Finite Time Thermodynamic Optimization of an Irreversible Proton Exchange Membrane Fuel Cell for Vehicle Use
Abstract
:1. Introduction
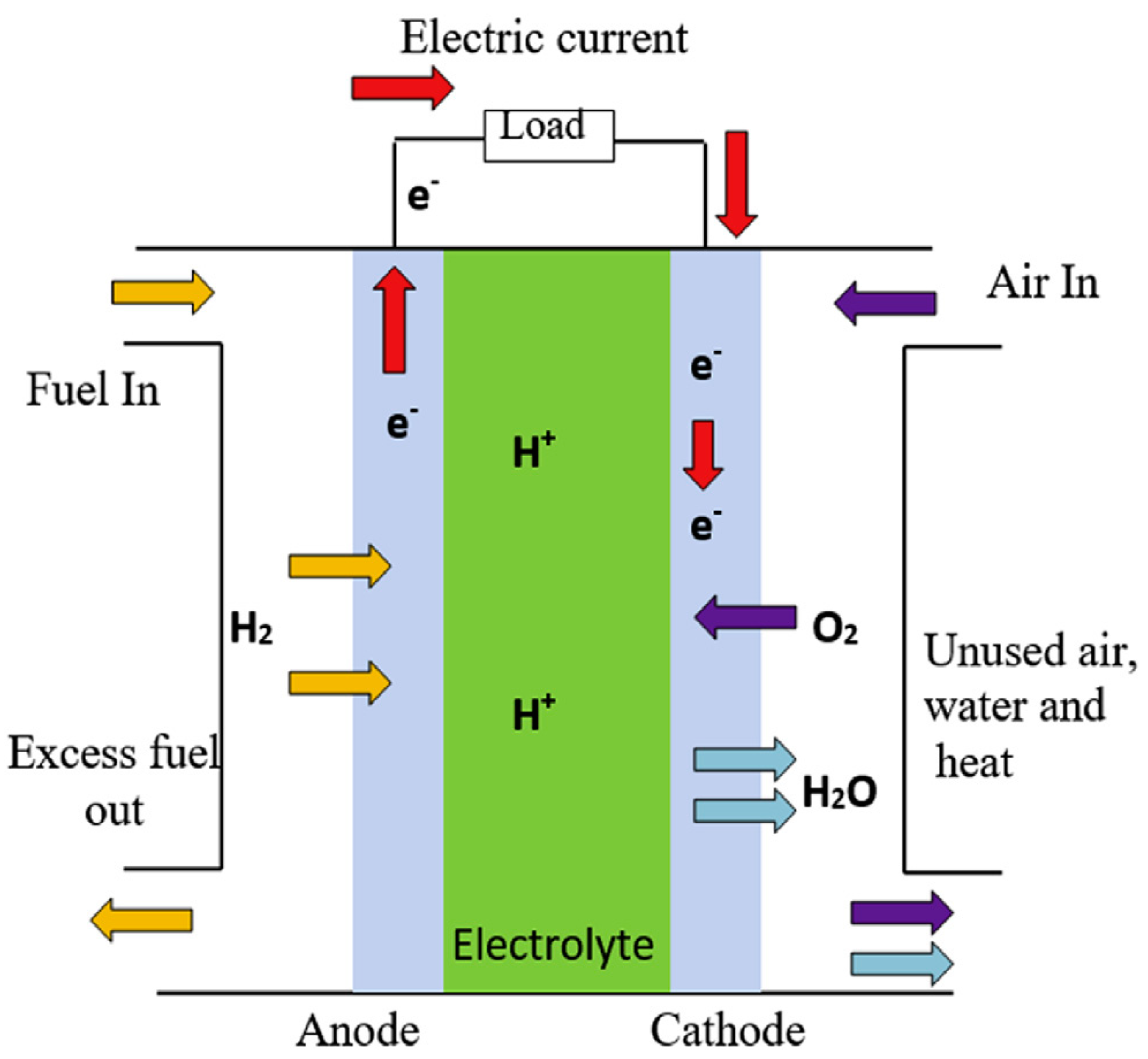
2. Finite Time Thermodynamic Model of Irreversible PEMFC
2.1. Reversible Output Voltage
2.2. The Irreversibility of PEMFC
2.2.1. Activation Overpotential
2.2.2. Ohmic Overpotential
2.2.3. Concentration Overpotential
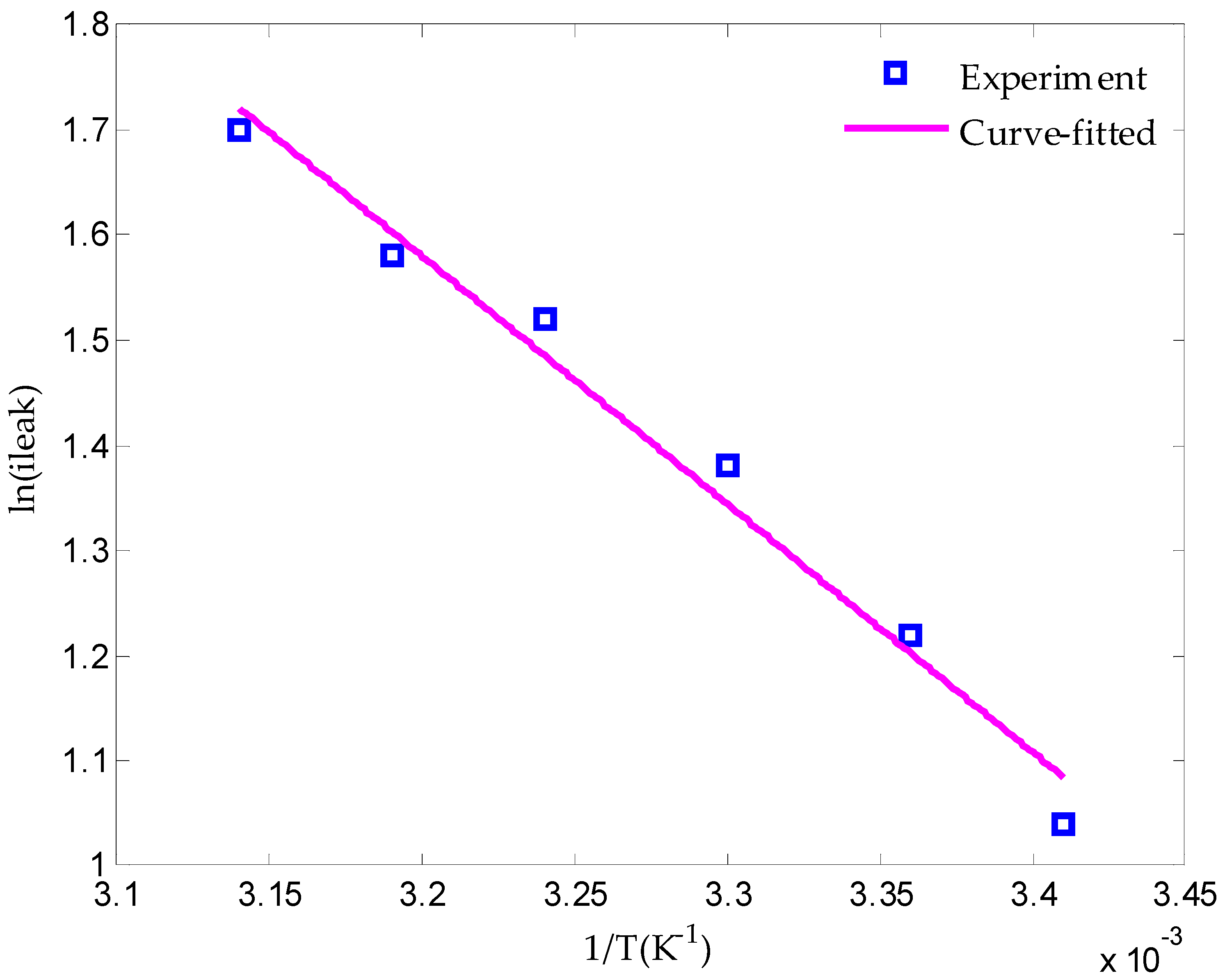
2.2.4. Leakage Current
2.2.5. Output Voltage
2.3. Evaluation of PEMFC Finite Time Thermodynamic Performance Indicators
2.3.1. Output Power Density
2.3.2. Output Efficiency
2.3.3. Entropy Production
2.3.4. Ecological Objective Function
2.3.5. Ecological Coefficient of Performance
3. Results and Discussion
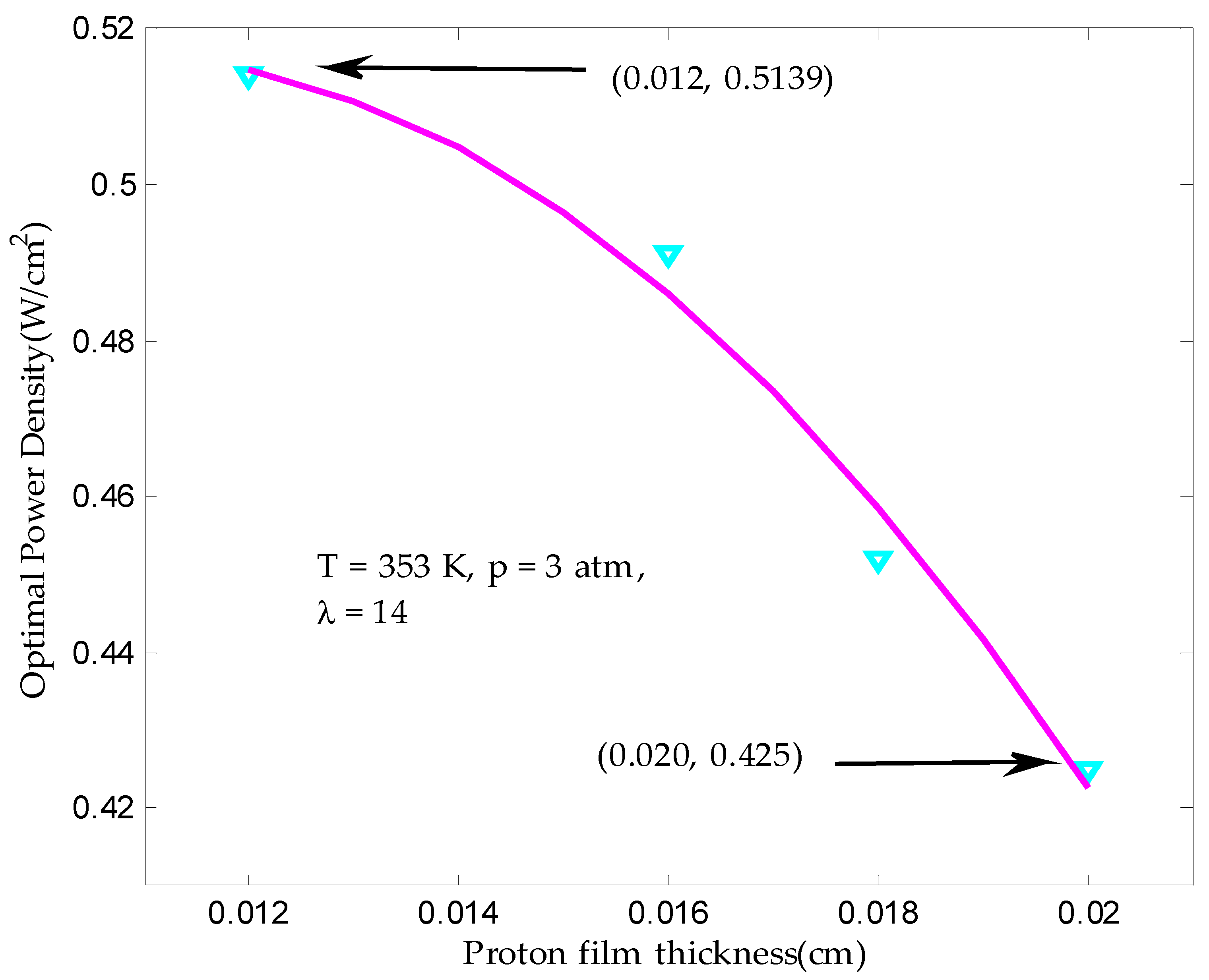
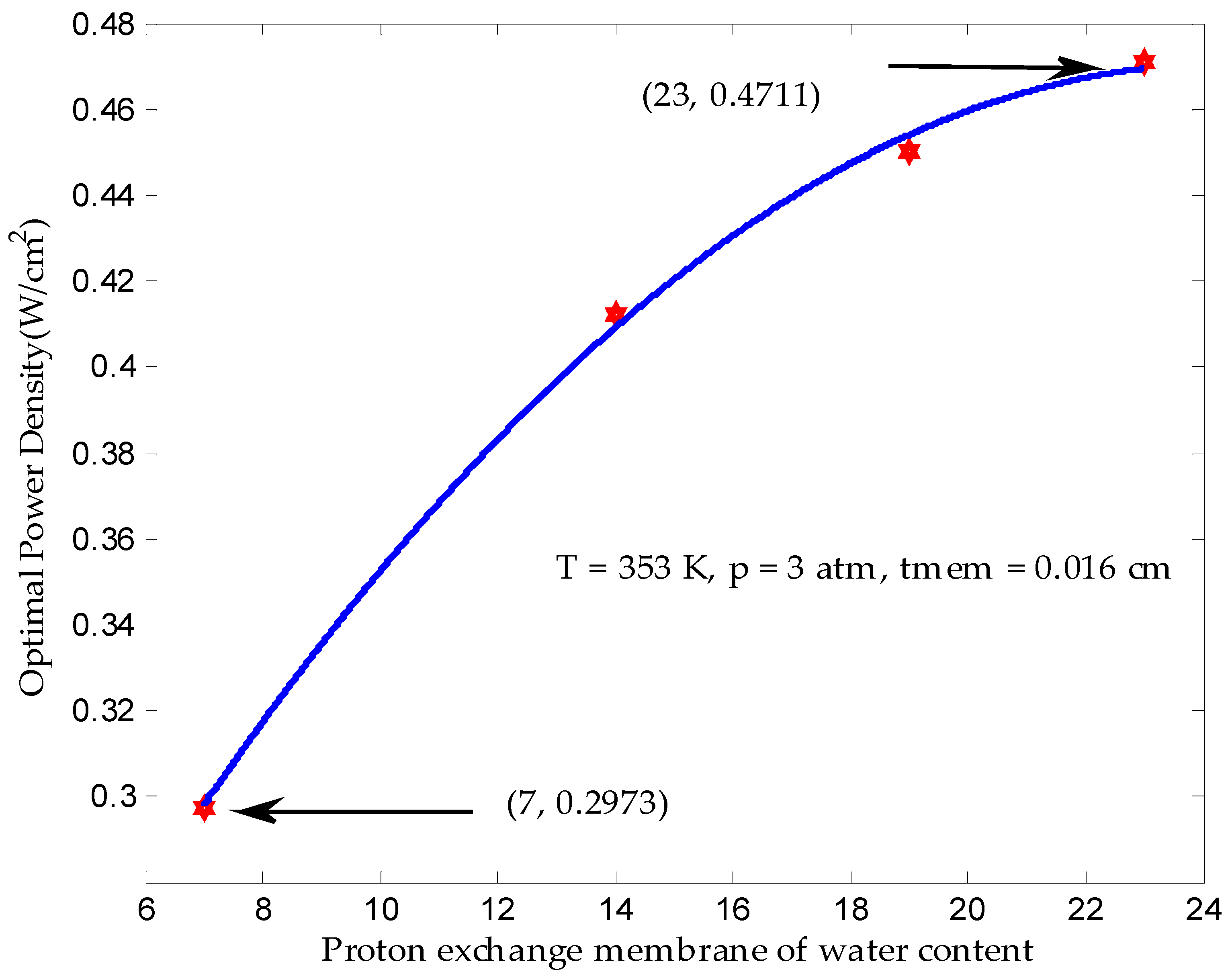
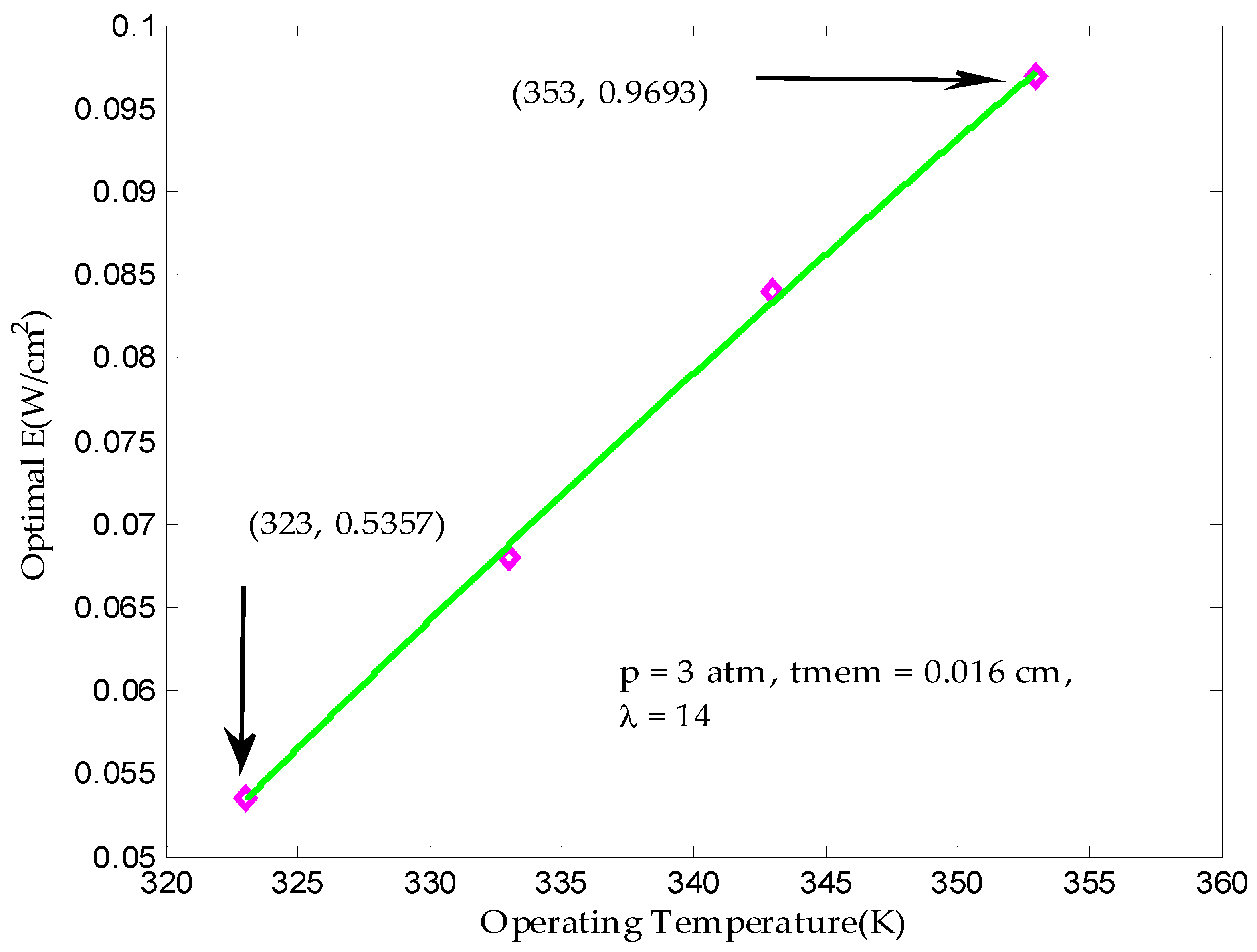
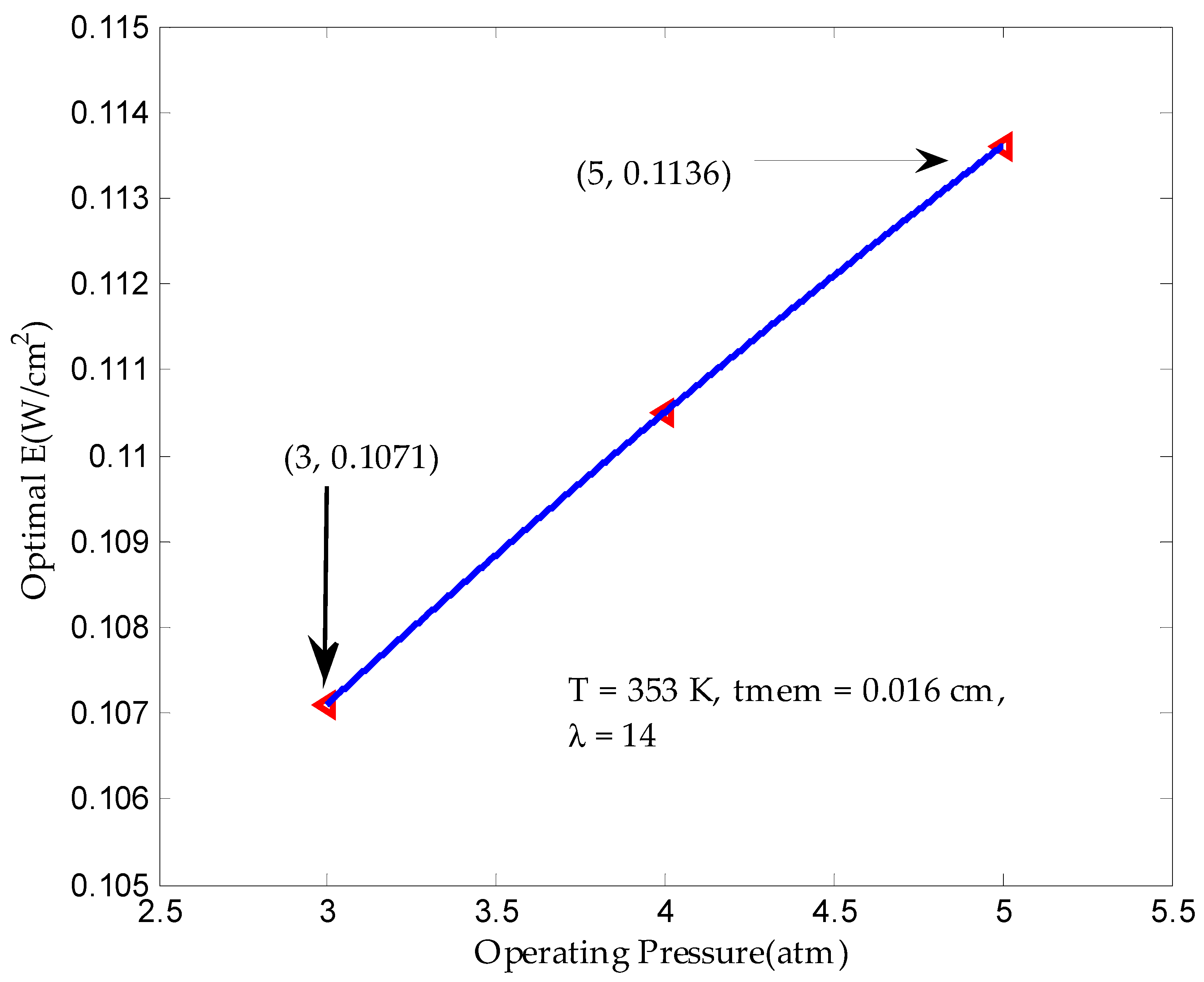
3.1. Maximum Output Power Density in Finite Time
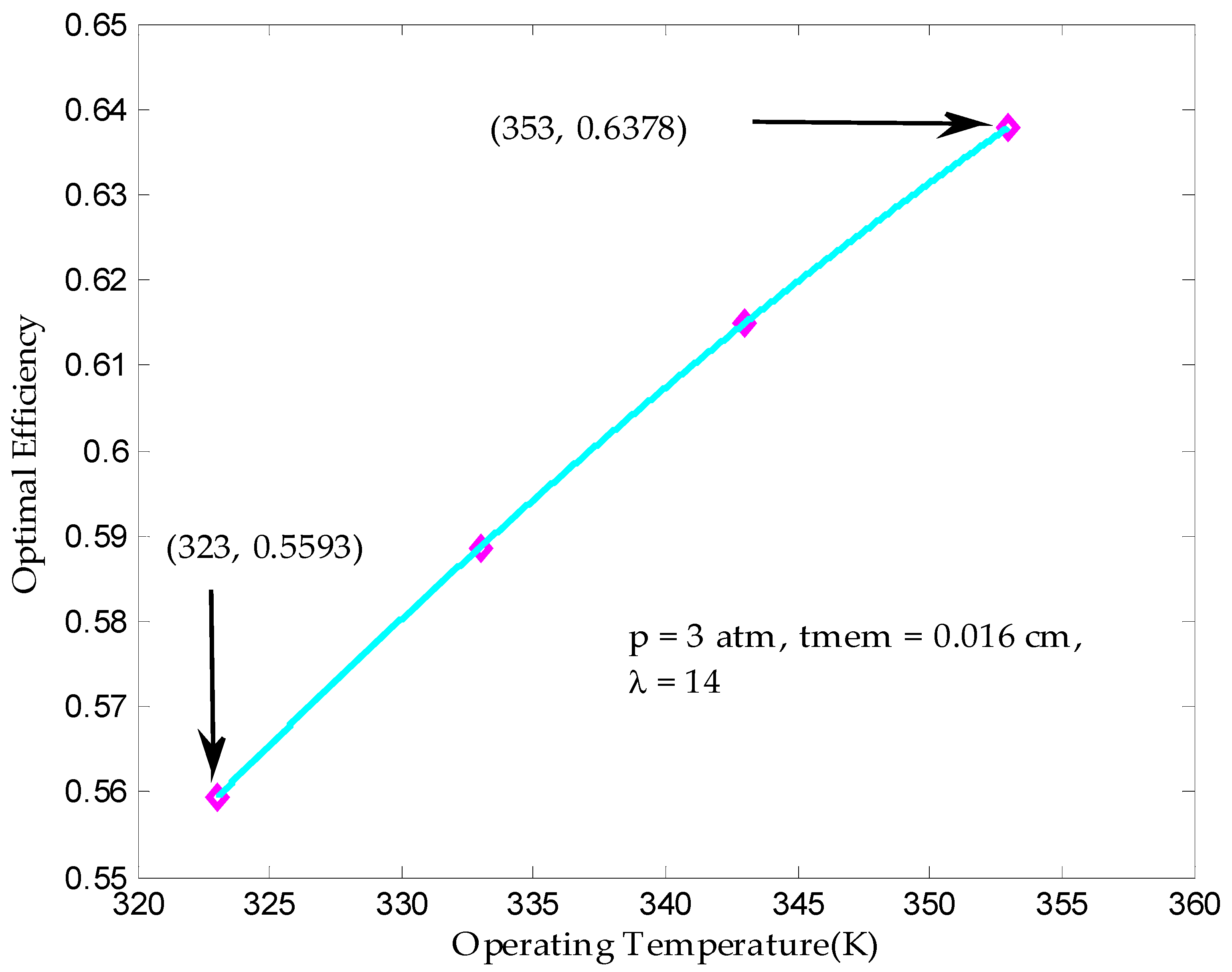
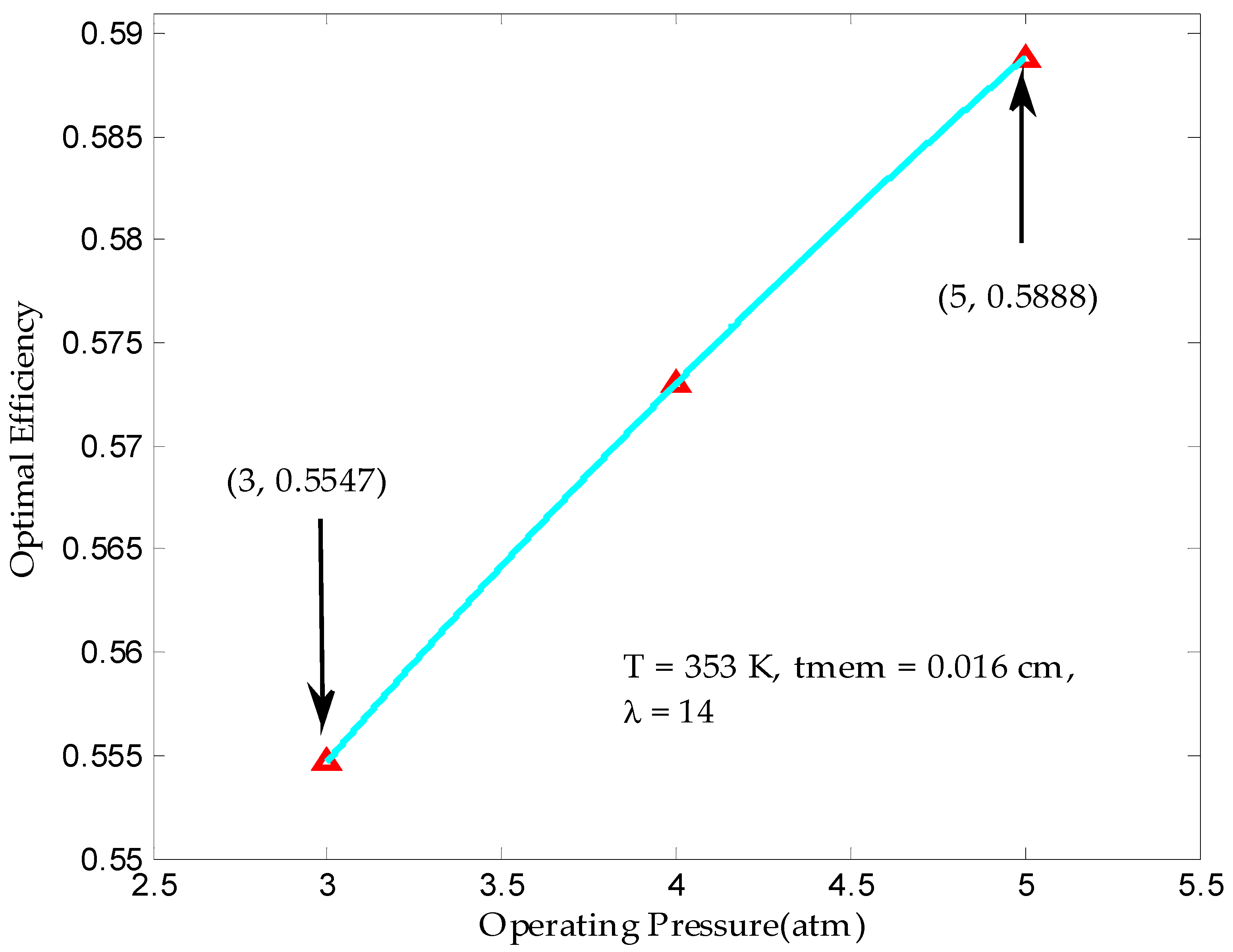
3.2. Maximum Output Efficiency
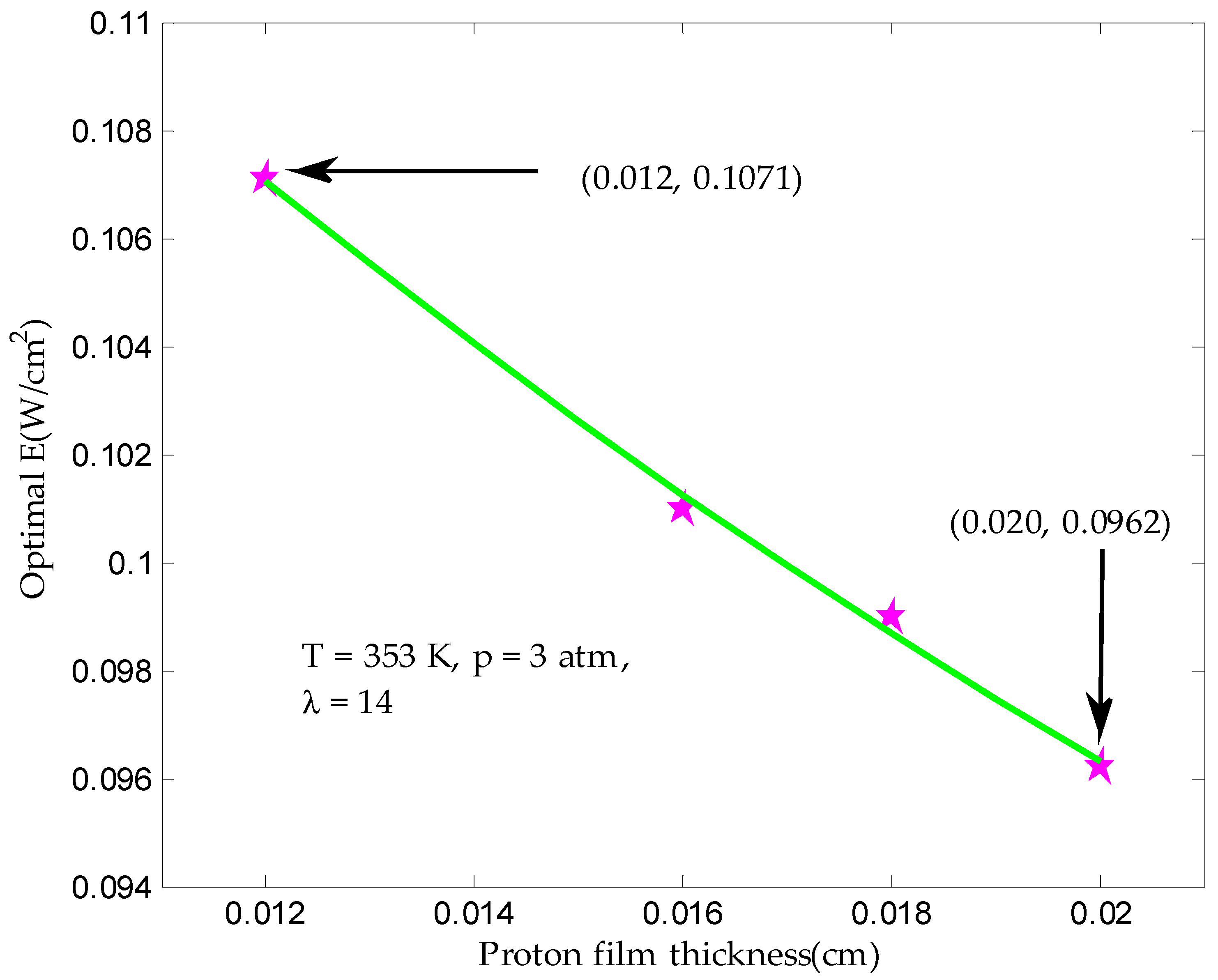
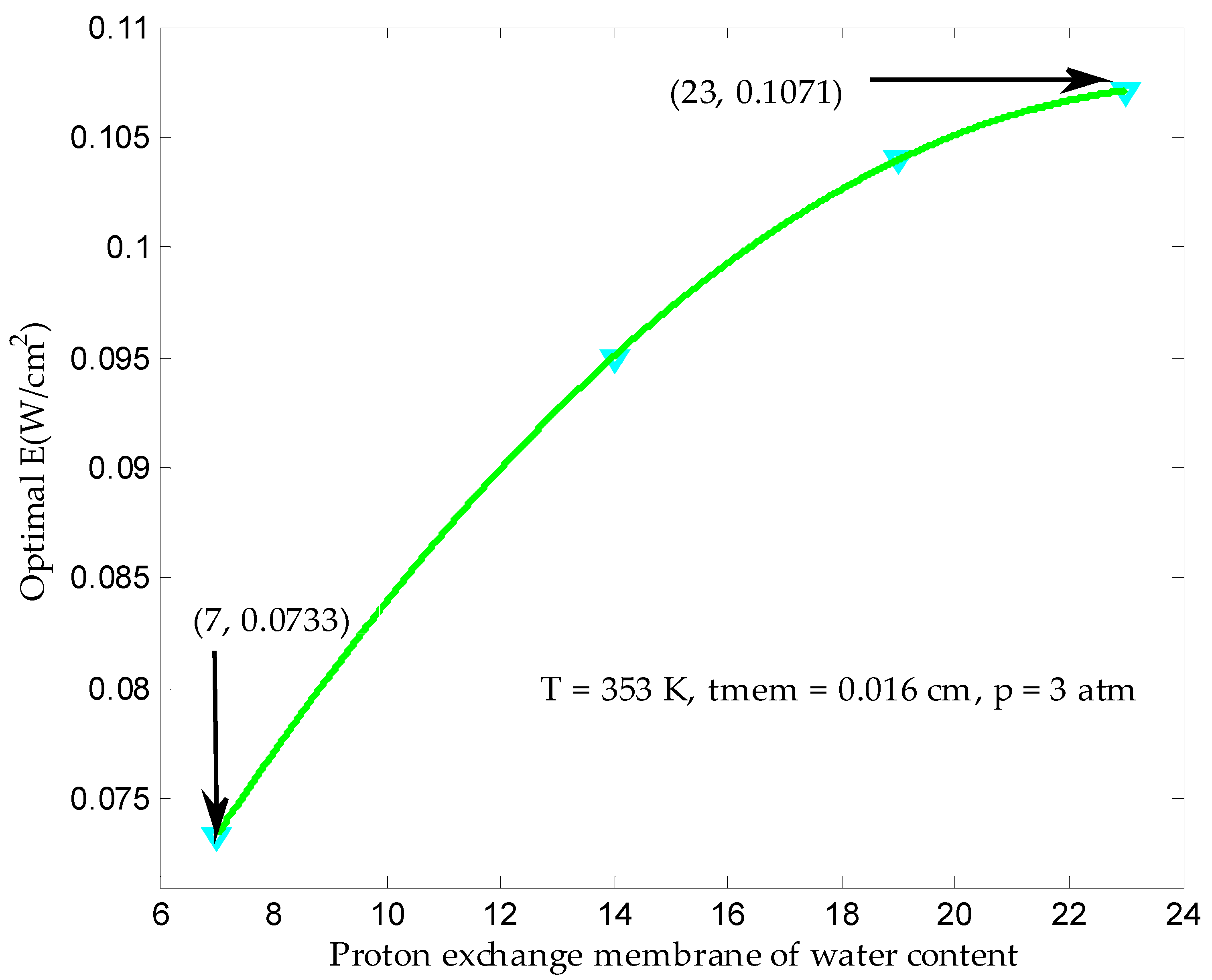
3.3. Maximum Ecological Objective Function in Finite Time
