1. Introduction
The tire is a kind of product which requires high safety performance. In order to ensure personal safety, tires must possess high strength, wear resistance, stability, and aging resistance. As a result, tires cannot degrade naturally for a long time (hundreds or even thousands of years) after abandonment. It is estimated that the number of used tires has exceeded 13 million tons in China and will continue to rise along with the rapid growth of the automobile industry [
1,
2]. Traditional ways of solid waste disposal such as landfills are not suited to used tires because they occupy a significant amount of land [
3,
4,
5]. Thus, removing this “black pollution” has become a worldwide problem. Due to environmental pressure, some countries in Europe have banned waste tire burning, landfilling, and piling since January 2014. Pyrolysis is one of the ways to process used tires and is considered the most effective and thorough treatment [
6,
7,
8,
9].
In the process of pyrolysis, adding a catalyst can reduce the activation energy and make the pyrolysis reaction more rapid and thorough [
10,
11,
12]. It can also improve the quality of pyrolysis oil and help enterprises specializing in cracking waste tires increase their economic benefits. However, the catalyst is disposable, as it cannot be separated from black carbon, making it extremely expensive to use catalysts. Therefore, the cost of catalysts has always been a bottleneck for the further development of the industry, and it also has become a particularly attractive field of research for scholars at home and abroad.
Fluid catalytic cracking (FCC) is the core process in petroleum processing [
13,
14,
15,
16]. It can effectively crack heavy oil and residue into small molecules and high-value products. In order to ensure a fast and efficient reaction in FCC, some catalysts must be replaced over time. These replaced catalysts are called spent FCC catalysts. A large quantity of waste catalysts (spent FCC catalysts) is produced every year. If there is no treatment of the spent catalyst for a long time, it will not only occupy valuable land resources but also cause serious pollution. It also poses a huge threat to human health [
17].
The disposal of waste catalysts has also attracted the attention of the Chinese government. In the 2016 edition of the National Hazardous Waste List, waste catalysts produced in the process of petroleum catalytic cracking were included, which greatly increased the cost of the disposal of waste catalysts. Spent FCC catalysts are still capable of high catalytic activity, although they cannot meet the requirements of petroleum catalytic cracking. New attempts have emerged at exploring the application of spent catalysts in waste tire pyrolysis. Using spent FCC catalysts for waste tire pyrolysis will improve the pyrolysis speed of waste tires and improve the quality of pyrolysis products. Research on spent FCC catalysts used for waste tire catalytic pyrolysis has not been published in the domestic and foreign literature.
In this study, relevant experimental research on the industrial application of spent FCC catalysts for the catalytic cracking of waste tires was carried out to solve the serious environmental problems caused by spent FCC catalysts and waste tires. By comparing the experimental results between waste tire pyrolysis and spent FCC catalysts for catalytic cracking, we have examined the feasibility of applying spent FCC catalysts in the cracking process of waste tires to realize the low-cost operation of waste tire catalytic cracking. At the same time, the treatment problem of spent FCC catalysts was solved.
2. Materials and Methods
2.1. Main Materials
A used KUMHO tire (215/65R16 98H) was the main material of our research. Spent FCC catalysts were provided by the Sinopec Jinan branch (Jinan, China) and its microactivity was 63.5% (following the Chinese Test Standard NB/SH/T 0952-2017). The other relevant characterization data of the spent FCC catalysts are shown in
Table 1 and
Table 2.
2.2. Experimental Device and Methods
2.2.1. Experimental Device
A pyrolysis furnace of DN1000 (
Figure 1) was made in our laboratory. It consisted of (a) a heating burner, (b) a gear drive, (c) water seal protection, (d) condensable gas combustion, (d) exhaust gas treatment, and so on. The cracking equipment could achieve a two-stage separation of the pyrolysis oil grade according to the oil condensation temperature. In the A tank, the cracking oil had a lower condensation temperature, which was defined as the light oil, and the cracking oil at the B outlet had a higher condensation temperature, which was defined as the heavy oil.
2.2.2. Analytical Instrument
The element analysis of the pyrolysis carbon black was tested by a VARIO MACRO element analyzer and the high calorific value of pyrolysis carbon black was tested by a C5000 calorimeter. The element of sulfur in pyrolysis oil was tested by an X-ray fluorescence sulfur meter. The ultimate analysis of the pyrolysis gas was characterized by gas chromatography–mass spectrometry (GC–MS).
