Highly Porous Graphitic Activated Carbons from Lignite via Microwave Pretreatment and Iron-Catalyzed Graphitization at Low-Temperature for Supercapacitor Electrode Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Process
2.3. Measurement Analysis
2.4. Electrochemical Measurements
3. Result and Discussion
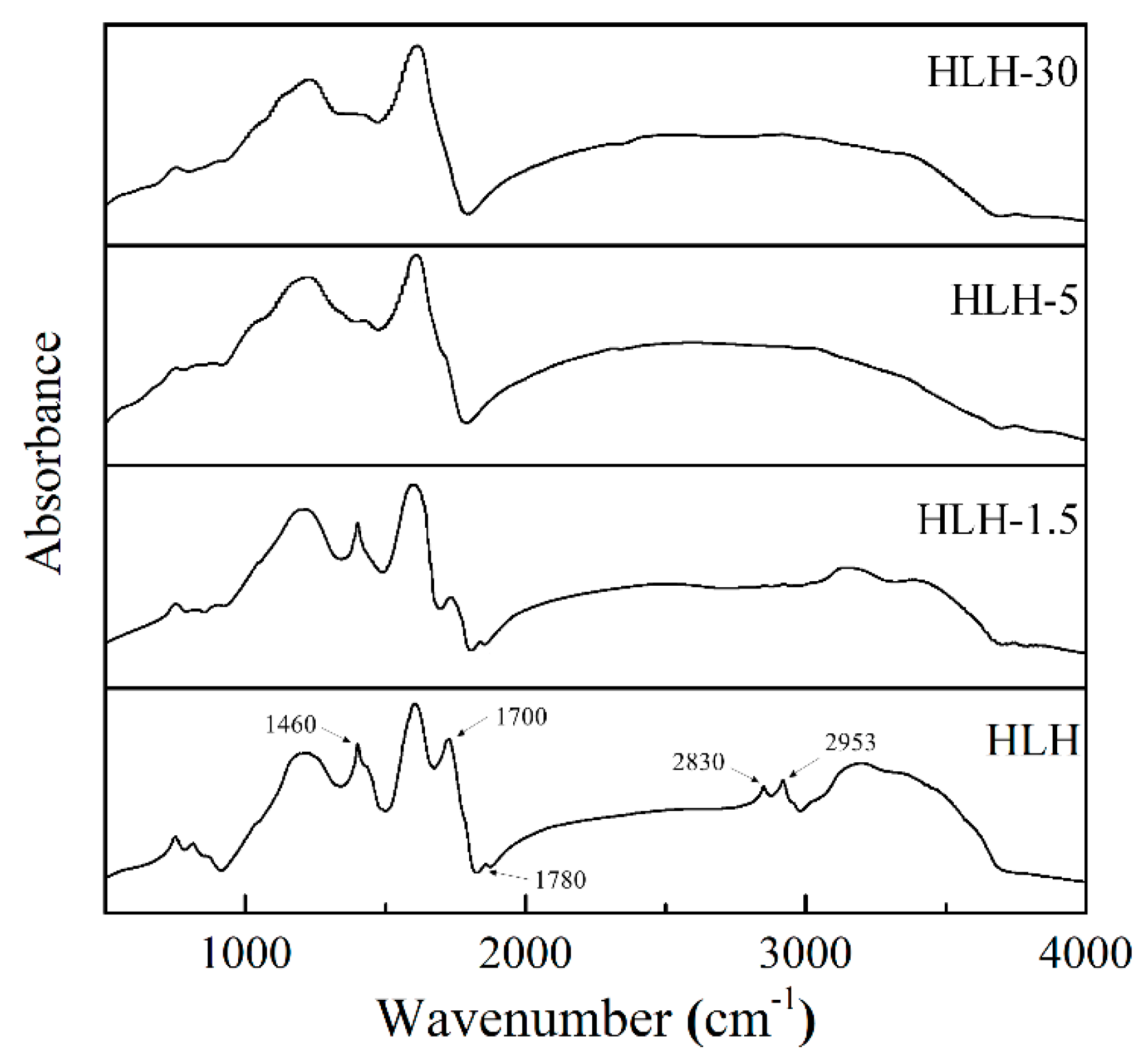
3.1. Chemical Structure Analysis by FTIR
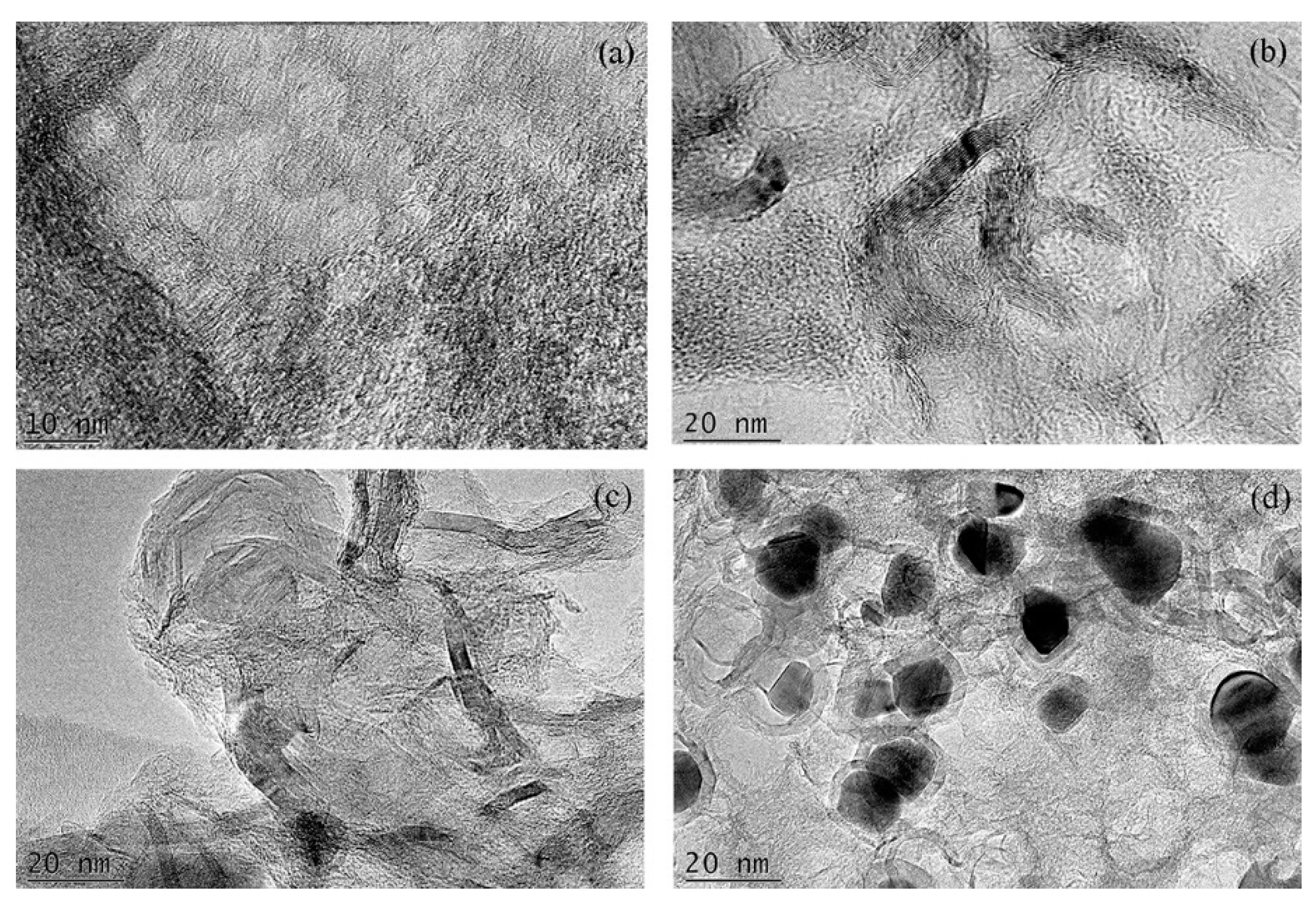
3.2. Microstructure Analysis by TEM
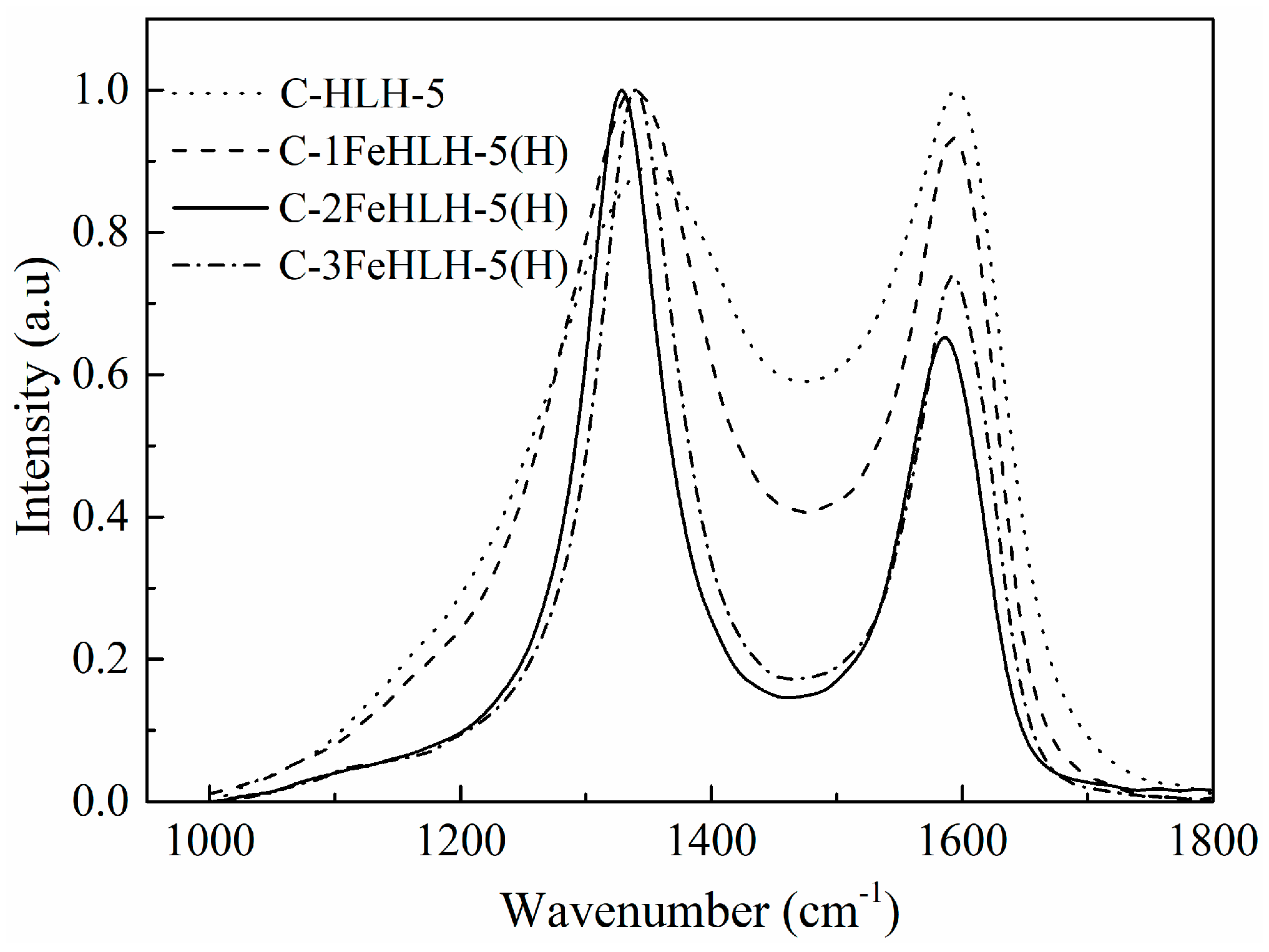
3.3. Hybrid Carbon Structure Analysis by Raman
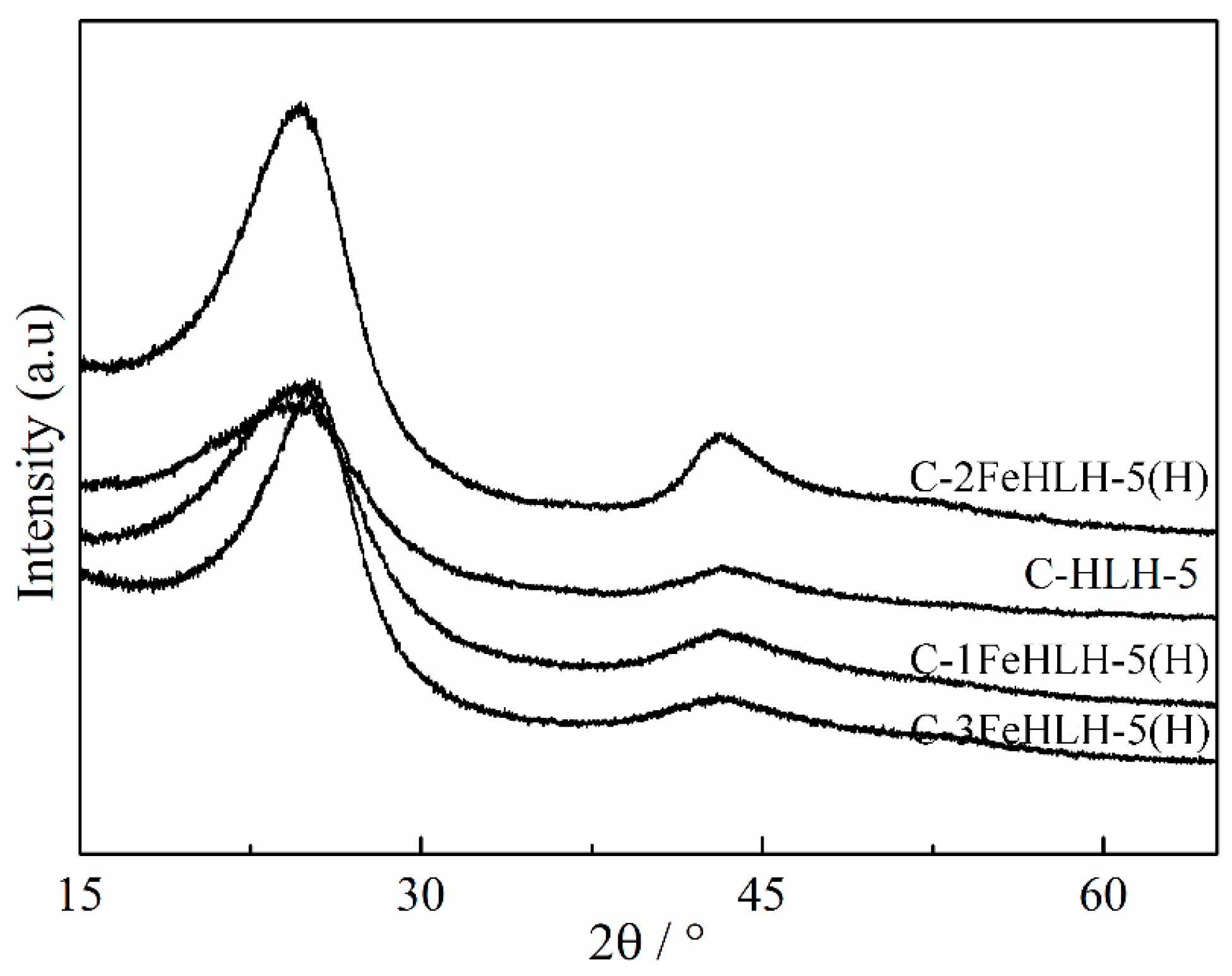
3.4. Crystal Structure Analysis by XRD
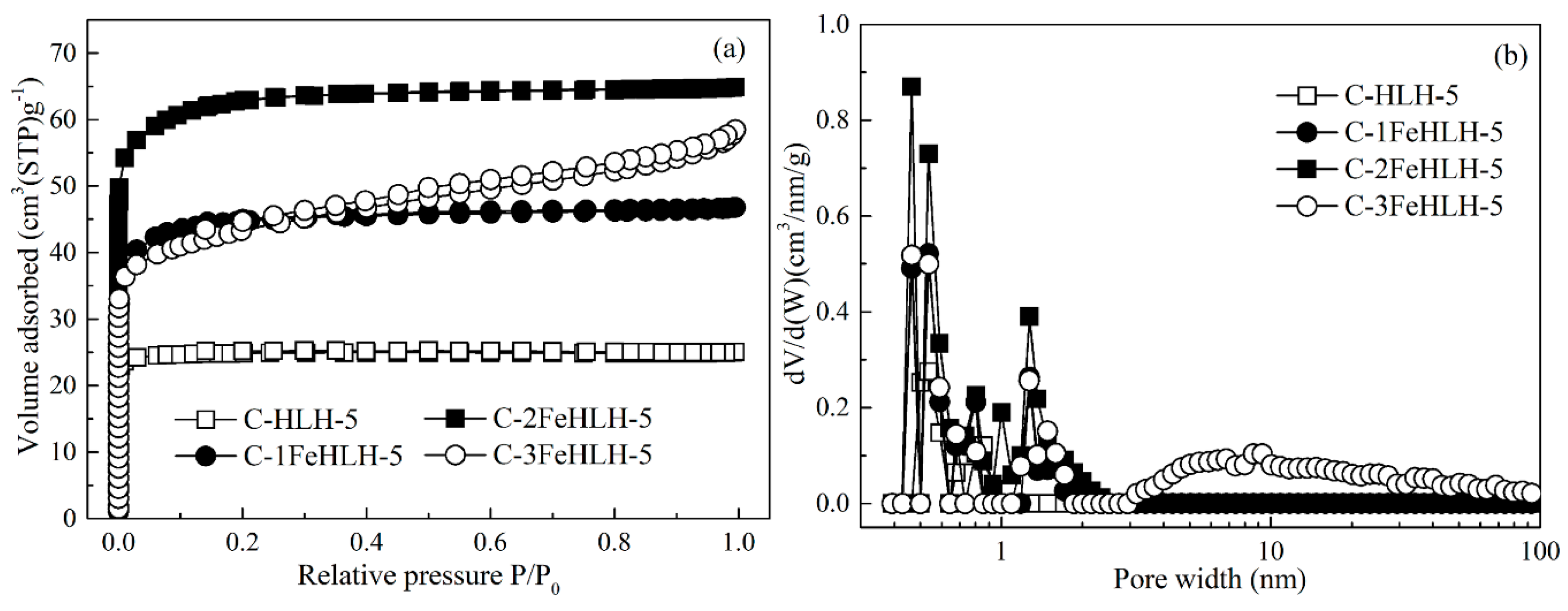
3.5. Pore Structure Analysis of the Nitrogen Adsorption Method
3.6. Pore Structure Development of Typical Chars during Activation
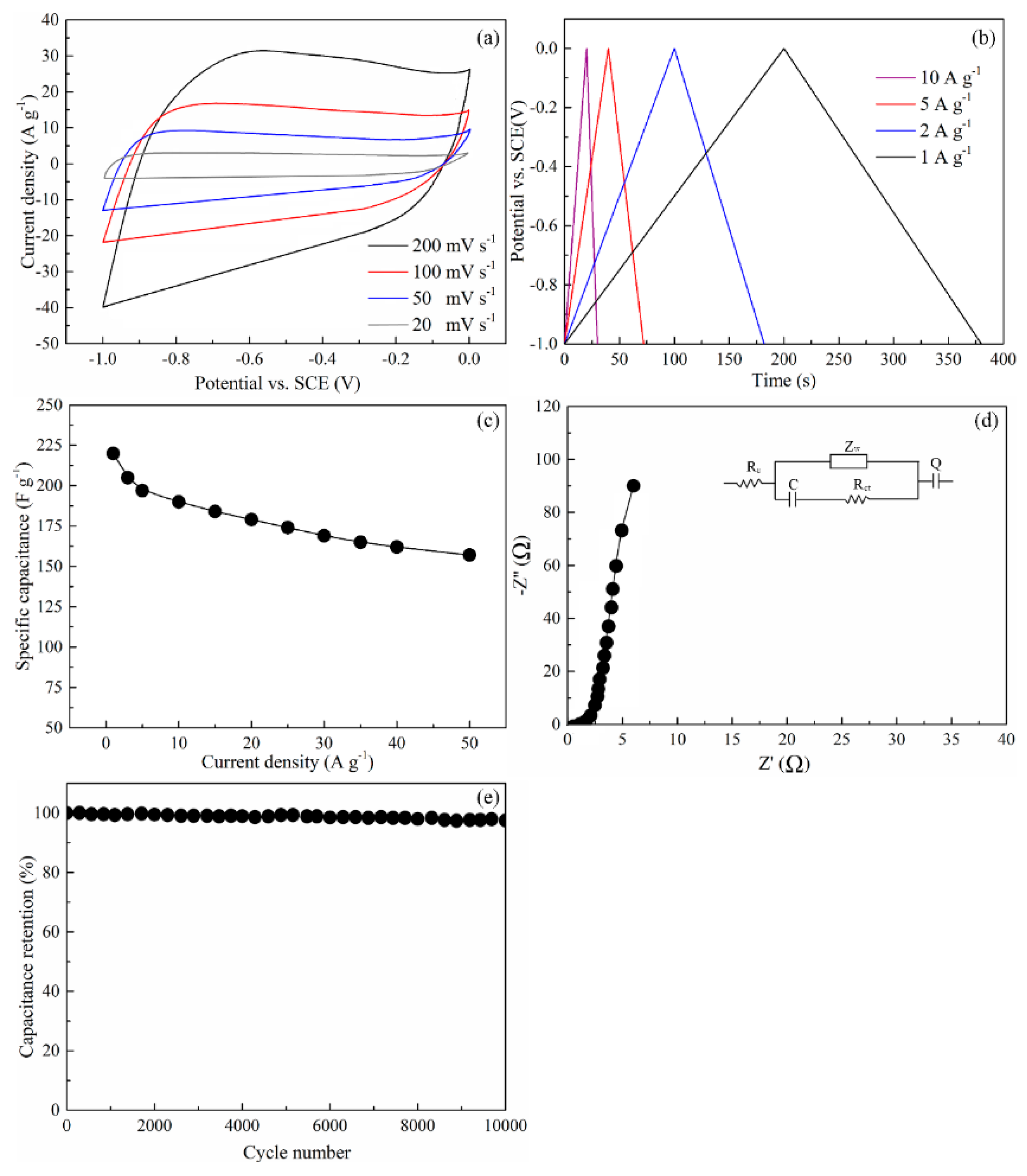
3.7. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Peng, L.L.; Wu, C.Z.; Xie, Y. Two dimensional nanomaterials for flexible supercapacitors. Chem. Soc. Rev. 2014, 43, 3303–3323. [Google Scholar] [CrossRef]
- Wang, D.W.; Li, F.; Liu, M.; Lu, G.Q.; Cheng, H.M. 3D aperiodic hierarchical porous graphitic carbon material for high-rate electrochemical capacitive energy storage. Angew. Chem. Int. Ed. 2008, 47, 373–376. [Google Scholar] [CrossRef]
- Ma, F.W.; Ma, D.; Wu, G.; Geng, W.D.; Shao, J.Q.; Song, S.J.; Wan, J.F.; Qiu, J.S. Construction of 3D nano-structure hierarchical porous graphitic carbons by charge-induced self-assembly and nanocrystal assisted catalytic graphitization for supercapacitors. Chem. Commun. 2016, 52, 6673–6676. [Google Scholar] [CrossRef]
