Proposal of a Learning Health System to Transform the National Health System of Spain
Abstract
:1. Introduction
1.1. Health Systems’ Challenges
1.2. Information and Communication Technologies Are the Tool to Transform Health System
1.3. Objectives
- Identify the current challenges of the Spanish National Health System.
- Propose a new Big Data based solution which will improve the quality, efficiency, and effectiveness of the Spanish National Health System to address these challenges. This process needs to use both clinical and non-clinical data to aid the decision-making processes related to patients’ health, research, and evaluation of the performance of the health system. The governance, architecture, legal framework, bioethical principles, data homogeneity, and technological tools needed for its creation, operation, and evolution must also be considered.
- Propose the creation of a Learning Health System (LHS) for the National Health System of Spain, envisioned as an integrated framework “in which progress in science, informatics, and care culture align to generate new knowledge as an ongoing, natural by-product of the care experience, and seamlessly refine and deliver best practices for continuous improvement in health and healthcare” [7].
2. Materials and Methods
3. Results
3.1. Dependency
3.2. Life Expectancy at Birth and Healthy Life Expectancy
3.3. Mortality and Morbidity
3.4. Health Expenditure
3.5. Out-of-Pocket Expenditure
3.6. Pharmaceutical Expenditure
3.7. Avoidable Mortality (Preventable and Amenable)
3.8. Share of Potentially Avoidable Hospital Admissions Due to Five Chronic Conditions
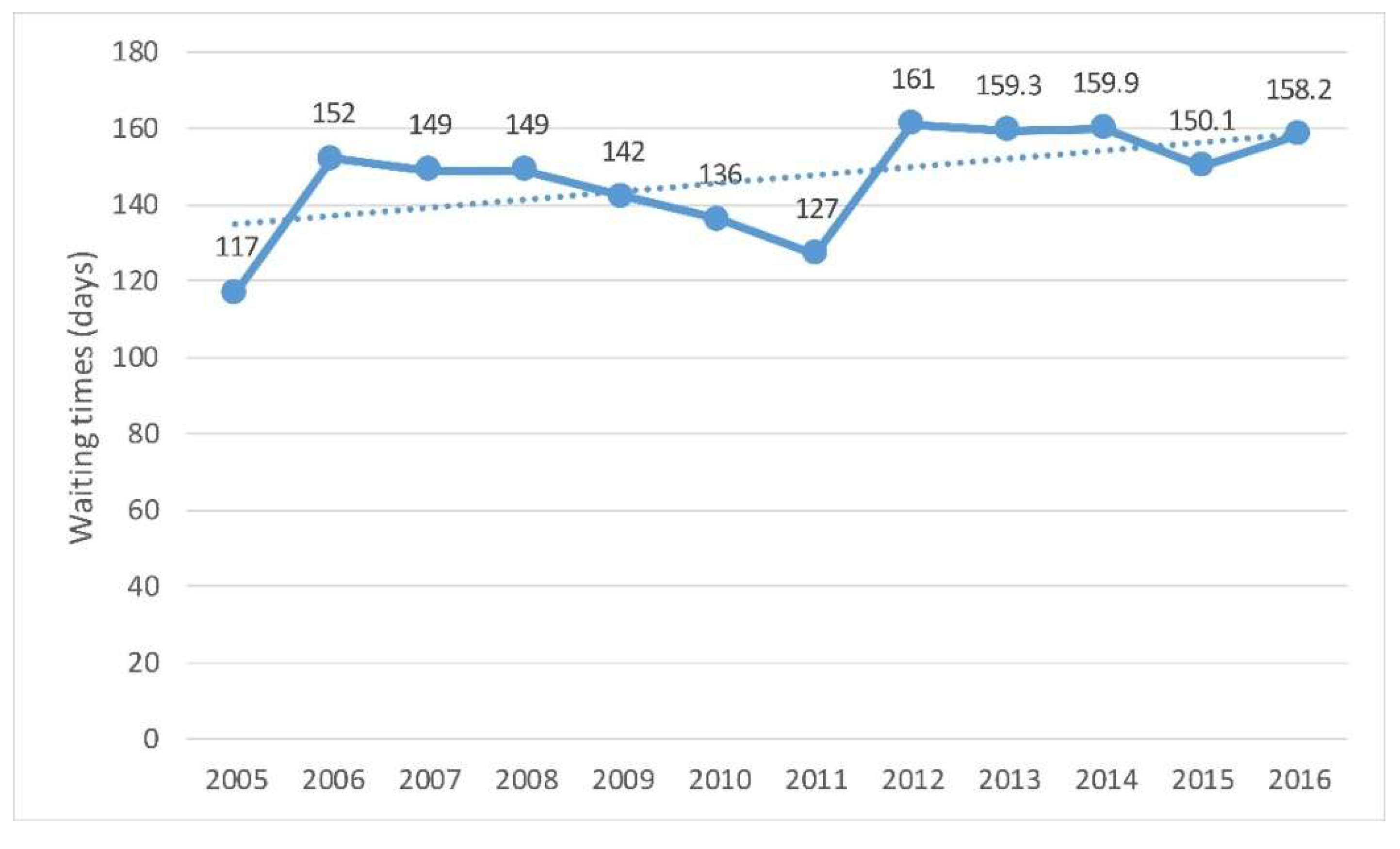
3.9. Waiting Times for Elective Surgery
3.10. Readiness of National Health Data Governance Frameworks to Support the Use of Electronic Health Record Data
4. Discussion
4.1. Main Challenges of the Spanish National Health System
- The rising old age dependency ratio. This growing proportion of elderly people means an increasing demand of healthcare services due to a prevalence of chronic diseases that will cause an increase in health expenditure.
