Comparison of Growth Characteristics and Properties of CVD TiN and TiO2 Anti-Coking Coatings
Abstract
:1. Introduction
2. Experimental Details
3. Results and Discussion
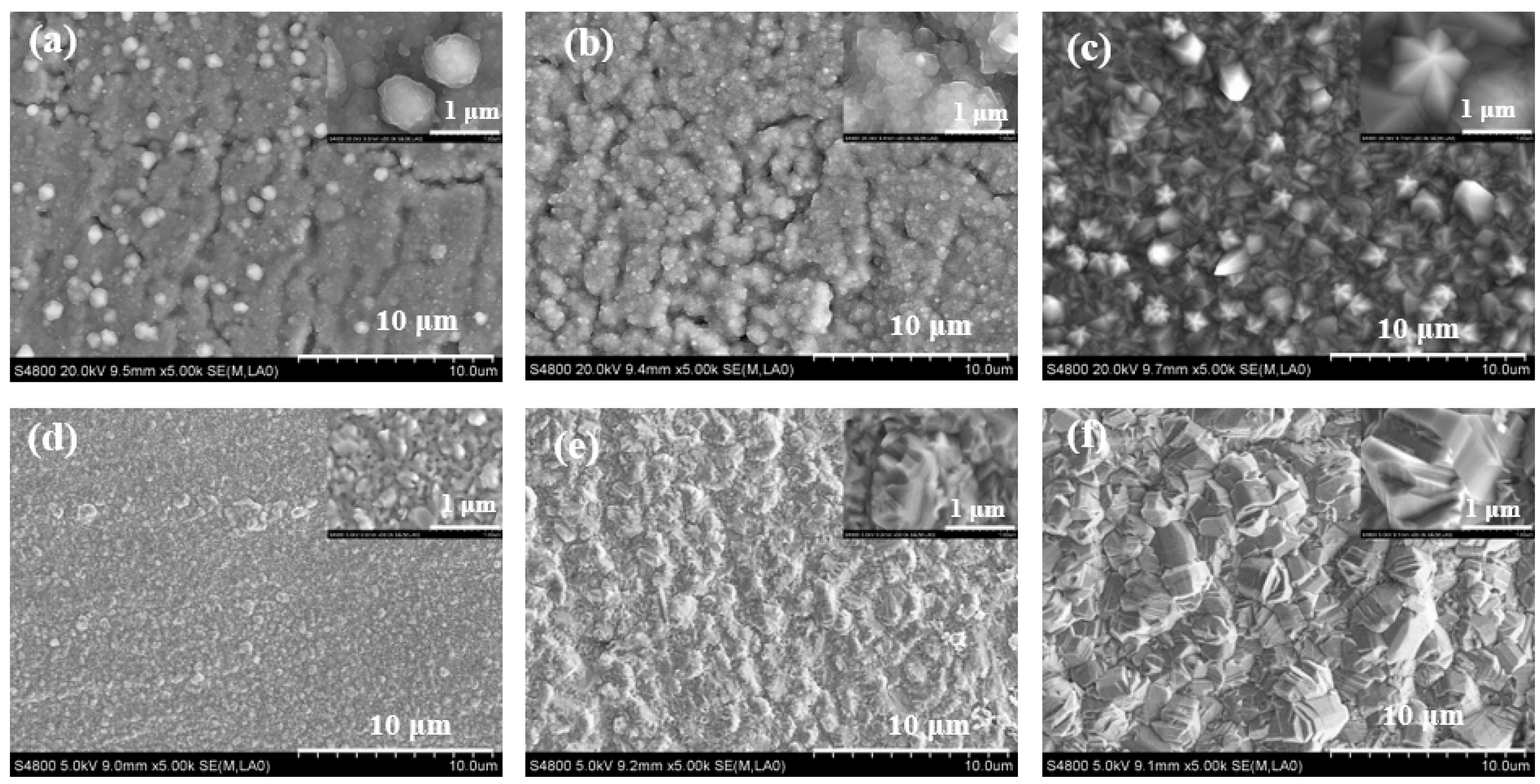
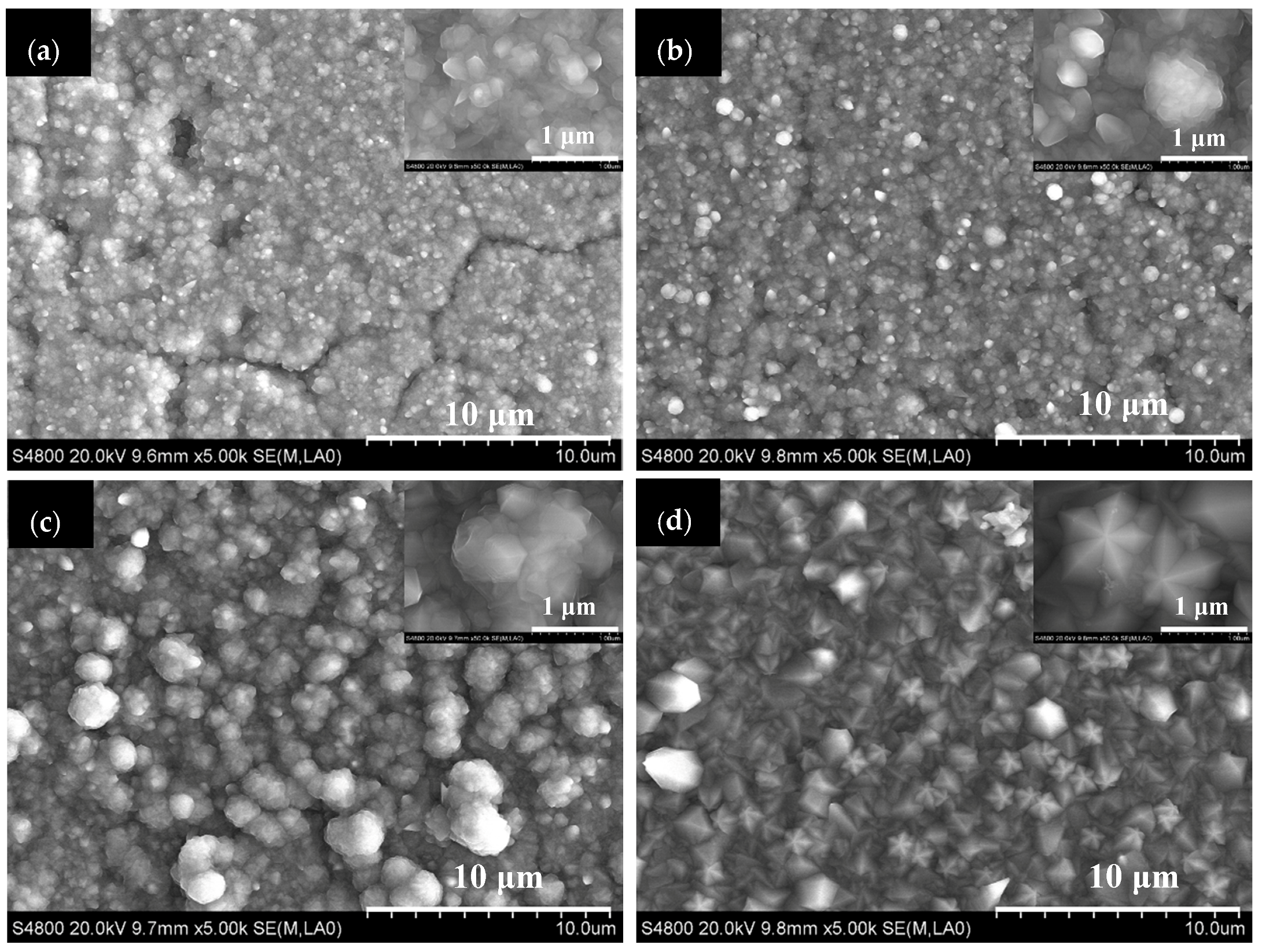
3.1. The Effect of Deposition Time
3.2. The Effect of Residence Time
3.3. The Effect of Partial Pressure
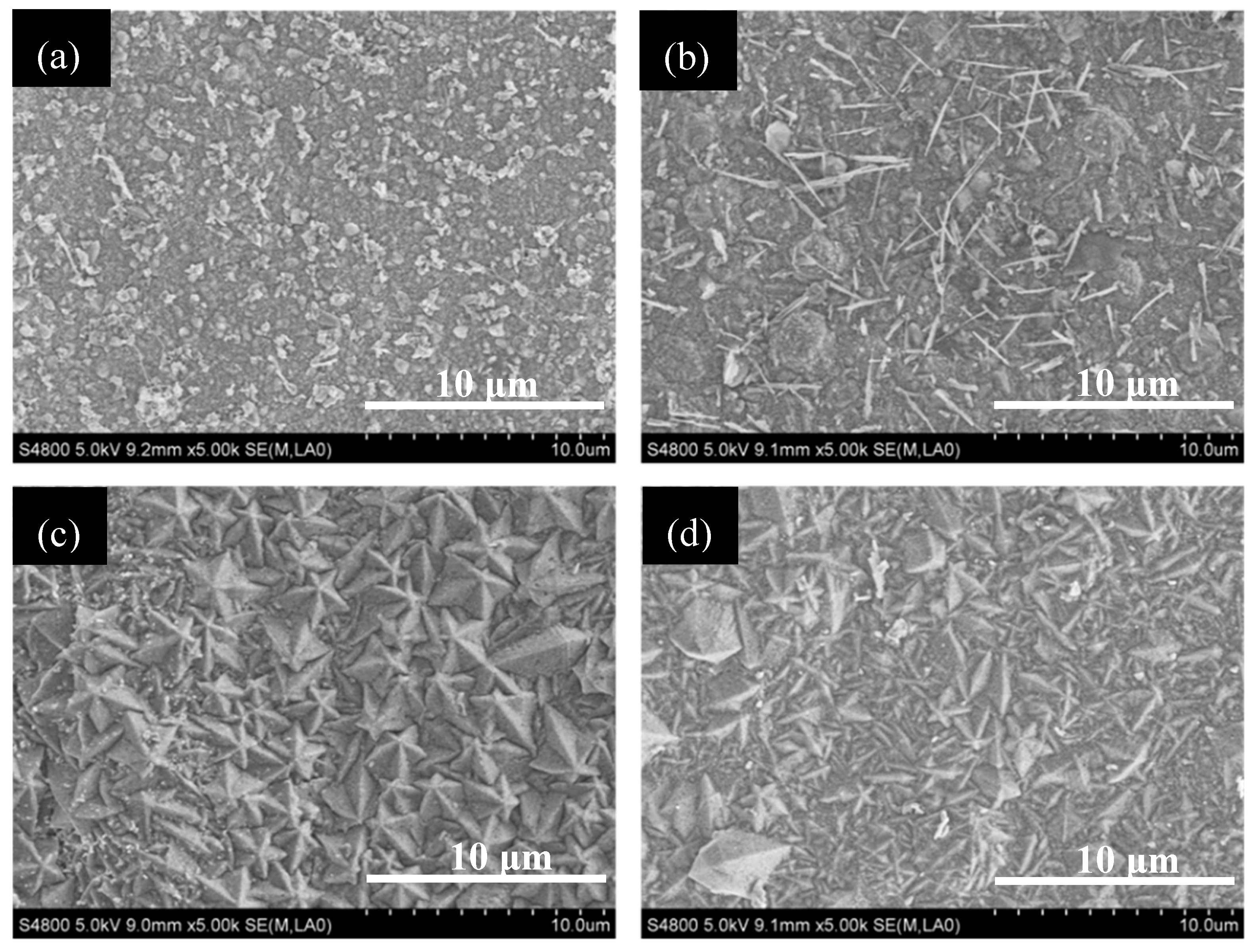
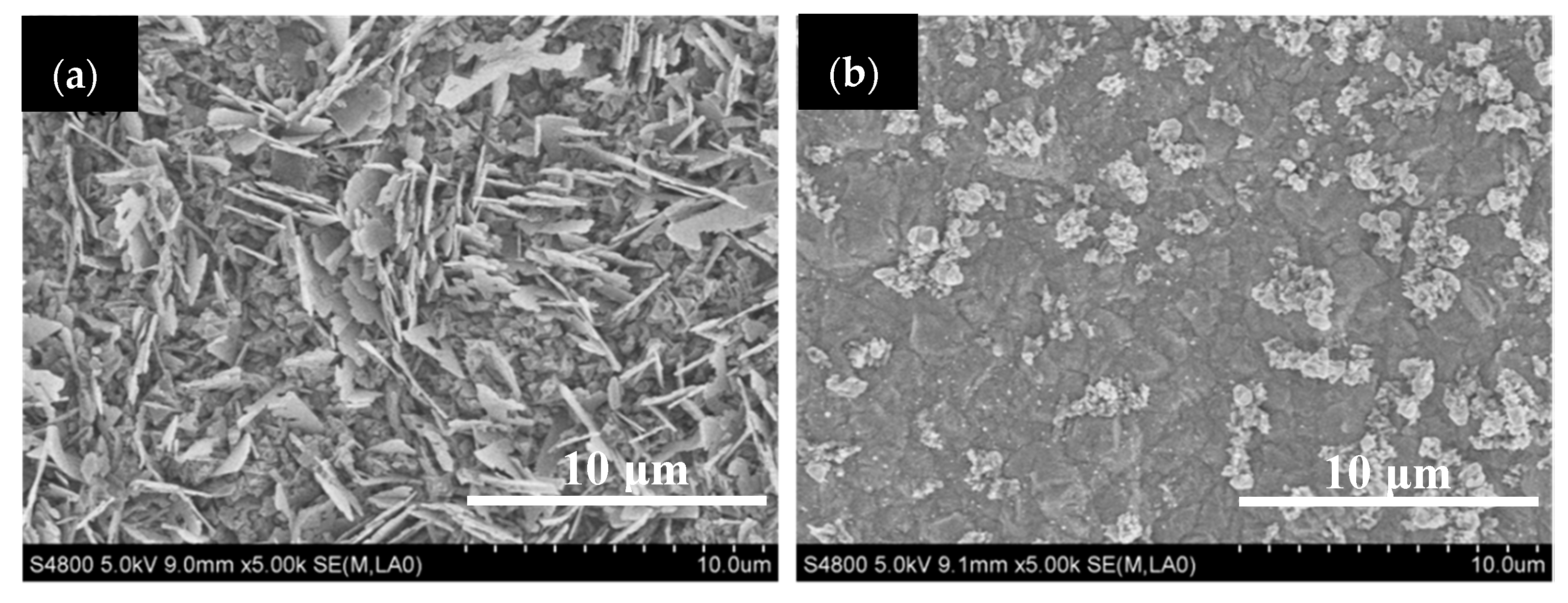
3.4. The Anti-Coking Properties of TiN and TiO2 Coatings
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jin, B.; Jing, K.; Liu, J.; Zhang, X.; Liu, G. Pyrolysis and coking of endothermic hydrocarbon fuel in regenerative cooling channel under different pressures. J. Anal. Appl. Pyrolysis 2017, 125, 117–126. [Google Scholar] [CrossRef]
- Edwards, T.; Propul, J. Liquid fuels and propellants for aerospace propulsion: 1903–2003. Power 2003, 19, 1089–1107. [Google Scholar] [CrossRef]
- Cheng, K.; Liang, S.; Huai, X. Effects of chemical heat sink generated by an oxalic acid cooling stream on film-cooling effectiveness. Exp. Heat Transf. 2016, 29, 113–123. [Google Scholar] [CrossRef]
- Wang, H.; Yang, W.; Tian, P.; Zhou, J.; Tang, R.; Wu, S. A highly active and anti-coking Pd-Pt/SiO2 catalyst for catalytic combustion of toluene at low temperature. Appl. Catal. A-Gen. 2017, 529, 60–67. [Google Scholar] [CrossRef]
- Masirana, N.; Vo, D.V.N.; Salam, M.A.; Abdullah, B. Improvement on coke formation of CaO-Ni/Al2O3 catalysts in ethylene production via dehydration of ethanol. Procedia Eng. 2016, 148, 1289–1294. [Google Scholar] [CrossRef]
