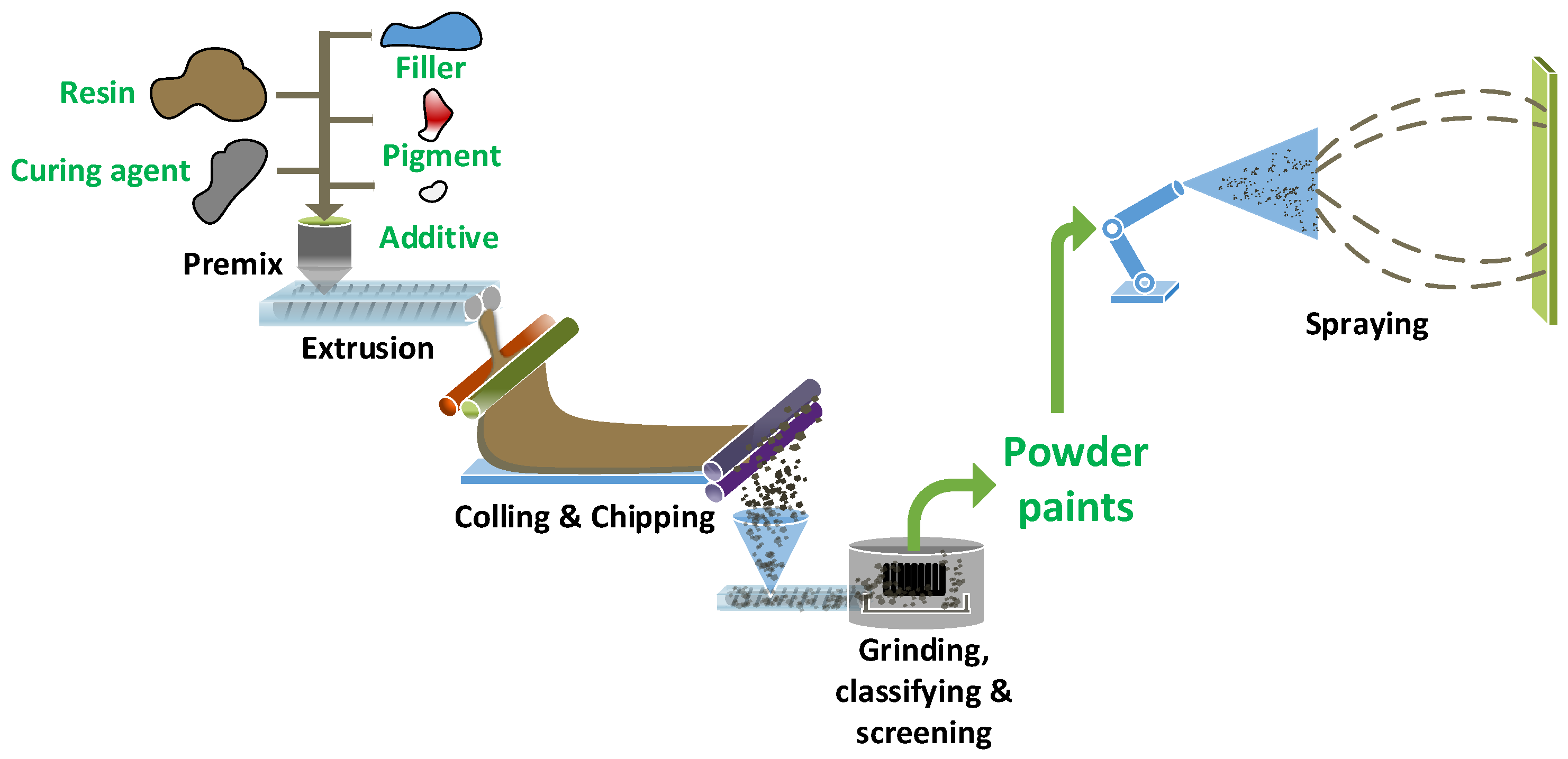
2.1. Preparation and Characterization of Powder Paints
As described earlier, powder coatings consist of five principal components, including polymer resins, curing agents, pigments, additives and fillers. The materials used in this study are shown in
Table 1. In this research, four different filler contents were tested in two widely used resin systems. Each sample contains different type and content of resins and curing agents, as shown in
Table 2. To determine the effects of type and content of fillers, the other components (pigments, degassing agents and flow agents) remained constant. Physical properties of fillers incorporated in the coatings are shown in
Table 3.
As illustrated in
Figure 1, the appropriate materials were premixed and then extruded by a twin-screw extruder (SLJ-10, Donghui Powder Coating Processing Equipment Co., Ltd., Yantai, China). The hot extrudates were cooled, crushed into small chips and added into an air classifier mill (ACM-02, Donghui Powder Coating Processing Equipment Co., Ltd., Yantai, China) for fine grinding, classifying and screening. The median particle size (D