- Liu, S.Q.; Zhang, Y.J.; Tuo, K.Y.; Wang, L.P.; Chen, G. Structure, electrical conductivity, and dielectric properties of semi-coke derived from microwave-pyrolyzed low-rank coal. Fuel Process. Technol. 2018, 178, 139–147. [Google Scholar] [CrossRef]
- Wang, Q.; Nie, Y.F.; Chen, X.Y.; Xiao, Z.H.; Zhang, Z.J. Controllable synthesis of 2D amorphous carbon and partially graphitic carbon materials: Large improvement of electrochemical performance by the redox additive of sulfanilic acid azochromotrop in KOH electrolyte. Electrochim. Acta 2016, 200, 247–258. [Google Scholar] [CrossRef]
- Zhu, Y.W.; Gao, J.H.; Li, Y. Preparation of activated carbons for SO2, adsorption by CO2, and steam activation. J. Taiwan Inst. Chem. Eng. 2012, 43, 112–119. [Google Scholar] [CrossRef]
- Zhu, Y.W.; Gao, J.H.; Li, Y.; Sun, F.; Qin, Y.K. Preparation and characterization of activated carbons for SO2 adsorption from taixi anthracite by physical activation with steam. Korean J. Chem. Eng. 2011, 28, 2344–2350. [Google Scholar] [CrossRef]
- San, M.G.; Fowler, G.D.; Sollars, C.J. A study of the characteristics of activated carbons produced by steam and carbon dioxide activation of waste tyre rubber. Carbon 2003, 41, 1009–1061. [Google Scholar]
- Wang, K.; Cao, Y.; Wang, X. Nickel catalytic graphitized porous carbon as electrode material for high performance supercapacitors. Energy 2016, 101, 9–15. [Google Scholar] [CrossRef]