- Ding, R.; Taylor, M.P.; Chiu, Y.L.; Smith, N.; Mowforth, C.W.; Evans, H.E. Influence of pre-oxidation on filamentary carbon deposition on 20Cr25Ni stainless steel. Oxid. Met. 2019, 91, 589–607. [Google Scholar] [CrossRef]
- Zeng, Z.; Natesan, K. Relationship of carbon crystallization to the metal-dusting mechanism of nickel. Chem. Mat. 2003, 15, 872–878. [Google Scholar] [CrossRef]
- Mohan, R.; Eser, S. Effectiveness of low-pressure metal–organic chemical vapor deposition coatings on metal surfaces for the mitigation of fouling from heated jet fuel. Ind. Eng. Chem. Res. 2011, 50, 7290–7304. [Google Scholar] [CrossRef]
- Yang, C.; Liu, G.; Wang, X.; Jiang, R.; Wang, L.; Zhang, X. Preparation and anti-coking performance of MOCVD alumina coatings for thermal cracking of hydrocarbon fuels under supercritical conditions. Ind. Eng. Chem. Res. 2012, 51, 1256–1263. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, H.; Zhou, J.; Luan, X. Simulation of SiO2/S coating deposition in a pilot plant set-up for coking inhibition. Chem. Eng. Res. Des. 2013, 91, 120–133. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, Z.; Luan, X.; Xu, H. Anti-coking property of the SiO2/S coating during light naphtha steam cracking in a pilot plant setup. Anal. J. Appl. Pyrol. 2011, 90, 7–12. [Google Scholar] [CrossRef]