2.3. Experimental Method and Design
2.3.1. Experimental Method
By comparing the experimental results between the pyrolysis of waste tires and the spent FCC catalysts for catalytic cracking, the feasibility of applying spent FCC catalysts in the cracking process of waste tires could be verified. Considering that the residual activity of spent FCC catalysts has only about half of that of new catalysts, the ratio of spent FCC catalysts to the waste tire was set as 3:100 in the catalytic cracking process. In the subsequent experiment, the pyrolysis of waste tires was defined as I, and the spent FCC catalyst for the catalytic cracking of waste tires was defined as II.
2.3.2. Experimental Process
(1) The well-cleaned waste tire was put into the pyrolysis furnace. (2) spent FCC catalysts were added into the pyrolysis furnace by the given ratio. (3) The pyrolysis furnace speed was set to 2 rpm. (4) At the beginning of the experiment, the emptying valve of the furnace was opened and run at the heating rate of 60 K/h to 120 °C. The aim was mainly to exhaust the air in the cracking furnace. (5) Then, the emptying valve was closed and the furnace was heated to 400 °C at 120 K/h and maintained at 400 °C until the end of the experiment.
3. Results and Discussion
3.1. Experimental Phenomena
The pyrolysis of waste tires is a complex physicochemical reaction process involving many interlacing reactions, including bond breaking, molecular rearrangement, hydrogen transfer, and various condensation reactions. The reaction mechanism is complicated [
18,
19,
20]. There are both endothermic and exothermic reactions in the pyrolysis process, and these reactions exhibit different reaction phenomena.
(1) In Experiment I, the pyrolysis gas was ignited at 170 °C and the flame lasted for 10 min. At 250 °C, the pressure in the pyrolysis furnace started to increase significantly, and the pyrolysis gas was ignited again. The pyrolysis gas continued to burn until the end of the experiment.
(2) In Experiment II, the pyrolysis gas was ignited when the temperature reached 140 °C, but it could not burn continuously. At 250 °C, the pressure in the pyrolysis furnace began to rise and the pyrolysis gas could be burned continuously. At 360 °C, the pressure in the pyrolysis furnace rose sharply (the rising rate was faster than that in Experiment I). With the pressure increasing, the pyrolysis gas was burned continuously, and the total combustion time was obviously shortened.
Experimental phenomena were different between Experiments I and II. From the aspect of reaction degree, Experiment II intensively reacted vigorously in the temperature range of 350–400 °C, during which the furnace pressure rose rapidly. From the aspect of reaction temperature, Experiment II was intense and concentrated at about 380 °C, while the temperature of Experiment I was between 250–400 °C, which was a large span. From the aspect of reaction time, the continuous combustion time of pyrolysis gas in Experiment II was obviously shorter than that of Experiment I.
3.2. Proximate Analysis of Material Balance
The essence of the waste rubber pyrolysis process is that organic macromolecular chains are broken into small molecules by heating [
7]. The main products include pyrolysis oil, pyrolysis gas, and pyrolysis carbon black. The yield of pyrolysis oil and pyrolysis carbon black has become one of the main indexes by which to evaluate the performance of the pyrolysis process and the equipment, as they are easy to transport and have high economic value.
Table 3 gives the detailed experimental data of the material balance of the two experiments.
As can be seen in
Table 3, the yield of pyrolysis oil in Experiment II decreased slightly compared with Experiment I, the yield of pyrolysis gas increased slightly, and the yields of carbon black and steel wire changed slightly. A tire is composed of multiple parts, such as the tread, sidewall, and belt. The quality of each component cannot be guaranteed to be exactly the same during the preparation process. At the same time, used tires have different degrees of wear. These reasons caused the yield changes of carbon black and steel. The main reasons for the different yields of pyrolysis oil and pyrolysis gas were that the addition of spent FCC catalysts made the waste tire pyrolysis process more intense, the breakage of macromolecular chains more thorough, and the production of small molecular gases increase. Thus, the pyrolysis gas increased and the yield of pyrolysis oil slightly decreased. The proportion of light oil in Experiment II is 11.25% higher than that of Experiment I. The spent FCC catalysts had a certain amount of influence on improving the quality grade of the pyrolysis oil.
3.3. Property Analysis of Pyrolysis Oil
Pyrolysis oil is a complex multicomponent mixture and has a wide distillation range [
21,
22,
23]. Its chemical composition is complicated. The physical and chemical properties, distillation characteristics, and oil appearance charts of the cracking oil were taken as the main indicators for evaluating the oil in the experiments. The relevant data are shown in
Table 4.
From the experimental data, it can be seen that the kinematic viscosity of oil in Experiment II decreased and the density increased. This was mainly because the spent FCC catalysts could reduce the activation energy of cracking, and at the same cracking temperature, the longer molecular chains could be broken. Thus, the hydrocarbon chain in the heavy oil tank was much longer and the oil density increased. In addition, under the action of the catalysts, polymer chain breakage was more severe, which reduced the proportion of cyclization compounds and aromatic hydrocarbons in oil and gas and increased the proportion of the chain structure, which led to a decrease of oil kinematic viscosity.
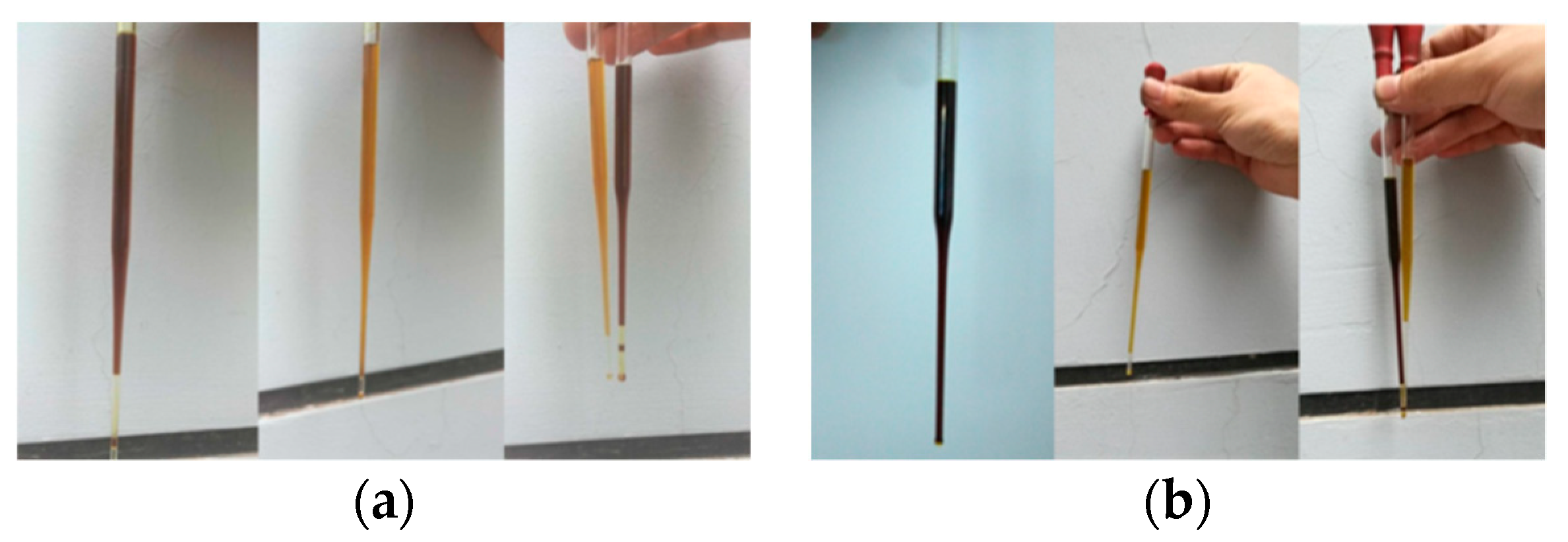
As can be seen from the distillation data of pyrolysis oil in Experiment II, for heavy oil B, the temperatures at the initial distillation point and most recovery volumes were lower than that of Experiment I, which indicates that under the catalysis of spent FCC catalysts, the fracture of the molecular chain is more violent, and the molecular chain structure of oil in the same distillation range is simpler. It can also be clearly seen from the comparison chart of oil appearance in
Figure 2, with spent FCC catalysts, the heavy oil pyrolysis oil was much darker than that of ordinary pyrolysis oil, which was in contrast to the light oil. It also shows that there were more large molecular chains in heavy oil B, and the cracking was more thorough under the same conditions in the Experiment II. From another point of view, it also shows that spent FCC catalysts can feasibly be used for the catalytic cracking of waste tires.
