Effect of Dissolved Organic Matter on Agglomeration and Removal of CuO Nanoparticles by Coagulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Experimental Procedure
2.2.1. Preparation of Stock Solution and Model Waters
2.2.2. Sedimentation Experiments
2.2.3. Coagulation Experiments with Synthetic Waters
2.3. Other Analytical Methods
3. Results and Discussion
3.1. Characterization of CuO NPs
3.2. Agglomeration of CuO NPs
3.3. Removal of CuO NPs
3.4. Removal of DOM and Turbidity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Vance, M.E.; Kuiken, T.; Vejerano, E.P.; McGinnis, S.P.; Hochella, M.F., Jr.; Rejeski, D.; Hull, M.S. Nanotechnology in the real world: Redeveloping the nanomaterial consumer products inventory. Beilstein J. Nanotechnol. 2015, 6, 1769–1780. [Google Scholar] [CrossRef]
- Gawande, M.B.; Goswami, A.; Felpin, F.-X.; Asefa, T.; Huang, X.; Silva, R.; Zou, X.; Zboril, R.; Varma, R.S. Cu and Cu-Based Nanoparticles: Synthesis and Applications in Catalysis. Chem. Rev. 2016, 116, 3722–3811. [Google Scholar] [CrossRef] [PubMed]
- Ju-Nam, Y.; Lead, J.R. Manufactured nanoparticles: An overview of their chemistry, interactions and potential environmental implications. Sci. Total Environ. 2008, 400, 396–414. [Google Scholar] [CrossRef] [PubMed]
- Keller, A.A.; Vosti, W.; Wang, H.; Lazareva, A. Release of engineered nanomaterials from personal care products throughout their life cycle. J. Nanoparticle Res. 2014, 16, 2489. [Google Scholar] [CrossRef]
- Westerhoff, P.; Song, G.; Hristovski, K.; Kiser, M.A. Occurrence and removal of titanium at full scale wastewater treatment plants: Implications for TiO2 nanomaterials. J. Environ. Monit. 2011, 13, 1195. [Google Scholar] [CrossRef] [PubMed]