- Zhou, J.; Xu, H.; Liu, J.; Qi, X.; Zhang, L.; Jiang, Z. Study of anti-coking property of SiO2/S composite coatings deposited by atmospheric pressure chemical vapor deposition. Mater. Lett. 2007, 29, 5087–5090. [Google Scholar] [CrossRef]
- Bao, B.; Liu, J.; Xu, H.; Liu, B.; Zhang, W. Inhibitory effect of MnCr2O4 spinel coating on coke formation during light naphtha thermal cracking. RSC Adv. 2016, 6, 68934–68941. [Google Scholar] [CrossRef]
- Tang, S.; Gao, S.; Hu, S.; Wang, J.; Zhu, Q.; Chen, Y.; Li, X. Inhibition effect of APCVD titanium nitride coating on coke growth during n-hexane thermal cracking under supercritical conditions. Ind. Eng. Chem. Res. 2014, 53, 5432–5442. [Google Scholar] [CrossRef]
- Tang, S.; Gao, S.; Wang, S.; Wang, J.; Zhu, Q.; Chen, Y.; Li, X. Characterization of CVD TiN coating at different deposition temperatures and its application in hydrocarbon pyrolysis. Surf. Coat. Technol. 2014, 258, 1060–1067. [Google Scholar] [CrossRef]
- Tang, S.; Wang, J.; Zhu, Q.; Chen, Y.; Li, X. Preparation of rutile TiO2 coating by thermal chemical vapor deposition for anti-coking applications. ACS Appl. Mater. Interfaces 2014, 6, 17157–17165. [Google Scholar] [CrossRef]
- Tang, S.; Shi, N.; Wang, J.; Tang, A. Comparison of the anti-coking performance of CVD TiN, TiO2 and TiC coatings for hydrocarbon fuel pyrolysis. Ceram. Int. 2017, 43, 3818–3823. [Google Scholar] [CrossRef]
- Altin, O.; Eser, S. Analysis of solid deposits from thermal stressing of a JP-8 fuel on different tube surfaces in a flow reactor. Ind. Eng. Chem. Res. 2001, 40, 596–603. [Google Scholar] [CrossRef]
