Phenomenological Over-Parameterization of the Triple-Fitting-Parameter Diffusion Models in Evaluation of Gas Diffusion in Coal
Abstract
:1. Introduction
2. Summary of the TFP Models
2.1. Ruckenstein Bidisperse Model
2.2. Fickian Diffusion-Relaxation Model
2.3. Double Exponential Model
3. Experimental Measurements of Fractional Sorption Curves
3.1. Sample
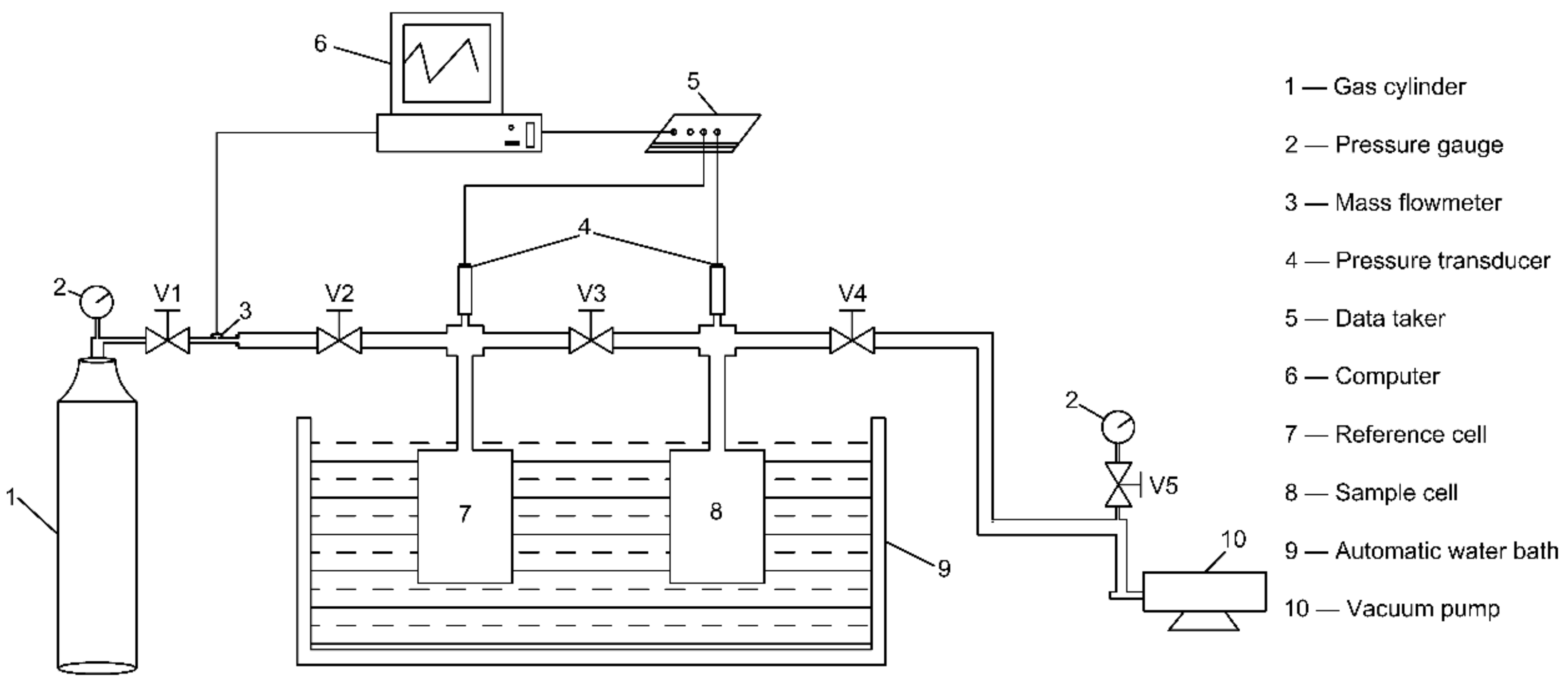
3.2. Experimental Setup
3.3. Measurement Procedures
- (1)
- Implement the sample into the sample cell.
- (2)
- Evacuate the air in the system and shut all valves.
- (3)
- Opening V1.
- (4)
- Open V2 slowly and inject CH4 gas from a gas cylinder into the reference cell.
- (5)
- When the gas pressure in the reference cell increased to about 1.0 MPa, close V2 and open V3 rapidly.
- (6)
- Record gas pressure change in the sample cell with a time interval of one second.
- (7)
- When the gas pressure in the sample cell kept invariant in two hours, terminate recording the gas pressure and close V3.
- (8)
- Repeat (3)–(6) two times.