- Savage, N.; Thomas, T.A.; Duncan, J.S. Nanotechnology applications and implications research supported by the US Environmental Protection Agency STAR grants program. J. Environ. Monit. 2007, 9, 1046–1054. [Google Scholar] [CrossRef] [PubMed]
- Von Moos, N.; Slaveykova, V.I. Oxidative stress induced by inorganic nanoparticles in bacteria and aquatic microalgae—State of the art and knowledge gaps. Nanotoxicology 2014, 8, 605–630. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Vijver, M.G.; Peijnenburg, W.J.G.M.; Galloway, T.S.; Tyler, C.R. A comparative analysis on the in vivo toxicity of copper nanoparticles in three species of freshwater fish. Chemosphere 2015, 139, 181–189. [Google Scholar] [CrossRef]
- Lee, S.; Chung, H.; Kim, S.; Lee, I. The genotoxic effect of ZnO and CuO nanoparticles on early growth of buckwheat, fagopyrum esculentum. Water. Air. Soil Pollut. 2013, 224, 1668. [Google Scholar] [CrossRef]
- Aruoja, V.; Dubourguier, H.-C.C.; Kasemets, K.; Kahru, A. Toxicity of nanoparticles of CuO, ZnO and TiO2 to microalgae Pseudokirchneriella subcapitata. Sci. Total Environ. 2009, 407, 1461–1468. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; McLean, J.E.; Latta, D.E.; Manangón, E.; Britt, D.W.; Johnson, W.P.; Boyanov, M.I.; Anderson, A.J. CuO and ZnO nanoparticles: Phytotoxicity, metal speciation, and induction of oxidative stress in sand-grown wheat. J. Nanoparticle Res. 2012, 14, 1125. [Google Scholar] [CrossRef]
- Dreher, K.L. Health and environmental impact of nanotechnology: Toxicological assessment of manufactured nanoparticles. Toxicol. Sci. 2004, 77, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Son, J.; Vavra, J.; Forbes, V.E. Effects of water quality parameters on agglomeration and dissolution of copper oxide nanoparticles (CuO-NPs) using a central composite circumscribed design. Sci. Total Environ. 