- Baker, R.T.K.; Chludzinski, J.J.; Lund, C.R.F. Further studies of the formation of filamentous carbon from the interaction of supported iron particles with acetylene. Carbon 1987, 25, 295–303. [Google Scholar] [CrossRef]
- Reyniers, M.S.G.; Froment, G.F. Influence of metal surface and sulfur addition on coke deposition in the thermal cracking of hydrocarbons. Ind. Eng. Chem. Res. 1995, 34, 773–785. [Google Scholar] [CrossRef]
- Crynes, L.L.; Crynes, B.L. Coke formation on polished and unpolished Incoloy 800 coupons during pyrolysis of light hydrocarbons. Ind. Eng. Chem. Res. 1987, 26, 2139–2144. [Google Scholar] [CrossRef]
- Marek, J.C.; Albright, L.F. Formation and removal of coke deposited on stainless steel and vycor surfaces from acetylene and ethylene. ACS Symp. 1982, 202, 123–149. [Google Scholar]
- Marek, J.C.; Albright, L.F. Surface phenomena during pyrolysis: The effects of treatments with various inorganic gases. ACS Symp. 1982, 202, 151–175. [Google Scholar]
- Gregg, S.J.; Leach, H.F. Reaction of nickel with carbon monoxide at elevated temperatures. J. Catal. 1966, 6, 308–313. [Google Scholar] [CrossRef]
- Durbin, M.J.; Castle, J.E. Carbon deposition from acetone during oxidation of iron—the effects of chromium and nickel. Carbon 1976, 14, 27–33. [Google Scholar] [CrossRef]
- Tang, S.; Wang, J.; Zhu, Q.; Chen, Y.; Li, X. Oxidation behavior of CVD star-shaped TiN coating in ambient air. Ceram. Int. 2015, 41, 9549–9554. [Google Scholar] [CrossRef]