- He, X.J.; Zhang, H.B.; Zhang, H. Direct synthesis of 3D hollow porous graphene balls from coal tar pitch for high performance supercapacitors. J. Mater. Chem. A 2014, 46, 19633–19640. [Google Scholar] [CrossRef]
- Li, Y.T.; Pi, Y.T.; Lu, L.M. Hierarchical porous active carbon from fallen leaves by synergy of K2CO3, and their supercapacitor performance. J. Power Sources 2015, 299, 519–528. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Preparation and characterization of activated carbon from sunflower seed oil residue via microwave assisted K2CO3 activation. Bioresour. Technol. 2010, 102, 9794–9799. [Google Scholar] [CrossRef]
- Ma, G.F.; Yang, Q.; Sun, K.J. Nitrogen-doped porous carbon derived from biomass waste for high-performance supercapacitor. Bioresour. Technol. 2015, 197, 137–142. [Google Scholar] [CrossRef]
- Elmouwahidi, A.; Bailón-García, E.; Pérez-Cadenas, A.F. Activated carbons from KOH and H3PO4-activation of olive residues and its application as supercapacitor electrodes. Electrochim. Acta 2017, 229, 219–228. [Google Scholar] [CrossRef]
- SeVilla, M.; Sanchís, C.; Valdés-Solís, T.; Morallõn, E.; Fuertes, A.B. Direct synthesis of graphitic carbon nanostructures from saccharides and their use as electrocatalytic supports. Carbon 2008, 46, 931–939. [Google Scholar] [CrossRef]
- Ōya, A.; Marsh, H. Phenomena of catalytic graphitization. J. Mater. Sci. 1982, 17, 309–322. [Google Scholar] [CrossRef]
- Li, S.; Tian, C.G.; Li, M.T.; Meng, X.Y.; Wang, L.; Wang, R.H.; Yin, J.; Fu, H.G. From coconut shell to porous graphene-like nanosheets for high-power supercapacitors. J. Mater. Chem. A 2013, 1, 6462–6470. [Google Scholar]