3.4. Data Analysis of Pyrolysis Carbon Black
In the pyrolysis process of waste tires, due to the continuous high temperature and complex pyrolysis environment, the pyrolysis carbon black has poor activity and more impurities. It needs further processing to reapply it as filler in tire manufacturing. Thus, reusing pyrolysis carbon black to manufacture tires is highly dependent on technology. Presently, further research work is still needed [
24,
25]. The metal elements, such as nickel and vanadium, contained in spent FCC catalysts can be conducive to combustion, and they also can improve the combustion performance of coal water slurry. The pyrolysis carbon black combustion properties are listed in
Table 5, which provides the basic preliminary research for the preparation of coal water slurry by mixing pyrolysis carbon black.
From the experimental data in
Table 5, it can be seen that the contents of carbon and ash dry base in Experiment II were slightly higher than those in Experiment I, while the hydrogen content, sulfur content, and high calorific value were lower. This was mainly due to the catalysis of the spent FCC catalysts, which made the cracking process of the waste rubber molecular chain more intense. The degree of the dehydrogenation reaction was intensified, and the cracking reaction was relatively sufficient. Thus, the combustion value was reduced and the ash content was increased.
3.5. Detection Data of Pyrolysis Gas
The composition of pyrolysis gas is complex. In the industrial pyrolysis process of waste tires, pyrolysis gas is usually burned directly [
26], which provides the heat required for the pyrolysis reaction. Therefore, it is critical to determine if there are sulfur, nitrogen, and other elements which can cause serious air pollution in pyrolysis gas.
In the experiment, GC–MS was applied to analyze the combustible gas collected by air bags. The instrument used was an Agilent 7890-5975c GC–MS, and the chromatographic column was a DB-5 (30 m × 0.25 mm × 0.25 um). The gas phase conditions were as follows: inlet temperature was 250 °C; split ratio was 5:1; starting temperature of heating program was 40 °C; and heating was to 250 °C at 8 °C/min. The mass spectrometry conditions were as follows: full scanning mode; scanning range was 30–400; ion source temperature was 230 °C; and quadrupole rod temperature was 150 °C. The relevant experimental data are shown in
Table 6 and
Table 7.
The abovementioned GC–MS data clearly shows that gas from the pyrolysis reaction was mainly composed of hydrocarbon gas. The gas composition in Experiment II was significantly less than that of Experiment I. Meanwhile, in Experiment II, the gas component fragment was mainly C4H8 (2-methyl-1-propene), accounting for 65.59% of the total gas. This was mainly due to the catalytic effect of spent FCC catalysts, which made the cracking intermediate active material convert to propylene and reduced the occurrence of other side reactions. Therefore, a new method is proposed that uses spent FCC catalysts for the catalytic cracking of waste tires to produce chemical raw materials. The feasibility of this method will be further verified in subsequent experiments.
3.6. Equilibrium Analysis of Sulfur Elements
The sulfur content in tires is 1–2% [
27]. Several studies have focused on the migration and transformation of sulfur elements during the pyrolysis process [
28,
29,
30]. From the above experimental data analysis, it can be seen that the sulfur content in pyrolysis gas was negligible. The sulfur content in the experimental products and the distribution of sulfur elements in the products are detailed in
Table 8.
Table 8 shows that the sulfur in pyrolysis carbon black accounted for about 73% of the total sulfur in waste tire raw materials and about 26% of that in pyrolysis oil. The sulfur element content in oil increased in Experiment II because the sulfur bond breaks first in a vulcanized rubber system. The C–H bond energy was 414 kJ·mol
−1, C–C bond energy was 347 kJ·mol
−1, C–S bond energy was 310 kJ·mol
−1, and S–S bond energy was 270 kJ·mol
−1, which decreased in turn, with sulfur bonds breaking first. Under the action of the catalysts, the intermediate active substance containing sulfur bonds reacted violently. Thus, the sulfur content increased in pyrolysis oil and decreased in carbon black.
4. Conclusions
1. Catalytic cracking of waste tires with spent FCC catalysts improved pyrolysis efficiency and the quality of cracking oil and reduced the content of sulfur and nitrogen in pyrolysis gas, which proves that the application of spent FCC catalysts in the pyrolysis of waste tires is effective.