50) of paint powders was within the range of 18 µm ± 0.5 µm, as shown in
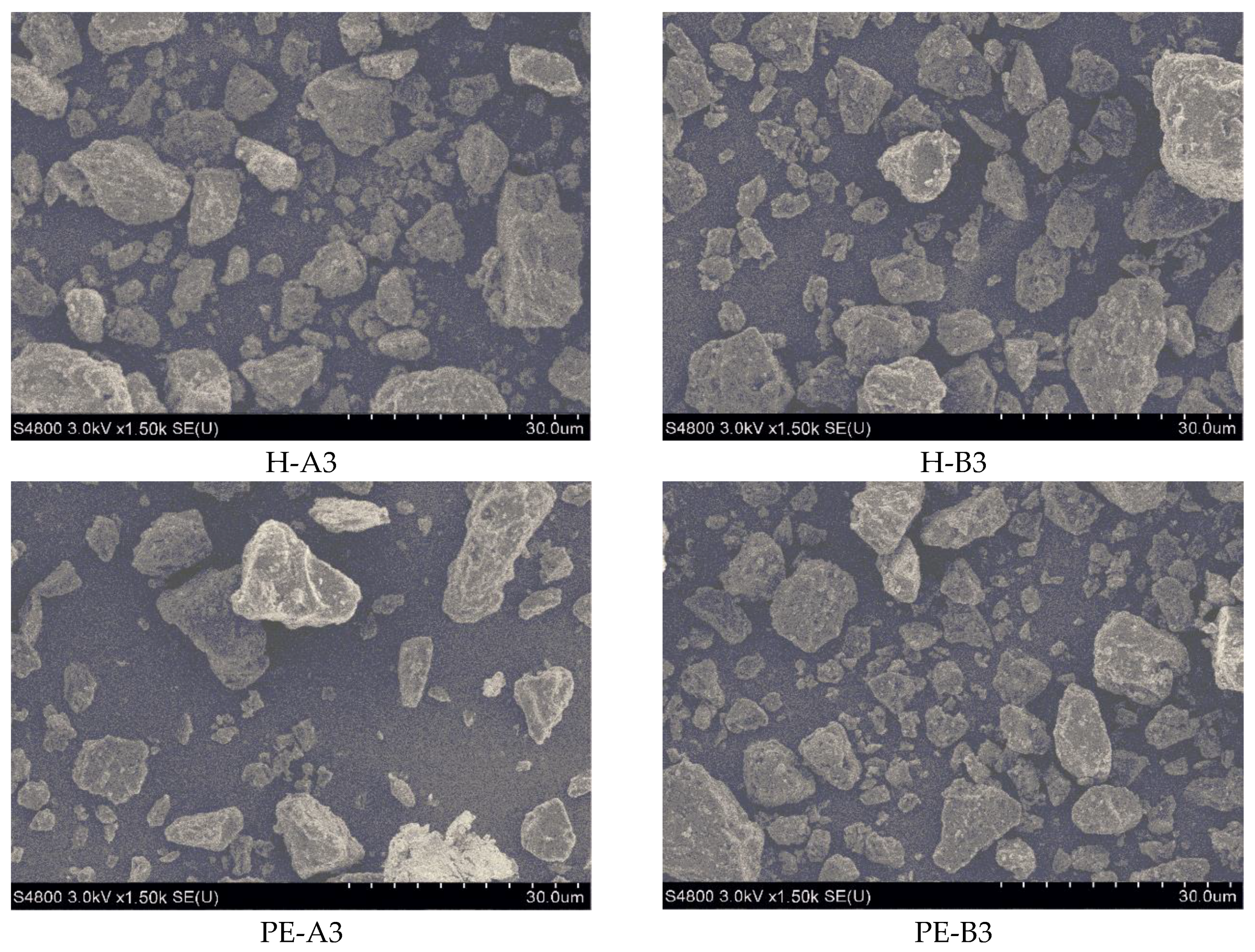
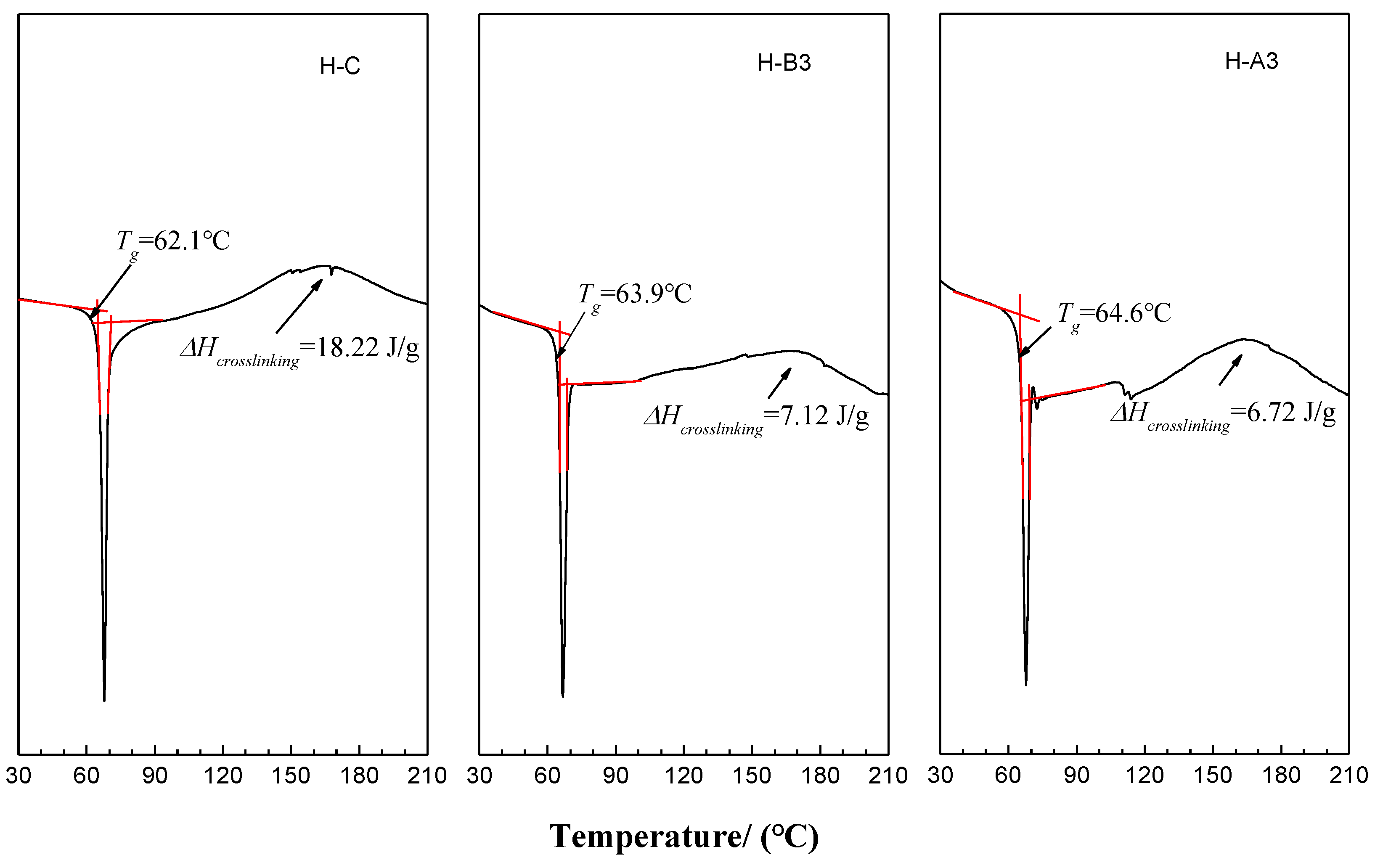
Table 4, which was measured by a laser particle size analyzer (BT2000B, Bettersize instruments Ltd., Dandong, China). Microstructure and morphology of the powder paints and coating films were characterized with scanning electron microscopy (SEM, S4800, Hitachi High-Technologies Global, Tokyo, Japan). Differential scanning calorimetric (DSC) was used to determine the glass transition temperature (T
g) of coatings and ΔH
crosslinking of the crosslinking events. The DSC curves were obtained by a calorimeter (DSC-3, Mettler Toledo Inc., Greifensee, Switzerland) at temperatures between 25 °C and 210 °C. It was operated at a heating rate of 10 °C/min under inert nitrogen atmosphere (N
2) with a flow rate of 50 mL/min.
