Green Synthesis of Potent Antimicrobial Silver Nanoparticles Using Different Plant Extracts and Their Mixtures
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical, Plants, Microorganism Strains and Media
2.2. Preparation of Plant Powder Aqueous Extract
2.3. Biosynthesis of AgNPs by Using the Plant Extracts
2.4. Characterizations of Synthesized AgNPs
2.4.1. Morphological Examination and Particle-Size Measurement
2.4.2. Size and Zeta-Potential Analysis
2.4.3. UV-Vis Spectrophotometry
2.4.4. Fourier-Transform Infrared Spectroscopy
2.4.5. In Vitro Antimicrobial Activity of AgNPs
2.4.6. Statistical Analysis
3. Results and Discussion
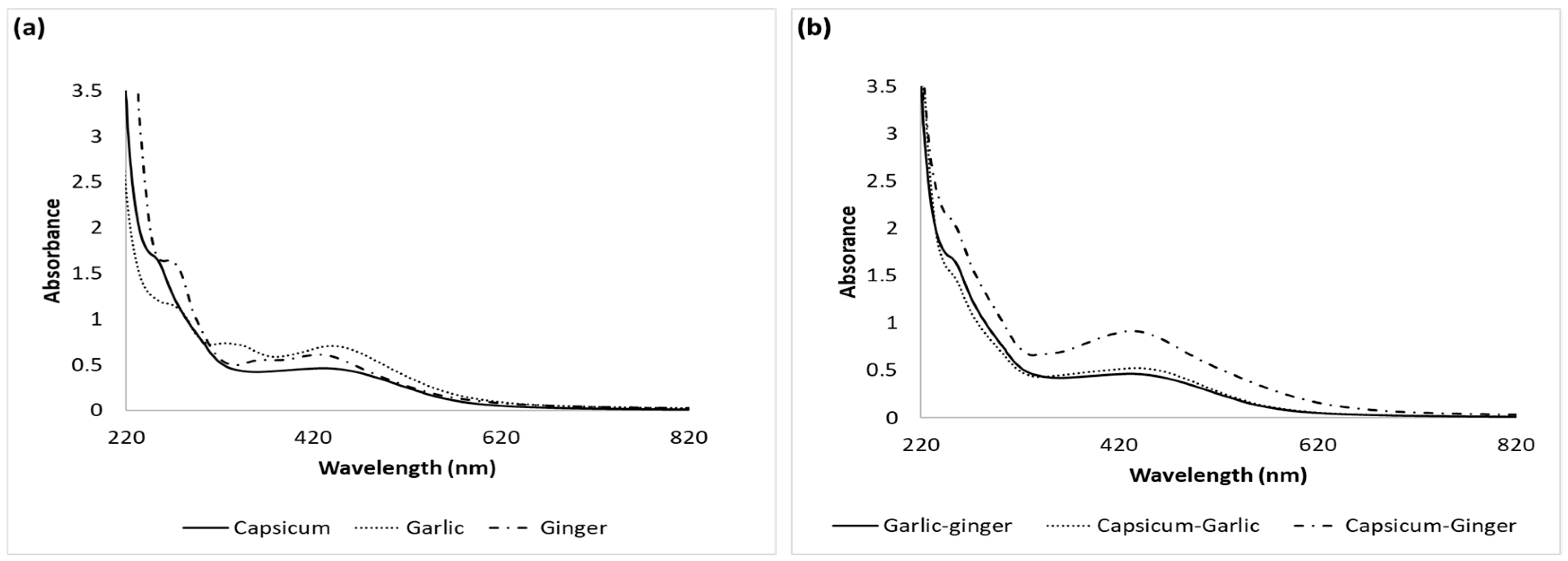
3.1. UV-Vis Absorbance Spectrophotometry
3.2. Measurement of DLS and Zeta-Potential Analysis
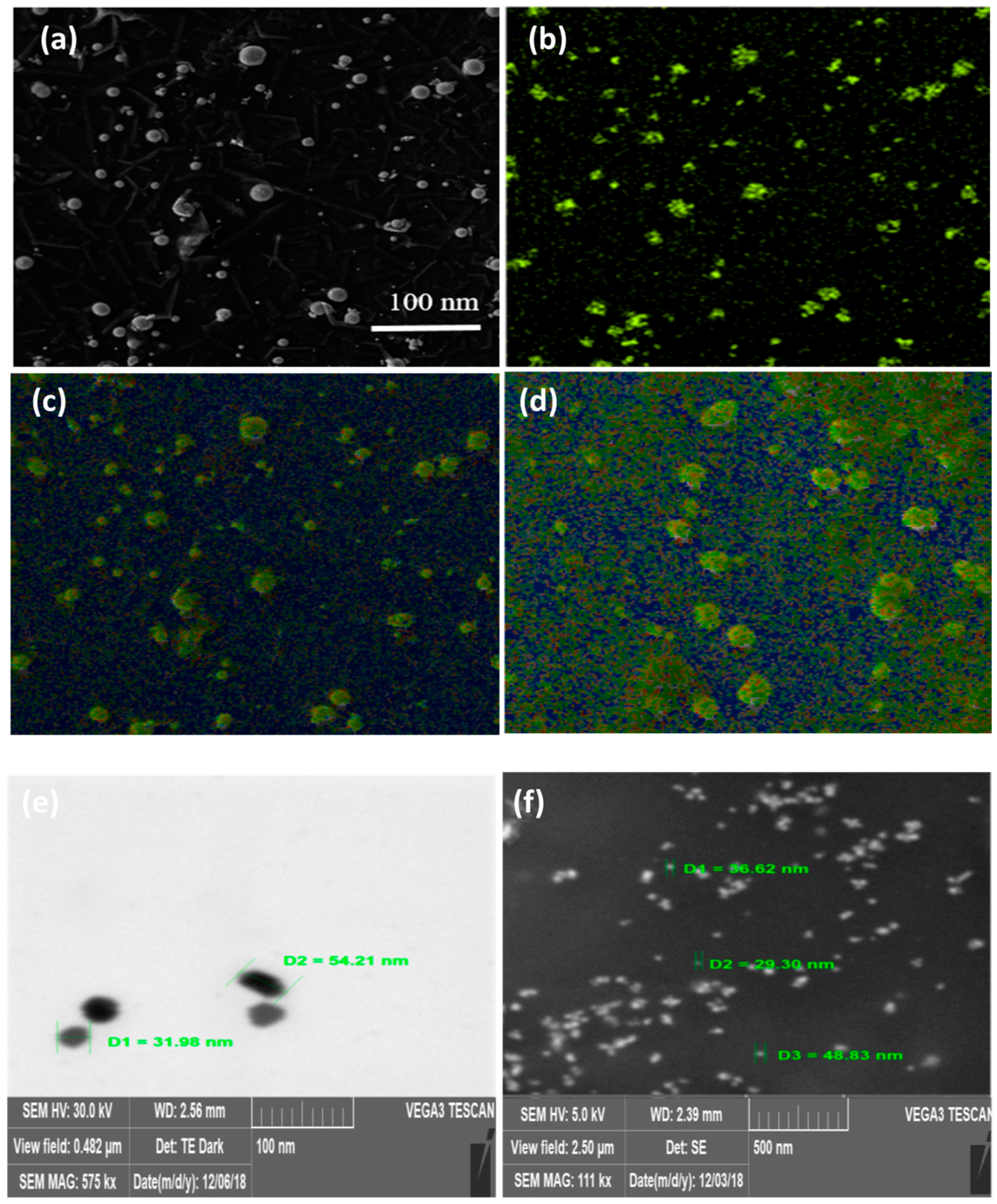
3.3. Morphological and Size Measurement by SEM
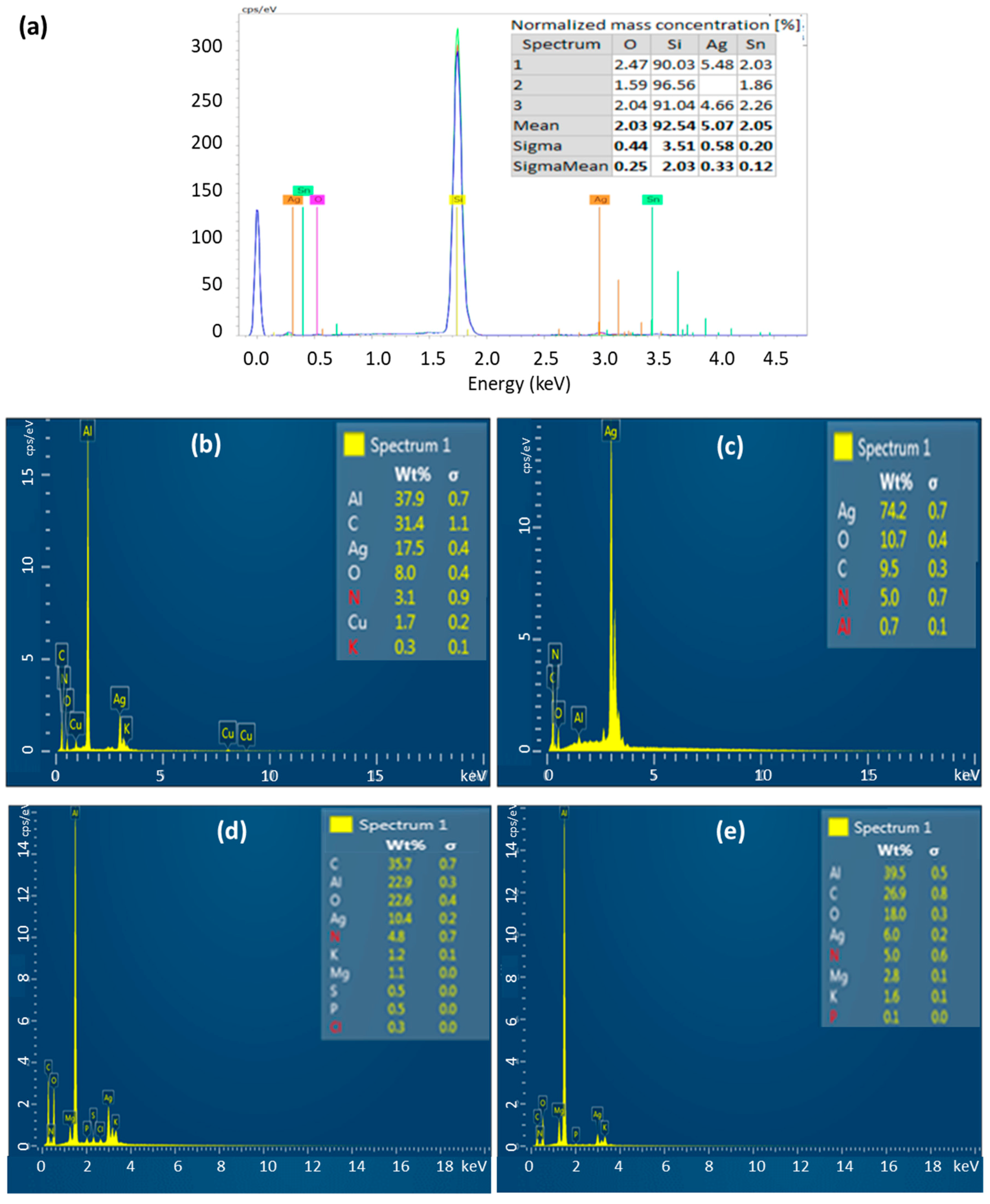
3.4. Elemental Analysis by EDX
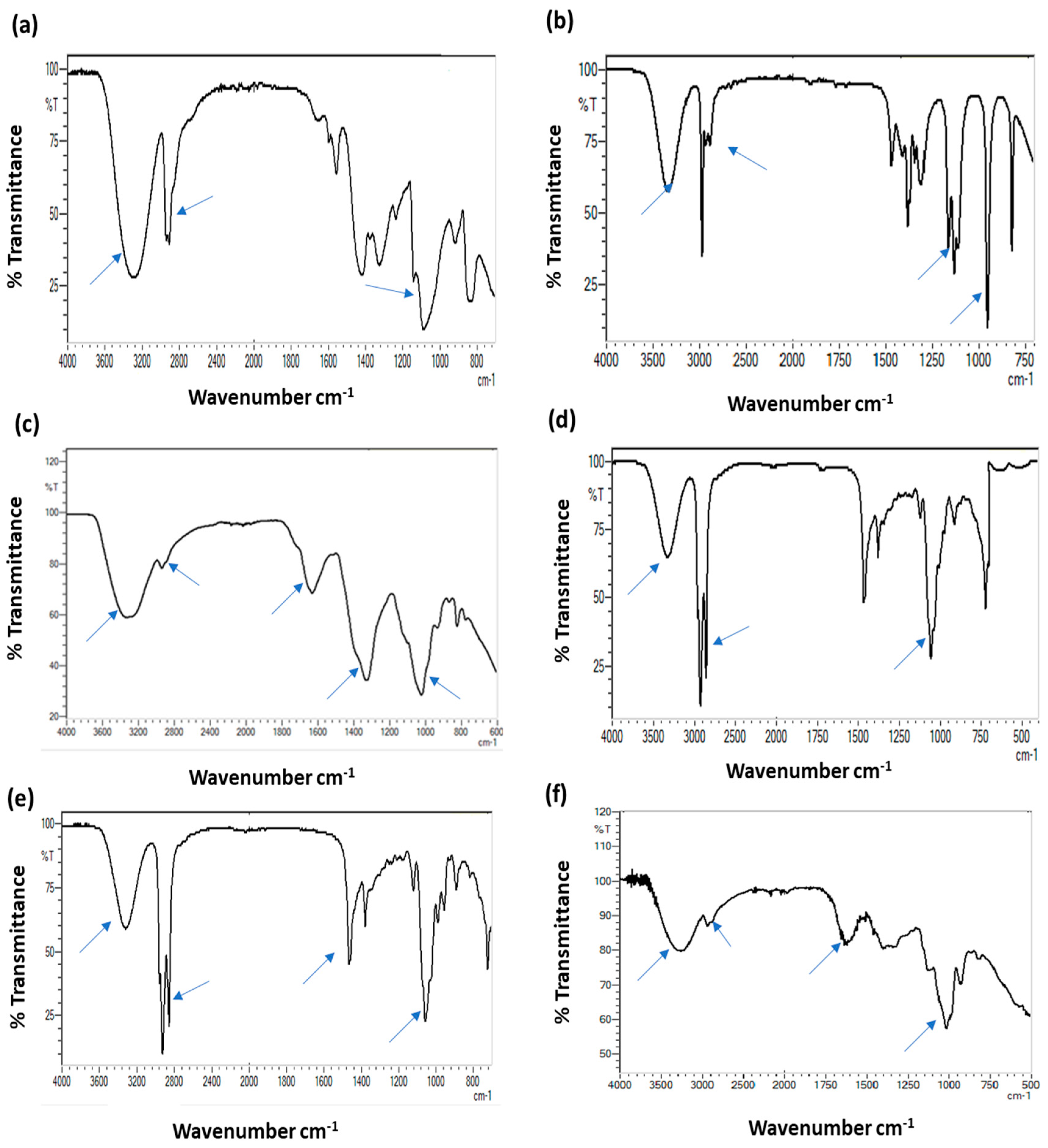
3.5. Fourier-Transform Infrared (FTIR) Spectroscopy
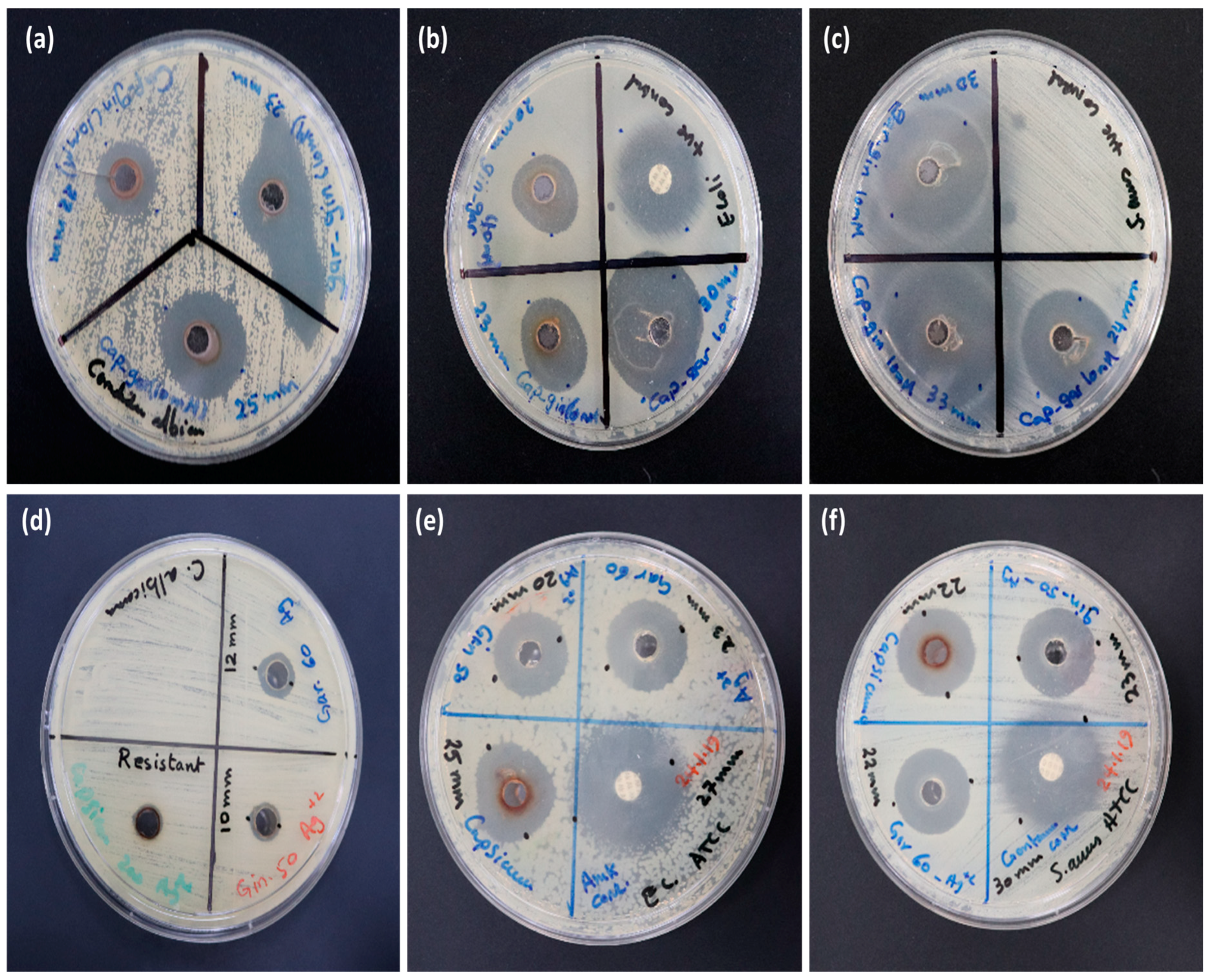
3.6. In Vitro Antimicrobial Activity of AgNPs
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AgNPs | Silver nanoparticles |
| DLS | Dynamic light scattering |
| UV-Vis | Ultra violet visible |
| FTIR | Fourier-Transform Infrared Radiometer |
| SEM | Scanning light microscope |
| ZOI | Zone of inhibition |
| EDX | Energy dispersed X-ray |
| PDI | Poly-dispersity Index |
| Gin | Ginger |
| Gar | Garlic |
| Cap | Capsicum |
| Gar-Gin | Garlic-Ginger |
| Gin-Cap | Ginger-capsicum |