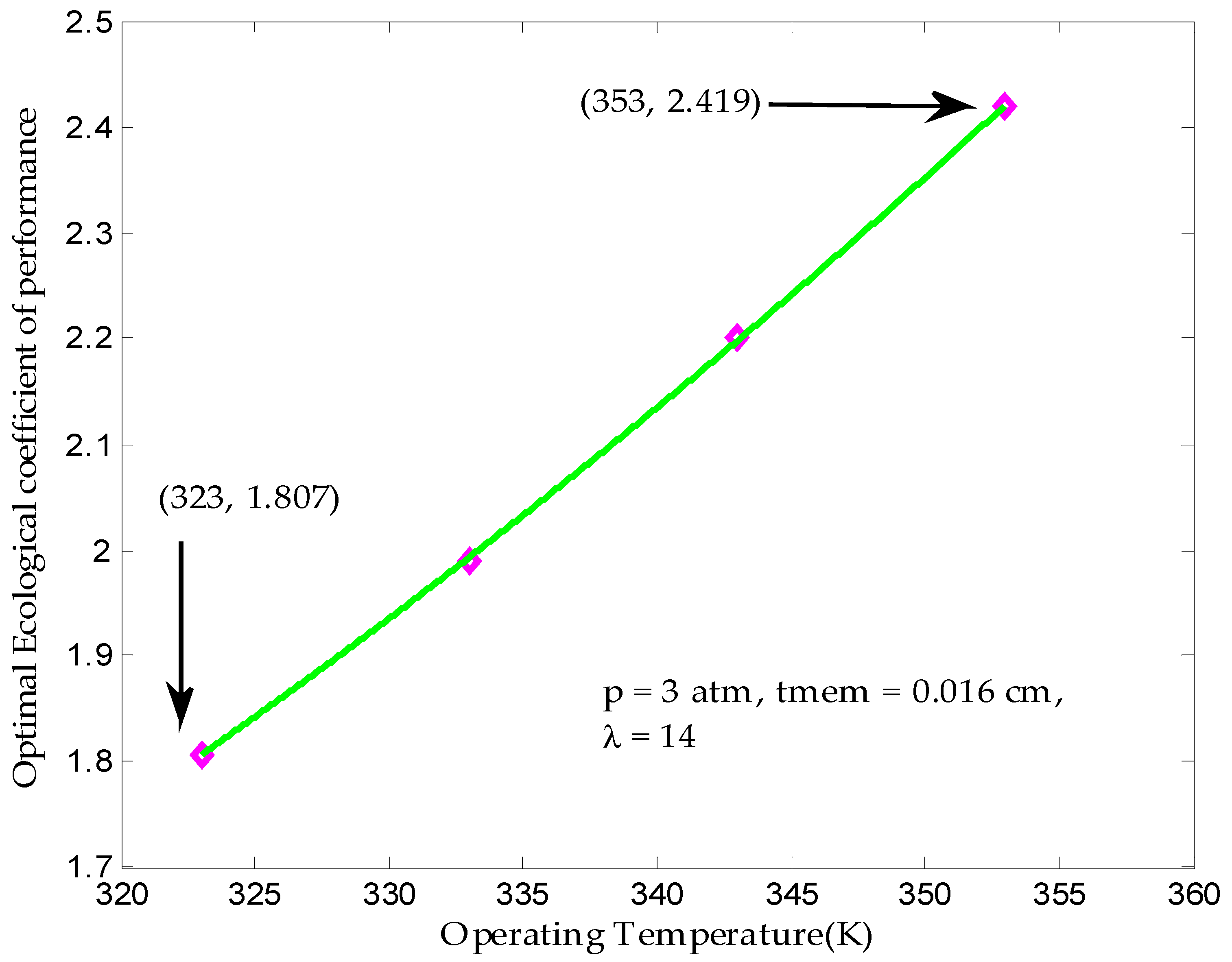
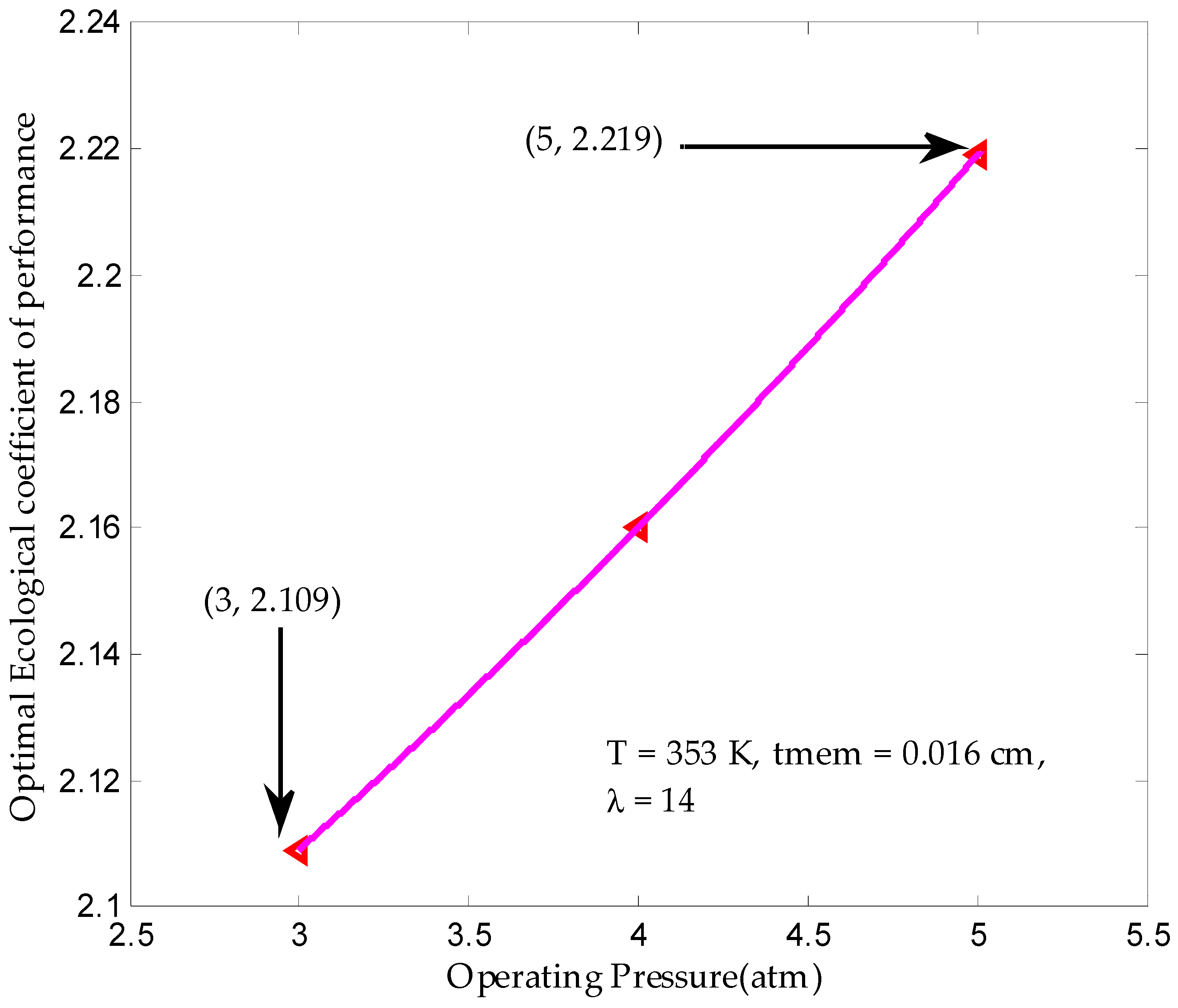
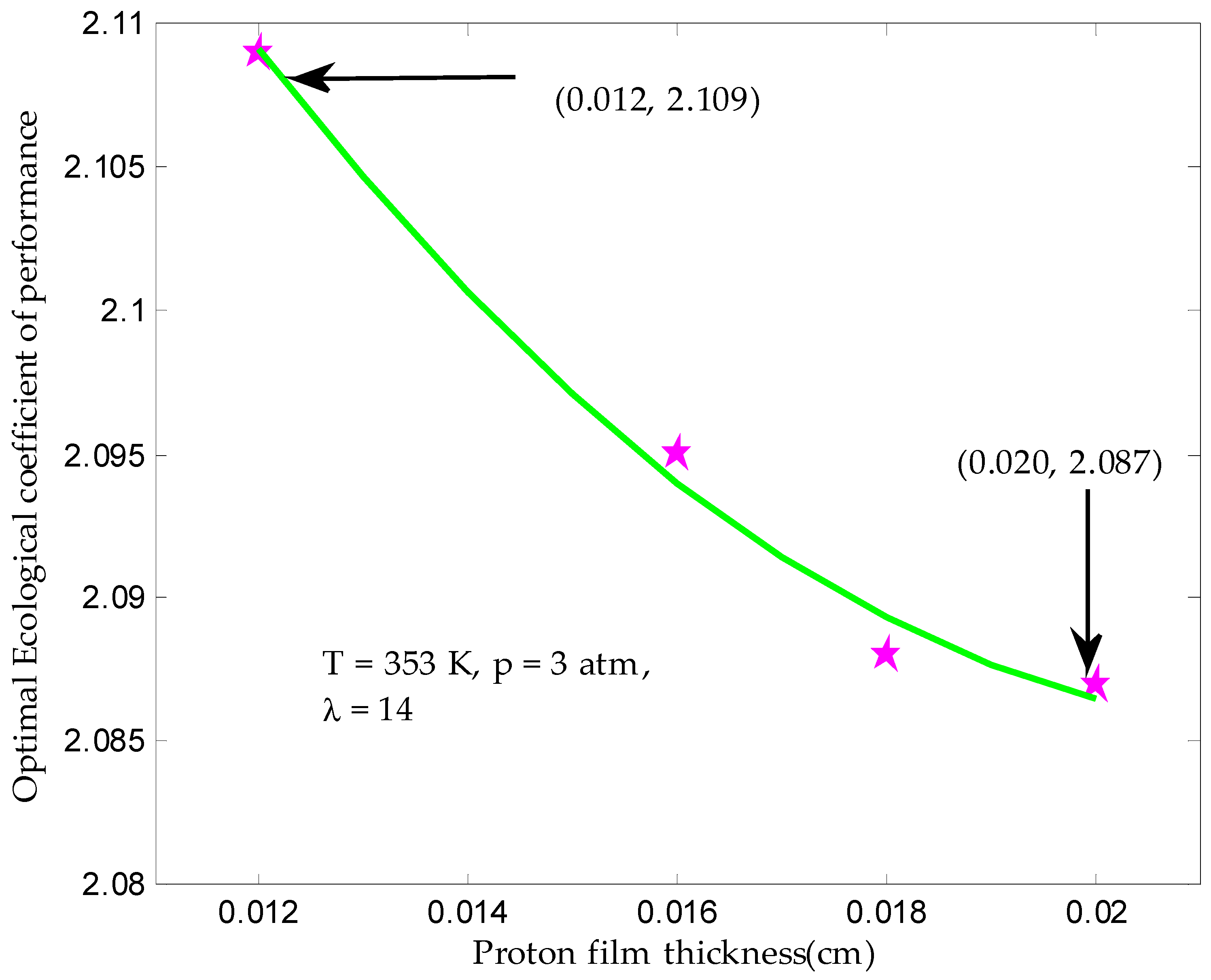
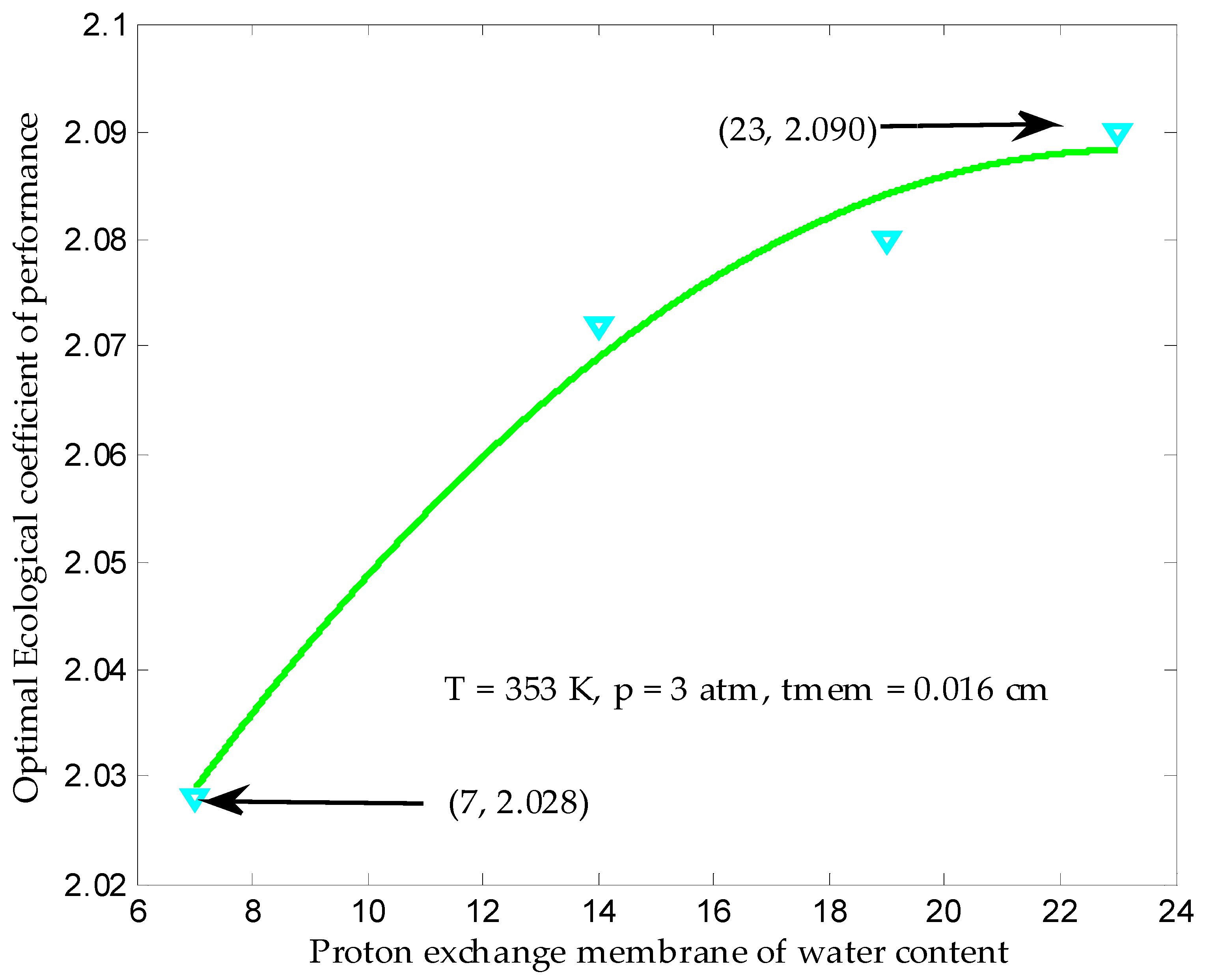
3.4. Maximum Ecological Performance Coefficient
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yuan, D.Q. The analytical method of finite time thermodynamics about the physical and the chemical performances of the fuel cell. Chin. J. Chem. Phys. 1999, 12, 63–66. [Google Scholar]
- Yan, Z.J. Comment on: The analytical method of finite time thermodynamics about the physical and the chemical performances of the fuel cell. Chin. J. Chem. Phys. 2001, 14, 378–380. [Google Scholar]
- Yuan, D.Q. Reply to Comment on: The analytical method of finite time thermodynamics about the physical and the chemical performances of the fuel cell. Chin. J. Chem. Phys. 2001, 14, 381–383. [Google Scholar]
- Yin, S.L. Ecological Optimization for a Hybrid System Integrating a Phosphoric Acid Fuel Cell with An Absorption Refrigerator. Master’s Thesis, Donghua University, Shanghai, China, 2017. [Google Scholar]
- Wei, F.F. The Optimal Thermodynamic Research and Application of Proton Exchange Membrane Fuel Cells. Master’s Thesis, Donghua University, Shanghai, China, 2013. [Google Scholar]