2015, 521, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Abbas, Z.; Labbez, C.; Nordholm, S.; Ahlberg, E. Size-dependent surface charging of nanoparticles. J. Phys. Chem. C 2008, 112, 5715–5723. [Google Scholar] [CrossRef]
- Peng, C.; Shen, C.; Zheng, S.; Yang, W.; Hu, H.; Liu, J.; Shi, J. Transformation of CuO Nanoparticles in the Aquatic Environment: Influence of pH, Electrolytes and Natural Organic Matter. Nanomaterials 2017, 7, 326. [Google Scholar] [CrossRef] [PubMed]
- Giasuddin, A.B.M.; Kanel, S.R.; Choi, H. Adsorption of humic acid onto nanoscale zerovalent iron and its effect on arsenic removal. Environ. Sci. Technol. 2007, 41, 2022–2027. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Vijver, M.G.; Peijnenburg, W.J.G.M. Impact of water chemistry on the behavior and fate of copper nanoparticles. Environ. Pollut. 2018, 234, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Springer, F.; Laborie, S.; Guigui, C. Removal of SiO2 nanoparticles from industry wastewaters and subsurface waters by ultrafiltration: Investigation of process efficiency, deposit properties and fouling mechanism. Sep. Purif. Technol. 2013, 108, 6–14. [Google Scholar] [CrossRef]
- Tiede, K.; Boxall, A.B.A.; Wang, X.; Gore, D.; Tiede, D.; Baxter, M.; David, H.; Tear, S.P.; Lewis, J. Application of hydrodynamic chromatography-ICP-MS to investigate the fate of silver nanoparticles in activated sludge. J. Anal. At. Spectrom. 2010, 25, 1149–1154. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Westerhoff, P.; Crittenden, J.C. Stability and removal of water soluble CdTe quantum dots in water. Environ. Sci. Technol. 