Novel Deep Eutectic Solvent Based on Levulinic Acid and 1,4-Butanediol as an Extraction Media for Bioactive Alkaloid Rutaecarpine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Preparation and Physicochemical Properties Tests of DESs
2.3. Extraction Procedure Using Different Solvents
2.4. Opimization of Extraction Conditions
2.5. HPLC-UV Analysis for Quantification of Rutaecarpine
2.6. NMR Spectroscopy
3. Results and Discussion
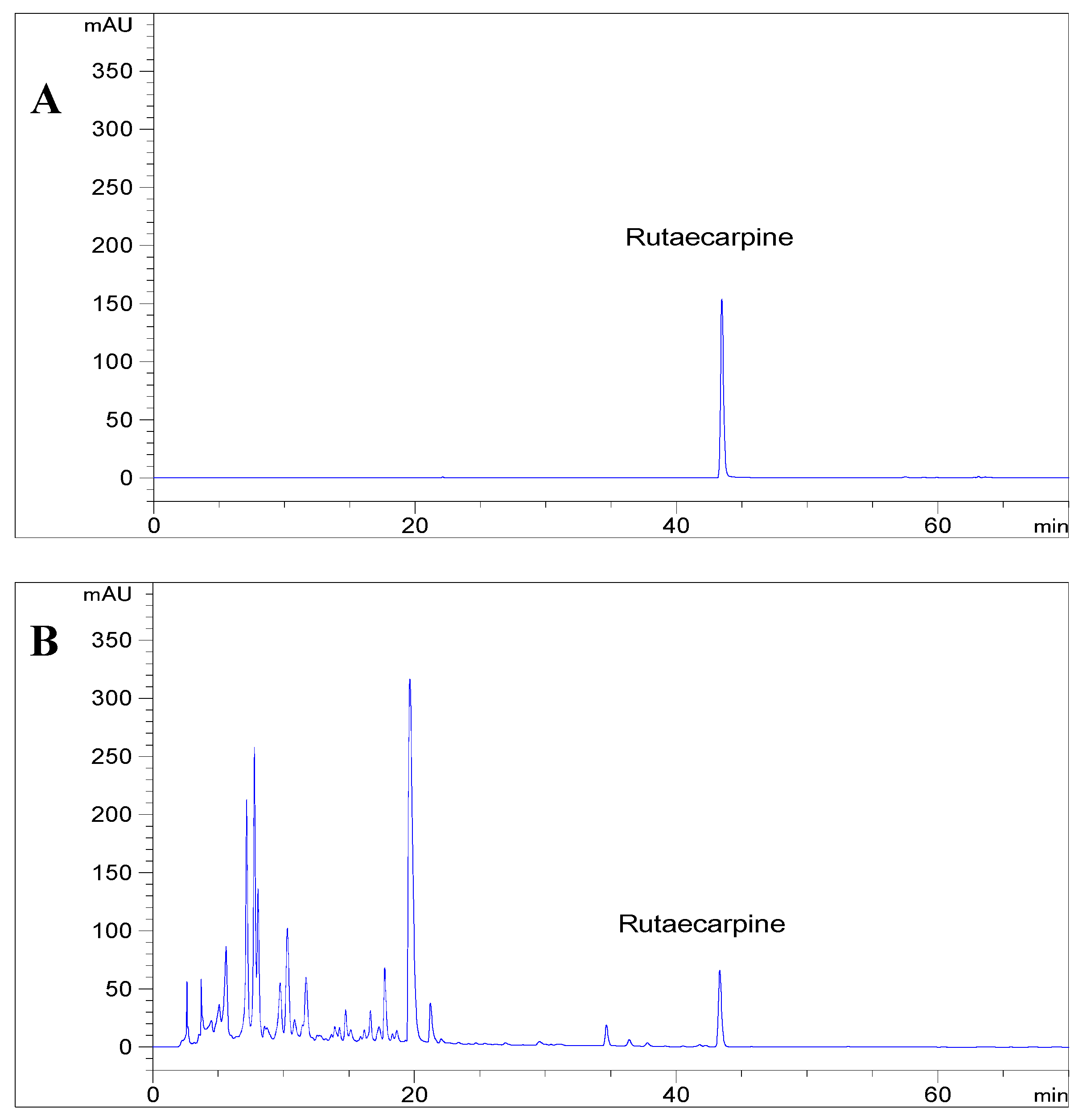
3.1. Evaluation of Analytical Method
3.2. Preparation of Various Types of DESs
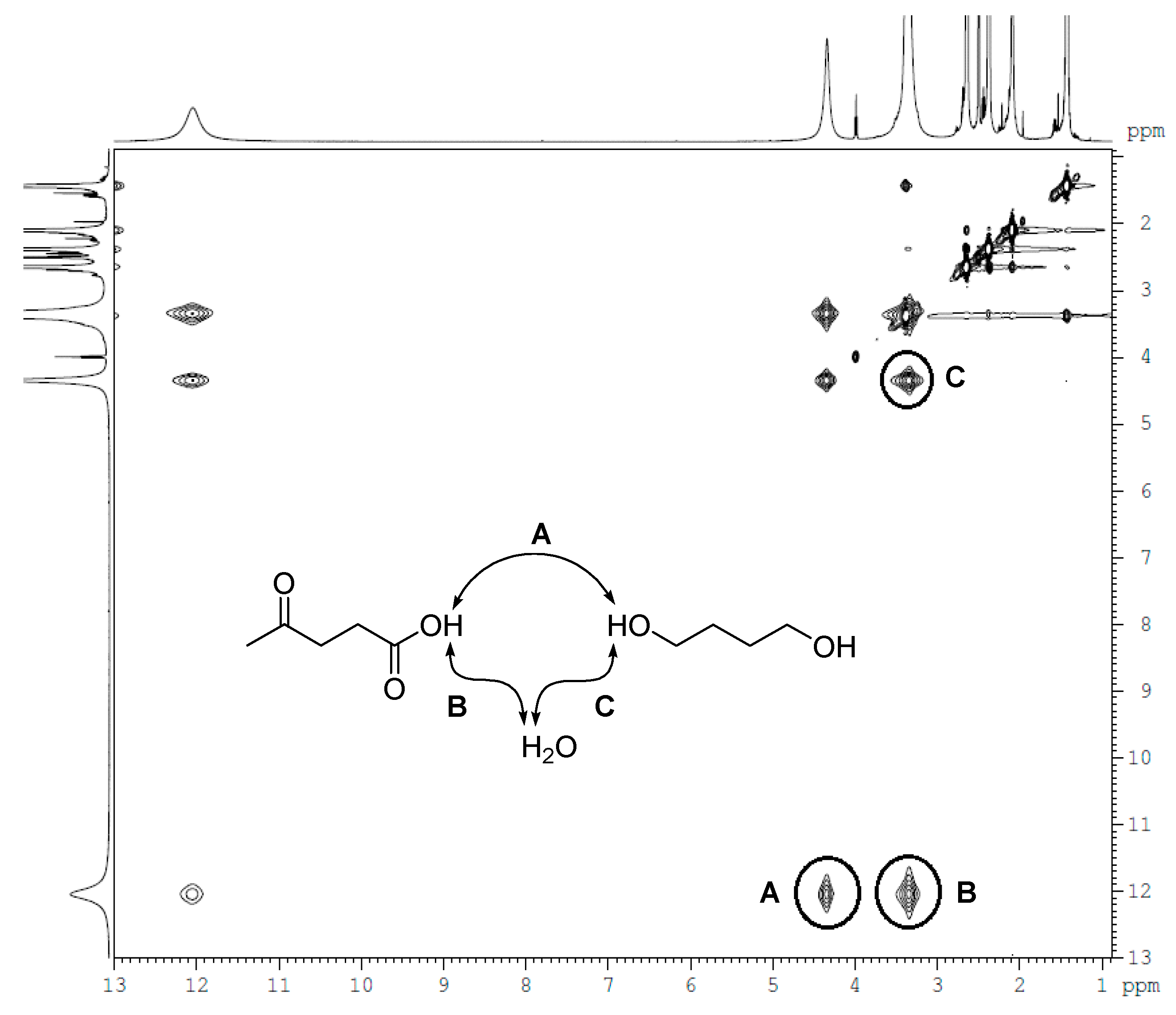
3.3. Supermolecular Structure of La-Buta DES
3.4. Selection of Initial Extraction Condition
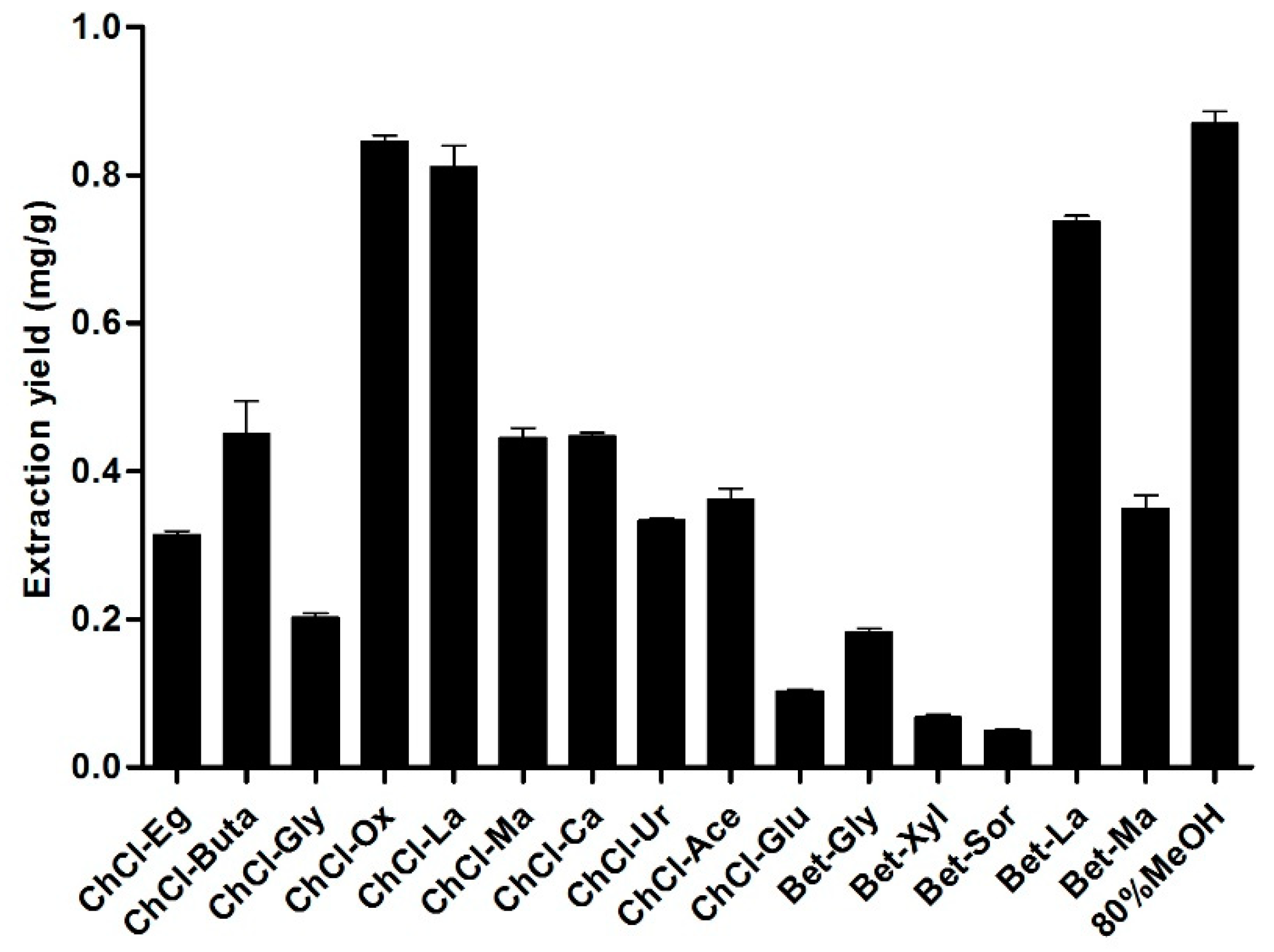
3.5. DESs Selection for Extraction
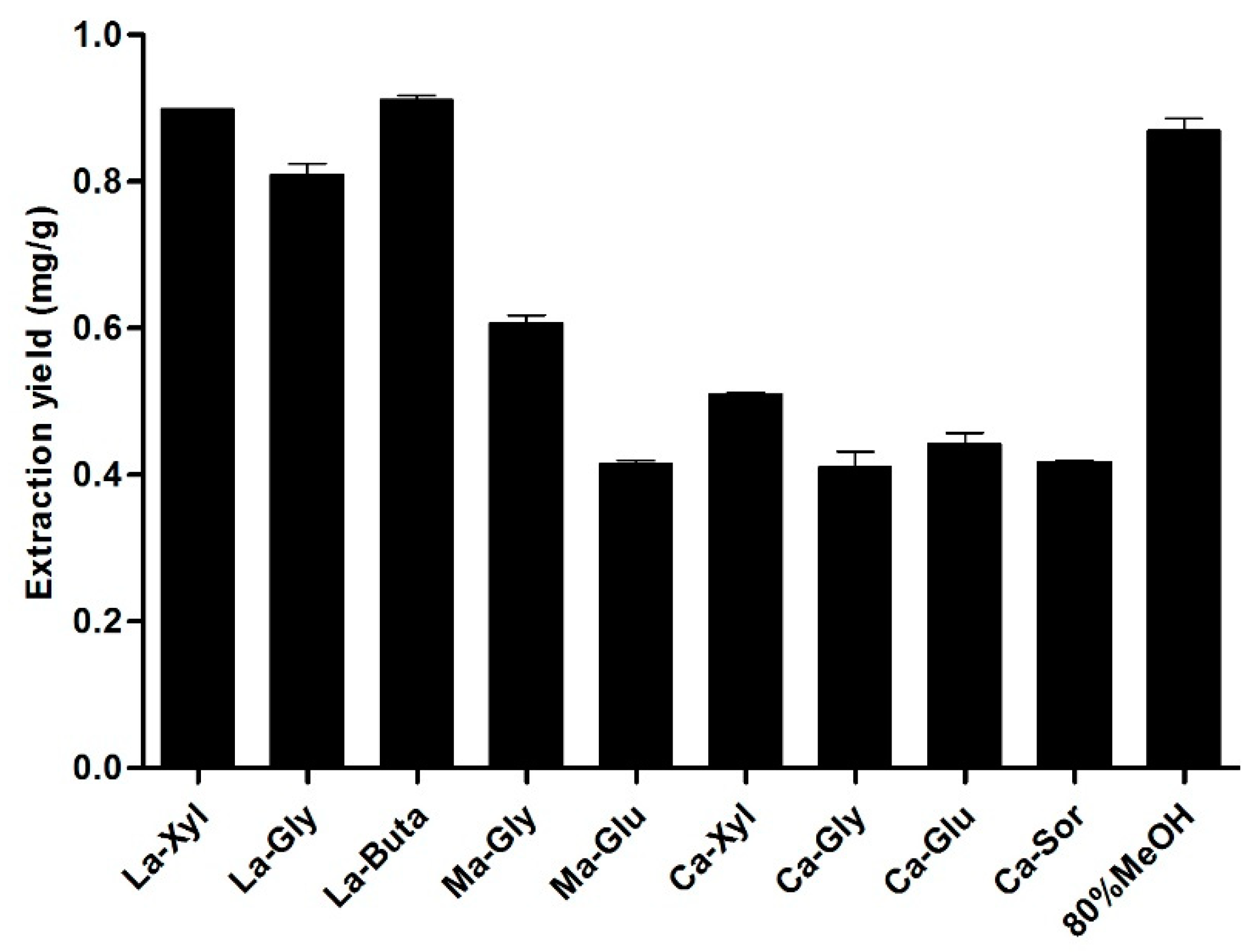
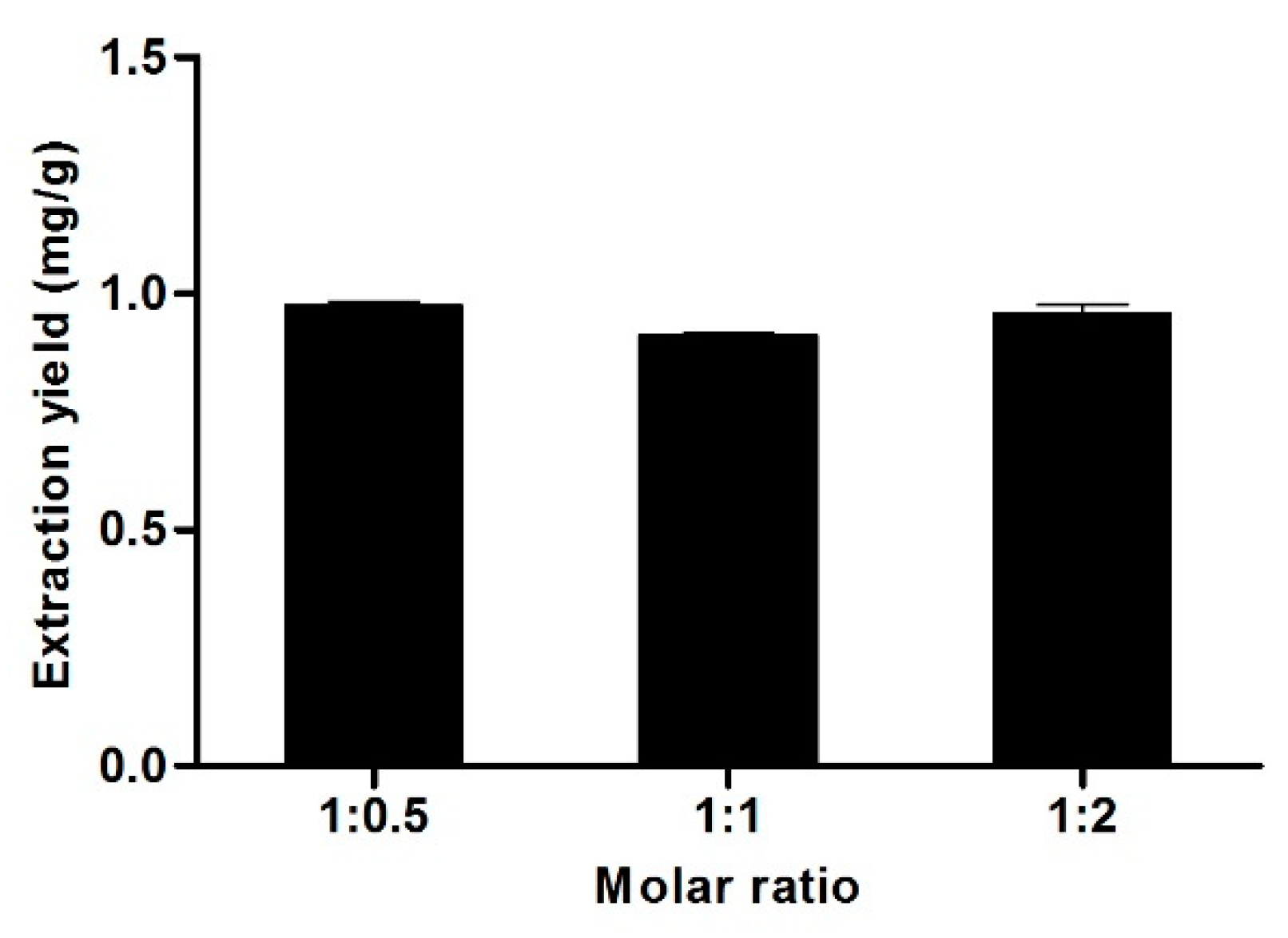
3.6. Effect of the Molar Ratio of La and Buta
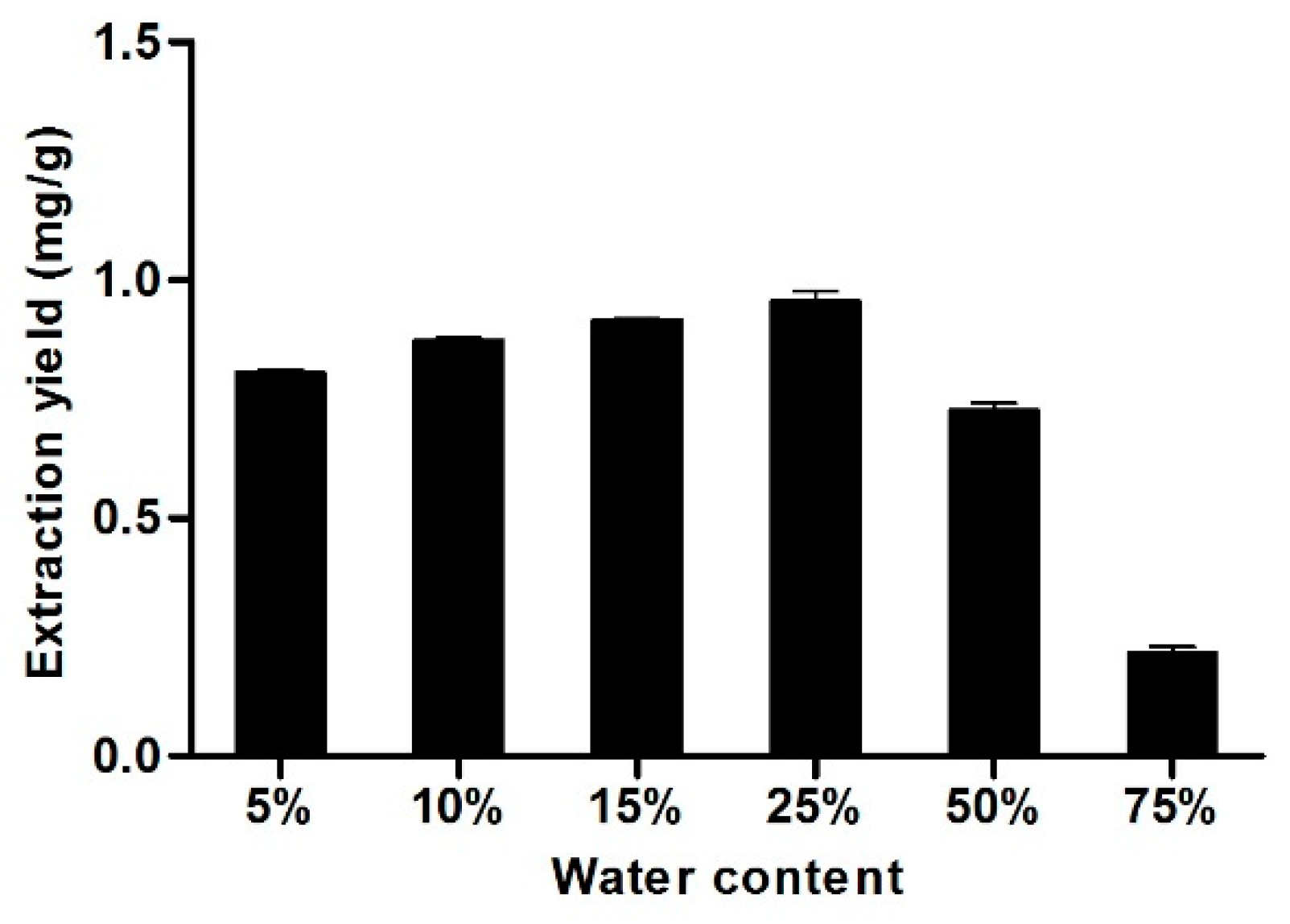
3.7. Effect of Water Content in La-Buta DES
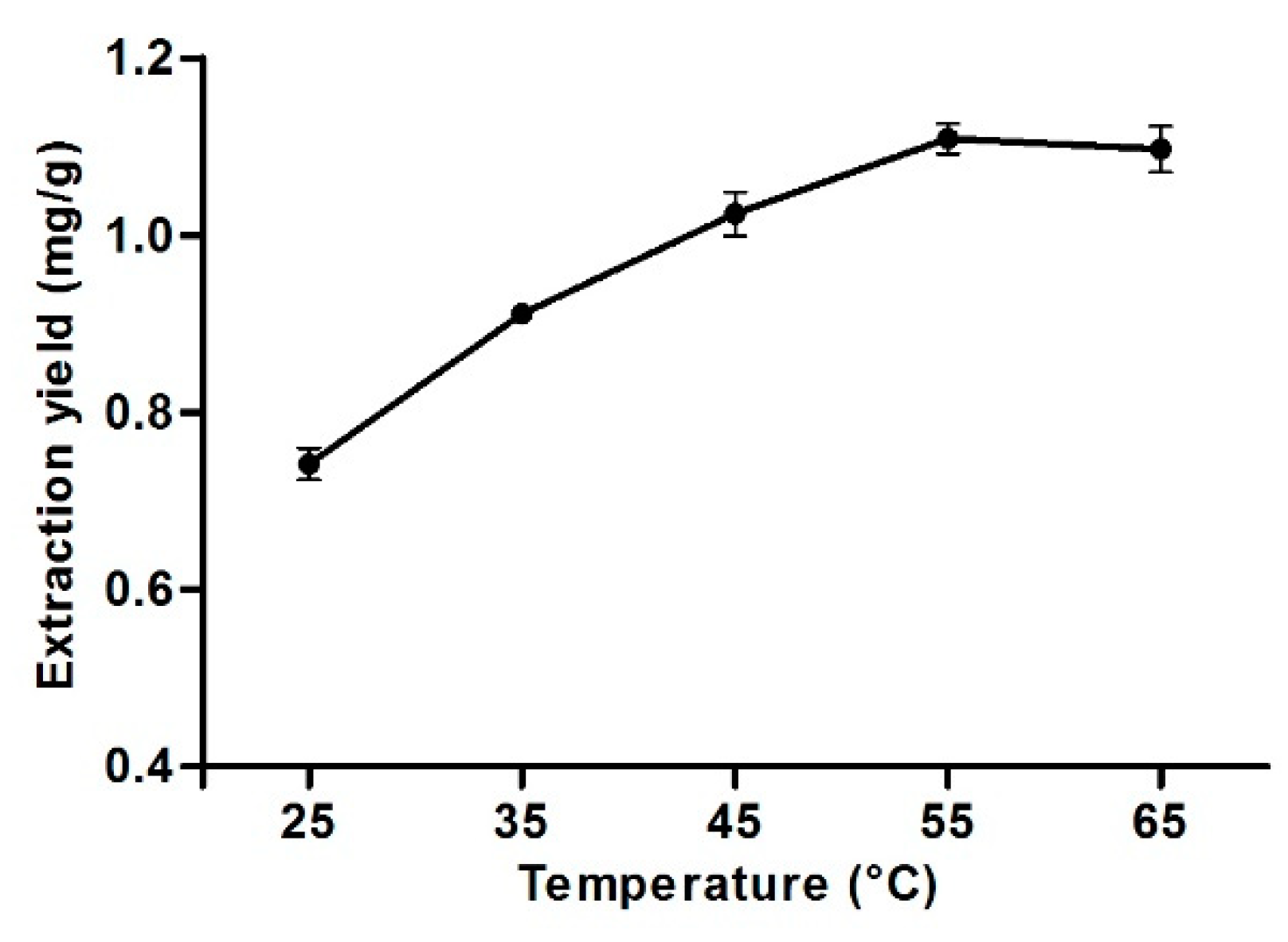
3.8. Effect of Extraction Temperature
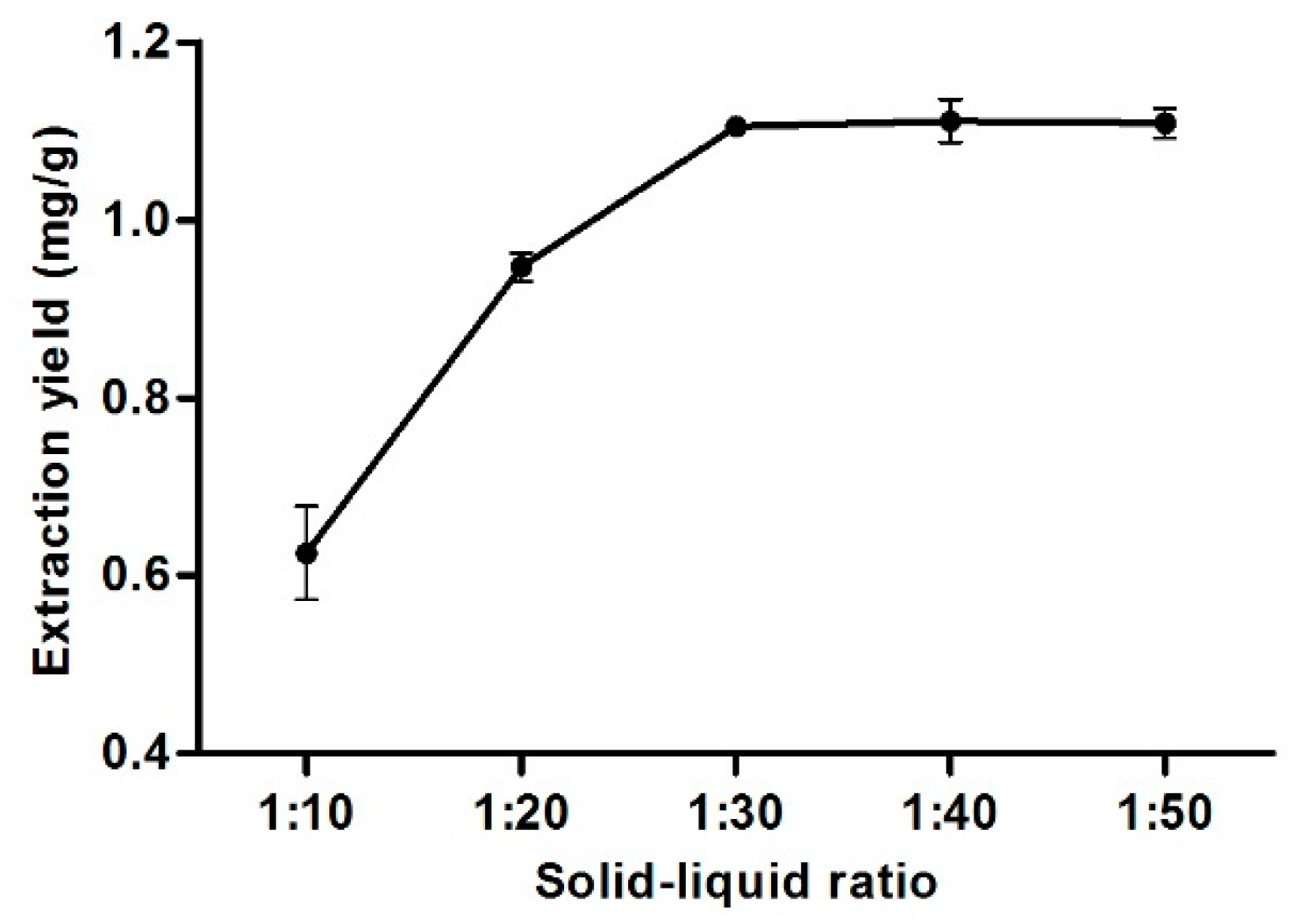
3.9. Effect of Solid-Liquid Ratio
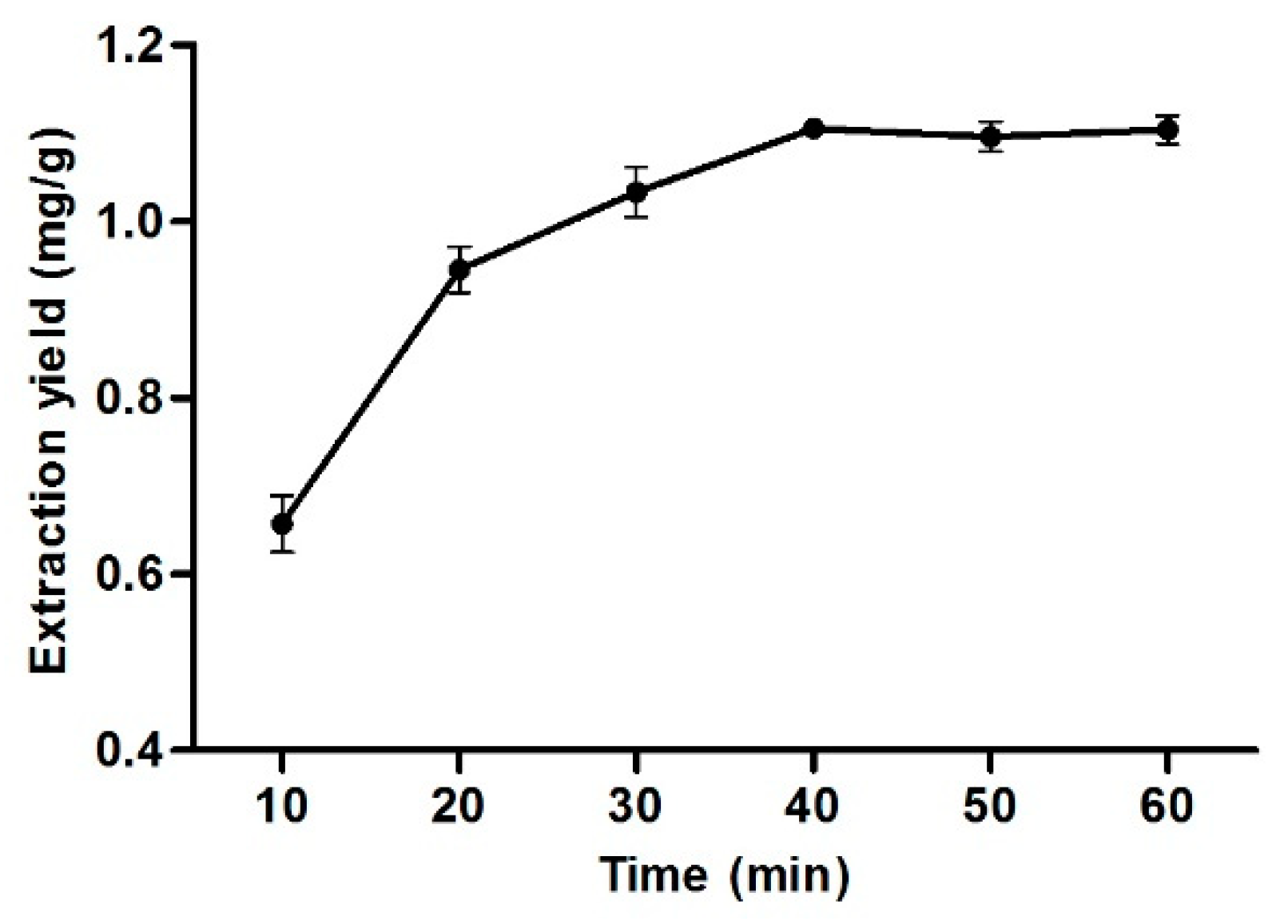
3.10. Effect of Extraction Time
3.11. Comparison of Current Extraction Method with Other Extraction Procedures
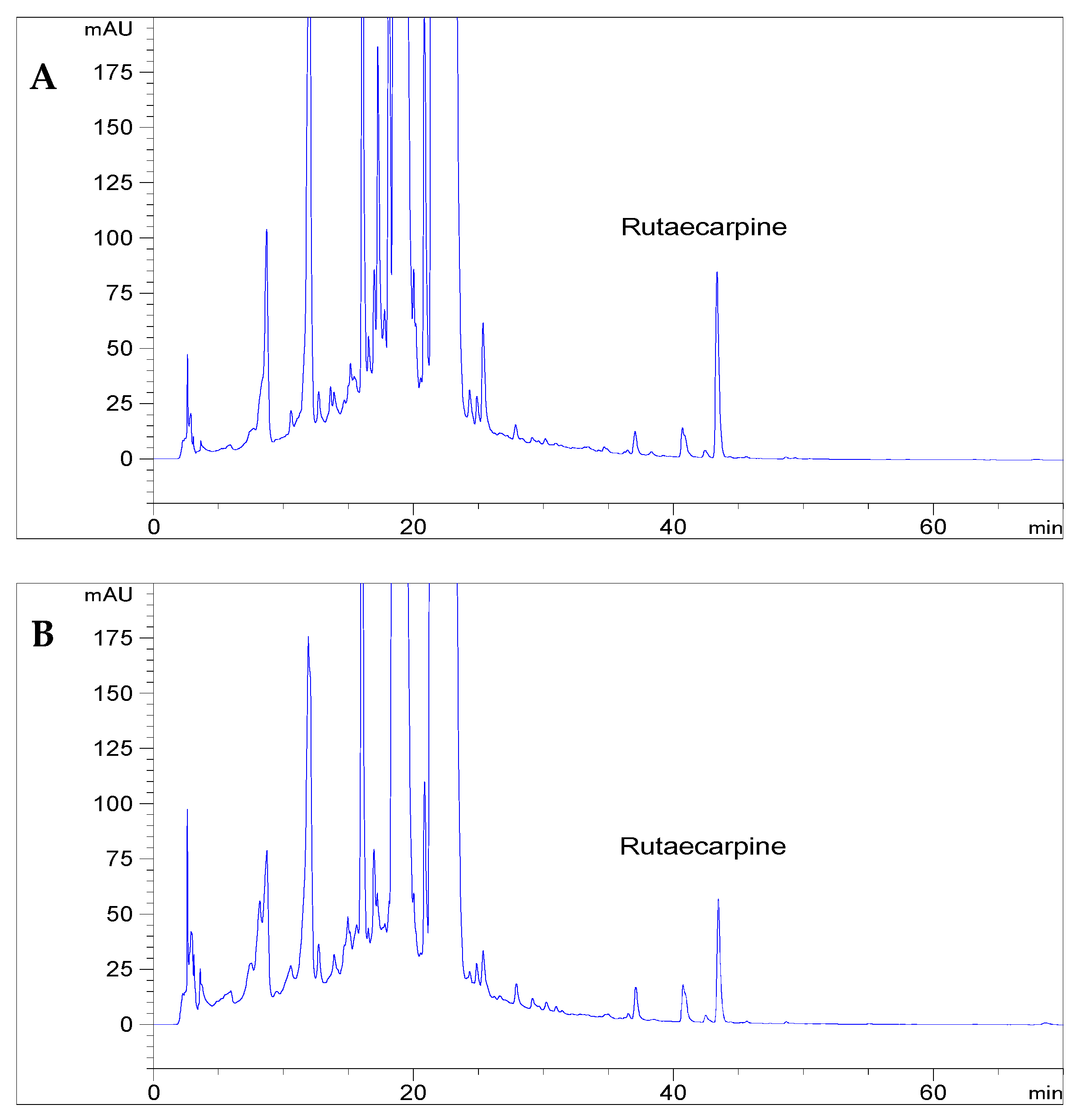
3.12. Application of rutaecarpine Extraction in Chinese Patent Medicines
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Piska, K.; Gunia-Krzyżak, A.; Koczurkiewicz, P.; Wójcik-Pszczoła, K.; Pękala, E. Piperlongumine (piplartine) as a lead compound for anticancer agents—Synthesis and properties of analogues: A mini-review. Eur. J. Med. Chem. 2018, 156, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Hadda, T.B.; Touzani, R. Diverse therapeutic potential of nitidine, a comprehensive review. Curr. Drug Metab. 2018, 19, 986–991. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Sharma, P.C.; Kumar, V. A mini review on pyridoacridines: Prospective lead compounds in medicinal chemistry. J. Adv. Res. 2015, 6, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.S.; Gao, R.; Li, D.; Peng, J.; Ran, L.L.; Li, Y.J. Solid dispersion of rutaecarpine improved its antihypertensive effect in spontaneously hypertensive rats. Biopharma. Drug Dispos. 2008, 29, 495–500. [Google Scholar] [CrossRef]