- Zhang, S.; Qian, X.R.; Shi, B. Multi-objective optimization for PEM fuel cell system. Comput. Appl. Chem. 2018, 35, 37–44. [Google Scholar]
- Xue, D.; Dong, Z. Optimal fuel cell system design considering functional performance and production costs. J. Power Sources 1998, 76, 69–80. [Google Scholar] [CrossRef]
- Frangopoulos, C.A.; Nakos, L.G. Development of a model for thermoeconomic design and operation optimization of a PEM fuel cell system. Energy 2006, 31, 1501–1519. [Google Scholar] [CrossRef]
- Me, M.; Sedighi, K.; Amidpour, M.; Alizadeh, E.; Miansari, M. Experimental and thermodynamic approach on proton exchange membrane fuel cell performance. J. Power Sources 2009, 190, 356–361. [Google Scholar]
- Tohidi, M.; Mansouri, S.H.; Amiri, H. Effect of primary parameters on the performance of PEM fuel cell. Int. J. Hydrogen Energy 2010, 17, 9338–9348. [Google Scholar] [CrossRef]
- Wei, F.F.; Huang, Y.W. Performance characteristics of an irreversible Proton Exchange Membrane (PEM) Fuel Cell. J. Donghua Univ. 2012, 29, 393–398. [Google Scholar]