2. When spent FCC catalysts were used in the catalytic cracking of waste tires, the yield of oil decreased slightly, but the proportion of light oil increased obviously and the quality of oil was improved.
3. A new idea was proposed to use spent FCC catalysts in the catalytic cracking of waste tires to produce high-value chemical raw materials.
4. The S element in pyrolysis carbon black accounted for about 73% of the total S elements in waste tires and about 26% of that in pyrolysis oil.
5. The distillation of pyrolysis oil has a wide range.
Author Contributions
Conceptualization, C.W. and X.T.; data curation, X.T., B.Z., and L.Z.; writing—original draft, X.T.; writing—review and editing, S.L.
Funding
This research was funded by the Shandong Provincial Natural Science Foundation, grant number ZR2016XJ003.
Conflicts of Interest
The authors declare no conflict of interests.
References
- Li, Q.; Li, F.; Meng, A.; Tan, Z.; Zhang, Y. Thermolysis of scrap tire and rubber in sub/super-critical water. Waste Manag. 2018, 71, 311–319. [Google Scholar] [CrossRef]
- Zeaiter, J.; Azizi, F.; Lameh, M.; Milani, D.; Ismail, H.Y.; Abbas, A. Waste tire pyrolysis using thermal solar energy: An integrated approach. Renew. Energy 2018, 123, 44–51. [Google Scholar] [CrossRef]
- Cai, W.; Lai, K.; Liu, C. Promoting sustainability of manufacturing industry through the lean energy-saving and emission-reduction strategy. Sci. Total Environ. 2019, 665, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Liu, C.; Zhang, C. Developing the ecological compensation criterion of industrial solid waste based on emergy for sustainable development. Energy 2018, 157, 940–948. [Google Scholar] [CrossRef]
- Cai, W.; Liu, C.; Lai, K.; Li, L.; Cunha, J.; Hu, L.K. Energy performance certification in mechanical manufacturing industry: A review and analysis. Energy Convers. Manag. 2019, 186, 415–432. [Google Scholar] [CrossRef]
- Williams, P.T. Pyrolysis of waste tyres: A review. Waste Manag. 2013, 33, 1714–1728. [Google Scholar] [CrossRef]
- Martinez, J.D.; Puy, N.; Murillo, R.; Garcia, T.; Navarro, M.V.; Mastral, A.M. Waste tyre pyrolysis—A review. Renew. Sustain. Energy Rev. 2013, 23, 179–213. [Google Scholar] [CrossRef]
- Chouaya, S.; Abbassi, M.A.; Younes, R.B.; Zoulalian, A. Scrap tires pyrolysis: Product yields, properties and chemical compositions of pyrolytic oil. Russ. J. Appl. Chem. 2018, 91, 1603–1611. [Google Scholar] [CrossRef]
- Aslan, D.I.; Parthasarathy, P.; Goldfarb, J.L.; Ceylan, S. Pyrolysis reaction models of waste tires: Application of master-plots method for energy conversion via devolatilization. Waste Manag. 2017, 68, 405–411. [Google Scholar] [CrossRef]
- Namchot, W.; Jitkarnka, S. Catalytic pyrolysis of waste tire using HY/MCM-41 core-shell composite. J. Anal. Appl. Pyrolysis 2016, 121, 297–306. [Google Scholar] [CrossRef]
- Kordoghli, S.; Paraschiv, M.; Kuncser, R.; Tazerout, M. Catalysts’ Influence on thermochemical decomposition of waste tires. Environ. Prog. Sustain. Energy 2017, 36, 1560–1567. [Google Scholar] [CrossRef]
- Miandad, R.; Barakat, M.A.; Rehan, M.; Aburiazaiza, A.S.; Gardy, J.; Nizami, A.S. Effect of advanced catalysts on tire waste pyrolysis oil. Process Saf. Environ. Prot. 2018, 116, 542–552. [Google Scholar] [CrossRef]
- Alvira, J.; Hita, I.; Rodriguez, E.; Arandes, J.M.; Castano, P. A data-driven reaction network for the fluid catalytic cracking of waste feeds. Processes 2018, 6, 243. [Google Scholar] [CrossRef]