- Borrelli, F.; Campagnuolo, C.; Capasso, R.; Fattorusso, E.; Taglialatela-Scafati, O. Iodinated indole alkaloids from Plakortis simplex—New plakohypaphorines and an evaluation of their antihistamine activity. Eur. J. Org. Chem. 2004, 2004, 3227–3232. [Google Scholar] [CrossRef]
- Shao, H.; Yang, Y.; Mi, Z.; Zhu, G.X.; Qi, A.P.; Ji, W.G.; Zhu, Z.R. Anticonvulsant effect of rhynchophylline involved in the inhibition of persistent sodium current and NMDA receptor current in the pilocarpine rat model of temporal lobe epilepsy. Neuroscience 2016, 337, 355–369. [Google Scholar] [CrossRef]
- De Castro Cunha, A.; Chierrito, T.P.C.; De Carvalho, G.M.; Leon, L.L.P.; da Silva, C.C.; Tanaka, J.C.; de Souza, L.M.; Gonçalves, R.A.C.; de Oliveira, A.J.B. Anti-leishmanial activity of alkaloidal extracts obtained from different organs of Aspidosperma ramiflorum. Phytomedicine 2012, 19, 413–417. [Google Scholar] [CrossRef]
- Nandini, H.S.; Naik, P.R. Antidiabetic, antihyperlipidemic and antioxidant effect of vincamine, in streptozotocin-induced diabetic rats. Eur. J. Pharmacol. 2019, 843, 233–239. [Google Scholar] [CrossRef]
- Liu, Y.P.; Liu, Q.L.; Zhang, X.L.; Niu, H.Y.; Guan, C.Y.; Sun, F.K.; Xu, W.; Fu, Y.H. Bioactive monoterpene indole alkaloids from Nauclea officinalis. Bioorg. Chem. 2019, 83, 1–5. [Google Scholar] [CrossRef]
- Huang, Y.X.; Tan, H.X.; Guo, Z.Y.; Wu, X.X.; Zhang, Q.L.; Zhang, L.; Diao, Y. The biosynthesis and genetic engineering of bioactive indole alkaloids in plants. J. Plant Biol. 2016, 59, 203–214. [Google Scholar] [CrossRef]
- Yang, Y.; Zuo, W.J.; Zhao, Y.X.; Dong, W.H.; Mei, W.L.; Dai, H.F. Indole alkaloids from Kopsia hainanensis and evaluation of their antimicrobial activity. Planta Med. 2012, 78, 1881–1884. [Google Scholar] [CrossRef] [PubMed]