- Springer, T.E.; Zawodzinski, T.A.; Gottesfeld, S. Polymer electrolyte fuel cell model. J. Electrochem. Soc. 1991, 138, 2334–2342. [Google Scholar] [CrossRef]
- Ahmed, F.; Hegazy, R. Multi-verse optimizer for identifying the optimal parameters of PEMFC. Energy 2018, 143, 634–644. [Google Scholar]
- Andreadis, G.M.; Podias, A.K.; Tsiakaras, P.E. The Effect of the Parasitic Current on the Direct Ethanol PEM Fuel Cell Operation. J. Power Sources 2008, 181, 214–227. [Google Scholar] [CrossRef]
- Zhao, Y.R.; Ou, C.J.; Chen, J.C. A new analytical approach to model and evaluate the performance of a class of irreversible fuel cells. Int. J. Hydrogen Energy 2011, 36, 15304–15312. [Google Scholar] [CrossRef]
- Yoon, K.J.; Gopalan, S.; Pal, U.B. Effect of Fuel Composition on Performance of Single-Step Cofired SOFCs. J. Electrochem. Soc. 2007, 154, 1080–1087. [Google Scholar] [CrossRef]
- Hamid, K.E. Temperature Effect on Proton Exchange Membrane Fuel Cell Performance Part I: Modelling and Validation. Energy Procedia 2014, 61, 2613–2616. [Google Scholar] [Green Version]
- Calise, F. Simulation and exergy analysis of a hybrid Solid Oxide Fuel Cell (SOFC)–Gas Turbine System. Energy 2006, 31, 3278–3299. [Google Scholar] [CrossRef]