- Ohtsuka, Y.; Xu, C.; Kong, D.; Tsubouchi, N. Decomposition of ammonia with iron and calcium catakysts supported on coal chars. Fuel 2004, 83, 685–692. [Google Scholar] [CrossRef]
- Mori, H.; Asmi, A.K.; Ohtsuka, Y. Role of iron catalyst in fate of fuel nitrogen during coal pyrolysis. Energy Fuel 1996, 10, 1022–1027. [Google Scholar] [CrossRef]
- Yu, Z.L.; Xin, S.; You, Y.; Yu, L.; Lin, Y.; Xu, D.W.; Qiao, C.; Huang, Z.H.; Yang, N.; Yu, S.H.; et al. Ion-catalyzed synthesis of microporous hard carbon embedded with expanded nanographite for enhanced lithium/sodium storage. J. Am. Chem. Soc. 2016, 138, 14915–14922. [Google Scholar] [CrossRef]
- Tahmasebi, A.; Yu, J.L.; Han, Y.N.; Li, X.C. A study of chemical structure changes of Chinese lignite during fluidized-bed drying in nitrogen and air. Fuel Process. Technol. 2012, 101, 85–93. [Google Scholar] [CrossRef]
- Aida, T. Reliable chemical determination of oxygen-containing functionalities in coal and coal products. Carboxylic acid and phenolic hydroxyl functionalities. Fuel Energy Abstr. 1996, 37, 411. [Google Scholar]
- Liu, D.D.; Gao, J.H.; Cao, Q.X.; Wu, S.H.; Qin, Y.K. Improvement of activated carbon from Jixi bituminous coal by air preoxidation. Energy Fuel 2017, 31, 1406–1415. [Google Scholar] [CrossRef]
- Liu, D.D.; Jia, B.Y.; Liu, X.J.; Zhao, B.J.; Gao, J.H.; Cao, Q.X.; Wu, S.H.; Qin, Y.K. Effects of oxygen functional groups and FeCl3 on the evolution of physico-chemical structure in activated carbon obtained from Jixi bituminous coal. RSC Adv. 2018, 8, 8569–8579. [Google Scholar] [CrossRef]
- Menéndez, J.A.; Arenillas, A.; Fidalgo, B.; Fernández, Y.; Zubizarreta, L.; Calvo, E.G.; Bermúdez, J.M. Microwave heating processes involving carbon materials. Fuel Process. Technol. 2010, 91, 1–8. [Google Scholar] [CrossRef]
- Menéndez, J.A.; Menéndez, E.M.; Iglesias, M.J.; García, A.; Pis, J.J. Modification of the surface chemistry of active carbons by means of microwave-induced treatments. Carbon 1999, 37, 1115–1121. [Google Scholar] [CrossRef]
- Valente Nabais, J.M.; Carrott, P.J.M.; Ribeiro Carrott, M.M.L.; Menéndez, J.A. Preparation and modification of activated carbon fibres by microwave heating. Carbon 2004, 42, 1315–1320. [Google Scholar] [CrossRef]
- Gong, X.Z.; Guo, Z.C.; Wang, Z. Variation of char structure during anthracite pyrolysis catalyzed by Fe2O3 and its influence on char combustion reactivity. Energy Fuels 2009, 23, 4547–4552. [Google Scholar] [CrossRef]
- Yang, K.B.; Peng, J.H.; Xia, H.Y.; Zhang, L.B.; Srinivasakannan, C.; Guo, S.H. Textural characteristics of activated carbon by single step CO2 activation from coconut shells. J. Taiwan Inst. Chem. Eng. 2010, 41, 367–372. [Google Scholar] [CrossRef]
- Pietrzak, R. XPS study and physico-chemical properties of nitrogen-enriched microporous activated carbon from high volatile bituminous coal. Fuel 2009, 88, 1871–1877. [Google Scholar] [CrossRef]
- Nomura, S.; Thomas, K.M. Fundamental aspects of coal structural changes in the thermoplastic phase. Fuel 1998, 77, 829–836. [Google Scholar] [CrossRef]
- Meng, F.R.; Yu, J.L.; Tahmasebi, A.; Han, Y.N.; Zhao, H.; Lucas, J. Characteristics of chars from low-temperature pyrolysis of lignite. Energy Fuels 2014, 28, 275–284. [Google Scholar] [CrossRef]
- Ibarra, J.; Muñoz, E.; Moliner, R. FTIR study of the evolution of coal structure during the coaification process. Org. Geochem. 1996, 24, 725–735. [Google Scholar] [CrossRef]
- Li, T.; Zhang, L.; Li, D. Effects of gasification atmosphere and temperature on char structural evolution during the gasification of collie sub-bituminous coal. Fuel 2014, 117, 1190–1195. [Google Scholar] [CrossRef]
- Sasezky, A.; Muckenhuber, H.; Grothe, H. Raman microspectroscopy of soot and related carbonaceous materials: Spectral analysis and structural information. Carbon 2005, 43, 1731–1742. [Google Scholar] [CrossRef]
- Wu, D.; Liu, G.; Chen, S.; Sun, R. An experimental investigation on heating rate effect in the thermal behavior of perhydrous bituminous coal during pyrolysis. J. Therm. Anal. Calorim. 2015, 119, 2195–2203. [Google Scholar] [CrossRef]
- Liu, D.D.; Gao, J.H.; Wu, S.H.; Qin, Y.K. Effect of char structures caused by varying the amount of FeCl3 on the pore development during activation. RSC Adv. 2016, 6, 87478–87485. [Google Scholar] [CrossRef]
- Wu, Y.Q.; Wang, J.J.; Wu, S.Y.; Huang, S.; Gao, J.S. Potassium-catalyzed steam gasification of petroleum coke for H2 production: Reactivity, selectivity and gas release. Fuel Process. Technol. 2011, 92, 523–530. [Google Scholar] [CrossRef]
- Gong, Y.N.; Li, D.L.; Luo, C.Z.; Fu, Q.; Pan, C.X. Highly porous graphitic biomass carbon as advanced electrode materials for supercapacitors. Green Chem. 2017, 19, 4132–4140. [Google Scholar] [CrossRef]
- Wang, L.J.; Sun, F.; Gao, J.H.; Pi, X.X.; Pei, T.; Qie, Z.P.; Zhao, G.B.; Qin, Y.K. A novel melt infiltration method promoting porosity development of low-rank coal derived activated carbon as supercapacitor electrode materials. J. Taiwan Inst. Chem. Eng. 2018, 91, 588–596. [Google Scholar] [CrossRef]

| Vad | FCad | Aad | Mad | Cdaf | Hdaf | Odaf * | Ndaf | Sdaf |
|---|---|---|---|---|---|---|---|---|
| 39.66 | 39.66 | 0.12 | 3.62 | 74.81 | 19.49 | 4.01 | 1.31 | 0.38 |
| HLH | HLH-1.5 | HLH-5 | HLH-30 | |
|---|---|---|---|---|
| C=O/Car | 2.10 | 1.73 | 0.15 | 0.12 |
| COOH/Car | 1.12 | 0.46 | 0.07 | 0.06 |
| C-O/Car | 2.31 | 1.83 | 0.14 | 0.11 |
| C-HLH-5 | C-1FeHLH-5(H) | C-2FeHLH-5(H) | C-3FeHLH-5(H) | |
|---|---|---|---|---|
| AD1/AG | 3.763 | 2.841 | 1.107 | 1.245 |
| AD3/AG | 2.707 | 1.811 | 0.505 | 0.614 |
| AD4/AG | 0.845 | 0.769 | 0.518 | 0.603 |
| AD1/AD3 | 1.390 | 1.568 | 2.137 | 2.028 |
| C-HLH-5 | C-1FeHLH-5(H) | C-2FeHLH-5(H) | C-3FeHLH-5(H) | |
|---|---|---|---|---|
| La (Å) | 23.66 | 25.71 | 31.28 | 28.55 |
| Lc(Å) | 12.33 | 13.17 | 15.85 | 14.01 |
| d002(Å) | 3.55 | 3.40 | 3.14 | 3.29 |
| N | 3.47 | 3.87 | 5.05 | 4.26 |
| Samples | SBET (m2/g) | Vt (m3/g) | Vmic (m3/g) | Non-Vmic (%) |
|---|---|---|---|---|
| AC-HLH-5-15.4(H) | 103.63 | 0.062 | 0.059 | 4.84 |
| AC-HLH-5-28.3(H) | 321.77 | 0.130 | 0.101 | 22.31 |