- Health expenditure pressures and the likely increase of out-of-pocket expenditure.
- Drug expenditures, both retail and consumed in hospitals.
- Waiting lists for surgery.
- The percentage of potentially preventable hospital admissions.
- The use of EHR data to fulfil national health information and research objectives.
4.1.1. The Rising Old Age Dependency Ratio
4.1.2. Healthcare Expenditure Pressures and the Likely Increase of Out-of-Pocket Expenditure
4.1.3. Drug Expenditures, Both Retail and Consumed in Hospitals
4.1.4. Waiting Lists for Surgery and the Preventable Hospital Admissions Ratio Represent Two Quality Issues That Also Should Be Considered
4.1.5. The Use of EHR Data to Fulfil National Health Information and Research Objectives
4.2. A Big Data Solution for the National Health System of Spain
4.2.1. Big Data Principles
- The economic properties of data suggest that data is an infrastructure-like resource which in theory can be used by an unlimited number of users and for an unlimited number of purposes as an input to produce goods and services.
- The value-creation mechanisms of data analytics, which include using data analytics to:
- gain insights (knowledge creation)
- automate decision-making.
- The use of high-performance IT infrastructure regarding the capacity of information management in terms of volume, speed, and variety.
- The extraction of information as a result of large-scale data processing.
- The generation of knowledge from that information and its use for the strategic management of an organization.
- Traditional: stored in clinical records and department files.
- Data generated by patients and citizens: patient reported outcomes, social networks, devices, and individual sensors
- Omics data: obtained from molecular biology.
4.2.2. Data Acquisition
- If the data is compatible with the system (format and compatibility).
- If the data responds to existing needs (suitability and appropriateness).
- If the data is complete (completeness).
- If the data is valid (integrity and consistency).
- If the data is detailed enough (precision and accuracy).
- If the data is structured in a way that the system can process (standardization and normalization).
4.2.3. Treatment of Data
- Centralized: it comprises a central node that works as the sole repository of the system data. This requires implementing a process for storing new data as they are generated, so that they are available for consultation and analysis by users of the system.
- Federated: each provider data center is at the same time the repository of its own dataset, which it only shares with the rest of the nodes of the system on demand, that is, when that data is required for analysis.
4.2.4. Data Interoperability
- Messaging standards (for data communication). These standards define the way the different IT systems communicate with each other, providing a set of rules that guarantees that the exchanged information is solid. These rules set the format, structures, and data types that build each of the allowed messages to be shared among the tools within the system and to communicate with external ones. Digital Imaging and Communications in Medicine (DICOM) [27] for digital imaging or Health Level Seven (HL7) [28] are two of the most used ones in worldwide health systems interoperability.
- Documentation standards (for human communication). If messages are intended for digital systems, documents structure information to be managed by humans. In a health system, everyday hundreds of documents are filled and read by professionals. These documents reflect, among others, valuable information about the patients and the processes they are subjected to. Standards here define common structures to capture and present information, from and to the system users. In turn, these structures enable software systems to analyze and process the information, build useful messages, and improve interoperability. Clinical Document Architecture (CDA® [29], Continuity of Care Record (CCR) [30], and Continuity of Care Document (CCD) [31] are extended clinical documentation standards.
- Terminology standards (to represent information). This set of standards provides a common lexis to define, represent, and code medical concepts, such diagnosis, disease, medication, etc. This health language will be later used to fill the clinical documents shared by the whole health system. Systematized Nomenclature of Medicine – Clinical Terms (SNOMED CT) [32] or 10th revision of the International Statistical Classification of Diseases and Related Health Problems, (ICD-10) [33] (for disease statistics) are two of the most relevant in this area.
4.2.5. Bioethical and Legal Aspects
- The health information system supports the monitoring and improvement of healthcare quality and system performance, as well as research innovations for better healthcare and outcomes.
- The processing and the secondary use of data for public health, research, and statistical purposes are permitted, subject to safeguards specified in the legislative framework for data protection.
- The public are consulted upon and informed about the collection and processing of personal health data.
- A certification/accreditation process for the processing of health data for research and statistics is implemented.
- The project approval process is fair and transparent and decision making is supported by an independent, multidisciplinary project review body.
- Best practices in data de-identification are applied to protect patient data privacy.
- Best practices in data security and management are applied to reduce reidentification and breach risks.
- Governance mechanisms are periodically reviewed at an international level to maximize societal benefits and minimize societal risks as new data sources and new technologies are introduced.
4.3. Proposal for the Learning Health System of Spain
4.3.1. Data Acquisition and Traceability
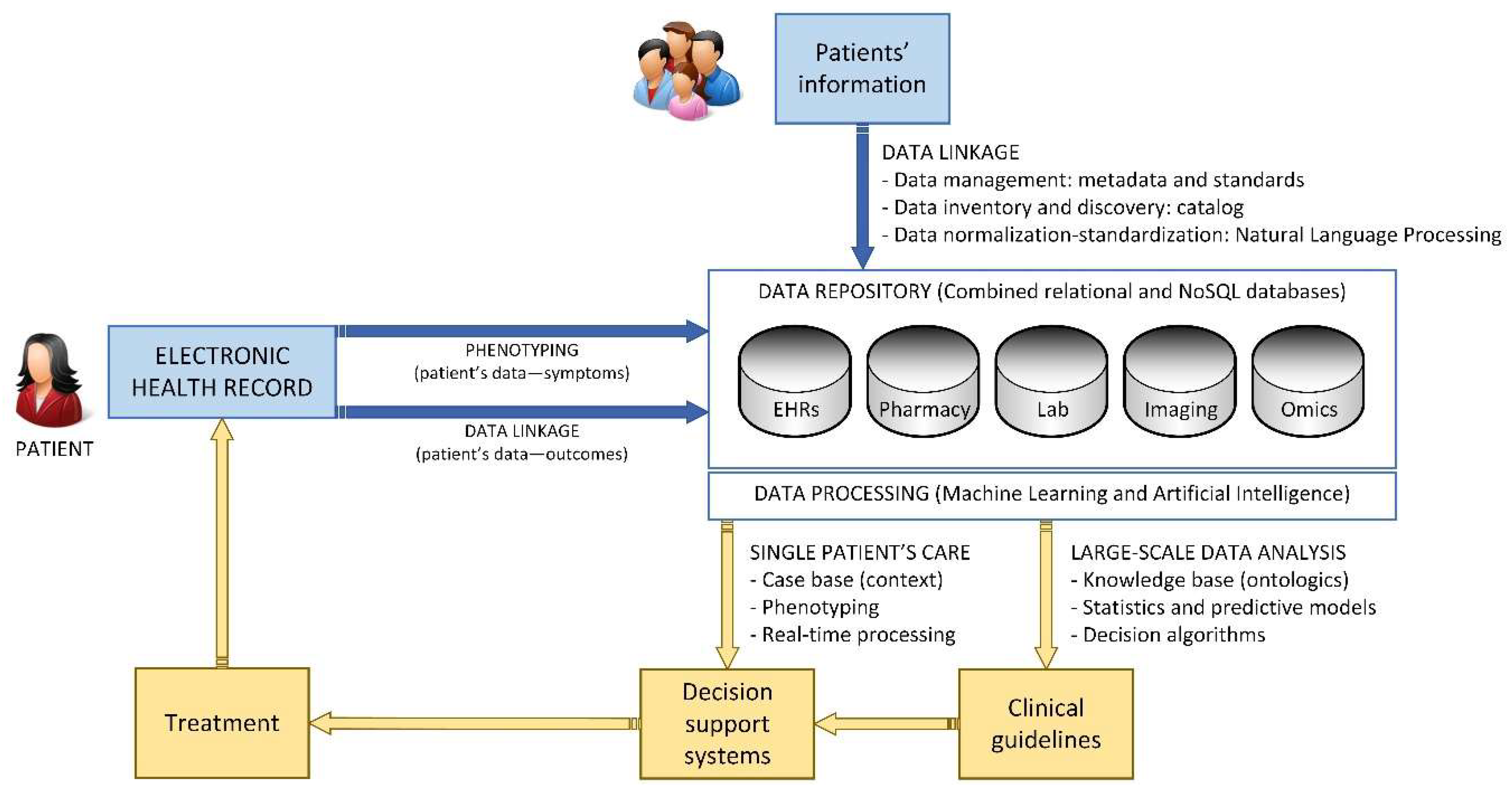
4.3.2. Architecture Overview
- Layer 1: Data Gathering and Data Homogenization
- Layer 2: Big Data and Machine Learning
- Layer 3: Presentation and Data Analysis
4.3.3. Road Map
- The definition of the BDNHS data models, considering the diversity of data that will be used; the information sources that will provide that data; and the use of that data inside the BDNHS.
- The standards policy on data management: acquisition, standardization, storage, exchange, and processing, among others.
- The election of a model of architecture for the system, which could be federated, centralized or hybrid. However, the most appropriate final architecture could end up being a hybrid model, with a centralized repository for clinical information, whose data structure would have a high degree of similarity, and federated repository for nonclinical information, which would cover diverse data originating in different sources.
4.3.4. Critical Success Elements
- The leadership of the Ministry of Health, which will allow the resistance that the regional health service could present to be overcome, by assigning a budget and handling the legal requirements for the creation of the bodies of the project, as well as modifying data protection rules and health research if necessary, and taking care of intellectual property matters.
- The agreement of the Interregional Healthcare Council creating the BDNHS, explicitly backing it and taking over the compromises required: data provision, and resource and budget allocation.
- The granting of a budget allowance for at least the first two years of execution of the project.
- The creation of a management body for the project, whose team must be comprised of renowned professionals with leadership skills. The director must have an executive profile but with flexibility and negotiating skills to reach agreements between the stakeholders of the project.
- The implication of all the Regional Health Services that comprise the National Health System of Spain, who must obtain value in the short term in order to promote and reward their participation.
- The training of professionals of the participating entities in IT health in order to have the best available human resources to execute the project.
- The creation of a management unit that will extend its control over data usage to improve public health as well as the health system. This management unit must determine the standards for joining the program. It must also sign the required agreements with the entities that decide to share their data once it is accredited that they comply with the requirements. This unit must be of permanent nature.
- The choice of an architecture model that considers the organization, technical, and budget conditions of the participants, especially those of the 18 regional health services.
- The consideration and thorough application of the recommendations provided by bioethics experts to provide trust to citizens and professional entities as well as patient associations.
- The compliance with data protection rules and the review of existing Spanish legislation in order to take advantage of the opportunities that it brings for projects such as the BDNHS.
- The participation of patients in the project. This requires a communication plan as well as making the project known to the broader society. The plan must convey trust regarding citizen’s privacy in line with the previous two recommendations. Trust requires transparency regarding data sources, their processing, usage, and results.
- The achievement of short-term milestones, bringing early benefits to users, and providing returns and visibility to the project.
5. Conclusions
- Identifying the required data and their sources.
- Implementing a repository of data to compile it before its analysis.
- Defining an architecture model according to the needs of the health system.
- Incorporating data protection measures in accordance with existing rules and applicable bioethics guidelines.
- Using the most adequate data tools for the defined system model.
- Creating the management unit required for putting the model to work and managing the system, with participation from regional health services, strong institutional backing, and the required budget allocation.
- Complying with bioethical legal and data transparency guidelines, and data processing and use.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Muir Gray, J.A. How to Get Better Value Healthcare, 2nd ed.; Oxford Press Ltd.: Oxford, UK, 2011. [Google Scholar]
- Porter, M.E.; Lee, T.H. The Strategy That Will Fix Health Care. Harvard Business Review. October 2013, pp. 50–67. Available online: https://hbr.org/2013/10/the-strategy-that-will-fix-health-care (accessed on 6 September 2019).
- Tremolada, M.; Schiavo, S.; Varotto, S.; Basso, G.; Pillon, M. Patient Satisfaction in Italian Childhood Cancer Survivors: Human Aspects of Treatment as a Key Factor in Patients’ Quality of Life. Health Soc. Work 2015, 40, e148–e155. [Google Scholar] [CrossRef]
- OECD Improving Health Sector Efficiency. The Role of Information and Communication Technologies; OECD Publishing: Paris, France, 2010. [Google Scholar]
- OECD. Strengthening Health Information Infrastructure for Health Care Quality Governance: Good Practices, New Opportunities and Data Privacy Protection Challenges; OECD Health Policy Studies; OECD Publishing: Paris, France, 2013. [Google Scholar] [CrossRef]
- OECD. Health Data Governance: Privacy, Monitoring and Research; OECD Health Policy Studies; OECD Publishing: Paris, France, 2015. [Google Scholar] [CrossRef]
- Bernstein, J.; Friedman, C.; Jacobson, P.; Rubin, J. Ensuring Public Health’s Future in a National-Scale Learning Health System. Am. J. Prev. Med. 2015, 48, 480–487. [Google Scholar] [CrossRef] [PubMed]
- WHO. Health Systems: Improving Performance; The World Health Report; World Health Organization: Geneva, Switzerland, 2000; Available online: https://www.who.int/healthinfo/paper30.pdf (accessed on 3 June 2019).
- OECD/EU. Health at a Glance: Europe 2018: State of Health in the EU Cycle; OECD Publishing: Paris, France; EU: Brussels, Belgium, 2018. [Google Scholar] [CrossRef]
- Ministerio de Sanidad, Consumo y Bienestar Social. Informe anual del Sistema Nacional de Salud. (Ministry of Health. Annual Report of National Health System). 2017. Available online: https://www.mscbs.gob.es/estadEstudios/estadisticas/sisInfSanSNS/tablasEstadisticas/InfAnSNS.htm (accessed on 3 June 2019).
- Instituto Nacional de Estadística. Indicadores Demográficos Básicos. (Spanish National Statistics Institute. Basic Demographic Indicators). Available online: http://www.ine.es/dynt3/inebase/es/index.htm?padre=2077&capsel=2081 (accessed on 6 September 2019).
- Ministerio de Sanidad. Estadística de Gasto Sanitario Público. (Ministry of Health. Public Health Expenditure Statistic). Available online: https://www.mscbs.gob.es/estadEstudios/estadisticas/inforRecopilaciones/gastoSanitario2005/home.htm (accessed on 6 September 2019).
- Ministerio de Hacienda. Estadística de Gasto Farmacéutico Y Sanitario (Ministry of Finance Pharmaceutical and Health Expenditure Statistics). 2019. Available online: http://www.hacienda.gob.es/es-ES/CDI/Paginas/EstabilidadPresupuestaria/InformacionAAPPs/Indicadores-sobre-Gasto-Farmacéutico-y-Sanitario.aspx (accessed on 6 September 2019).
- Ministerio de Sanidad. Implantación de la Receta Electrónica en el Sistema Nacional de Salud (Ministry of Health. Implementation of Electronic Prescription in National Health System). Available online: http://www.mscbs.gob.es/profesionales/recetaElectronicaSNS/NIVEL_DE_IMPLANTACION_febrero_2019.pdf (accessed on 6 September 2019).
- OECD. New Health Technologies: Managing Access, Value and Sustainability; OECD Publishing: Paris, France, 2017. [Google Scholar] [CrossRef]
- Informe Sobre la Actualización del Programa de Estabilidad 2019–2022. Informe 32/19. AIREF. Autoridad Independiente de Responsabilidad Fiscal 2019 (Report about the Update of the Stability Programme. Report 32/19. AIREF. Independent Authority for Fiscal Responsibility). Available online: https://www.airef.es/wp-content/uploads/2019/05/informe-ape/Informe_AIReF_APE-2019-2022.pdf (accessed on 6 September 2019).