- Chan, S.H.; Xia, Z.T. Polarization effects in electrolyte/electrode-supported solid oxide fuel cells. J. Appl. Electrochem. 2002, 32, 339–347. [Google Scholar] [CrossRef]
- Calise, F.; Palombo, A. Design and partial load exergy analysis of hybrid SOFC–GT power plant. J. Power Sources 2006, 158, 225–244. [Google Scholar] [CrossRef]
- Chan, S.H.; Khor, K.A.; Xia, Z.T. A complete polarization model of a solid oxide fuel cell and its sensitivity to the change of cell component thickness. J. Power Sources 2001, 93, 130–140. [Google Scholar] [CrossRef]
- Shaker, H. Analytical modeling of PEM fuel cell i-V curve. Renew. Energy 2011, 36, 451–458. [Google Scholar]
- Zawodzinski, T.A.; Smith, V.T.; Springer, T.A. Water uptake by and transport through Nafion 117 membranes. J. Electrochem. Soc. 1992, 140, 1041–1047. [Google Scholar] [CrossRef]
- Liu, H. Modeling and Simulation of Proton Exchange Membrane Fuel Cell. Master’s Thesis, School of North China Electric Power University, Beijing, China, 2012. [Google Scholar]
- Mann, R.F.; Hooper, M.; Jensen, H.M.; Peppley, B.A.; Roberge, P.R. Development and application of a generalized steady-state electrochemical model for a PEM fuel cell. J. Power Sources 2000, 86, 173–180. [Google Scholar] [CrossRef]