According to Geldart’s Powder Classification, the ultrafine powder paints used in this study can be categorized in Group C (particle size under 25 µm–30 µm), which is difficult to fluidize due to its cohesive nature [
31]. The cohesion is attributed to the relatively larger interparticle attractive forces, which can be overcome by the addition of nanoparticles (fluidization additives), thus improving the flowability of Group C particles [
32,
33,
34,
35]. Fluidization additive used in this study is listed in
Table 1, which is dry blended (0.7 wt.%) before the powder paints are applied onto the substrates.
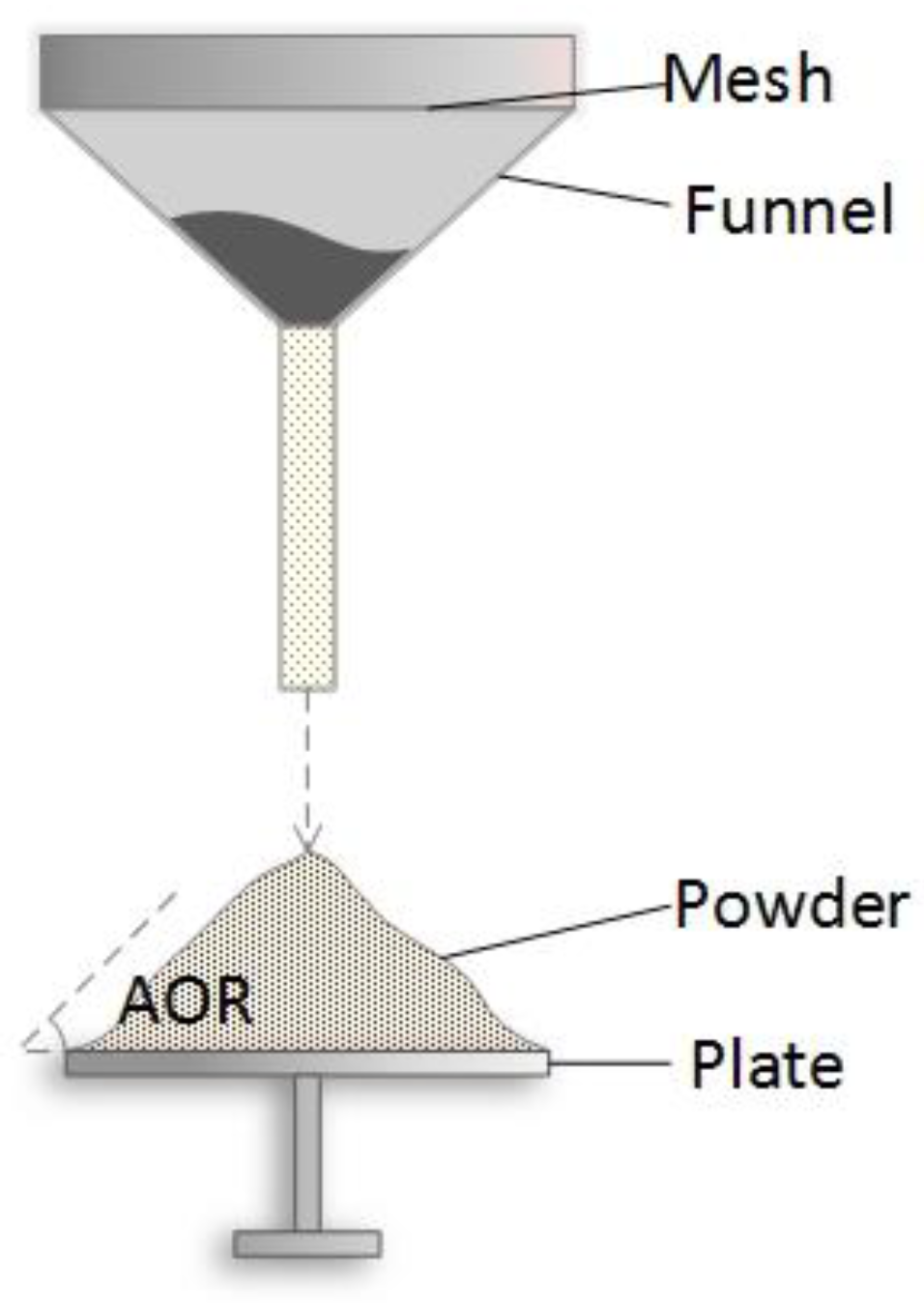
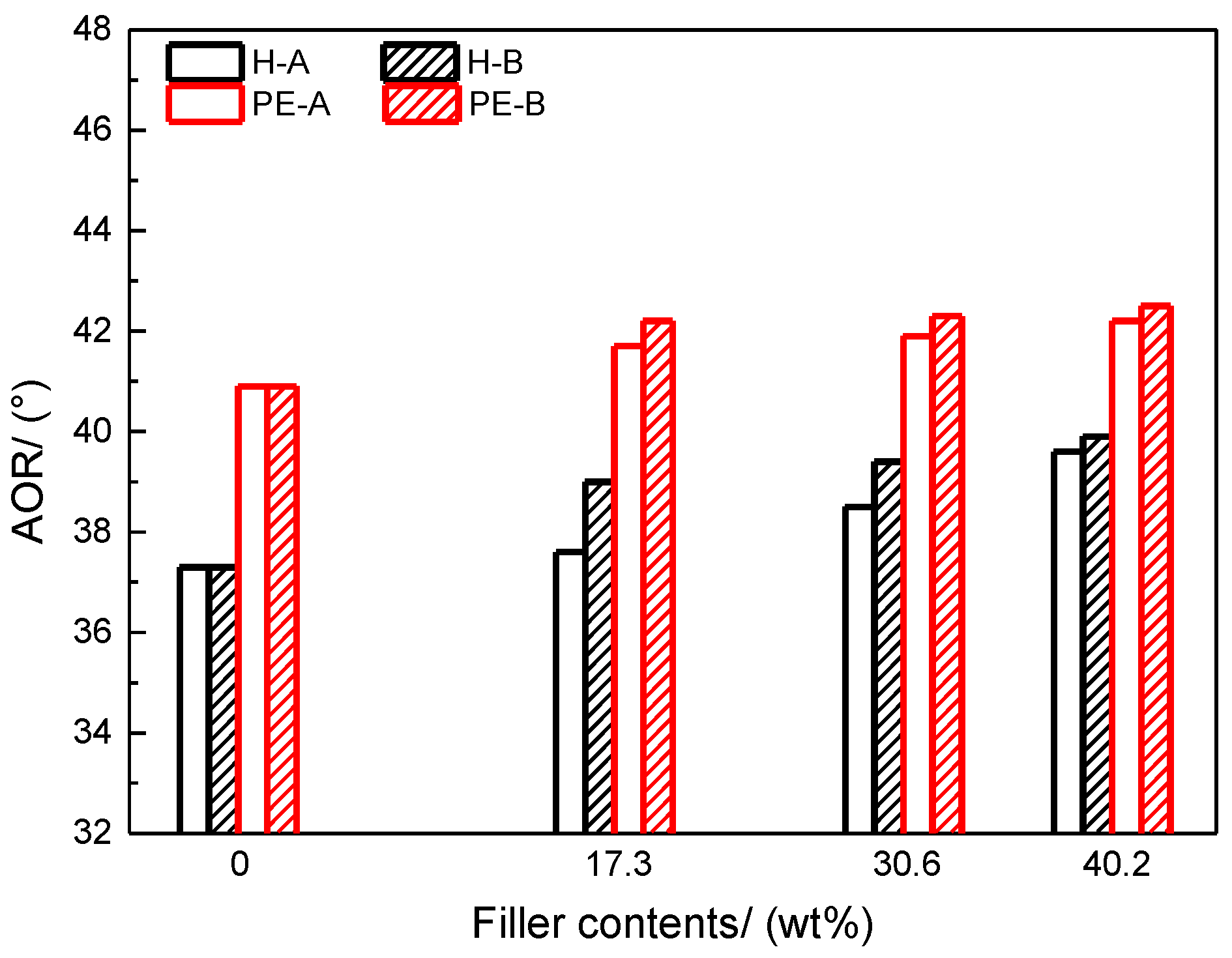
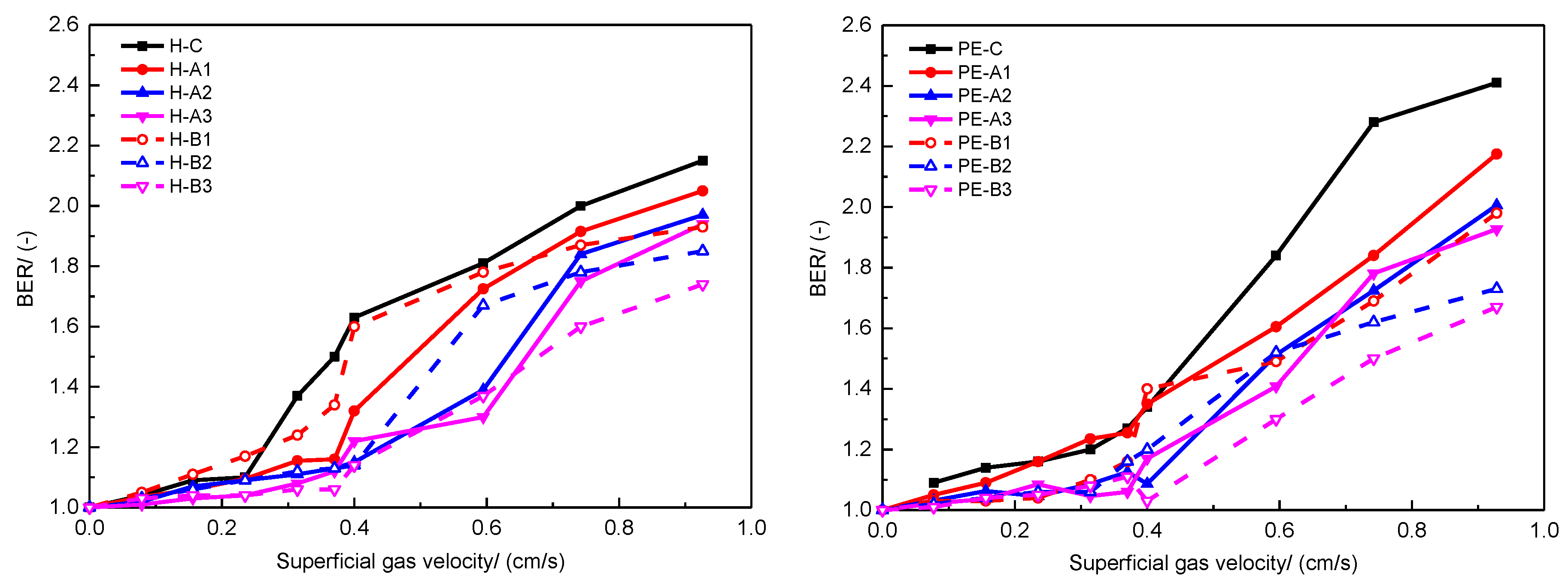
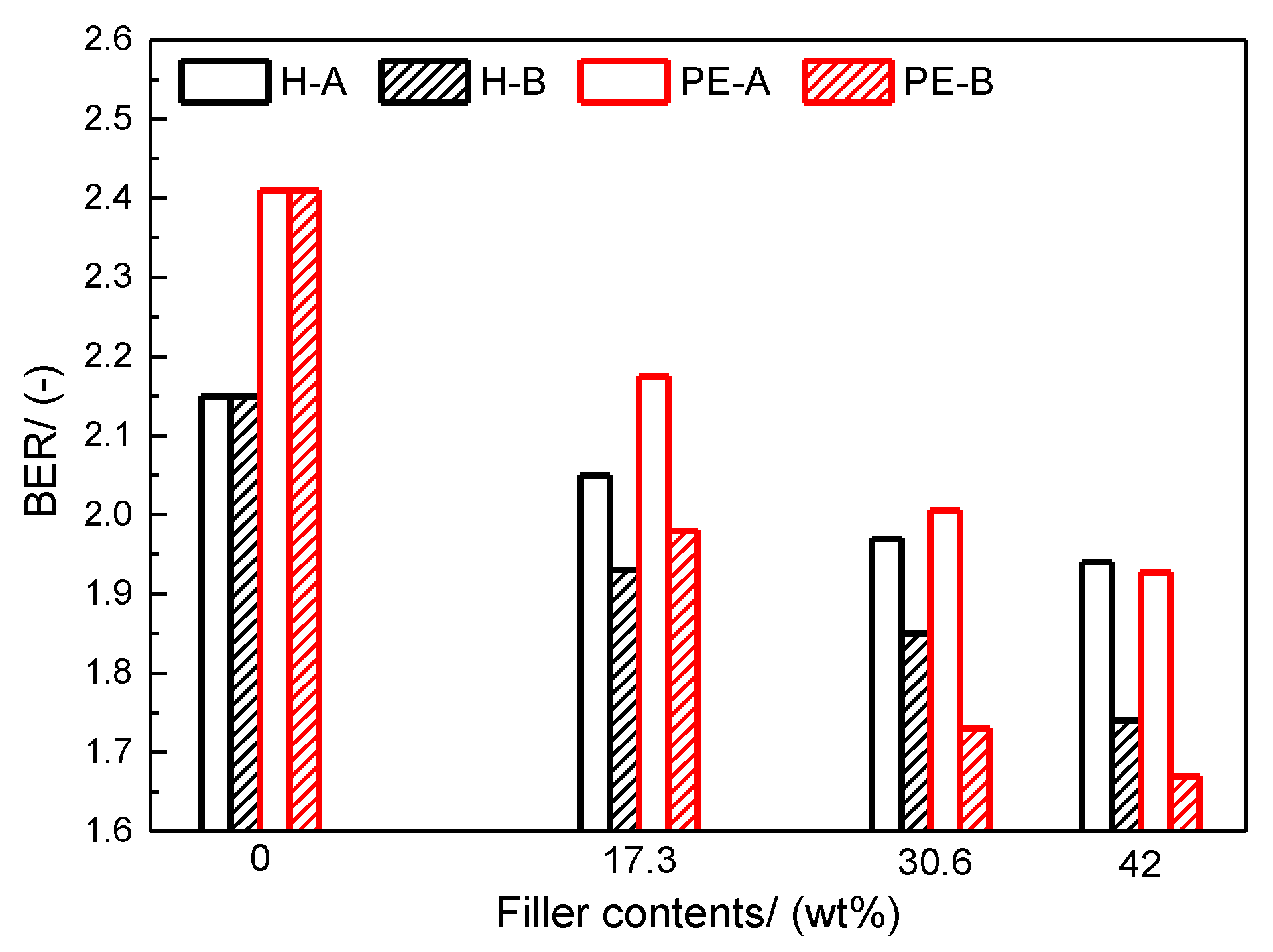
Flow behavior of powder paints was characterized with angle of repose (AOR) and bed expansion ratio (BER) tests [
36]. As shown in