- Cheng, H.; Lin, T.; Hon, M. Multiple twins induced preferred growth in TiN and SiC films prepared by CVD. Scripta Mater. 1996, 35, 113–116. [Google Scholar] [CrossRef]
- Cheng, H.; Hon, M. Growth mechanism of star-shaped TiN crystals. J. Cryst. Growth 1994, 142, 117–123. [Google Scholar] [CrossRef]
- Venables, J.A.; Spiller, G.; Hanbucken, M. Nucleation and growth of thin films. Rep. Prog. Phys. 1992, 47, 5–29. [Google Scholar]
- Tingey, G.L. Kinetics of the water-gas equilibrium reaction. I. the reaction of carbon dioxide with hydrogen. J. Phys. Chem. 1966, 70, 1406–1412. [Google Scholar] [CrossRef]
- Cheng, H.E.; Chiang, M.J.; Hon, M.H. Growth characteristics and properties of TiN coating by chemical vapor deposition. J. Electrochem. Soc. 1995, 142, 1573–1578. [Google Scholar] [CrossRef]
- Xie, W.; Fang, W.; Li, D.; Xing, Y.; Guo, Y.; Lin, R. Coking of model hydrocarbon fuels under supercritical condition. Energy Fuels 2009, 23, 2997–3001. [Google Scholar] [CrossRef]
- Eser, S.; Venkataraman, A.R.; Altin, O. Deposition of carbonaceous solids on different substrates from thermal stressing of JP-8 and jet-A fuels. Ind. Eng. Chem. Res. 2006, 45, 8946–8955. [Google Scholar] [CrossRef]



| Conditions | TiN Coating | TiO2 Coating |
|---|---|---|
| Flow rate of H2 diluent gas (L/min) | 1.83 | 1.88 |
| Flow rate of H2 carrier gas (L/min) | 0.95 | 0.88 |
| Flow rate of N2 (L/min) | 1.40 | none |