| Cap-Gar | Capsicum-garlic |
| NPs | Nanoparticles |
| PVA | Polyvinyl alcohol |
| PAA | Polyacrylic acid |
| MSA | Mercaptosuccinic acid |
| PEG | Polyethylene glycol |
| ANOVA | Analysis of variance |
| SPR | Surface plasmonic resonance |
| MHB | Mueller Hinton Broth |
| MHA | Mueller Hinton Agar |
References
- Ashour, A.A.; Raafat, D.; El-Gowelli, H.M.; El-Kamel, A.H. Green synthesis of silver nanoparticles using cranberry powder aqueous extract: Characterization and antimicrobial properties. Int. J. Nanomed. 2015, 10, 7207–7221. [Google Scholar]
- Chernousova, S.; Epple, M. Silver as antibacterial agent: Ion, nanoparticle, and metal. Angew. Chem. Int. Ed. 2013, 52, 1636–1653. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-R.; Xie, X.-B.; Shi, Q.-S.; Zeng, H.-Y.; Ou-Yang, Y.-S.; Chen, Y.-B. Antibacterial activity and mechanism of silver nanoparticles on Escherichia coli. Appl. Microbiol. Biotechnol. 2009, 85, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Morones-Ramirez, J.R.; Winkler, J.A.; Spina, C.S.; Collins, J.J. Silver Enhances Antibiotic Activity Against Gram-negative Bacteria. Sci. Transl. Med. 2013, 5, 190ra81. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-J.; Sung, W.S.; Suh, B.K.; Moon, S.-K.; Choi, J.-S.; Kim, J.G.; Lee, D.G. Antifungal activity and mode of action of silver nano-particles on Candida albicans. BioMetals 2008, 22, 235–242. [Google Scholar] [CrossRef]