- Son, J.; Chang, H.W.; Jahng, Y. Progress in studies on rutaecarpine. II.—Synthesis and structure-biological activity relationships. Molecules 2015, 20, 10800–10821. [Google Scholar] [CrossRef]
- Wang, C.H.; Hao, Z.Y.; Zhou, J.; Zhang, L.; Sun, Y.X.; Liang, C.Z. Rutaecarpine alleviates renal ischemia reperfusion injury in rats by suppressing the JNK/p38 MAPK signaling pathway and interfering with the oxidative stress response. Mol. Med. Rep. 2017, 16, 922–928. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.M.; Chen, Q.Q.; Jia, S.J.; He, L.M.; Wang, A.P.; Li, D.; Li, Y.J.; Li, X.H. Involvement of TRPV1 in the expression and release of calcitonin gene-related peptide induced by tutaecarpine. Mol. Med. Rep. 2018, 17, 5168–5174. [Google Scholar] [CrossRef]
- Tian, K.M.; Li, J.J.; Wu, S.W. Rutaecarpine: A promising cardiovascular protective alkaloid from Evodia Rutaecarpa (Wu Zhu Yu). Pharmacol. Res. 2019, 141, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Ruesgas-Ramón, M.; Figueroa-Espinoza, M.C.; Durand, E. Application of deep eutectic solvents (DES) for phenolic compounds extraction: Overview, challenges, and opportunities. J. Agric. Food Chem. 2017, 65, 3591–3601. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.T.; Huang, S.X.; Xu, W.G.; Wu, S.J. Optimization of ultrasonic-assisted extracting of evodiamine and rutaecarpine. Food Res. Dev. 2016, 37, 54–57. [Google Scholar] [CrossRef]
- Lu, S.F.; Wang, Y.R. Optimization of the extraction procedure of Evodia fruits by the uniform design and orthogonal design. J. Beijing Univ. Tradit. Chin. Med. 2004, 27, 66–70. [Google Scholar] [CrossRef]
- Pellati, F.; Benvenuti, S.; Yoshizaki, F.; Melegari, M. Development and validation of HPLC methods for the analysis of phenethylamine and indoloquinazoline alkaloids in Evodia species. J. Sep. Sci. 2006, 29, 641–649. [Google Scholar] [CrossRef]
- Tang, X.L.; Huang, Z.F.; Chen, Y.; Liu, Y.H.; Liu, Y.H.; Zhao, J.N.; Yi, J.H. Simultaneous determination of six bioactive compounds in Evodiae Fructus by high-performance liquid chromatography with diode array detection. J. Chromatogr. Sci. 2014, 52, 149–156. [Google Scholar] [CrossRef]