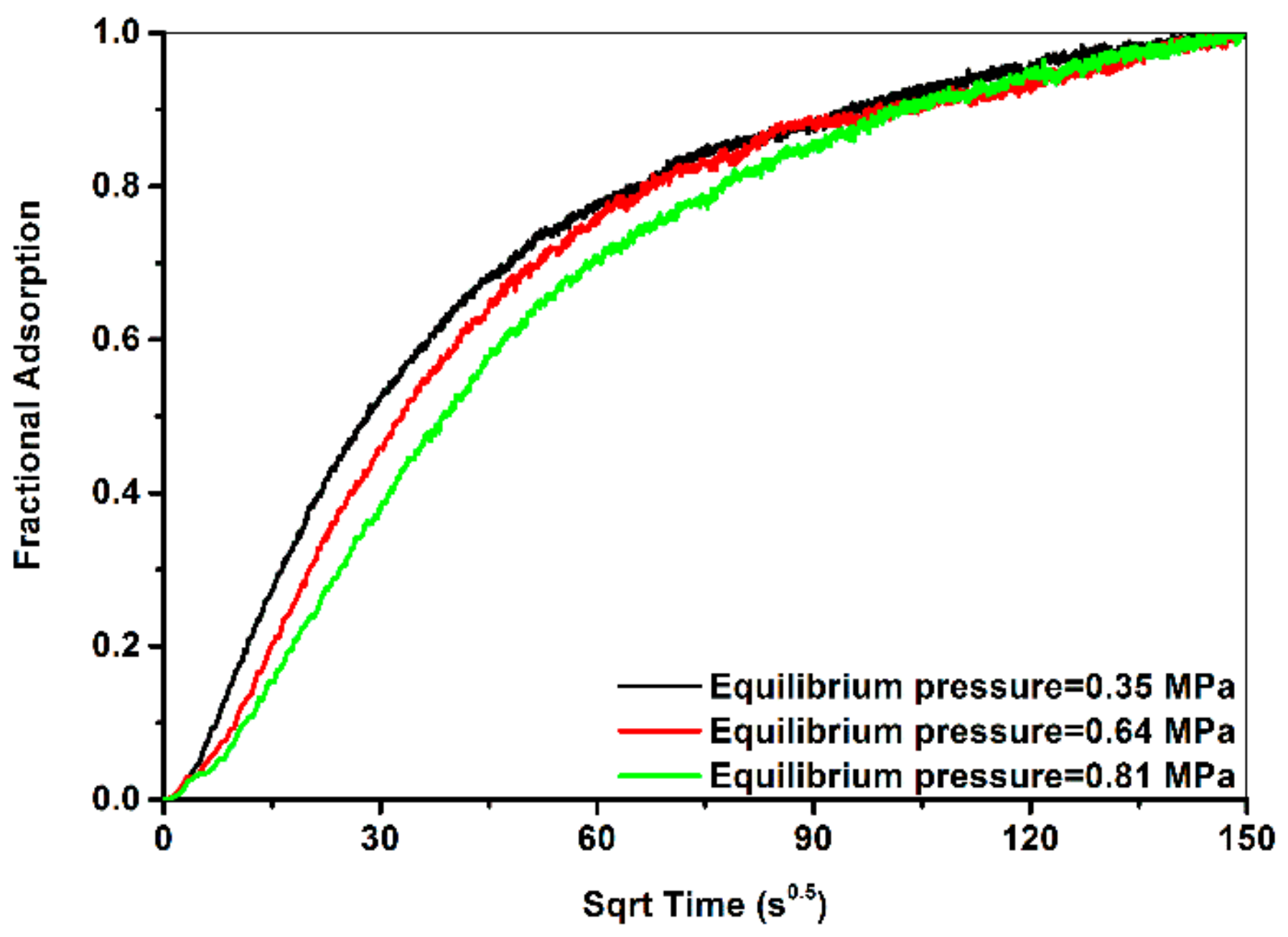
3.4. Measured Fractional Adsorption Curves
4. Modeling the Measured Fractional Adsorption Curves with the TFP Models
4.1. Modeling Configuration
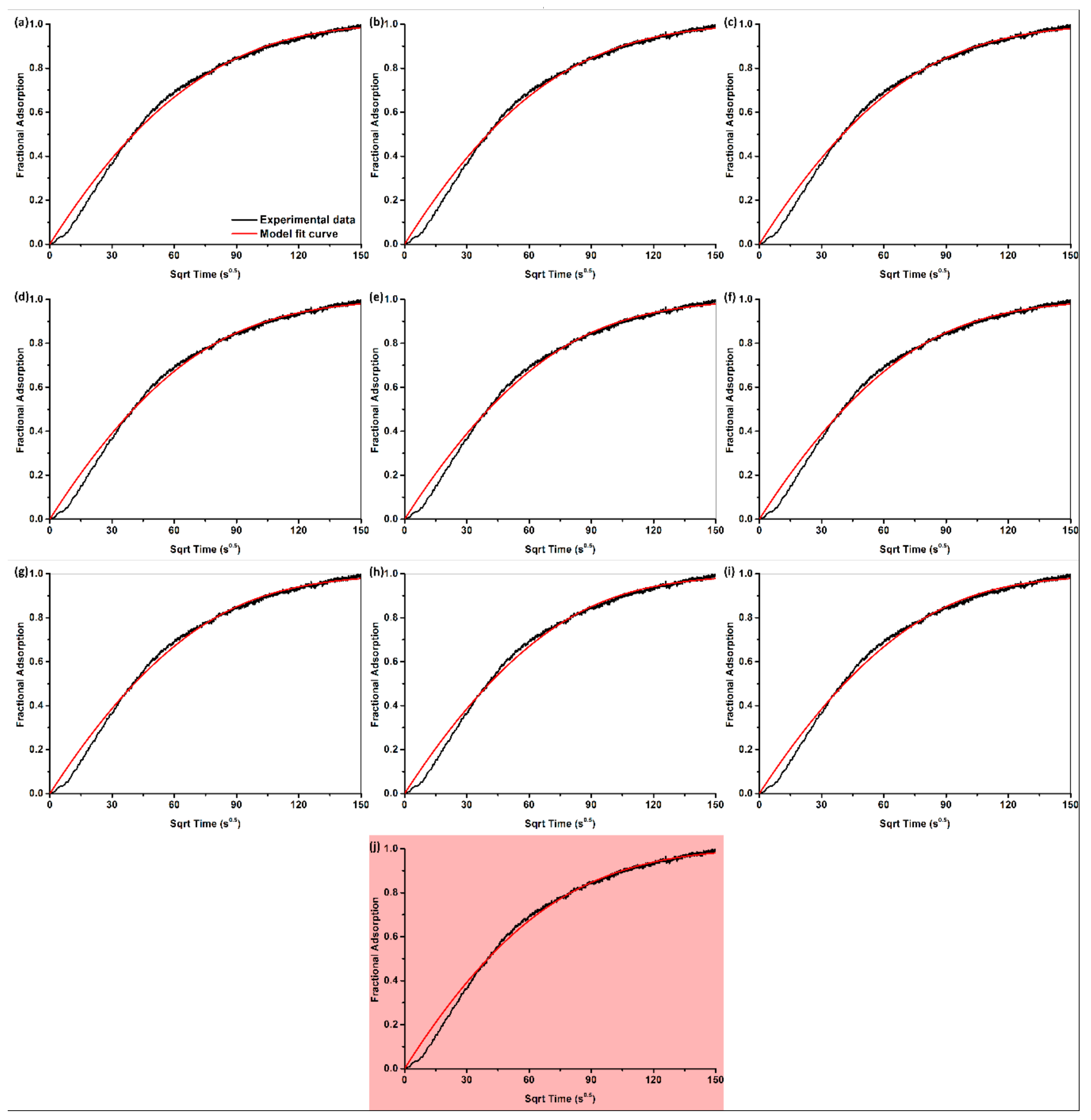
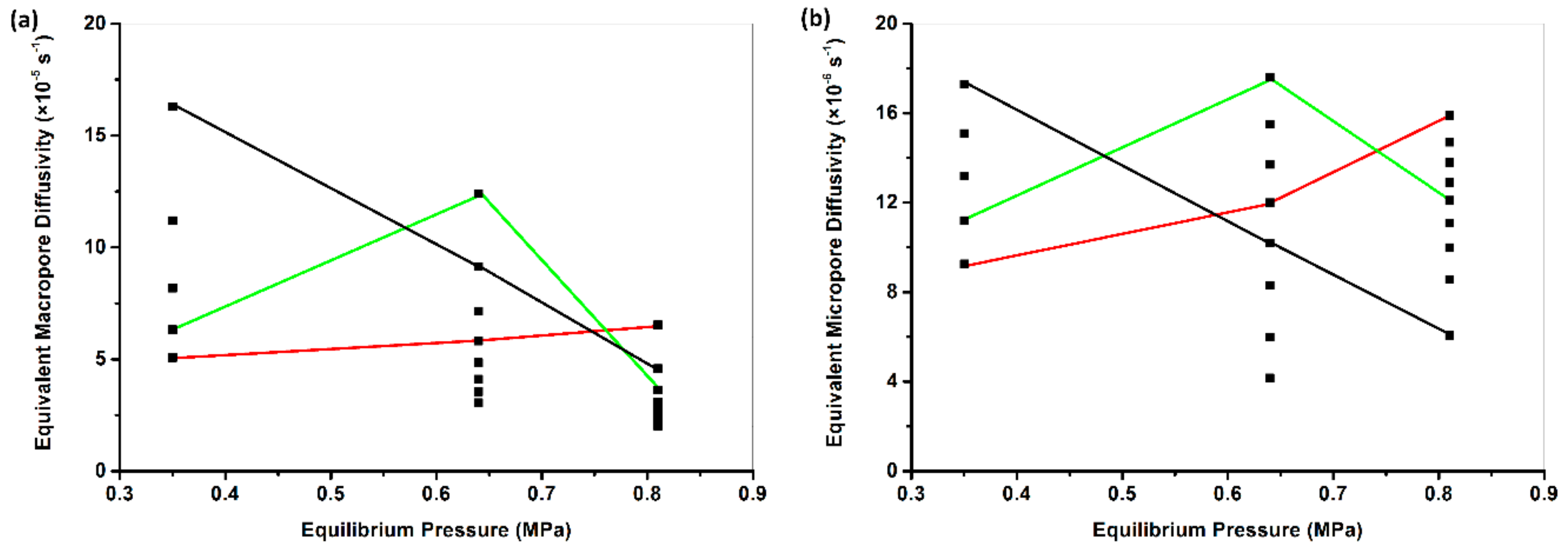
4.2. Modeling Results
5. Discussion
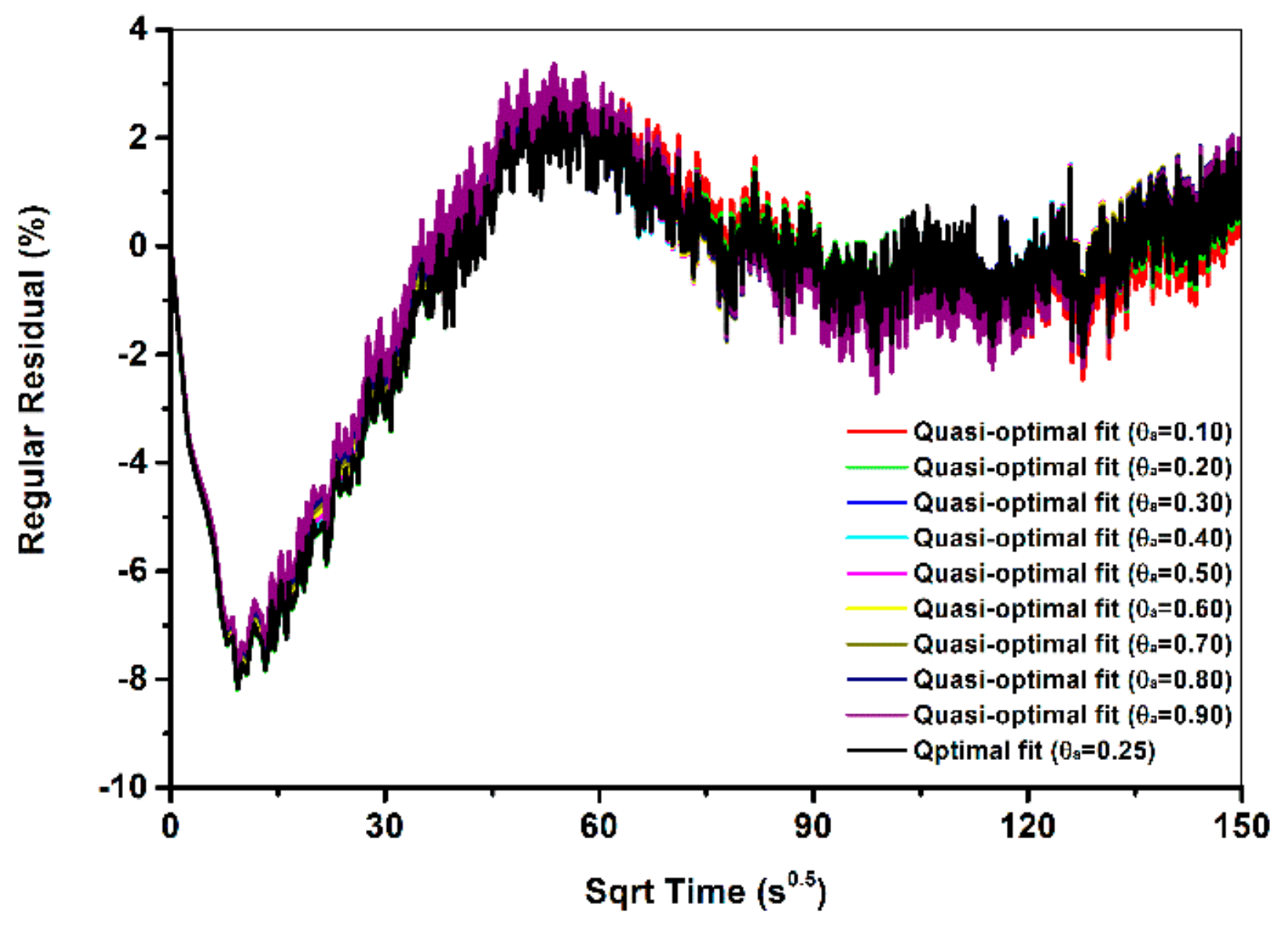
5.1. Phenomenological Over-Parameterization of the TFP Models
5.2. Implications for Future Work
6. Conclusions
- (1)
- The measured adsorption rate was negatively proportional to gas pressure. This correlation can be attributed to coal swelling and decreasing molecular diffusion rate with increasing gas pressure.
- (2)
- The TFP models had better performance in curve fitting than the UP model did. The gas pressure dependence of diffusivity/rate parameter was dependent on the chosen model. The UP-diffusivity and the RB-diffusivity with the assumption of 0 < < 10−3 decreased with increasing gas pressure. The RB-macropore diffusivity with the assumption of 0 < < 1.0 and the DE-fast rate parameter were also negatively proportional to gas pressure. However, the RB-micropore diffusivity with the assumption of 0 < < 1.0, the FDR-diffusivity and relaxation rate, and the DE-slow rate parameter showed non-monotonic dependence on gas pressure.
- (3)
- In addition to optimal fits, the TFP model produced multiple quasi-optimal fits that were highly close to the corresponding optimal fit in fit curves. This issue was defined as phenomenological over-parameterization in this paper. Phenomenological over-parameterization makes optimal fit non-unique and all optimal and quasi-optimal fits re potentially optimal. Phenomenological over-parameterization also makes the modeling results of the TFP models uninterpretable in physics.
- (4)
- Expanding the upper bound of made the RB model phenomenologically over-parameterized in curve fitting. This misusage induced the literature-reported anomalous non-monotonic dependence of diffusivity on gas and coal properties such as gas pressure, moisture content, and particle size. Although the correct usage of RB model with the assumption of 0 < < 10−3 is absent of phenomenological over-parameterization, anomalously high macropore adsorption fraction was regressed when fitting to the fractional adsorption curves measured in this study.
- (5)
- Although the TFP models have better performance in curve fitting than the UP model does, their modeling results are seemingly uninterpretable in physics. Their excellent curve fitting performance may be due to the usage of three fitting parameters. Clarkson and Bustin [14] developed a numerical bidisperse diffusion model that has only two fitting parameters. This also has excellent performance in curve fitting and the results can be physically interpretable. Therefore, this numerical bidisperse diffusion model may be a good alternative to the TFP models in future studies.
Author Contributions
Funding
Acknowledgement
Conflicts of Interest
Nomenclature
| Sorbate concentration in macropores mol·m−3 | |
| Sorbate concentration in micropores, mol·m−3 | |
| Sorbate concentration on macropore surfaces, mol·m−2 | |
| Sorbate concentration on micropore surfaces, mol·m−2 | |
| Macropore diffusion coefficient, m2·s−1 | |
| Equivalent macropore diffusivity, s−1 | |
| Equivalent Fickian diffusivity in the FDR model, s−1 | |
| Micropore diffusion coefficient, m2·s−1 | |
| Equivalent micropore diffusivity, s−1 | |
| Equivalent unipore diffusivity, s−1 | |
| Henry constant, m3·m−2 | |
| Fast sorption rate parameter in the DE model, s−1 | |
| Relaxation rate parameter in the FDR model, s−1 | |
| Slow sorption rate parameter in the DE model, s−1 | |
| Initial sorption amount, mol | |
| Sorption amount at time t, mol | |
| Sorption amount at infinite time, mol | |
| Initial gas pressure, MPa | |
| Gas pressure at time , MPa | |
| Final gas pressure, MPa | |
| Residual (unoccupied) sorption capacity in the DE model, mol | |
| Universal gas constant, J·mol−1·K−1 | |
| Macropore radius, m | |
| Microsphere radius, m | |
| Macropore surface area, m2 | |
| Temperature, K | |
| Void volume of the experimental system, m3 | |
| Compressibility factors at , dimensionless | |
| Compressibility factors at , dimensionless | |
| Compressibility factors at , dimensionless | |
| Ratio of equivalent micropore diffusivity to equivalent macropore diffusivity, fraction | |
| Macropore sorption fraction in the RB model, fraction | |
| Sorption fraction of the fast sorption stage in the DE model, fraction | |
| Sorption fraction due to Fickian diffusion in the FDR model, fraction | |
| Macroporosity, fraction | |
| Microporosity, fraction |
References
- Moore, T. Coalbed methane: A review. Int. J. Coal Geol. 2012, 101, 36–81. [Google Scholar] [CrossRef]
- Busch, A.; Gensterblum, Y. CBM and CO2-ECBM related sorption processes in coal: A review. Int. J. Coal Geol. 2011, 87, 49–71. [Google Scholar] [CrossRef]
- Bustin, R.; Clarkson, C. Geological controls on coalbed methane reservoir capacity and gas content. Int. J. Coal Geol. 1998, 38, 3–26. [Google Scholar] [CrossRef]
- Bodden, W.R.; Ehrlich, R. Permeability of coals and characteristics of desorption tests: Implications for coalbed methane production. Int. J. Coal Geol. 1998, 35, 333–347. [Google Scholar] [CrossRef]
- Li, D.Y.; Liu, Q.F.; Weniger, P.; Gensterblum, Y.; Busch, A.; Krooss, B. High-pressure sorption isotherms and sorption kinetics of CH4 and CO2 on coals. Fuel 2010, 89, 569–580. [Google Scholar] [CrossRef]
- Crosdale, P.; Beamish, B.; Valix, M. Coalbed methane sorption related to coal composition. Int. J. Coal Geol. 1998, 35, 147–158. [Google Scholar] [CrossRef]
- Smith, D.M.; Williams, F.L. Diffusion models for gas production from coal: Determination of diffusion parameters. Fuel 1984, 63, 256–261. [Google Scholar] [CrossRef]
- Bhowmik, S.; Dutta, P. Adsorption rate characteristics of methane and CO2 in coal samples from Raniganj and Jharia coalfields of India. Int. J. Coal Geol. 2013, 113, 50–59. [Google Scholar] [CrossRef]
- Pan, Z.J.; Connell, L. Modelling permeability for coal reservoirs: A review of analytical models and testing data. Int. J. Coal Geol. 2012, 92, 1–44. [Google Scholar] [CrossRef]
- Wang, K.; Zang, J.; Wang, G.D.; Zhou, A.T. Anisotropic permeability evolution of coal with effective stress variation and gas sorption: Model development and analysis. Int. J. Coal Geol. 2014, 130, 53–65. [Google Scholar] [CrossRef]
- Zang, J.; Wang, K. A numerical model for simulating single-phase gas flow in anisotropic coal. J. Nat. Gas Sci. Eng. 2016, 28, 153–172. [Google Scholar] [CrossRef]
- Zang, J.; Wang, K. Gas sorption-induced coal swelling kinetics and its effects on coal permeability evolution: Model development and analysis. Fuel 2017, 189, 164–177. [Google Scholar] [CrossRef]
- Wang, K.; Zang, J.; Feng, Y.; Wu, Y. Effects of moisture on diffusion kinetics in Chinese coals during methane desorption. J. Nat. Gas Sci. Eng. 2014, 21, 1005–1014. [Google Scholar] [CrossRef]
- Clarkson, C.; Bustin, R. The effect of pore structure and gas pressure upon the transport properties of coal: A laboratory and modeling study. 2. Adsorption rate modeling. Fuel 1999, 78, 1345–1362. [Google Scholar] [CrossRef]
- Nandi, S.P.; Walker, J.; Philip, L. Activated diffusion of methane from coals at elevated pressures. Fuel 1975, 54, 81–86. [Google Scholar] [CrossRef]
- Pillalamarry, M.; Harpalani, S.; Liu, S. Gas diffusion behavior of coal and its impact on production from coalbed methane reservoirs. Int. J. Coal Geol. 2011, 86, 342–348. [Google Scholar] [CrossRef]
- Staib, G.; Sakurovs, R.; Gray, E. Dispersive diffusion of gases in coals. Part II: An assessment of previously proposed physical mechanisms of diffusion in coal. Fuel 2015, 143, 620–629. [Google Scholar] [CrossRef]
- Staib, G.; Sakurovs, R.; Gray, E. Dispersive diffusion of gases in coals. Part I: Model development. Fuel 2015, 143, 612–619. [Google Scholar] [CrossRef]
- Charrière, D.; Pokryszka, Z.; Behra, P. Effect of pressure and temperature on diffusion of CO2 and CH4 into coal from the Lorraine basin (France). Int. J. Coal Geol. 2010, 81, 373–380. [Google Scholar] [CrossRef]
- Švábová, M.; Weishauptová, Z.; Přibyl, O. The effect of moisture on the sorption process of CO2 on coal. Fuel 2012, 92, 187–196. [Google Scholar] [CrossRef]
- Zhang, J. Experimental Study and Modeling for CO2 Diffusion in Coals with Different Particle Sizes: Based on Gas Absorption (Imbibition) and Pore Structure. Energy Fuels 2016, 30, 531–543. [Google Scholar] [CrossRef]
- Pan, Z.J.; Connell, L.; Camilleri, M.; Connelly, L. Effects of matrix moisture on gas diffusion and flow in coal. Fuel 2010, 89, 3207–3217. [Google Scholar] [CrossRef]
- Zheng, G.Q.; Pan, Z.J.; Tang, S.H.; Ling, B.C.; Lv, D.W.; Connell, L. Laboratory and modeling study on gas diffusion with pore structures in different-rank Chinese coals. Energy Explor. Exploit. 2013, 31, 859–877. [Google Scholar] [CrossRef]
- Guo, J.; Kang, T.; Kang, J.; Zhao, G.; Huang, Z. Effect of the lump size on methane desorption from anthracite. J. Nat. Gas Sci. Eng. 2014, 20, 337–346. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion; Clarendon Press: Gloucestershire, UK, 1975. [Google Scholar]
- Ruckenstein, E.; Vaidyanathan, A.; Youngquist, G. Sorption by solids with bidisperse pore structures. Chem. Eng. Sci. 1971, 26, 1305–1318. [Google Scholar] [CrossRef]
- Berens, A.; Hopfenberg, H. Diffusion and relaxation in glassy polymer powders: 2. Separation of diffusion and relaxation parameters. Polymer 1978, 19, 489–496. [Google Scholar] [CrossRef]
- Busch, A.; Gensterblum, Y.; Krooss, B.; Littke, R. Methane and carbon dioxide adsorption–diffusion experiments on coal: Upscaling and modeling. Int. J. Coal Geol. 2004, 60, 151–168. [Google Scholar] [CrossRef]
- Karacan, C.O. An effective method for resolving spatial distribution of adsorption kinetics in heterogeneous porous media: Application for carbon dioxide sequestration in coal. Chem. Eng. Sci. 2003, 58, 4681–4693. [Google Scholar] [CrossRef]
- Cui, X.J.; Bustin, R.; Dipple, G. Selective transport of CO2, CH4, and N2 in coals: Insights from modeling of experimental gas adsorption data. Fuel 2004, 83, 293–303. [Google Scholar] [CrossRef]
- Staib, G.; Sakurovs, R.; Gray, E. A pressure and concentration dependence of CO2 diffusion in two Australian bituminous coals. Int. J. Coal Geol. 2013, 116–117, 106–116. [Google Scholar] [CrossRef]
- OriginLab Corp. OriginPro 9.0 User Guide. 2015. Available online: http://www.originlab.com/doc/User-Guide (accessed on 20 March 2019).
- Radliński, A.P.; Busbridge, T.L.; Gray, E.M.A.; Blach, T.P.; Cookson, D.J. Small angle X-ray scattering mapping and kinetics study of sub-critical CO2 sorption by two Australian coals. Int. J. Coal Geol. 2009, 77, 80–89. [Google Scholar]
| Model Classification | Model Name | Authors |
|---|---|---|
| Single-fitting-parameter model | Unipore model | Crank [25] |
| Numerical unipore model | Clarkson and Bustin [14] | |
| Linear driving force model | Charrière et al. [19] | |
| Double-fitting-parameter model | Stretched exponential model | Staib et al. [17], Staib et al. [18] |
| Numerical bidisperse model | Clarkson and Bustin [14] | |
| Triple-fitting-parameter model | Ruckenstein bidisperse model | Ruckenstein et al. [26] |
| Fickian diffusion-relaxation model | Berens and Hopfenberg [27] | |
| Double exponential model | Busch et al. [28] |
| True Density (g/cm3, Dry) | Apparent Density (g/cm3, Dry) | Proximate Analysis | |||
|---|---|---|---|---|---|
| Mad (%) | Ad (%) | Vdaf (%) | Fixed Carbon (%) | ||
| 1.49 | 1.40 | 0.61 | 11.02 | 7.50 | 80.87 |
| First Injection | Second Injection | Third Injection | |||
|---|---|---|---|---|---|
| Maximum gas pressure (MPa) | Equilibrium gas pressure (MPa) | Maximum gas pressure (MPa) | Equilibrium gas pressure (MPa) | Maximum gas pressure (MPa) | Equilibrium gas pressure (MPa) |
| 0.60 | 0.35 | 0.80 | 0.64 | 0.89 | 0.81 |
| Model | Fit Parameters | Equilibrium Pressure (MPa) | ||
|---|---|---|---|---|
| 0.35 | 0.64 | 0.81 | ||
| UP model | (s−1) | 2.59 × 10−5 | 2.24 × 10−5 | 1.75 × 10−5 |
| (%) | 94.81 | 96.01 | 99.15 | |
| RB model (0 < < 10−3) | θa | 0.962 | 0.956 | 0.986 |
| (s−1) | 3.18 × 10−5 | 2.82 × 10−5 | 1.86 × 10−5 | |
| (s−1) | 3.18 × 10−8 | 2.82 × 10−8 | 1.86 × 10−8 | |
| (%) | 97.02 | 98.12 | 99.27 | |
| RB model (0 < < 1.0) | θa | 0.416 | 0.527 | 0.250 |
| (s−1) | 1.06 × 10−4 | 5.52 × 10−5 | 4.03 × 10−5 | |
| (s−1) | 1.48 × 10−5 | 1.15 × 10−5 | 1.42 × 10−5 | |
| (%) | 99.71 | 99.21 | 99.42 | |
| FDR model | θF | 0.63 | 0.69 | 0.52 |
| (s−1) | 6.45 × 10−4 | 3.74 × 10−4 | 3.75 × 10−4 | |
| (s−1) | 9.36 × 10−4 | 7.35 × 10−4 | 8.99 × 10−4 | |
| (%) | 99.75 | 99.28 | 99.56 | |
| DE model | θf | 0.58 | 0.62 | 0.48 |
| (s−1) | 1.28 × 10−2 | 7.58 × 10−3 | 6.87 × 10−3 | |
| (s−1) | 1.03 × 10−3 | 8.43 × 10−4 | 9.25 × 10−4 | |
| (%) | 99.53 | 99.46 | 99.84 | |
| Equilibrium Pressure (MPa) | θa | |||
|---|---|---|---|---|
| 0.01 | 1.08 × 10−1 | 1.08 × 10−4 | 99.15 |
| Equilibrium Pressure (MPa) | θa | |||
|---|---|---|---|---|
| 0.35 | 0.30 | 1.63 × 10−4 | 1.73 × 10−5 | 99.45 |
| 0.40 | 1.12 × 10−4 | 1.51 × 10−5 | 99.71 | |
| 0.50 | 8.18 × 10−5 | 1.32 × 10−5 | 99.61 | |
| 0.60 | 6.32 × 10−5 | 1.12 × 10−5 | 99.29 | |
| 0.70 | 5.07 × 10−5 | 9.26 × 10−6 | 98.84 | |
| 0.64 | 0.20 | 1.24 × 10−4 | 1.76 × 10−5 | 98.25 |
| 0.30 | 9.14 × 10−5 | 1.55 × 10−5 | 98.80 | |
| 0.40 | 7.14 × 10−5 | 1.37 × 10−5 | 99.10 | |
| 0.50 | 5.81 × 10−5 | 1.20 × 10−5 | 99.21 | |
| 0.60 | 4.85 × 10−5 | 1.02×10−5 | 99.18 | |
| 0.70 | 4.11 × 10−5 | 8.30 × 10−6 | 99.04 | |