- Pozdnyakov, A.S.; Emel’Yanov, A.I.; Kuznetsova, N.P.; Ermakova, T.G.; Fadeeva, T.V.; Sosedova, L.M.; Prozorova, G.F. Nontoxic hydrophilic polymeric nanocomposites containing silver nanoparticles with strong antimicrobial activity. Int. J. Nanomed. 2016, 11, 1295–1304. [Google Scholar] [CrossRef]
- Jaiswal, S.; Bhattacharya, K.; McHale, P.; Duffy, B. Dual effects of β-cyclodextrin-stabilised silver nanoparticles: Enhanced biofilm inhibition and reduced cytotoxicity. J. Mater. Sci. Mater. Electron. 2015, 26, 52. [Google Scholar] [CrossRef]
- Ajitha, B.; Reddy, Y.A.K.; Reddy, P.S.; Jeon, H.-J.; Ahn, C.W. Role of capping agents in controlling silver nanoparticles size, antibacterial activity and potential application as optical hydrogen peroxide sensor. RSC Adv. 2016, 6, 36171–36179. [Google Scholar] [CrossRef]
- Wei, L.; Lu, J.; Xu, H.; Patel, A.; Chen, Z.S.; Chen, G. Silver nanoparticles: Synthesis, properties, and therapeutic applications. Drug Discov. Today 2015, 20, 595–601. [Google Scholar] [CrossRef]
- Rafique, M.; Sadaf, I.; Rafique, M.S.; Tahir, M.B. A review on green synthesis of silver nanoparticles and their applications. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1272–1291. [Google Scholar] [CrossRef]
- Sathishkumar, M.; Sneha, K.; Won, S.; Cho, C.-W.; Kim, S.; Yun, Y.-S. Cinnamon zeylanicum bark extract and powder mediated green synthesis of nano-crystalline silver particles and its bactericidal activity. Colloids Surf. B Biointerfaces 2009, 73, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Khatami, M.; Pourseyedi, S. Phoenix dactylifera (date palm) pit aqueous extract mediated novel route for synthesis high stable silver nanoparticles with high antifungal and antibacterial activity. IET Nanobiotechnol. 