2008, 42, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Hyung, H.; Kim, J.-H.H. Dispersion of C60in natural water and removal by conventional drinking water treatment processes. Water Res. 2009, 43, 2463–2470. [Google Scholar] [CrossRef] [PubMed]
- Holbrook, R.D.; Kline, C.N.; Filliben, J.J. Impact of source water quality on multiwall carbon nanotube coagulation. Environ. Sci. Technol. 2010, 44, 1386–1391. [Google Scholar] [CrossRef] [PubMed]
- Sousa, V.S.; Corniciuc, C.; Teixeira, M.R. The effect of TiO2 nanoparticles removal on drinking water quality produced by conventional treatment C/F/S. Water Res. 2017, 109, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Abbott Chalew, T.E.; Ajmani, G.S.; Huang, H.; Schwab, K.J. Evaluating nanoparticle breakthrough during drinking water treatment. Environ. Health Perspect. 2013, 121, 1161–1166. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Li, Y.; Tang, T.; Yuan, Z.; Yu, C.-P. Removal of silver nanoparticles by coagulation processes. J. Hazard. Mater. 2013, 261, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xue, N.; Chu, Y.; Sun, Y.; Yan, H.; Han, Q. CuO nanoparticle-humic acid (CuONP-HA) composite contaminant removal by coagulation/ultrafiltration process: The application of sodium alginate as coagulant aid. Desalination 2015, 367, 265–271. [Google Scholar] [CrossRef]
- Khan, R.; Inam, M.; Park, D.; Zam Zam, S.; Shin, S.; Khan, S.; Akram, M.; Yeom, I. Influence of Organic Ligands on the Colloidal Stability and Removal of ZnO Nanoparticles from Synthetic Waters by Coagulation. Processes 2018, 6, 170. [Google Scholar] [CrossRef]
- Campinas, M.; Rosa, M.J. The ionic strength effect on microcystin and natural organic matter surrogate adsorption onto PAC. J. Colloid Interface Sci. 2006, 299, 520–529. [Google Scholar] [CrossRef]