Figure 2, AOR is defined as the angle between the horizontal and the side of the cone when the powder falls freely on the plate. It reflects the cohesiveness and internal friction of a powder and is commonly employed to characterize the flow behavior of powder samples. In general, powder samples with AOR over 45° are usually considered cohesive, while samples with a smaller AOR have better flowability [
37].
BER is a useful parameter that indicates the extent to which powders expand at a specific superficial gas velocity. It is the ratio of the bed height at operating conditions (expanded) to the fixed bed height, as defined in Equation (1). Generally, BER is used to characterize fluidization quality based on the belief that a higher BER indicates a better gas-solid contact [
38]. A higher BER implies a large amount of gas trapped among the paint powders, which contributes to adequate gas-solid contact during the following spraying process, resulting in a smoother visual appearance. In this study, fluidization of the paint powders was conducted in a homemade fluidized bed as schematically shown in
Figure 3. The fluidized bed column was made of Plexiglas that was 75 cm tall with I.D. of 5 cm. The expanded bed height was measured simultaneously when the paint powders were fluidized steadily at each set of superficial gas velocity. In this study, the BER is reported using a gas velocity ranging from 0 cm/s to near 1.0 cm/s, which is representative fluidization in a powder coating process [
1,
39].
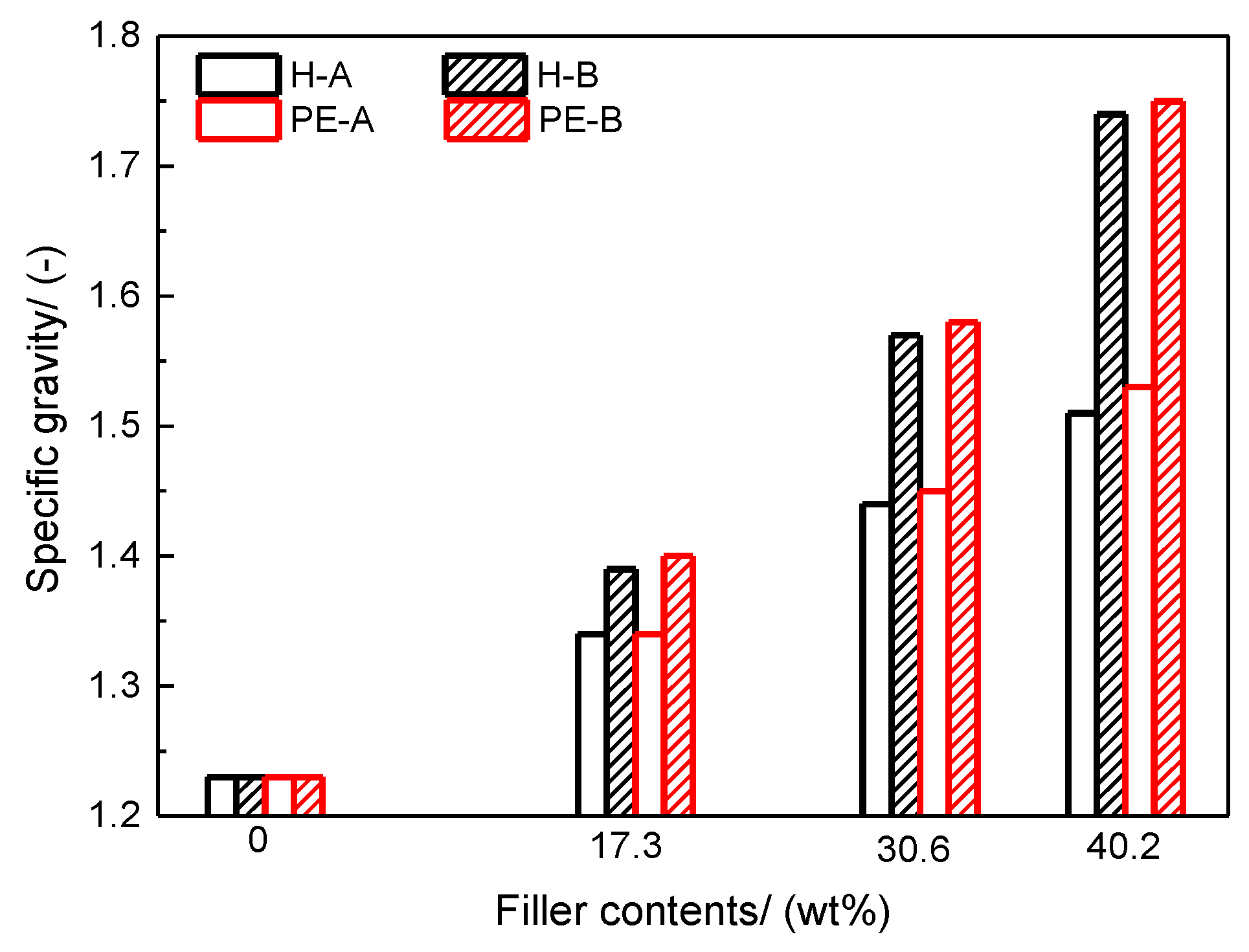
The specific gravity is a significant parameter for calculating the cost benefit of powder coatings, usually in the form of square meters/kg powder coating in a given film thickness. It can provide theoretical usage and coverage of powder coatings.