2015, 9, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Mohan Kumar, K.; Siva Kumar, K.; Sinha, M.; Mandal, B.K.; Sreedhara Reddy, P.; Ghosh, A.R. Green synthesis of silver nanoparticles using Terminalia chebula extract at room temperature and their antimicrobial studies. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 91, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Reddy, N.J.; Vali, D.N.; Rani, M.; Rani, S.S. Evaluation of antioxidant, antibacterial and cytotoxic effects of green synthesized silver nanoparticles by Piper longum fruit. Mater. Sci. Eng. C 2014, 34, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; Yngard, R.A.; Lin, Y. Silver nanoparticles: Green synthesis and their antimicrobial activities. Adv. Colloid Interface Sci. 2009, 145, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Shankar, T.; Karthiga, P.; Swarnalatha, K.; Rajkumar, K. Green synthesis of silver nanoparticles using Capsicum frutescens and its intensified activity against E. coli. Resour. Technol. 2017, 3, 303–308. [Google Scholar]
- Otunola, G.A.; Afolayan, A.J.; Ajayi, E.O.; Odeyemi, S.W. Characterization, antibacterial and antioxidant properties of silver nanoparticles synthesized from aqueous extracts of Allium sativum, Zingiberofficinale, and Capsicum frutescens. Pharmacogn. Mag. 2017, 13, 201–208. [Google Scholar] [CrossRef]
- Yang, N.; Li, F.; Jian, T.; Liu, C.; Sun, H.; Wang, L.; Xu, H. Biogenic synthesis of silver nanoparticles using ginger (Zingiberofficinale) extract and their antibacterial properties against aquatic pathogens. Acta Oceanol. Sin. 2017, 36, 95–100. [Google Scholar] [CrossRef]