- Takla, S.S.; Shawky, E.; Hammoda, H.M.; Darwish, F.A. Green techniques in comparison to conventional ones in the extraction of Amaryllidaceae alkaloids: best solvent selection and parameters optimization. J. Chromatogr. A 2018, 1567, 99–110. [Google Scholar] [CrossRef]
- Liu, B.; Guo, F.; Chang, Y.L.; Jiang, H.L.; Wang, Q. Optimization of extraction of evodiamine and rutaecarpine from fruit of Evodia rutaecarpa using modified supercritical CO2. J. Chromatogr. A 2010, 1217, 7833–7839. [Google Scholar] [CrossRef]
- Su, E.Z.; Yang, M.; Cao, J.; Lu, C.; Wang, J.H.; Cao, F.L. Deep eutectic solvents as green media for efficient extraction of terpene trilactones from Ginkgo biloba leaves. J. Liq. Chromatogr. Relat. Technol. 2017, 40, 385–391. [Google Scholar] [CrossRef]
- Dai, Y.T.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Tailoring properties of natural deep eutectic solvents with water to facilitate their applications. Food Chem. 2015, 187, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.M.; Wang, L.J.; Gao, Z.; Zhuang, B.; Yin, Q.; Liu, E.H. Green and efficient extraction of different types of bioactive alkaloids using deep eutectic solvents. Microchem. J. 2019, 145, 345–353. [Google Scholar] [CrossRef]
- Tang, W.Y.; Li, G.Z.; Chen, B.Q.; Zhu, T.; Row, K.H. Evaluating ternary deep eutectic solvents as novel media for extraction of flavonoids from Ginkgo biloba. Sep. Sci. Technol. 2017, 52, 91–99. [Google Scholar] [CrossRef]
- Zhuang, B.; Dou, L.L.; Li, P.; Liu, E.H. Deep eutectic solvents as green media for extraction of flavonoid glycosides and aglycones from Platyladi Cacumen. J. Pharm. Biomed. 2017, 134, 214–219. [Google Scholar] [CrossRef]
- Jeong, K.M.; Ko, J.; Zhao, J.; Jin, Y.; Yoo, D.E.; Han, S.Y. Mult-functioning deep eutectic solvents as extraction and storage media for bioactive natural products that are readily applicable to cosmetic products. J. Clean. Prod. 2017, 151, 87–95. [Google Scholar] [CrossRef]
- Huang, Y.; Feng, F.; Jiang, J.; Qiao, Y.; Wu, T.; Voglmeir, J.; Chen, Z.G. Green and efficient extraction of rutin from tartary buckwheat hull by using natural deep eutectic solvents. Food Chem. 2017, 221, 1400–1405. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Dou, L.L.; Guo, L.; Li, P.; Liu, E.H. Comprehensive evaluation of deep eutectic solvents in extraction of bioactive natural products. ACS Sustain. Chem. Eng. 2016, 4, 2405–2411. [Google Scholar] [CrossRef]
- Craveiro, R.; Aroso, I.; Flammia, V.; Carvalho, T.; Viciosa, M.T.; Dionísio, M.; Barreiros, S.; Reis, R.L.; Duarte, A.R.C.; Paiva, A. Properties and thermal behavior of natural deep eutectic solvents. J. Mol. Liq. 2016, 215, 534–540. [Google Scholar] [CrossRef]
- Dai, Y.; van Spronsen, J.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef]
- Wang, M.; Wang, J.Q.; Zhou, Y.Y.; Zhang, M.Y.; Xia, Q.; Bi, W.T.; Chen, D.D.Y. Ecofriendly mechanochemical extraction of bioactive compounds from plant with deep eutectic solvents. ACS Sustain. Chem. Eng. 2017, 5, 6297–6303. [Google Scholar] [CrossRef]
- Liu, Y.; Friesen, J.B.; McAlpine, J.B.; Lankin, D.C.; Chen, S.N.; Pauli, G.F. Natural deep eutectic solvent: Properties applications, and perspectives. J. Nat. Prod. 2018, 81, 679–690. [Google Scholar] [CrossRef]
- Francisco, M.; van den Bruinhorst, A.; Kroon, M.C. Low-transition-temperature mixtures (LTTMs): A new generation of designer solvent. Angew. Chem. Int. Ed. 2013, 52, 3074–3085. [Google Scholar] [CrossRef] [PubMed]
- Bubalo, M.C.; Ćurko, N.; Tomašević, M.; Ganić, K.K.; Redovniković, R. Green extraction of grape skin phenolics by using deep eutectic solvents. Food Chem. 2016, 200, 159–166. [Google Scholar] [CrossRef]
- Zhang, P.T.; Pan, B.Y.; Liao, Q.F.; Yao, M.C.; Xu, X.J.; Wan, J.Z.; Liu, D.; Xie, Z.Y. Simultaneous quantification of limonin, two indolequinazoline alkaloids, and four quinolone alkaloids in Evodia rutaecarpa (Juss.) Benth by HPLC-DVD method. J. Anal. Methods Chem. 2013, 2013, 827361. [Google Scholar] [CrossRef] [PubMed]
- Nam, M.W.; Zhao, J.; Lee, M.S.; Jeong, J.H.; Lee, J. Enhanced extraction of bioactive natural products using tailor-made deep eutectic solvents: Application to flavonoid extraction from Flos sophorae. Green Chem. 2015, 17, 1718–1727. [Google Scholar] [CrossRef]
- Cui, Q.; Peng, X.; Yao, X.H.; Wei, Z.F.; Luo, M.; Wang, W.; Zhao, C.J.; Fu, Y.J.; Zu, Y.G. Deep eutectic solvent-based microwave-assisted extraction of genistin, genis–tein and apigenin from pigeon pea roots. Sep. Purif. Technol. 2015, 150, 63–72. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, F.; Jiang, L.; Jiang, R.; Tan, S.J. Determination of evodiamine and rutaecarpine in Wiji Wan by HPLC. Chin. J. Chin. Mater. Med. 2003, 38, 1193–1194. [Google Scholar] [CrossRef]
- Zhang, X.F.; Qiu, F.R.; Jiang, J.; Gao, C.L.; He, M. Simultaneous determination of 6 alkaloids in Zuojin Pill and Xianglian Pill by LC-MS/MS. Chin. Traditi. Pat. Med. 2010, 32, 597–600. [Google Scholar] [CrossRef]
- Tan, S.J.; Zhao, B.C.; Wang, H.T.; Li, J.L.; Chu, F. Determination of evodiamine and rutaecarpine in Wiji Wan by HPLC. Pharm. J. Chin. P. L. A. 2002, 18, 201–204. [Google Scholar] [CrossRef]
- Luo, D.L.; Xi, X.R.; Gao, Y.M.; Qi, Y.D.; Huang, P. Determination of evodiamine and rutaecarpine in Wiji Wan by micellar electrokinetic capillary chromatography. Chin. J. Chin. Mater. Med. 2007, 32, 1936–1938. [Google Scholar] [CrossRef]

| Abbreviation | HBA | HBD | Molar Ratio | Water Content (%) |
|---|---|---|---|---|
| ChCl-Eg | choline chloride | ethylene glycol | 1:2 | 25 |
| ChCl-Buta | choline chloride | 1,4-butanediol | 1:2 | 25 |
| ChCl-Gly | choline chloride | glycerol | 1:2 | 25 |
| ChCl-Ox | choline chloride | oxalic acid | 1:1 | 25 |
| ChCl-La | choline chloride | levulinic acid | 1:2 | 25 |
| ChCl-Ma | choline chloride | dl-malic acid | 3:2 | 25 |
| ChCl-Ca | choline chloride | citric acid | 2:1 | 25 |
| ChCl-Ur | choline chloride | urea | 1:2 | 25 |
| ChCl-Ace | choline chloride | acetamide | 1:2 | 25 |
| ChCl-Glu | choline chloride | d-glucose | 1:1 | 25 |
| Abbreviation | HBA | HBD | Molar Ratio | Water Content (%) |
|---|---|---|---|---|
| Bet-Gly | betaine | glycerol | 1:2 | 25 |
| Bet-Xyl | betaine | xylitol | 1:1 | 25 |
| Bet-Sor | betaine | d-sorbitol | 5:6 | 25 |
| Bet-La | betaine | levulinic acid | 1:2 | 25 |
| Bet-Ma | betaine | dl-malic acid | 1:2 | 25 |
| Abbreviation | Molar Ratio | Water Content (%) | pH | Viscosity (25 °C) (mm2/s) | ENR (kcal/mol) |
|---|---|---|---|---|---|
| La-Xyl | 1:1 | 25 | 2.02 | 47.59 | 49.13 |
| La-Gly | 1:1 | 25 | 1.83 | 11.00 | 49.29 |
| La-Buta | 1:1 | 25 | 2.23 | 9.60 | 49.47 |
| Ma-Gly | 1:1 | 25 | 1.05 | 45.39 | 47.89 |
| Ma-Glu | 1:1 | 25 | 0.82 | 107.20 | 48.46 |
| Ca-Xyl | 1:1 | 25 | 0.74 | 160.00 | 47.65 |
| Ca-Gly | 1:2 | 25 | 1.06 | 63.79 | 47.89 |
| Ca-Glu | 1:1 | 25 | 0.62 | 157.00 | 48.21 |
| Ca-Sor | 1:1 | 25 | 1.13 | 196.00 | 47.81 |
| Molar Ratio | Levulinic Acid | 1,4-Butanediol | |||||
|---|---|---|---|---|---|---|---|
| COOH 1 | CH2 2 | CH2 3 | CH3 4 | CH2OH 1 | CH2OH 2 | CH2 3 | |
| 1:0.5 | 12.05 a | 2.65 | 2.38 | 2.09 | 4.34 | 3.38 | 1.43 |
| (1.08H) b | (3H) | (1.09H) c | (2H) | ||||
| 1:1 | 12.08 | 2.64 | 2.38 | 2.09 | 4.35 | 3.38 | 1.43 |
| (0.80H) | (3H) | (0.89H) | (2H) | ||||
| 1:2 | 12.04 | 2.65 | 2.38 | 2.09 | 4.34 | 3.38 | 1.43 |
| (0.98H) | (3H) | (0.98H) | (2H) | ||||
| Sample | Solvent | Method | Extraction Yield (mg/g) | Relative Recovery (%) a |
|---|---|---|---|---|
| 1 | La-Buta DES aqueous solution with 1:0.5 molar ratio containing 25% water | Ultrasound-assisted extraction at 55 °C for 40 min | 1.105 ± 0.009 | 100 |
| 2 | La-Buta DES aqueous solution with 1:0.5 molar ratio containing 25% water | Magnetic stirring extraction at 55 °C for 40 min | 1.037 ± 0.026 | 93.8 |
| 3 | La-Buta DES aqueous solution with 1:0.5 molar ratio containing 25% water | Ultrasound-assisted extraction at 35 °C for 40 min | 0.911 ± 0.011 | 82.4 |
| 4 | Water | Ultrasound-assisted extraction at 35 °C for 40 min | 0.080 ± 0.001 | 7.2 |
| 5 | Methanol [19] | Ultrasound-assisted extraction at 35 °C for 40 min | 0.847 ± 0.010 | 76.6 |
| 6 | 80% Aqueous methanol (v/v) | Ultrasound-assisted extraction at 35 °C for 40 min | 0.869 ± 0.002 | 78.6 |
| 7 | Methanol | Solvent boiling reflux extraction for 30 min | 0.852 ± 0.006 | 77.1 |