| Flow rate of CO2 (mL/min) | none | 40 |
| Deposition press (atm) | 1 | 1 |
| Preheating temperature (°C) | 650 | 650 |
| Substrate temperature (°C) | 850 | 800 |
| Vaporizer temperature (°C) | 28 | 28 |
| Gas line temperature (°C) | 100 | 100 |
| Deposition time (min) | 90 | 90 |
| Conditions | Value |
|---|---|
| Flow rate of N2 diluent gas (mL/min) | 17 |
| Flow rate of N2 carrier gas (mL/min) | 73 |
| Substrate temperature (°C) | 770 |
| Pre-heater temperature (°C) | 400 |
| Vaporizer temperature (°C) | 50 |
| Experimental time (h) | 1.5 |
| Gas line temperature (°C) | 130 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, S.; Liu, T.; Duan, S.; Guo, J.; Tang, A. Comparison of Growth Characteristics and Properties of CVD TiN and TiO2 Anti-Coking Coatings. Processes 2019, 7, 574. https://doi.org/10.3390/pr7090574
Tang S, Liu T, Duan S, Guo J, Tang A. Comparison of Growth Characteristics and Properties of CVD TiN and TiO2 Anti-Coking Coatings. Processes. 2019; 7(9):574. https://doi.org/10.3390/pr7090574
Chicago/Turabian StyleTang, Shiyun, Tao Liu, Shuiping Duan, Junjiang Guo, and Anjiang Tang. 2019. "Comparison of Growth Characteristics and Properties of CVD TiN and TiO2 Anti-Coking Coatings" Processes 7, no. 9: 574. https://doi.org/10.3390/pr7090574
APA StyleTang, S., Liu, T., Duan, S., Guo, J., & Tang, A. (2019). Comparison of Growth Characteristics and Properties of CVD TiN and TiO2 Anti-Coking Coatings. Processes, 7(9), 574. https://doi.org/10.3390/pr7090574