In this study, all the powder paints were fabricated by an ACM. The operating parameters are virtually the same, with minor adjustments to ensure all the paint samples’ D
50 fall in the range of 18 µm ± 0.5 µm and are in the same particle shape. Consequently, the differences of AORs and BERs caused by particle size and shape can be neglected. The characterizations mentioned above were performed by devices and instruments listed in
Table 5.
2.2. Preparation and Characterization of Finished Films
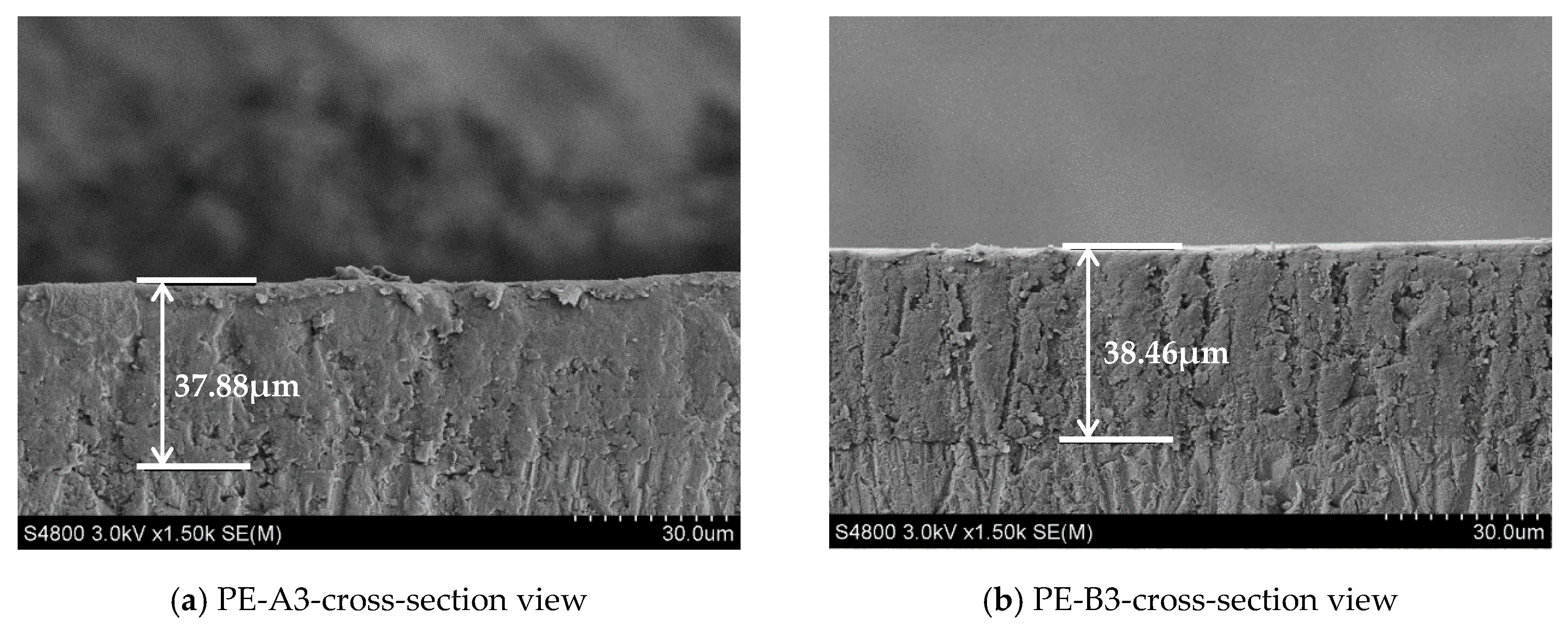
After measurements of powder paints described before, each of the powder samples was sprayed on two different types of panels from Q-Lab (Q-Lab Corporation Ltd., Westlake, OH, USA): aluminum panel (88.9/63.5/0.8, L/W/T, mm) and steel panel (127.0/76.2/0.8, L/W/T, mm). All panels were degreased with acetone prior to spraying. Powder samples were sprayed using a Sure coat corona gun (Nordson Corporation, Westlake, OH, USA). All the sprayed panels were cured at 204 °C for 10 min to ensure complete curing. After curing, a film thickness of 38 µm ± 5% were retained for all the subsequent measurements. The thickness of the coating film was measured by a PosiTector 6000 thickness gage (DeFelsko Corporation, Ogdensburg, NY, USA).