- Edzwald, J.K.; Van Benschoten, J.E. Aluminum coagulation of natural organic matter. In Chemical Water and Wastewater Treatment; Springer: Berlin, Germany, 1990; pp. 341–359. [Google Scholar]
- USEPA—United States Environmental Protection Agency. Enhanced Coagulation and Enhanced Precipitative Softening Guidance Manual; USEPA—United States Environmental Protection Agency: Washington, DC, USA, 1999. [Google Scholar]
- Letterman, R. Water Quality and Treatment, a Handbook of Community Water Supplies, 5th ed.; American Water Works Association: Denver, CO, USA, 1999. [Google Scholar]
- Jiang, J.; Oberdörster, G.; Biswas, P. Characterization of size, surface charge, and agglomeration state of nanoparticle dispersions for toxicological studies. J. Nanoparticle Res. 2009, 11, 77–89. [Google Scholar] [CrossRef]
- Romanello, M.B.; de Cortalezzi, M.M.F. An experimental study on the aggregation of TiO2 nanoparticles under environmentally relevant conditions. Water Res. 2013, 47, 3887–3898. [Google Scholar] [CrossRef]
- Baalousha, M. Aggregation and disaggregation of iron oxide nanoparticles: Influence of particle concentration, pH and natural organic matter. Sci. Total Environ. 2009, 407, 2093–2101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Mao, J.; Zhao, Q.; He, S.; Ma, J. Effect of AlCl3 concentration on nanoparticle removal by coagulation. J. Environ. Sci. 2015, 38, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Pinotti, A.; Zaritzky, N. Effect of aluminum sulfate and cationic polyelectrolytes on the destabilization of emulsified wastes. Waste Manag. 2001, 21, 535–542. [Google Scholar] [CrossRef]