| 0.80 | 3.55 × 10−5 | 5.98 × 10−6 | 98.80 | |
| 0.90 | 3.06 × 10−5 | 4.18 × 10−6 | 98.44 | |
| 0.81 | 0.10 | 6.53 × 10−5 | 1.59 × 10−5 | 99.37 |
| 0.20 | 4.58 × 10−5 | 1.47 × 10−5 | 99.41 | |
| 0.30 | 3.63 × 10−5 | 1.38 × 10−5 | 99.42 | |
| 0.40 | 3.09 × 10−5 | 1.29 × 10−5 | 99.40 | |
| 0.50 | 2.75 × 10−5 | 1.21 × 10−5 | 99.39 | |
| 0.60 | 2.51 × 10−5 | 1.11 × 10−5 | 99.38 | |
| 0.70 | 2.32 × 10−5 | 1.00 × 10−5 | 99.36 | |
| 0.80 | 2.16 × 10−5 | 8.56 × 10−6 | 99.34 | |
| 0.90 | 2.02 × 10−5 | 6.06 × 10−6 | 99.32 |
| Equilibrium Pressure (MPa) | θF | |||
|---|---|---|---|---|
| 0.35 | 0.50 | 1.14 × 10−3 | 1.21 × 10−3 | 98.96 |
| 0.60 | 7.26 × 10−4 | 9.93 × 10−4 | 99.71 | |
| 0.70 | 4.84 × 10−4 | 7.98 × 10−4 | 99.57 | |
| 0.80 | 3.36 × 10−4 | 6.05 × 10−4 | 98.90 | |
| 0.64 | 0.50 | 7.28 × 10−4 | 1.09 × 10−3 | 98.34 |
| 0.60 | 5.08 × 10−4 | 9.02 × 10−4 | 99.08 | |
| 0.70 | 3.68 × 10−4 | 7.26 × 10−4 | 99.28 | |
| 0.80 | 2.74 × 10−4 | 5.44 × 10−4 | 99.10 | |
| 0.90 | 2.07 × 10−4 | 3.05 × 10−4 | 98.65 | |
| 0.81 | 0.30 | 9.97 × 10−4 | 1.21 × 10−3 | 98.63 |
| 0.40 | 6.06 × 10−4 | 1.05 × 10−3 | 99.36 | |
| 0.50 | 4.01 × 10−4 | 9.20 × 10−4 | 99.56 | |
| 0.60 | 2.85 × 10−4 | 8.08 × 10−4 | 99.52 | |
| 0.70 | 2.15 × 10−4 | 7.06 × 10−4 | 99.43 | |
| 0.80 | 1.70 × 10−4 | 6.00 × 10−4 | 99.36 | |
| 0.90 | 1.39 × 10−4 | 4.54 × 10−4 | 99.32 |
| Equilibrium Pressure (MPa) | θf | |||
|---|---|---|---|---|
| 0.35 | 0.50 | 1.74 × 10−2 | 1.20 × 10−3 | 99.09 |
| 0.60 | 1.18 × 10−2 | 9.82 × 10−4 | 99.48 | |
| 0.64 | 0.50 | 1.09 × 10−2 | 1.08 × 10−3 | 98.80 |
| 0.60 | 8.00 × 10−3 | 8.78 × 10−4 | 99.45 | |
| 0.70 | 6.04 × 10−3 | 6.86 × 10−4 | 99.12 | |
| 0.81 | 0.40 | 9.07 × 10−3 | 1.04 × 10−3 | 99.65 |
| 0.50 | 6.45 × 10−3 | 8.96 × 10−4 | 99.83 | |
| 0.60 | 4.85 × 10−3 | 7.58 × 10−4 | 99.49 |
| Equilibrium Pressure (MPa) | ORB Model | ERB Model | FDR Model | DE Model | ||||
|---|---|---|---|---|---|---|---|---|
| 0.35 | 1.00 | 1.00 | 3.21 | 1.87 | 3.39 | 2.00 | 1.47 | 1.22 |
| 0.64 | 1.00 | 1.00 | 4.04 | 6.75 | 3.52 | 3.58 | 1.81 | 1.57 |
| 0.81 | 927.80 | 927.80 | 3.25 | 2.62 | 7.18 | 2.67 | 1.87 | 1.37 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zang, J.; Wang, K.; Liu, A. Phenomenological Over-Parameterization of the Triple-Fitting-Parameter Diffusion Models in Evaluation of Gas Diffusion in Coal. Processes 2019, 7, 241. https://doi.org/10.3390/pr7040241
Zang J, Wang K, Liu A. Phenomenological Over-Parameterization of the Triple-Fitting-Parameter Diffusion Models in Evaluation of Gas Diffusion in Coal. Processes. 2019; 7(4):241. https://doi.org/10.3390/pr7040241
Chicago/Turabian StyleZang, Jie, Kai Wang, and Ang Liu. 2019. "Phenomenological Over-Parameterization of the Triple-Fitting-Parameter Diffusion Models in Evaluation of Gas Diffusion in Coal" Processes 7, no. 4: 241. https://doi.org/10.3390/pr7040241
APA StyleZang, J., Wang, K., & Liu, A. (2019). Phenomenological Over-Parameterization of the Triple-Fitting-Parameter Diffusion Models in Evaluation of Gas Diffusion in Coal. Processes, 7(4), 241. https://doi.org/10.3390/pr7040241