- El-Refai, A.A.; Ghoniem, G.A.; El-Khateeb, A.Y.; Hassaan, M.M. Eco-friendly synthesis of metal nanoparticles using ginger and garlic extracts as biocompatible novel antioxidant and antimicrobial agents. J. Nanostruct. Chem. 2018, 8, 71–81. [Google Scholar] [CrossRef]
- Chung, L.Y. The Antioxidant Properties of Garlic Compounds: Allyl Cysteine, Alliin, Allicin, and Allyl Disulfide. J. Med. Food 2006, 9, 205–213. [Google Scholar] [CrossRef]
- Von White, G.; Kerscher, P.; Brown, R.M.; Morella, J.D.; McAllister, W.; Dean, D.; Kitchens, C.L. Green synthesis of robust, biocompatible silver nanoparticles using garlic extract. J. Nanomater. 2012, 2012, 55. [Google Scholar] [CrossRef]
- Kharissova, O.V.; Dias, H.V.R.; Kharisov, B.I.; Pérez, B.O.; Pérez, V.M.J. The greener synthesis of nanoparticles. Trends Biotechnol. 2013, 31, 240–248. [Google Scholar] [CrossRef]
- Govindappa, M.; Hemashekhar, B.; Arthikala, M.K.; Ravishankar Rai, V.; Ramachandra, Y.L. Characterization, antibacterial, antioxidant, antidiabetic, anti-inflammatory and antityrosinase activity of green synthesized silver nanoparticles using Calophyllumtomentosum leaves extract. Results Phys. 2018, 9, 400–408. [Google Scholar] [CrossRef]
- Pasupuleti, V.R.; Prasad, T.N.V.K.V.; Shiekh, R.A.; Balam, S.K.; Narasimhulu, G.; Reddy, C.S.; Gan, S.H. Biogenic silver nanoparticles using Rhinacanthusnasutus leaf extract: Synthesis, spectral analysis, and antimicrobial studies. Int. J. Nanomed. 2013, 8, 3355–3364. [Google Scholar] [CrossRef]