- Edzwald, J.K.; Tobiason, J.E. Enhanced coagulation: US requirements and a broader view. Water Sci. Technol. 1999, 40, 63–70. [Google Scholar] [CrossRef]
- Sharp, E.L.; Jarvis, P.; Parsons, S.A.; Jefferson, B. Impact of fractional character on the coagulation of NOM. Colloids Surf. A Physicochem. Eng. Asp. 2006, 286, 104–111. [Google Scholar] [CrossRef]
- Bond, T.; Goslan, E.H.; Parsons, S.A.; Jefferson, B. Disinfection by-product formation of natural organic matter surrogates and treatment by coagulation, MIEX® and nanofiltration. Water Res. 2010, 44, 1645–1653. [Google Scholar] [CrossRef] [PubMed]
- Matilainen, A.; Vepsäläinen, M.; Sillanpää, M. Natural organic matter removal by coagulation during drinking water treatment: a review. Adv. Colloid Interface Sci. 2010, 159, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Joseph, L.; Flora, J.R.V.; Park, Y.-G.; Badawy, M.; Saleh, H.; Yoon, Y. Removal of natural organic matter from potential drinking water sources by combined coagulation and adsorption using carbon nanomaterials. Sep. Purif. Technol. 2012, 95, 64–72. [Google Scholar] [CrossRef]
- Zhao, Y.X.; Gao, B.Y.; Zhang, G.Z.; Phuntsho, S.; Shon, H.K. Coagulation by titanium tetrachloride for fulvic acid removal: Factors influencing coagulation efficiency and floc characteristics. Desalination 2014, 335, 70–77. [Google Scholar] [CrossRef]
- Shon, H.K.; Vigneswaran, S.; Kim, I.S.; Cho, J.; Kim, G.I.; Kim, J.B.; Kim, J.H. Preparation of titanium dioxide (TiO2) from sludge produced by titanium tetrachloride (TiCl4) flocculation of wastewater. Environ. Sci. Technol. 2007, 41, 1372–1377. [Google Scholar] [CrossRef]
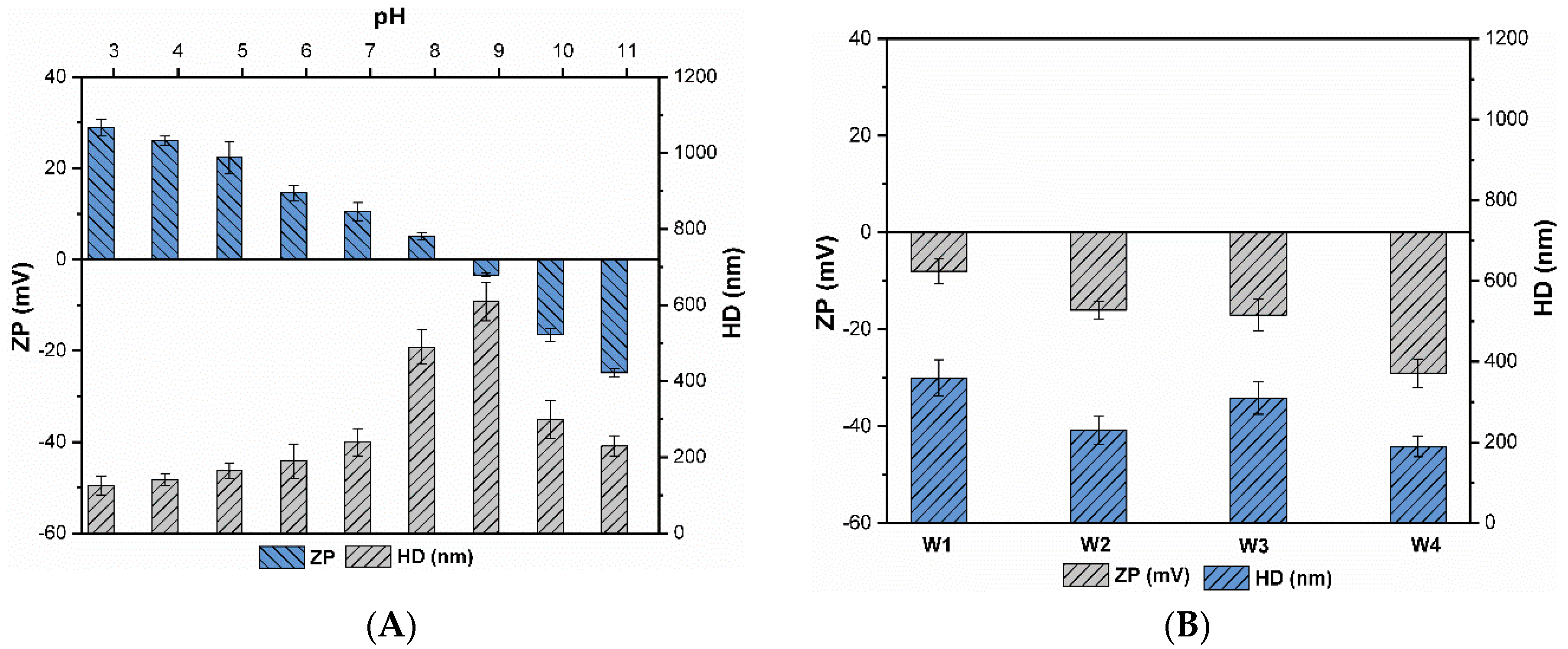
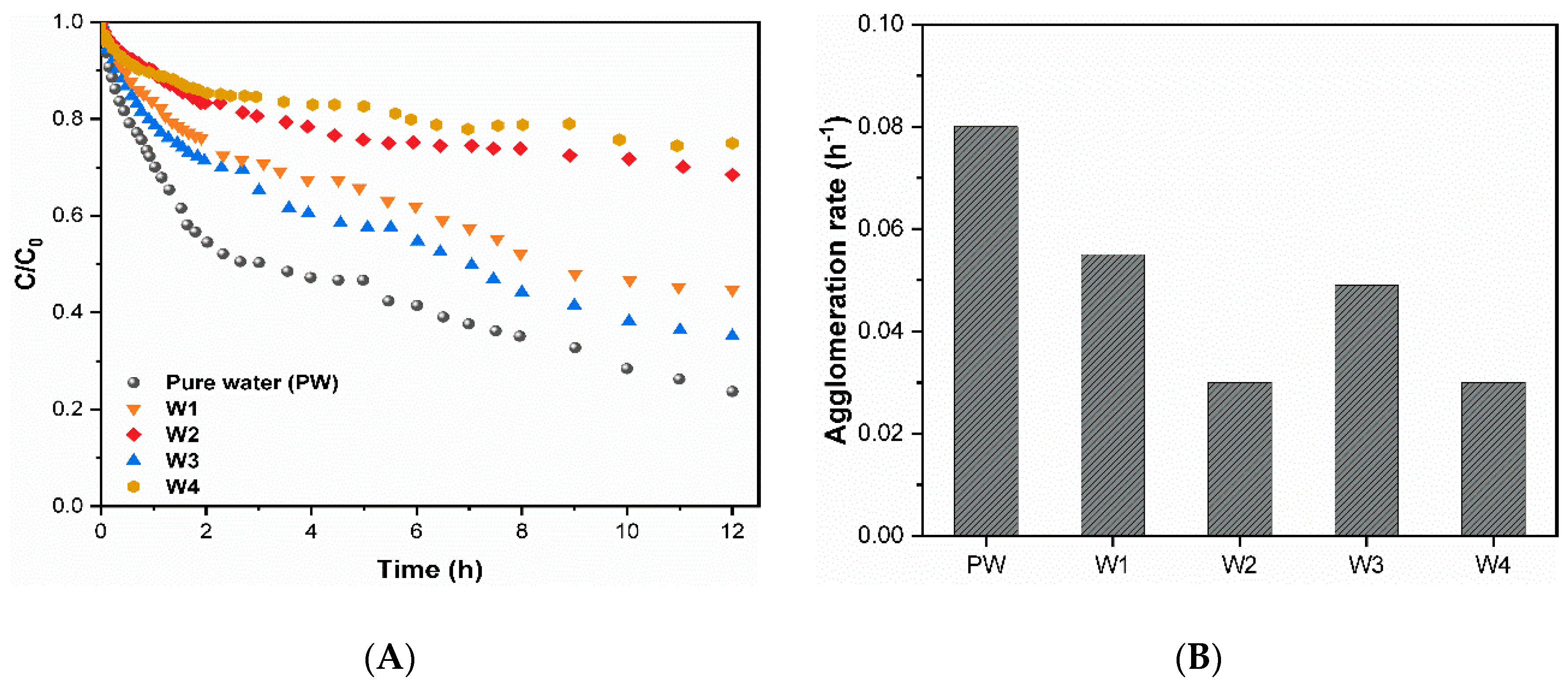
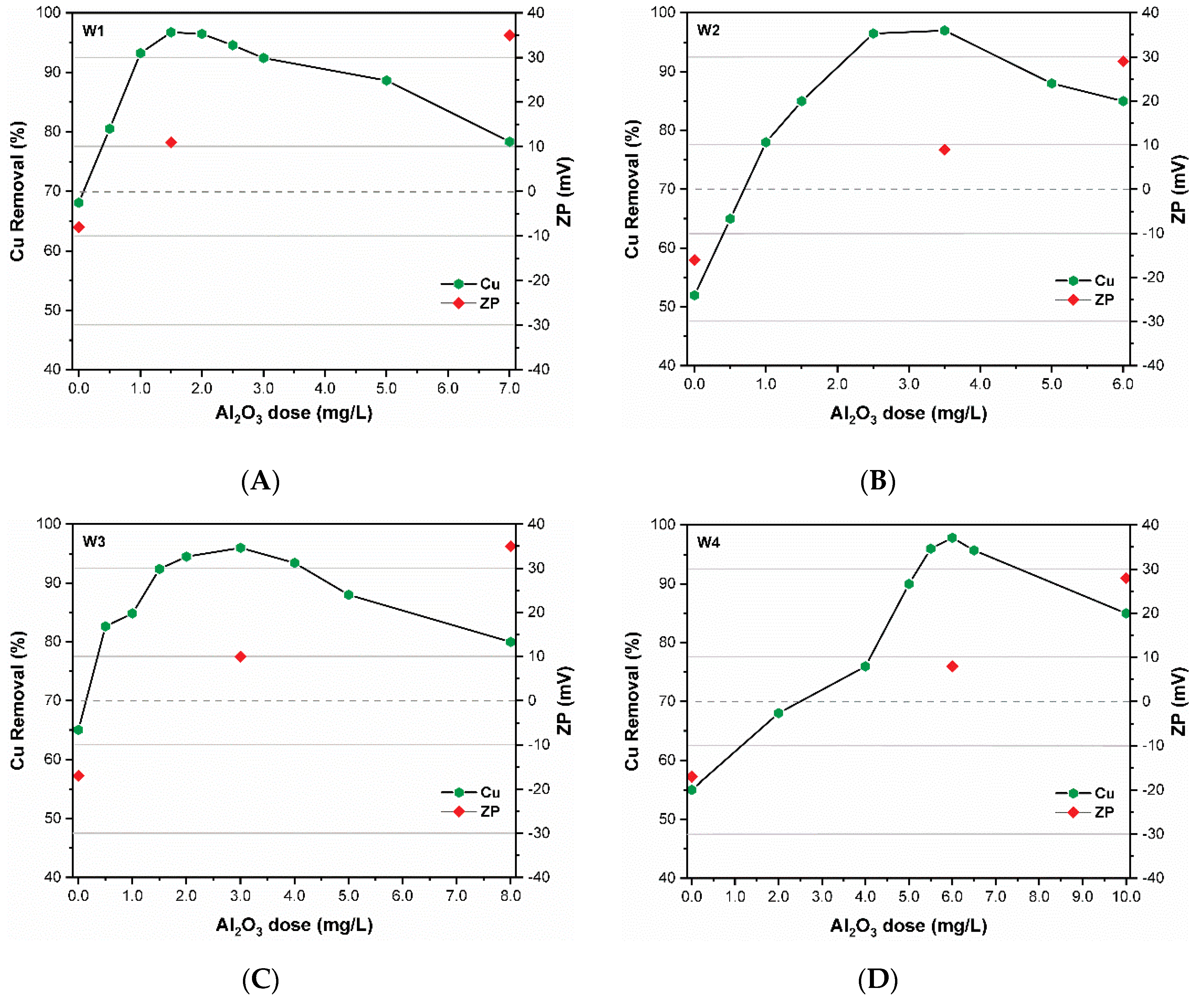