The performance of coating films depends on many factors; for instance, the properties of the resin systems, environment exposed and other factors. Many test methods for developing and monitoring film properties are available to simulate the application conditions and accelerate the degradation process of powder coatings. In this study, for evaluating the pencil scratch hardness, impact resistance, conical mandrel bend test, specular gloss, UV and corrosion resistances of the film surface by instrumental measurements, detailed evaluation procedures are followed according to appropriate ASTM standards. Characterizations of the coating films were conducted 24 h after cured. Instruments used and ASTM standards followed in the measurements are presented in
Table 5.
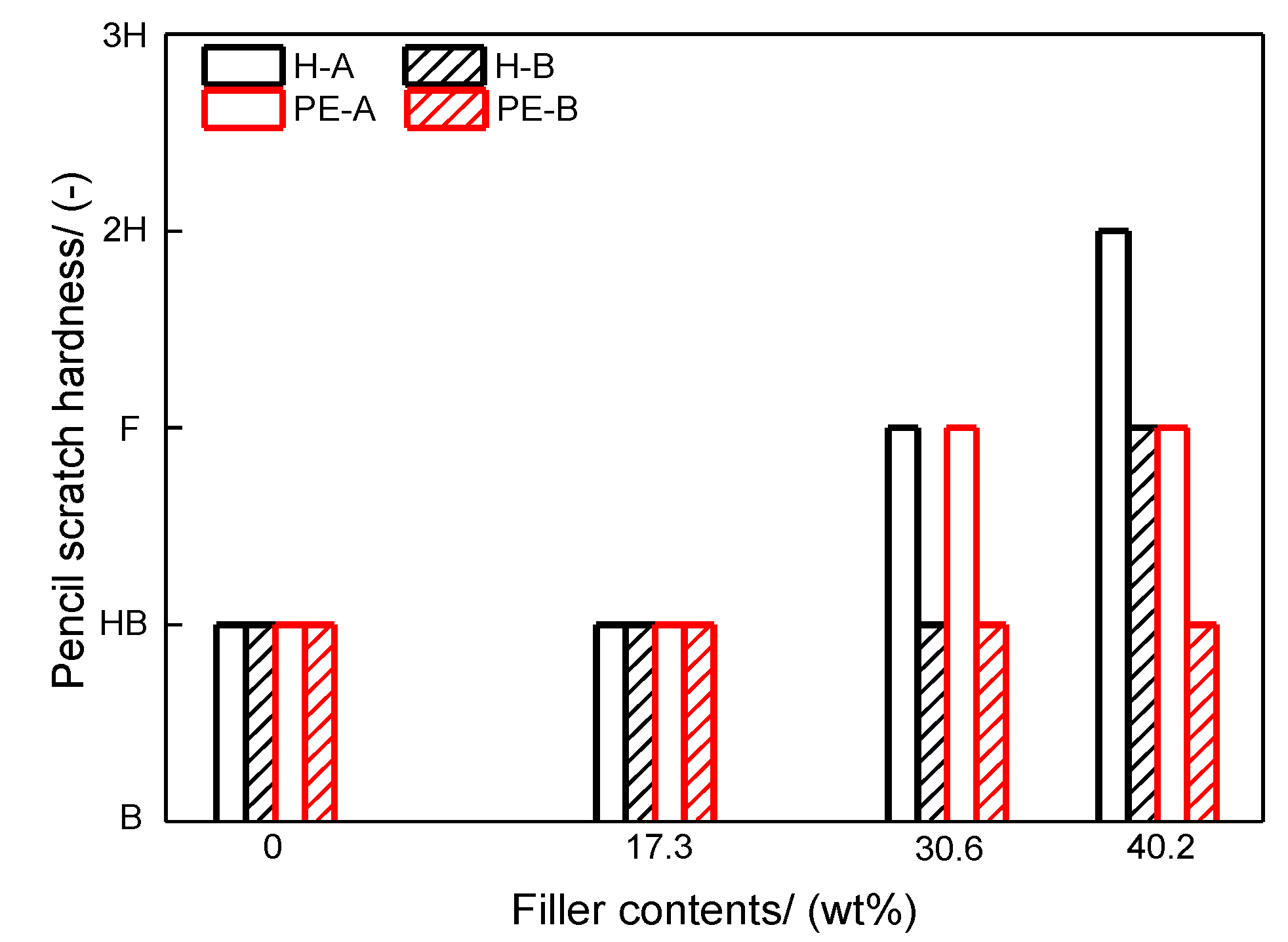
Pencil scratch hardness test uses constant pressure and special pencils (from 9H to 9B degrees) to scratch the finished film to determine the hardness of the film. The Impact resistances of the coated films were characterized by an impact tester. A steel weight with a hemispherical head (diameter: 13 mm, weight: 1.8 kg) was dropped from a height between 20 and 120 cm onto the coated panels. The same procedure is repeated by increasing the height by 25 mm increments until cracks appear, which presents the failure of the coating. Flexibility of the coating films is measured by a conical mandrel bend apparatus. The panels are bent for about 135°. The radius of the cone varies from 1.5 mm to 19 mm. The