- Mo, Z.J. Parameter optimization for a PEMFC model with a hybrid genetic algorithm. Int. J. Energy Res. 2006, 30, 585–897. [Google Scholar] [CrossRef]
- Chen, X.; Li, W.B.; Gong, G.C. Parametric analysis and optimization of PEMFC system for maximum power and efficiency using MOEA/D. Appl. Therm. Eng. 2017, 121, 400–409. [Google Scholar] [CrossRef]
- Li, C.J.; Liu, Y.; Ma, Z.S. Thermodynamic analysis of the performance of an irreversible PEMFC. Defect Diffus. Forum 2018, 388, 350–360. [Google Scholar] [CrossRef]
- Barbir, F.; Gorgun, H.; Wang, X. Relationship between pressure drop and cell resistance as a diagnostic tool for PEM fuel cells. J. Power Sources 2017, 141, 96–101. [Google Scholar] [CrossRef]
- Santarelli, M.G.; Torchio, M.F.; Cochis, P. Parameters estimation of a PEM fuel cell polarization curve and analysis of their behavior with temperature. J. Power Sources 2006, 159, 824–835. [Google Scholar] [CrossRef]
- Angulo-Brown, F. An ecological optimization criterion for finite time heat engines. J. Appl. Phys. 1991, 69, 7465. [Google Scholar] [CrossRef]
- Chen, L.; Sun, F.; Chen, W. On the ecological figures of merits for thermodynamic cycles. J. Eng. Therm. Energy Power 1994, 9, 374–376. [Google Scholar]
- Yasin, U.; Bahri, S.; Ali, K. Performance analysis and optimization of an irreversible dual-cycle based on an ecological coefficient of performance criterion. Appl. Energy 2005, 82, 23–29. [Google Scholar]
- Khalil, B.; Ali, C. Analysis of proton exchange membrane fuel cells voltage drops for different operating parameters. Int. J. Hydrogen Energy 2018, 43, 3512–3519. [Google Scholar]
- Berning, T.; Djilali, N. Three-dimensional computational analysis of transport phenomena in a PEM fuel cell—A parametric study. J. Power Sources 2003, 124, 440–452. [Google Scholar] [CrossRef]
- Ay, M.; Midilli, A.; Dincer, I. Thermodynamic modeling of a proton exchange membrane fuel cell. Int. J. Energy 2006, 3, 16–44. [Google Scholar]
- Rowe, A.; Li, X.G. Mathematical modeling of proton exchange membrane fuel cells. J. Power Sources 2001, 102, 82–96. [Google Scholar] [CrossRef] [Green Version]




| Parameters | Values |
|---|---|
| Operating temperature, T (K) | 323–353 |
| Operating pressure, a (atm) | 3–5 |
| Faraday constant, F (C/mol) | 96485 |
| Gas constant, R (J/mol.K) | 8.314 |