- Khatami, M.; Mehnipor, R.; Poor, M.H.S.; Jouzani, G.S. Facile Biosynthesis of Silver Nanoparticles Using Descurainiasophia and Evaluation of Their Antibacterial and Antifungal Properties. J. Clust. Sci. 2016, 27, 1601–1612. [Google Scholar] [CrossRef]
- Sun, Q.; Cai, X.; Li, J.; Zheng, M.; Chen, Z.; Yu, C.-P. Green synthesis of silver nanoparticles using tea leaf extract and evaluation of their stability and antibacterial activity. Colloids Surf. A Physicochem. Eng. Asp. 2014, 444, 226–231. [Google Scholar] [CrossRef]
- Bindhu, M.; Umadevi, M. Synthesis of monodispersed silver nanoparticles using Hibiscus cannabinus leaf extract and its antimicrobial activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 101, 184–190. [Google Scholar] [CrossRef]
- Wang, L.; Wu, Y.; Xie, J.; Wu, S.; Wu, Z. Characterization, antioxidant and antimicrobial activities of green synthesized silver nanoparticles from Psidium guajava L. leaf aqueous extracts. Mater. Sci. Eng. C 2018, 86, 1–8. [Google Scholar] [CrossRef]
- Anandalakshmi, K.; Venugobal, J.; Ramasamy, V. Characterization of silver nanoparticles by green synthesis method using Pedalium murex leaf extract and their antibacterial activity. Appl. Nanosci. 2016, 6, 399–408. [Google Scholar] [CrossRef]
- Sre, P.R.; Reka, M.; Poovazhagi, R.; Kumar, M.A.; Murugesan, K. Antibacterial and cytotoxic effect of biologically synthesized silver nanoparticles using aqueous root extract of Erythrina indica lam. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 135, 1137–1144. [Google Scholar]