- Mehla, S.; Kukade, S.; Kumar, P.; Rao, P.V.C.; Sriganesh, G.; Ravishankar, R. Fine tuning H-transfer and beta-scission reactions in VGO FCC using metal promoted dual functional ZSM-5. Fuel 2019, 242, 487–495. [Google Scholar] [CrossRef]
- Xu, Y.H. Advance in China fluid catalytic cracking (FCC) process. Sci. Sin. Chim. 2014, 44, 13–24. [Google Scholar] [CrossRef]
- Zhang, H.Z. Study on the treatment of FCC spent catalysts for low-carbon cycle and comprenensive utilization. Chem. Ind. Eng. Prog. 2016, 35, 358–362. [Google Scholar]
- Liu, T.; Qiu, Z.F.; Yang, J.; Cao, L.M.; Zhang, W. Output hazard and treatment methods of spent refinery catalysts in China. Environ. Prot. Chem. Ind. 2015, 35, 159–164. [Google Scholar]
- Xu, F.F.; Wang, B.; Ming, X.; Jiang, Y.; Hao, J.H. TG-FTIR and Py-GC/MS study on pyrolysis mechanism and products distribution of waste bicycle tire. Energy Convers. Manag. 2018, 175, 288–297. [Google Scholar] [CrossRef]
- Gauthier-Maradei, P.; Valderrama, Y.C.; Nabarlatz, D. Mathematical model of scrap tire rubber pyrolysis in a non-isothermal fixed bed reactor: Definition of a chemical mechanism and determination of kinetic parameters. Waste Biomass Valoriz. 2019, 10, 561–573. [Google Scholar] [CrossRef]
- Yazdani, E.; Hashemabadi, S.H.; Taghizadeh, A. Study of waste tire pyrolysis in a rotary kiln reactor in a wide range of pyrolysis temperature. Waste Manag. 2019, 85, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Costa, G.A.; dos Santos, R.G. Fractionation of tire pyrolysis oil into a light fuel fraction by steam distillation. Fuel 2019, 241, 558–563. [Google Scholar] [CrossRef]
- Dogan-Saglamtimur, N.; Bilgil, A.; Guven, A.; Otgun, H. Producing of qualified oil and carbon black from waste tyres and pet bottles in a newly designed pyrolysis reactor. J. Therm. Anal. Calorim. 2019, 135, 3339–3351. [Google Scholar] [CrossRef]
- Wang, K.; Xu, Y.P.; Duan, P.G.; Wang, F.; Xu, Z.X. Thermo-chemical conversion of scrap tire waste to produce gasoline fuel. Waste Manag. 2019, 86, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.D.; Cardona-Uribe, N.; Murillo, R.; Garcia, T. Carbon black recovery from waste tire pyrolysis by demineralization: Production and application in rubber compounding. Waste Manag. 2019, 85, 574–584. [Google Scholar] [CrossRef] [PubMed]
- Sugatri, R.I.; Wirasadewa, Y.C.; Saputro, K.E.; Muslih, E.Y. Recycled carbon black from waste of tire industry: Thermal study. Microsyst. Technol. 2018, 24, 749–755. [Google Scholar] [CrossRef]
- Czajczynska, D.; Krzyzynska, R.; Jouhara, H.; Spencer, N. Use of pyrolytic gas from waste tire as a fuel: A review. Energy 2017, 134, 1121–1131. [Google Scholar] [CrossRef]
- Liu, S.; Yu, J.; Bikane, K.; Ma, C.; Wang, B. Rubber pyrolysis: Kinetic modeling and vulcanization effects. Energy 2018, 155, 215–225. [Google Scholar] [CrossRef]
- Unapumnuk, K.; Keener, T.C.; Lu, M.M.; Liang, F.Y. Investigation into the removal of sulfur from tire derived fuel by pyrolysis. Fuel 2008, 87, 951–956. [Google Scholar] [CrossRef]
- Lanteigne, J.R.; Laviolette, J.P.; Chaouki, J. Behavior of Sulfur during the Pyrolysis of Tires. Energy Fuel 2015, 29, 763–774. [Google Scholar] [CrossRef]
- Hu, H.Y.; Fang, Y.; Liu, H.; Luo, G.Q.; Liu, W.Q.; Li, A.J.; Yao, H. The fate of sulfur during rapid pyrolysis of scrap tires. Chemosphere 2014, 97, 102–107. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).