| Current density, I (A/cm2) | 0–1.4 |
| Proton film thickness, tmem (cm) | 0.012–0.020 |
| Limit current density, iL (A/cm2) | 1.4 |
| Standard mole, ΔS (J/mol) | −163.4 |
| Standard Gibbs free energy, Δg (KJ/mol) | −273.3 |
| Ambient temperature, T0 (K) | 298.15 |
| Transfer coefficient, α | 0.5 |
| Electronic number, n | 2 |
| Resistance generated by electron flow, Rele (Ω) | 0.005 |
| Proton membrane water content, λ | 7, 14, 19, 23 |
| Oxygen to nitrogen molar ratio | 0.21/0.79 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Liu, Y.; Xu, B.; Ma, Z. Finite Time Thermodynamic Optimization of an Irreversible Proton Exchange Membrane Fuel Cell for Vehicle Use. Processes 2019, 7, 419. https://doi.org/10.3390/pr7070419
Li C, Liu Y, Xu B, Ma Z. Finite Time Thermodynamic Optimization of an Irreversible Proton Exchange Membrane Fuel Cell for Vehicle Use. Processes. 2019; 7(7):419. https://doi.org/10.3390/pr7070419
Chicago/Turabian StyleLi, Changjie, Ye Liu, Bing Xu, and Zheshu Ma. 2019. "Finite Time Thermodynamic Optimization of an Irreversible Proton Exchange Membrane Fuel Cell for Vehicle Use" Processes 7, no. 7: 419. https://doi.org/10.3390/pr7070419