- Mallmann, E.J.J.; Cunha, F.A.; Castro, B.N.; Maciel, A.M.; Menezes, E.A.; Fechine, P.B.A. Antifungal Activity of Silver Nanoparticles Obtained by Green Synthesis. Rev. Inst. Med. Trop. Sao Paulo 2015, 57, 165–167. [Google Scholar] [CrossRef]
- Mariselvam, R.; Ranjitsingh, A.J.A.; Usha Raja Nanthini, A.; Kalirajan, K.; Padmalatha, C.; MosaeSelvakumar, P. Green synthesis of silver nanoparticles from the extract of the inflorescence of Cocos nucifera (Family: Arecaceae) for enhanced antibacterial activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 129, 537–541. [Google Scholar] [CrossRef]
- Bindhu, M.; Umadevi, M. Antibacterial and catalytic activities of green synthesized silver nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 135, 373–378. [Google Scholar] [CrossRef]
- Tippayawat, P.; Phromviyo, N.; Boueroy, P.; Chompoosor, A. Green synthesis of silver nanoparticles in aloe vera plant extract prepared by a hydrothermal method and their synergistic antibacterial activity. PeerJ 2016, 4, e2589. [Google Scholar] [CrossRef]
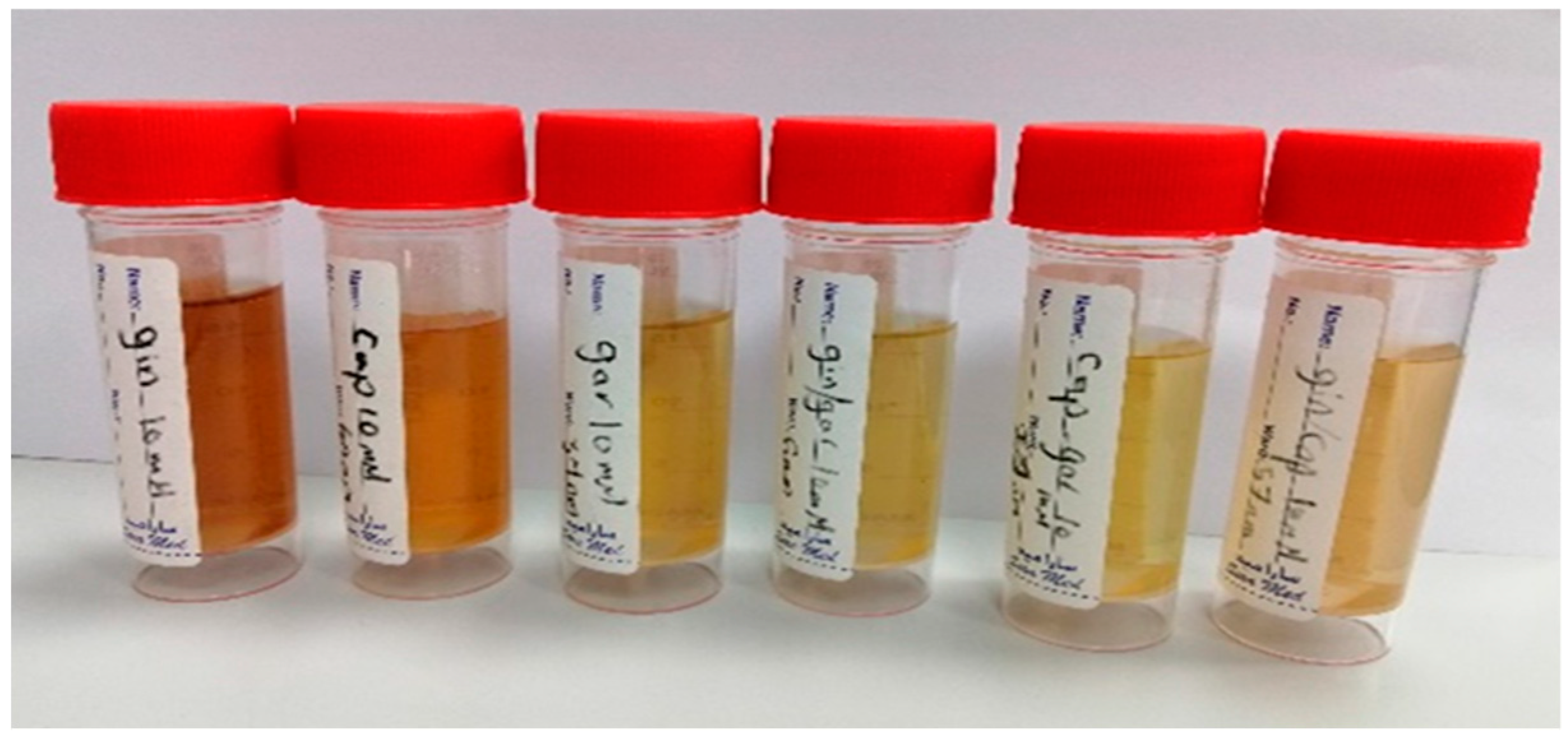
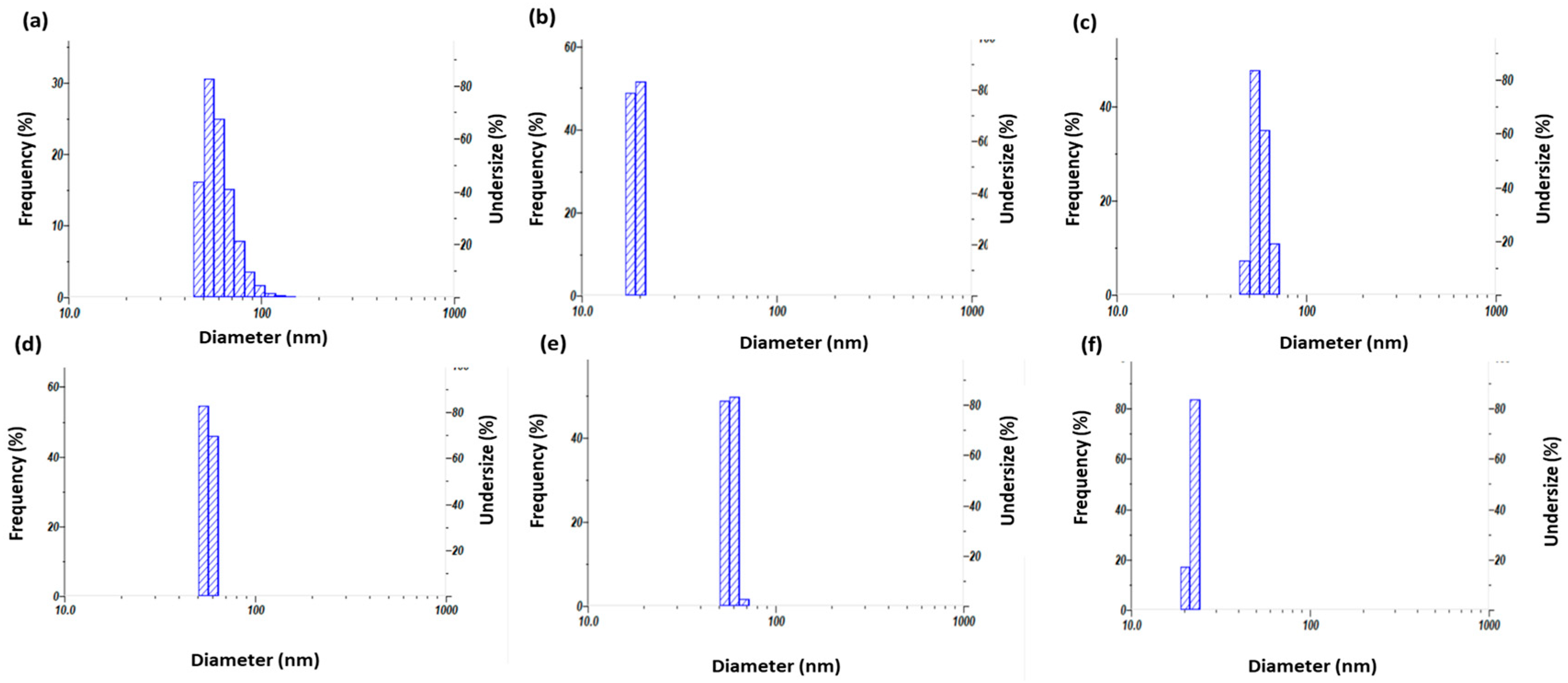


| Plant Extract Used (5% v/v) Silver Nitrate 910 mM | Zeta-Potential (mV) | Particle Size Mean (nm) | Poly-Dispersity Index (PDI) |
|---|---|---|---|
| Cap | −34.5 | 60.9 ± 12.2 | 0.149 |
| Gar | −23.5 | 19.1 ± 1.2 | 0.982 |
| Gin | −36.8 | 57.3 ± 5.5 | 0.718 |
| Cap-Gar | −18.8 | 56.9 ± 3.5 | 0.982 |
| Cap-Gin | −37.1 | 57.4 ± 3.4 | 0.788 |
| Gar-Gin | −34.0 | 22.4 ± 1.0 | 1.190 |
| Silver Nanoparticle Samples | ZOI (mm) against Pathogenic Bacteria | |||
|---|---|---|---|---|
| Sample No. | Plant Extract(s) | E. coli | S. aureus | C. albicans |
| 1 | Cap | 22 ± 0.12 | 27 ± 0.41 | 12 ± 0.36 |
| 2 | Gar | 22 ± 0.44 | 24 ± 0.16 | 11 ± 0.19 |
| 3 | Gin | 21 ± 0.53 | 26 ± 0.32 | 11 ± 0.48 |
| 4 | Cap-Gar | 30 ± 0.26 | 24 ± 0.48 | 25 ± 0.20 |
| 5 | Gin-Cap | 23 ± 0.09 | 33 ± 0.29 | 22 ± 0.53 |
| 6 | Gar-Gin | 20 ± 0.05 | 30 ± 0.36 | 23 ± 0.33 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reda, M.; Ashames, A.; Edis, Z.; Bloukh, S.; Bhandare, R.; Abu Sara, H. Green Synthesis of Potent Antimicrobial Silver Nanoparticles Using Different Plant Extracts and Their Mixtures. Processes 2019, 7, 510. https://doi.org/10.3390/pr7080510
Reda M, Ashames A, Edis Z, Bloukh S, Bhandare R, Abu Sara H. Green Synthesis of Potent Antimicrobial Silver Nanoparticles Using Different Plant Extracts and Their Mixtures. Processes. 2019; 7(8):510. https://doi.org/10.3390/pr7080510
Chicago/Turabian StyleReda, May, Akram Ashames, Zehra Edis, Samir Bloukh, Richie Bhandare, and Hamed Abu Sara. 2019. "Green Synthesis of Potent Antimicrobial Silver Nanoparticles Using Different Plant Extracts and Their Mixtures" Processes 7, no. 8: 510. https://doi.org/10.3390/pr7080510
APA StyleReda, M., Ashames, A., Edis, Z., Bloukh, S., Bhandare, R., & Abu Sara, H. (2019). Green Synthesis of Potent Antimicrobial Silver Nanoparticles Using Different Plant Extracts and Their Mixtures. Processes, 7(8), 510. https://doi.org/10.3390/pr7080510