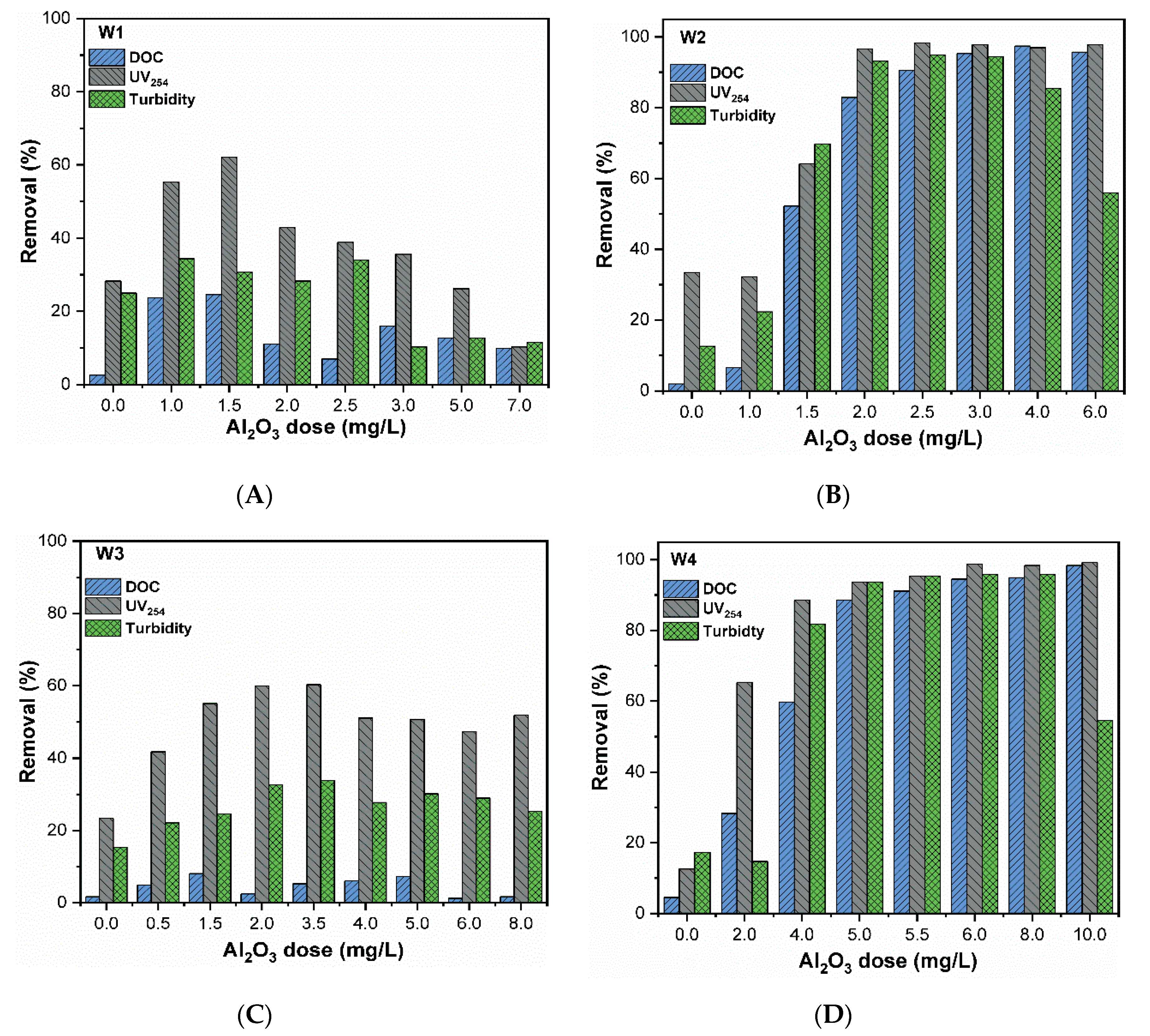
| DOC Concentration a | DOC Type b | Water Code | DOC (mgCL−1) | UV254nm (cm−1) | SUVA (L(m.mg)−1) | Turbidity (NTU) | EC (µScm−1) | pH |
|---|---|---|---|---|---|---|---|---|
| Moderate (ca. 2–3 mg CL−1) | Hydrophilic SUVA < 3 | W1 | 2.69 ± 0.27 | 0.031 ± 0.002 | 1.34 ± 0.07 | 1.17 ± 0.01 | 375 ± 1.7 | 4.60 |
| Hydrophobic SUVA > 4 | W2 | 2.54 ± 0.12 | 0.192 ± 0.039 | 7.93 ± 1.31 | 4.96 ± 0.21 | 378 ± 1.0 | 6.39 | |
| Moderate to high (ca. 6 mg CL−1) | Hydrophilic SUVA < 3 | W3 | 6.21 ± 0.25 | 0.041 ± 0.011 | 0.68 ± 0.08 | 1.18 ± 0.25 | 382 ± 1.5 | 4.21 |
| Hydrophobic SUVA > 4 | W4 | 5.80 ± 1.54 | 0.490 ± 0.020 | 8.59 ± 0.68 | 9.45 ± 0.65 | 388 ± 1.0 | 6.83 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, R.; Inam, M.A.; Akram, M.; Uddin, A.; Khan, S.; Yeom, I.T. Effect of Dissolved Organic Matter on Agglomeration and Removal of CuO Nanoparticles by Coagulation. Processes 2019, 7, 455. https://doi.org/10.3390/pr7070455
Khan R, Inam MA, Akram M, Uddin A, Khan S, Yeom IT. Effect of Dissolved Organic Matter on Agglomeration and Removal of CuO Nanoparticles by Coagulation. Processes. 2019; 7(7):455. https://doi.org/10.3390/pr7070455
Chicago/Turabian StyleKhan, Rizwan, Muhammad Ali Inam, Muhammad Akram, Ahmed Uddin, Sarfaraz Khan, and Ick Tae Yeom. 2019. "Effect of Dissolved Organic Matter on Agglomeration and Removal of CuO Nanoparticles by Coagulation" Processes 7, no. 7: 455. https://doi.org/10.3390/pr7070455
APA StyleKhan, R., Inam, M. A., Akram, M., Uddin, A., Khan, S., & Yeom, I. T. (2019). Effect of Dissolved Organic Matter on Agglomeration and Removal of CuO Nanoparticles by Coagulation. Processes, 7(7), 455. https://doi.org/10.3390/pr7070455