| AC-HLH-5-55.6(H) | 666.25 | 0.201 | 0.113 | 43.78 |
| AC-2FeHLH-5-16.3(H) | 652.55 | 0.168 | 0.160 | 4.76 |
| AC-2FeHLH-5-29.4(H) | 1048.15 | 0.259 | 0.245 | 5.41 |
| AC-2FeHLH-5-41.4(H) | 1852.43 | 0.378 | 0.356 | 5.82 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, D.; Zhao, X.; Su, R.; Hao, Z.; Jia, B.; Li, S.; Dong, L. Highly Porous Graphitic Activated Carbons from Lignite via Microwave Pretreatment and Iron-Catalyzed Graphitization at Low-Temperature for Supercapacitor Electrode Materials. Processes 2019, 7, 300. https://doi.org/10.3390/pr7050300
Liu D, Zhao X, Su R, Hao Z, Jia B, Li S, Dong L. Highly Porous Graphitic Activated Carbons from Lignite via Microwave Pretreatment and Iron-Catalyzed Graphitization at Low-Temperature for Supercapacitor Electrode Materials. Processes. 2019; 7(5):300. https://doi.org/10.3390/pr7050300
Chicago/Turabian StyleLiu, Dongdong, Xiaoman Zhao, Rui Su, Zhengkai Hao, Boyin Jia, Song Li, and Liangjie Dong. 2019. "Highly Porous Graphitic Activated Carbons from Lignite via Microwave Pretreatment and Iron-Catalyzed Graphitization at Low-Temperature for Supercapacitor Electrode Materials" Processes 7, no. 5: 300. https://doi.org/10.3390/pr7050300
APA StyleLiu, D., Zhao, X., Su, R., Hao, Z., Jia, B., Li, S., & Dong, L. (2019). Highly Porous Graphitic Activated Carbons from Lignite via Microwave Pretreatment and Iron-Catalyzed Graphitization at Low-Temperature for Supercapacitor Electrode Materials. Processes, 7(5), 300. https://doi.org/10.3390/pr7050300