- Carnicero, J. La Prestación Farmacéutica en el Sistema Nacional de Salud y en la Comunidad Foral de Navarra. In Pharmaceutical provision in National Health System and in the Autonomous Community of Navarra; Gobierno de Navarra, Departamento de Salud: Pamplona, Spain, 1996. [Google Scholar]
- Eichler, H.G.; Bloechl-Daum, B.; Broich, K.; Kyrle, P.A.; Oderkirk, J.; Rasi, G.; Wenzl, M. Data rich, information poor: Can we use electronic health records to create a learning healthcare system for pharmaceuticals? Clin. Pharmacol. Ther. 2019, 105, 912–922. [Google Scholar] [CrossRef] [PubMed]
- OECD. Data-Driven Innovation: Big Data for Growth and Well-Being; OECD Publishing: Paris, France, 2015. [Google Scholar] [CrossRef]
- Rojas, D.; Carnicero, J. Big Data and Public Health Systems: Issues and Opportunities. Int. J. Interact. Multimed. Artif. Intell. 2018, 4, 53–59. [Google Scholar] [CrossRef]
- Weng, C.; Kahn, M.G. Clinical Research Informatics for Big Data and Precision Medicine. Yearb. Med. Inform. 2016, 10, 211–218. [Google Scholar]
- Martin-Sanchez, F.; Verspoor, K. Big data in medicine is driving big changes. Yearb. Med. Inform. 2014, 9, 14–20. [Google Scholar] [PubMed]
- Carnicero, J.; Rojas, D. Healthcare Decision-Making Support Based on the Application of Big Data to Electronic Medical Records: A Knowledge Management Cycle. In Leveraging Biomedical and Healthcare—Semantics, Analytics and Knowledge; Kobeissy, F., Wang, K., Zaraket, F., Alawieh, A., Eds.; Elsevier: San Diego, CA, USA, 2018. [Google Scholar]
- Ahmed, M.; Ullah, A.S.B. Infrequent pattern mining in smart healthcare environment using data summarization. J. Supercomput. 2018, 74, 5041–5059. [Google Scholar] [CrossRef]
- Modoni, G.E.; Sacco, M.; Terkaj, W. A survey of RDF store solutions. In Proceedings of the 2014 International Conference on Engineering, Technology and Innovation (ICE), Bergamo, Italy, 23–25 June 2014; IEEE: Piscataway, NJ, USA, 2014; pp. 1–7. [Google Scholar]
- Modoni, G.E.; Veniero, M.; Sacco, M. Semantic knowledge management and integration services for AAL. In Italian Forum of Ambient Assisted Living; Springer: Cham, Switzerland, 2016; pp. 287–299. [Google Scholar]
- DICOM Standard. DICOMWeb™ Publications. Available online: https://www.dicomstandard.org/current/ (accessed on 22 June 2019).
- Introduction to HL7 Standards. Health Level Seven (HL7) International. Available online: http://www.hl7.org/implement/standards/index.cfm?ref=nav (accessed on 22 June 2019).
- Clinical Document Architecture, Release 2 Health Level Seven (HL7) International. Available online: https://www.hl7.org/implement/standards/product_brief.cfm?product_id=7 (accessed on 22 June 2019).
- Standard Specification for Continuity of Care Record (CCR). Active Standard ASTM E2369. Available online: https://www.astm.org/Standards/E2369.htm (accessed on 22 June 2019).
- Continuity of Care Document (CCD): Changing the Landscape of Healthcare Information Exchange. Corepoint Health. Available online: https://corepointhealth.com/resource-center/white-papers/continuity-care-document-ccd-changing-landscape-healthcare-information/continuity-care-document-ccd-changing-landscape-healthcare-information-exchange/ (accessed on 22 June 2019).
- SNOMED CT: 5-Step Briefing. © SNOMED International 2019. Available online: http://www.snomed.org/snomed-ct/five-step-briefing (accessed on 22 June 2019).
- International Classification of Diseases (ICD). © World Health Organization. Available online: https://www.who.int/classifications/icd/factsheet/en/ (accessed on 22 June 2019).
- León-Sanz, P. Key Points for an Ethical Evaluation of Healthcare Big Data. Processes 2019, 7, 493. [Google Scholar] [CrossRef]
- Char, D.S.; Shah, N.H.; Magnus, D. Implementing machine learning in health care—Addressing ethical challenges. N. Engl. J. Med. 2018, 378, 981–983. [Google Scholar] [CrossRef] [PubMed]
- UNESCO. Report of the International Bioethics Committee of UNESCO on Big Data and Health; UNESCO: Paris, France, 2017; Available online: https://unesdoc.unesco.org/ark:/48223/pf0000248724 (accessed on 3 June 2019).
