the corresponding simulation method is also presented. On the basis of the simulation, the proposed SRDC process is compared to the traditional two-column process, and the results show the economical superiority of the SRDC. A more detailed analysis of the proposed process will be presented in the future.
Author Contributions
Conceptualization, S.T.; methodology, S.T.; software, F.J.; validation, G.C. and S.X.; formal analysis, F.J. and X.G.; investigation, G.C. and S.X.; resources, S.T.; data curation, F.J.; writing-original draft preparation, S.T. and F.J.; writing-review and editing, G.C., F.J., X.G., S.X., and S.T.; supervision, S.X.; project administration, G.C., F.J., X.G., S.X. and S.T. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by the National Natural Science Foundation of China (No. 21776145). The Innovation and Training Plan of the University Students in Qingdao University of Science and Technology (No. 201810426125).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Nomenclature
| AcAn | Acetic Anhydride |
| AC | the heat transfer area of condenser |
| AR | the heat transfer area of reboiler |
| B | bottom rate |
| BuAc | butyric acid |
| BuAn | butyric anhydride |
| BuAnAc | butyric anhydride acetate |
| D | distillate rate |
| F | the fresh feed of the reactive distillation column |
| HAC | acetic acid |
| HX | heater exchanger |
| IDr | the internal diameter of the rectifying section |
| IDrxn | the internal diameter of the reaction section |
| IDs | the internal diameter of the stripping section |
| kn + | the forward reaction rate constants, dm3·mol−1·min−1 |
| kn− | the backward reaction rate constants, dm3·mol−1·min−1 |
| N | the number of trays |
| NT | the total number of trays |
| NF | the feed stage of the fresh feed |
| Nr | the number of trays in rectifying section |
| Nrxn | the number of trays in reactive section |
| Ns | the number of trays in stripping section |
| NT | the total number of trays |
| QR | reboiler duty |
| QC | condenser duty |
| RR | the reflux ratio |
| RF | the recycled flow of BuAc |
| RD | reactive distillation |
| SRDC | single reactive distillation column |
| TAC | total annual cost |
| Tb | boiling point |
References
- Gao, F. Development of Continuous Reactive Distillation Process for Production of Butyric Anhydride. Master’s Thesis, Qingdao University of Science and Technology, Qingdao, China, 2007. [Google Scholar]
- Sahlodin, A.-M.; Barton, P.-I. Optimal Campaign Continuous Manufacturing. Ind. Eng. Chem. Res. 2015, 54, 11344–11359. [Google Scholar] [CrossRef]
- Tian, H.; Zhao, S.; Zheng, H.; Haung, Z. Optimization of coproduction of ethyl acetate and butyl acetate by reactive distillation. Chin. J. Chem. Eng. 2015, 23, 667–674. [Google Scholar] [CrossRef]
- Mallaiah, M.; Kishore, K.-A.; Reddy, G.-V. Catalytic Reactive Distillation for the Esterification Process: Experimental and Simulation. Chem. Biochem. Eng. Q. 2017, 31, 293–302. [Google Scholar] [CrossRef]
- Xu, H.; Ye, Q.; Zhang, H.; Qin, J.-W.; Li, N. Design and control of reactive distillation–recovery distillation flowsheet with a decanter for synthesis of N-propyl propionate. Chem. Eng. Process. 2014, 85, 38–47. [Google Scholar] [CrossRef]
- Gonzalez, D.-R.; Bastidas, P.; Rodriguez, G.; Gil, I. Design alternatives and control performance in the pilot scale production of isoamyl acetate via reactive distillation. Chem. Eng. Res. Des. 2017, 123, 347–359. [Google Scholar] [CrossRef]
- Chung, Y.-H.; Peng, T.-H.; Lee, H.-Y.; Chen, C.-L.; Chien, I.-L. Design and control of reactive distillation system for esterification of levulinic Acid and n-Butanol. Ind. Eng. Chem. Res. 2015, 54, 3341–3354. [Google Scholar] [CrossRef]
- Chua, W.-J.; Rangaiah, G.-P.; Hidajat, K. Design and optimization of isopropanol process based on two alternatives for reactive distillation. Chem. Eng. Process. 2017, 118, 108–116. [Google Scholar] [CrossRef]
- Li, B.-C.; Han, X.-P.; Zhang, W.-L.; Geng, C.-X. Simulation and experimental research on butyric anhydride synthesis by reactive distillation. J. Chem. Ind. Eng. 2017, 45, 72–78. [Google Scholar]
- Li, B.-C.; Xue, X.-X.; Zhang, W.-L.; Geng, C.-X. Study on the kinetics of preparation of butyric anhydride by acylation. J. Chem. Ind. Eng. 2017, 45, 49–53. [Google Scholar]
- Renon, H.; Prausnitz, J.-M. Local compositions in thermodynamic excess functions for liquid mixtures. AI ChE J. 1968, 14, 135–144. [Google Scholar]
- Luyben, W.-L. Design and control of a methanol reactor/column process. Ind. Eng. Chem. Res. 2010, 49, 6150–6163. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).