| 8 | 80% Aqueous methanol (v/v) [20] | Solvent boiling reflux extraction for 30 min | 0.858 ± 0.013 | 77.6 |
| Entry | Sample | Solvent | Method | Extraction Yield (mg/g) | Ref. |
|---|---|---|---|---|---|
| 1 | Zuojin Pill | La-Buta DES aqueous solution with 1:0.5 molar ratio containing 25% water | Ultrasound-assisted extraction at 55 °C for 40 min | 0.346 ± 0.006 | This work |
| 2 | Zuojin Pill | Ethanol | Solvent boiling reflux extraction for 60 min | 0.204 ± 0.031 | [40] |
| 3 | Zuojin Pill | Methanol | Ultrasound-assisted extraction at 25 °C for 45 min | 0.256 ± 0.002 | [41] |
| 4 | Wuji Pill | La-Buta DES aqueous solution with 1:0.5 molar ratio containing 25% water | Ultrasound-assisted extraction at 55 °C for 40 min | 0.146 ± 0.006 | This work |
| 5 | Wuji Pill | Ethanol | Solvent boiling reflux extraction for 60 min | 0.065 ± 0.006 | [42] |
| 6 | Wuji Pill | Methanol | Ultrasound-assisted extraction at 25 °C for 45 min | 0.052 ± 0.002 | [43] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Si, Y.-Y.; Sun, S.-W.; Liu, K.; Liu, Y.; Shi, H.-L.; Zhao, K.; Wang, J.; Wang, W. Novel Deep Eutectic Solvent Based on Levulinic Acid and 1,4-Butanediol as an Extraction Media for Bioactive Alkaloid Rutaecarpine. Processes 2019, 7, 171. https://doi.org/10.3390/pr7030171
Si Y-Y, Sun S-W, Liu K, Liu Y, Shi H-L, Zhao K, Wang J, Wang W. Novel Deep Eutectic Solvent Based on Levulinic Acid and 1,4-Butanediol as an Extraction Media for Bioactive Alkaloid Rutaecarpine. Processes. 2019; 7(3):171. https://doi.org/10.3390/pr7030171
Chicago/Turabian StyleSi, Yue-Yue, Shi-Wei Sun, Kun Liu, Yang Liu, Hai-Lin Shi, Ke Zhao, Jin Wang, and Wei Wang. 2019. "Novel Deep Eutectic Solvent Based on Levulinic Acid and 1,4-Butanediol as an Extraction Media for Bioactive Alkaloid Rutaecarpine" Processes 7, no. 3: 171. https://doi.org/10.3390/pr7030171
APA StyleSi, Y.-Y., Sun, S.-W., Liu, K., Liu, Y., Shi, H.-L., Zhao, K., Wang, J., & Wang, W. (2019). Novel Deep Eutectic Solvent Based on Levulinic Acid and 1,4-Butanediol as an Extraction Media for Bioactive Alkaloid Rutaecarpine. Processes, 7(3), 171. https://doi.org/10.3390/pr7030171