- Ministerio de Sanidad, Consumo y Bienestar Social. Proyecto de Historia Clínica Digital del Sistema Nacional de Salud. Informe de situación. Available online: http://www.mscbs.gob.es/profesionales/hcdsns/contenidoDoc/HCDSNS_Informe_de_situacion_20190401.pdf (accessed on 3 June 2019).




| Indicator | Spain | European Union |
|---|---|---|
| Population under 15 years old/Total population | 15% | 16% |
| Population older than 65/Total population | 19.0% | 19.0% |
| Life expectancy at birth (years) | 82.8 | 81.0 |
| Life expectancy at birth (women) (years) | 86.3 | 83.6 |
| Healthy life years (women) | 66.5 | 64.2 |
| Life expectancy at birth (men) (years) | 80.5 | 78.2 |
| Healthy life years (men) | 65.9 | 63.5 |
| Spending on healthcare (% GDP) | 8.8 | 9.6 |
| Share of total health spending financed by out-of-pocket payments | 23.8% | 18.2% |
| Pharmaceutical expenditure (retail) per capita and as a share of health expenditure | 19.1% | 16.8% |
| Pharmaceutical expenditure (retail) per capita financed by government/compulsory schemes as a share of pharmaceutical expenditure | 57% | 64% |
| Share of potentially avoidable hospital admissions due to five chronic conditions: diabetes, hypertension, heart failure, COPD and bronchiectasis, and asthma. | 6.3% | 5.5% |
| Indicator | Spain |
|---|---|
| % Pharmaceutical expenditure (retail) | 9.35 |
| % Pharmaceutical expenditure (hospitals) | 15.65 |
| % Total pharmaceutical expenditure as a share of National Health System | 25.00 |
| Precision and Validity of Data and Algorithms |
|---|
| Consent to data sharing and new ways to obtain them, such as automated sharing, voluntary communication, amplified consent, or dynamic consent. |
| Confidentiality, which deals with the repercussion of coding or pseudo anonymization, and the anonymization of data in the analysis of information, as well as the dilemma between right to privacy and public interest. |
| Step | Phase | Description |
|---|---|---|
| 1 | Approval by institutions | The project must be approved by the Health Ministry and the Interterritorial Board, who must explicitly back it. |
| 2 | Budget funding | The project must be awarded a budget. It will be covered with Ministry and regional funds and other sources, such as EU funds. |
| 3 | Project team selection | The team will be comprised of scientific groups, patients, and research centers, among others. |
| 4 | Operational goals definition | The following goals must be considered: clinical assistance, investigation, and management of health services and systems. |
| 5 | Digital health platform creation | An online bio health center dedicated to digital health must be created |
| 6 | Legal and institutional frame | Government units, task units, procurement, agreements, and partnerships. Ministry and Interterritorial Board accountability. Data protection rules, biomedical research, intellectual property. |
| 7 | Technical requirements definition | Definition of data modeling. Data protection rules. System architecture model |
| 8 | Information security plan | System Access control. System Access registry. Information use control. Registry of information. Access, auditing of system access and data use. Information integrity. Information availability |
| 9 | Technical proposal | Technical proposal that details the necessary steps for system implementation |
| 10 | Management | Management groups, with Ministry of Health and regional representatives. Usage rules. Protocols to access and use data |
| 11 | Pilot execution | Required to optimize developed tools |
| 12 | Global evaluation | Use of the system and impact on efficacy, effectiveness, and efficiency of the National Health System, which should impact the health of the population |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carnicero, R.; Rojas, D.; Elicegui, I.; Carnicero, J. Proposal of a Learning Health System to Transform the National Health System of Spain. Processes 2019, 7, 613. https://doi.org/10.3390/pr7090613
Carnicero R, Rojas D, Elicegui I, Carnicero J. Proposal of a Learning Health System to Transform the National Health System of Spain. Processes. 2019; 7(9):613. https://doi.org/10.3390/pr7090613
Chicago/Turabian StyleCarnicero, Rafael, David Rojas, Ignacio Elicegui, and Javier Carnicero. 2019. "Proposal of a Learning Health System to Transform the National Health System of Spain" Processes 7, no. 9: 613. https://doi.org/10.3390/pr7090613
APA StyleCarnicero, R., Rojas, D., Elicegui, I., & Carnicero, J. (2019). Proposal of a Learning Health System to Transform the National Health System of Spain. Processes, 7(9), 613. https://doi.org/10.3390/pr7090613