coating film is deemed flexible if no crack appears. Specular gloss describes the power of a test surface to reflect light specularly. A complete specular light reflection is 100, while a complete diffuse reflection is 0. The surface roughness test is conducted to characterize the discontinuities over some distance (15 mm in this study) on the specimen surface. To ensure the reliability and reproducibility of the test results, the measurements were performed in three different regions for each of the finished films. The salt spray test is an essential method to evaluate corrosion resistance of a coating film in terms of loss of adhesion under long-term exposure to salt fog. An X-form-scribe was made on the surface of the coated panel leaving the metal substrate exposed. The representative mean rust creepages can be determined by a rating grade from 10 to 1 (
Table 6). The resistance of a powder coated film against UV exposure (UV accelerate test) is one of the most important parameters to determine the applicability of a particular coating for external conditions. The UV tester used in this study is equipped with two xenon-arc lamps with a maximum wavelength of 340 nm. A cycle consists of 102 min of UV irradiation (0.35 W/m
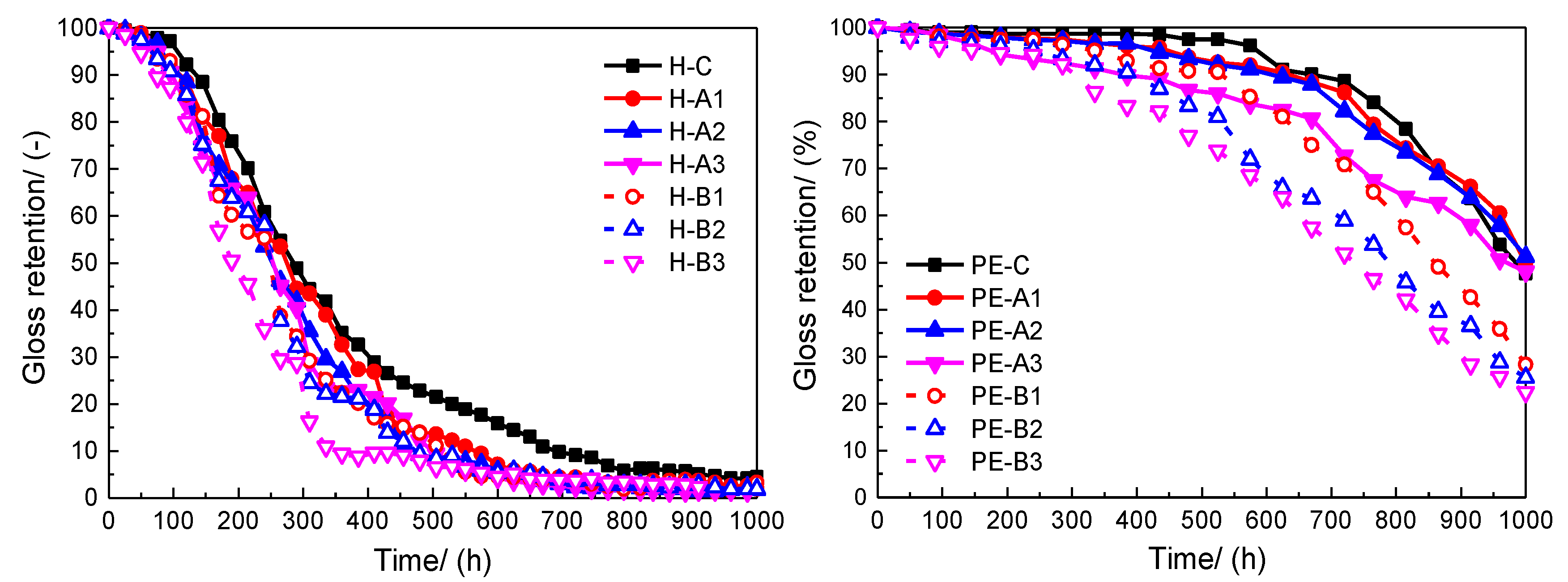
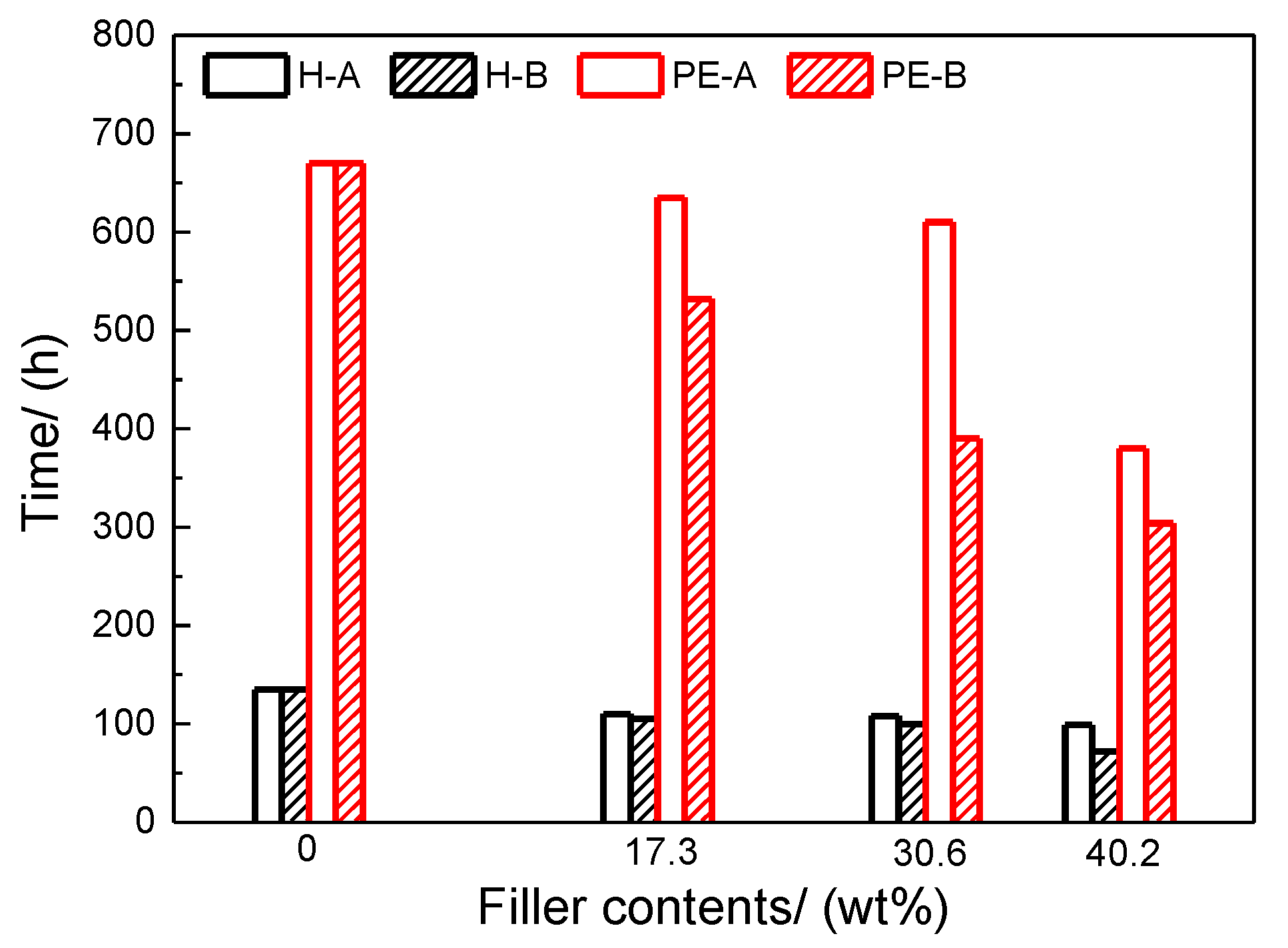
2, 63 °C) in dry conditions and 18 min in water spray conditions. The measured specular gloss at 60° was used to calculate the gloss retention of the coating film. The gloss retention is defined as: