Exploitation of Wheat Straw Biorefinery Side Streams as Sustainable Substrates for Microorganisms: A Feasibility Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
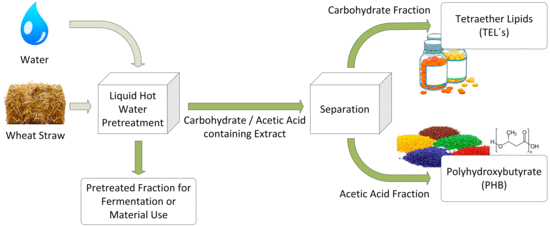
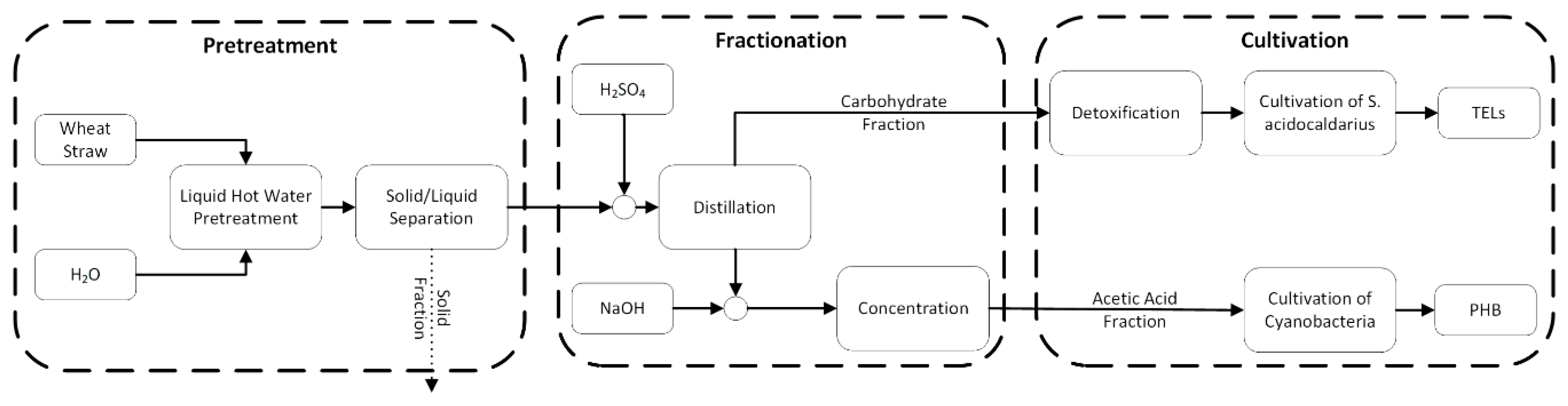
2.2. Process Description
2.3. Wheat Straw Pretreatment
2.4. Extract Separation
2.5. Cultivation of S. acidocaldarius
2.6. Cultivation of Cyanobacteria
2.7. Analytics
3. Results & Discussion
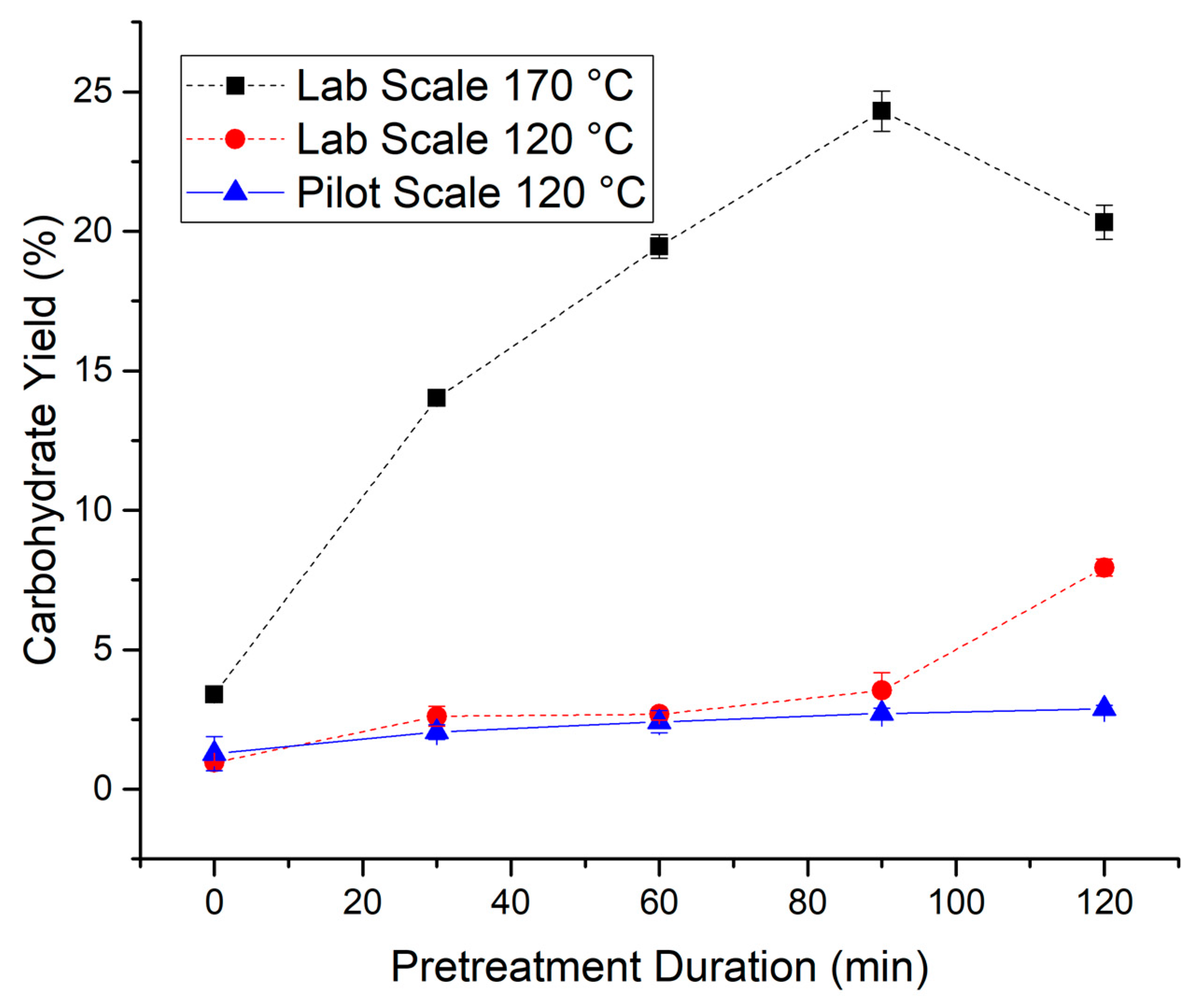
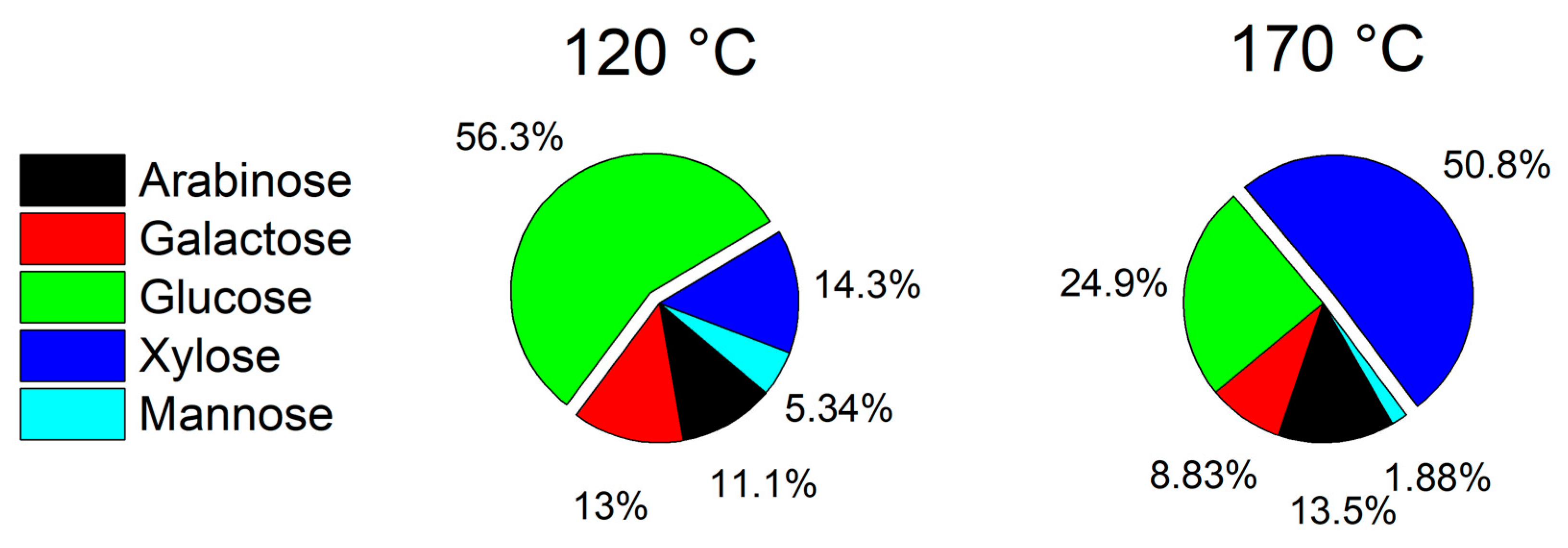
3.1. Wheat Straw Pretreatment
3.2. Fractionation of the Extract
3.3. Cultivation of S. acidocaldarius
3.4. Cultivation of Cyanobacteria
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sánchez, Ó.J.; Cardona, C.A. Trends in biotechnological production of fuel ethanol from different feedstocks. Bioresour. Technol. 2008, 99, 5270–5295. [Google Scholar] [CrossRef] [PubMed]
- Kamm, B.; Kamm, M. Principles of biorefineries. Appl. Microbiol. Biotechnol. 2004, 64, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.L.; Fan, Y.T.; Xing, Y.; Pan, C.M.; Zhang, G.S.; Lay, J.J. Enhanced biohydrogen production from cornstalk wastes with acidification pretreatment by mixed anaerobic cultures. Biomass Bioenergy 2007, 31, 250–254. [Google Scholar] [CrossRef]
- Cherubini, F.; Strømman, A.H. Chemicals from lignocellulosic biomass: Opportunities, perspectives, and potential of biorefinery systems. Biofuels Bioprod. Biorefining 2011, 5, 548–561. [Google Scholar] [CrossRef]
- FitzPatrick, M.; Champagne, P.; Cunningham, M.F.; Whitney, R.A. A biorefinery processing perspective: Treatment of lignocellulosic materials for the production of value-added products. Bioresour. Technol. 2010, 101, 8915–8922. [Google Scholar] [CrossRef]
- Yang, B.; Wyman, C.E. Pretreatment: The key to unlocking low-cost cellulosic ethanol. Biofuels Bioprod. Biorefining 2008, 2, 26–40. [Google Scholar] [CrossRef]
- Kim, Y.; Hendrickson, R.; Mosier, N.S.; Ladisch, M.R. Liquid Hot Water Pretreatment of Cellulosic Biomass. In Biofuels; Humana Press: Totowa, NJ, USA, 2009; Volume 581, pp. 93–102. [Google Scholar]
- Kamravamanesh, D.; Lackner, M.; Herwig, C. Bioprocess Engineering Aspects of Sustainable Polyhydroxyalkanoate Production in Cyanobacteria. Bioengineering 2018, 5, 111. [Google Scholar] [CrossRef]
- Katayama, N.; Iijima, H.; Osanai, T. Production of Bioplastic Compounds by Genetically Manipulated and Metabolic Engineered Cyanobacteria. In Synthetic Biology of Cyanobacteria; Zhang, W., Song, X., Eds.; Advances in Experimental Medicine and Biology; Springer: Singapore, 2018; pp. 155–169. [Google Scholar]
- Quehenberger, J.; Shen, L.; Albers, S.-V.; Siebers, B.; Spadiut, O. Sulfolobus—A Potential Key Organism in Future Biotechnology. Front. Microbiol. 2017, 8, 2474. [Google Scholar] [CrossRef]
- Steinbüchel, A. PHB and Other Polhydroxyalkanoic Acids. In Biotechnology Set; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2008; pp. 403–464. [Google Scholar]
- Liebergesell, M.; Sonomoto, K.; Madkour, M.; Mayer, F.; Steinbüchel, A. Purification and Characterization of the Poly(Hydroxyalkanoic Acid) Synthase from Chromatium vinosum and Localization of the Enzyme at the Surface of Poly(Hydroxyalkanoic Acid) Granules. Eur. J. Biochem. 1994, 226, 71–80. [Google Scholar] [CrossRef]
- Samantaray, S.; Mallick, N. Production and characterization of poly-β-hydroxybutyrate (PHB) polymer from Aulosira fertilissima. J. Appl. Phycol. 2012, 24, 803–814. [Google Scholar] [CrossRef]
- Lackner, M. Bioplastics Kirk-Othmer Encyclopedia of Chemical Technology; Wiley: New York, NY, USA, 2015. [Google Scholar]
- Madison, L.L.; Huisman, G.W. Metabolic Engineering of Poly(3-Hydroxyalkanoates): From DNA to Plastic. Microbiol. Mol. Biol. Rev. 1999, 63, 21. [Google Scholar] [PubMed]
- Grothe, E.; Chisti, Y. Poly(β-hydroxybutyric acid) thermoplastic production by Alcaligenes latus: Behavior of fed-batch cultures. Bioprocess Eng. 2000, 22, 441–449. [Google Scholar] [CrossRef]
- Schubert, P.; Steinbüchel, A.; Schlegel, H.G. Cloning of the Alcaligenes eutrophus genes for synthesis of poly-beta-hydroxybutyric acid (PHB) and synthesis of PHB in Escherichia coli. J. Bacteriol. 1988, 170, 5837–5847. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.F.; Wu, Q.Y.; Shen, Z.Y. Accumulation of poly-β-hydroxybutyrate in cyanobacterium Synechocystis sp. PCC6803. Bioresour. Technol. 2001, 76, 85–90. [Google Scholar] [CrossRef]
- Hänsel, S. Handbook of Climate Change. Mitigation and Adaptation, Second Edition. Environ. Earth Sci. 2018, 77, 554. [Google Scholar] [CrossRef]
- He, H.; Lu, Y.; Qi, J.; Zhu, Q.; Chen, Z.; Wu, W. Adapting liposomes for oral drug delivery. Acta Pharm. Sin. B 2019, 9, 36–48. [Google Scholar] [CrossRef]
- Jacobsen, A.-C.; Jensen, S.M.; Fricker, G.; Brandl, M.; Treusch, A.H. Archaeal lipids in oral delivery of therapeutic peptides. Eur. J. Pharm. Sci. 2017, 108, 101–110. [Google Scholar] [CrossRef]
- Kaur, G.; Garg, T.; Rath, G.; Goyal, A.K. Archaeosomes: An excellent carrier for drug and cell delivery. Drug Deliv. 2016, 23, 2497–2512. [Google Scholar] [CrossRef]
- Mahmoud, G.; Jedelská, J.; Omar, S.M.; Strehlow, B.; Schneider, M.; Bakowsky, U. Stabilized tetraether lipids based particles guided prophyrins photodynamic therapy. Drug Deliv. 2018, 25, 1526–1536. [Google Scholar] [CrossRef]
- Uhl, P.; Pantze, S.; Storck, P.; Parmentier, J.; Witzigmann, D.; Hofhaus, G.; Huwyler, J.; Mier, W.; Fricker, G. Oral delivery of vancomycin by tetraether lipid liposomes. Eur. J. Pharm. Sci. 2017, 108, 111–118. [Google Scholar] [CrossRef]
- Schiraldi, C.; Marulli, F.; Lernia, I.D.; Martino, A.; De Rosa, M. A microfiltration bioreactor to achieve high cell density in Sulfolobus solfataricus fermentation. Extremophiles 1999, 3, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Quehenberger, J.; Albersmeier, A.; Glatzel, H.; Hackl, M.; Kalinowski, J.; Spadiut, O. A defined cultivation medium for Sulfolobus acidocaldarius. J. Biotechnol. 2019, 301, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Romano, I.; Calandrelli, V.; Pagnotta, E.; Esposito, E.; Di Maso, R. Whey as a medium for biomass production ofSulfolobus solfataricus. Biotechnol. Tech. 1992, 6, 391–392. [Google Scholar] [CrossRef]
- Brock, T.D.; Brock, K.M.; Belly, R.T.; Weiss, R.L. Sulfolobus: A new genus of sulfur-oxidizing bacteria living at low pH and high temperature. Arch. Mikrobiol. 1972, 84, 54–68. [Google Scholar] [CrossRef] [PubMed]
- Rippka, R.; Deruelles, J.; Waterbury, J.B.; Herdman, M.; Stanier, R.Y. Generic Assignments, Strain Histories and Properties of Pure Cultures of Cyanobacteria. Microbiology 1979, 111, 1–61. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C. Determination of Sugars, Byproducts, and Degradation Products in Liquid Fraction Process Samples. National Renewable Energy Laboratory (NREL): Denver, CO, USA, 2008. [Google Scholar]
- Weinwurm, F.; Cunha, J.; Friedl, A. Pretreatment of wheat straw by liquid hot water and organosolv processes. Chem. Eng. Trans. 2012, 29, 541–546. [Google Scholar]
- Carvalheiro, F.; Silva-Fernandes, T.; Duarte, L.C.; Gírio, F.M. Wheat straw autohydrolysis: Process optimization and products characterization. Appl. Biochem. Biotechnol. 2009, 153, 84–93. [Google Scholar] [CrossRef]
- Ertas, M.; Han, Q.; Jameel, H.; Chang, H. min Enzymatic hydrolysis of autohydrolyzed wheat straw followed by refining to produce fermentable sugars. Bioresour. Technol. 2014, 152, 259–266. [Google Scholar] [CrossRef]
- Han, Q.; Jin, Y.; Jameel, H.; Chang, H.M.; Phillips, R.; Park, S. Autohydrolysis Pretreatment of Waste Wheat Straw for Cellulosic Ethanol Production in a Co-located Straw Pulp Mill. Appl. Biochem. Biotechnol. 2014, 175, 1193–1210. [Google Scholar] [CrossRef]
- Ruiz, H.A.; Vicente, A.A.; Teixeira, J.A. Kinetic modeling of enzymatic saccharification using wheat straw pretreated under autohydrolysis and organosolv process. Ind. Crops Prod. 2012, 36, 100–107. [Google Scholar] [CrossRef][Green Version]
- Boyer, L.J.; Vega, J.L.; Klasson, K.T.; Clausen, E.C.; Gaddy, J.L. The effects of furfural on ethanol production by saccharomyces cereyisiae in batch culture. Biomass Bioenergy 1992, 3, 41–48. [Google Scholar] [CrossRef]
- Rasmussen, H.; Sørensen, H.R.; Meyer, A.S. Formation of degradation compounds from lignocellulosic biomass in the biorefinery: Sugar reaction mechanisms. Carbohydr. Res. 2014, 385, 45–57. [Google Scholar] [CrossRef]
- Palmqvist, E.; Grage, H.; Meinander, N.Q.; Hahn-Hägerdal, B. Main and interaction effects of acetic acid, furfural, andp-hydroxybenzoic acid on growth and ethanol productivity of yeasts. Biotechnol. Bioeng. 1999, 63, 46–55. [Google Scholar] [CrossRef]
- Grogan, D.W. Phenotypic characterization of the archaebacterial genus Sulfolobus: comparison of five wild-type strains. J. Bacteriol. 1989, 171, 6710–6719. [Google Scholar] [CrossRef] [PubMed]
- De Jaeger, L. Strain Improvement of Oleaginous Microalgae. Ph.D. Thesis, Wageningen University, Wageningen, the Netherlands, 2015; p. 177. [Google Scholar]
- Sharma, L.; Kumar Singh, A.; Panda, B.; Mallick, N. Process optimization for poly-β-hydroxybutyrate production in a nitrogen fixing cyanobacterium, Nostoc muscorum using response surface methodology. Bioresour. Technol. 2007, 98, 987–993. [Google Scholar] [CrossRef] [PubMed]
- Panda, B.; Jain, P.; Sharma, L.; Mallick, N. Optimization of cultural and nutritional conditions for accumulation of poly-β-hydroxybutyrate in Synechocystis sp. PCC 6803. Bioresour. Technol. 2006, 97, 1296–1301. [Google Scholar] [CrossRef]
- Kamravamanesh, D.; Pflügl, S.; Nischkauer, W.; Limbeck, A.; Lackner, M.; Herwig, C. Photosynthetic poly-β-hydroxybutyrate accumulation in unicellular cyanobacterium Synechocystis sp. PCC 6714. AMB Express 2017, 7, 143. [Google Scholar] [CrossRef]
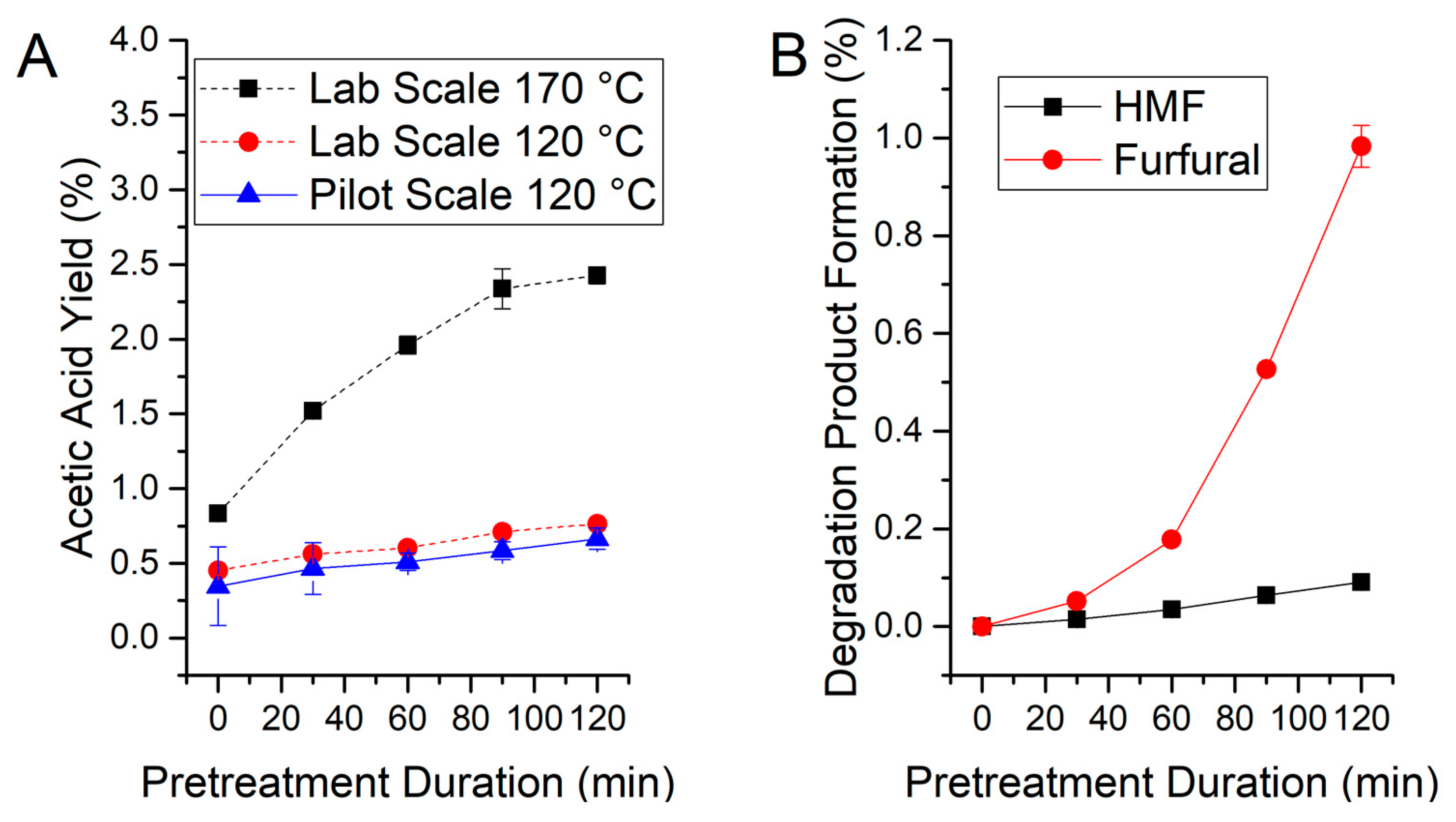
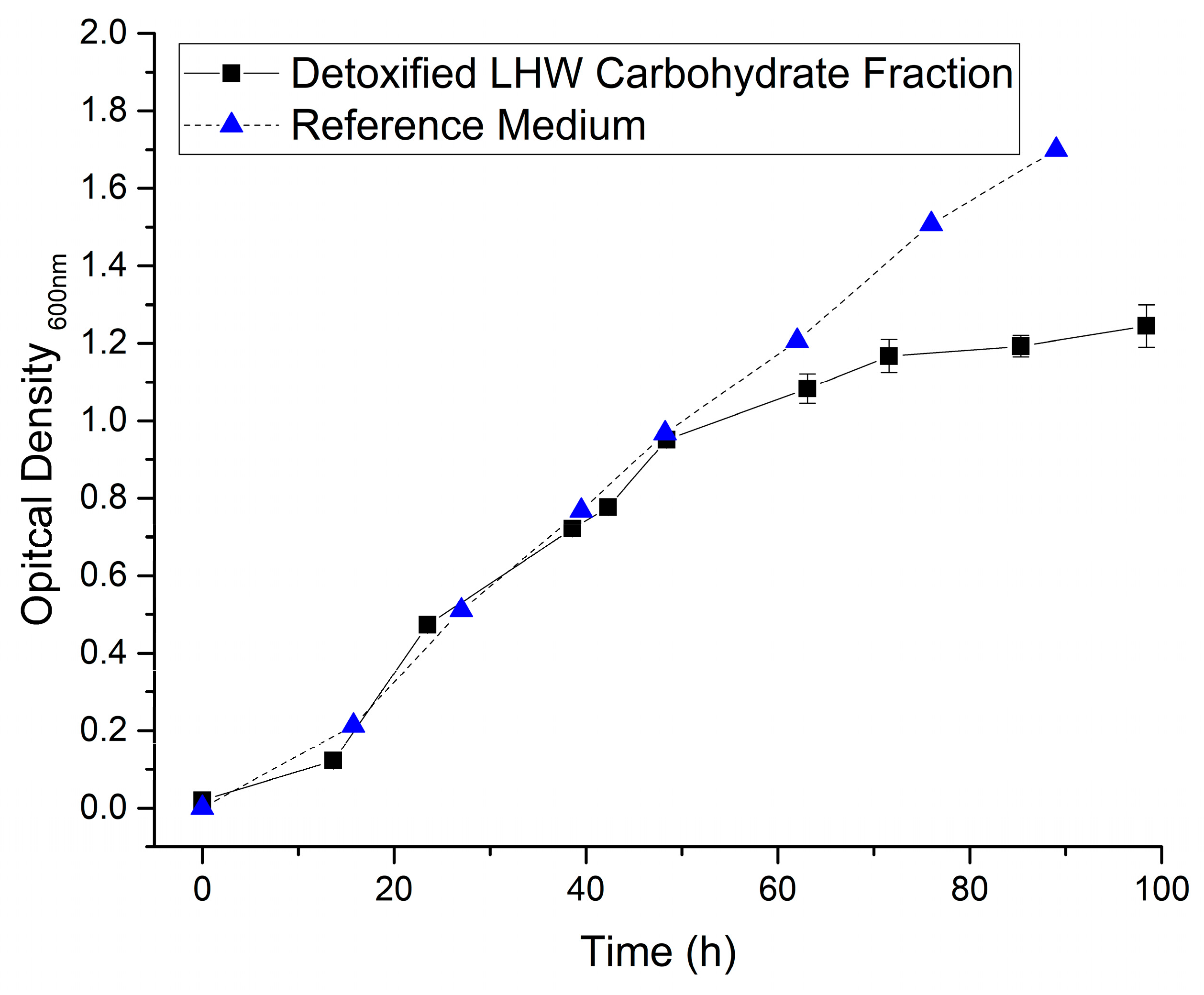
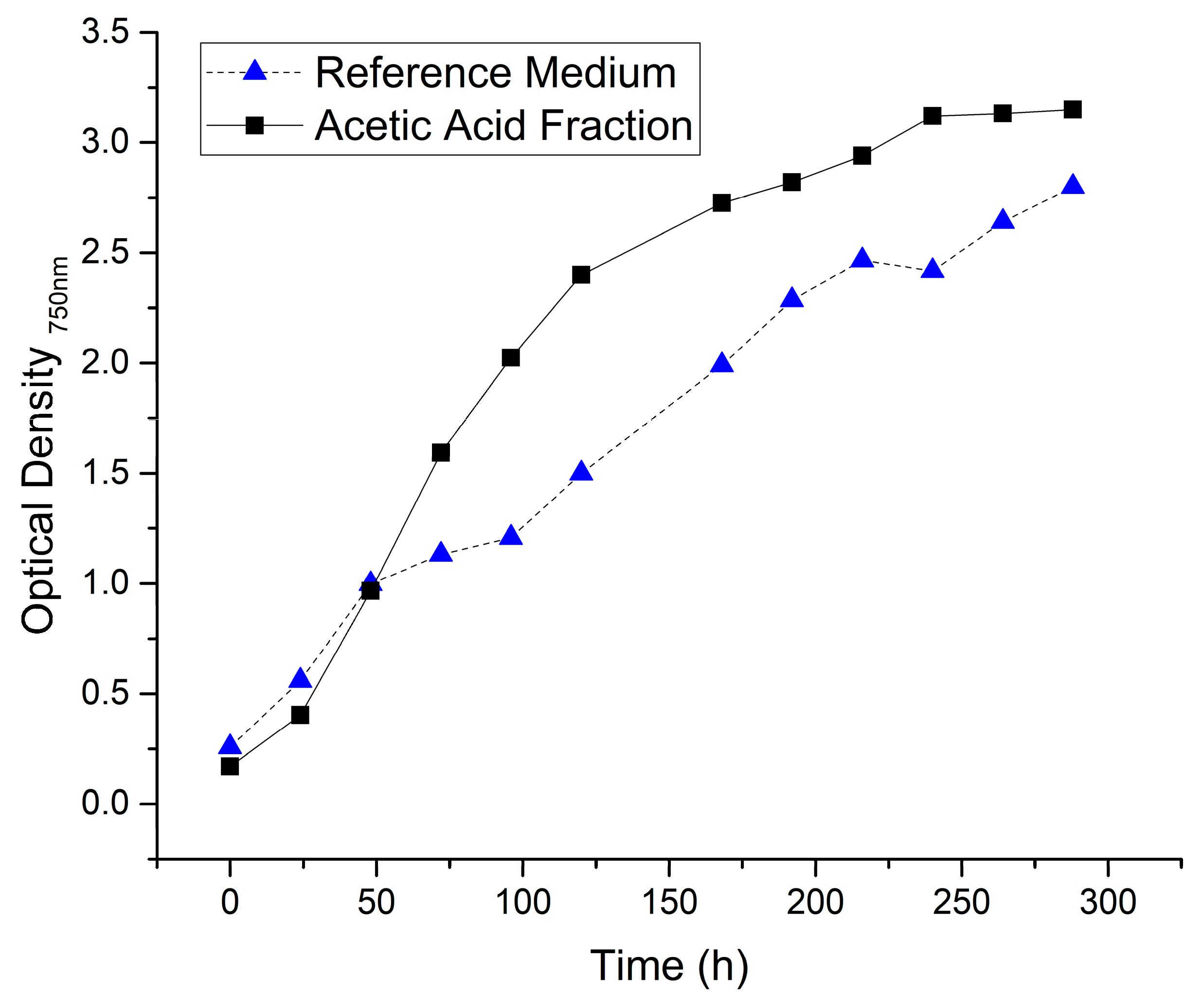
| Arabinan 1 | Galactan 1 | Cellulose 1,2 | Xylane 1 | Mannan 1 | Lignin | Ash |
|---|---|---|---|---|---|---|
| 2.68 | 0.872 | 32.5 | 19.8 | 0.409 | 16.1 | 0.539 |
| ±0.0717 | ±0.0360 | ±0.985 | ±0.432 | ±0.00937 | ±0.518 | ±0.261 |
| Arabinose | Galactose | Glucose | Xylose | Mannose | Acetic Acid | |
|---|---|---|---|---|---|---|
| Monomeric Carbohydrates (g/L) | 0.549 | 0.176 | 5.32 | 0.459 | 0.533 | - |
| Total Carbohydrates (g/L) | 0.833 | 0.953 | 9.03 | 2.17 | 0.732 | - |
| Degradation Products 1 (g/L) | - | - | - | - | - | 1.84 |
| Arabinose | Galactose | Glucose | Xylose | Mannose | Acetic Acid | |
|---|---|---|---|---|---|---|
| Monomeric Carbohydrates (g/L) | 1.22 | 0.090 | 3.67 | 0.0860 | 0.254 | - |
| Total Carbohydrates (g/L) | 2.39 | 2.08 | 11.4 | 2.56 | 0.664 | - |
| Degradation Products 1 (g/L) | - | - | - | - | - | 0.128 |
| mg/L | Arabinose | Galactose | Glucose | Xylose | Mannose | Acetic Acid |
|---|---|---|---|---|---|---|
| Before AC Treatment | 609 | 45 | 1835 | 43 | 127 | 64 |
| After AC Treatment | 167 | 25 | 1534 | - | 41 | 56 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beisl, S.; Quehenberger, J.; Kamravamanesh, D.; Spadiut, O.; Friedl, A. Exploitation of Wheat Straw Biorefinery Side Streams as Sustainable Substrates for Microorganisms: A Feasibility Study. Processes 2019, 7, 956. https://doi.org/10.3390/pr7120956
Beisl S, Quehenberger J, Kamravamanesh D, Spadiut O, Friedl A. Exploitation of Wheat Straw Biorefinery Side Streams as Sustainable Substrates for Microorganisms: A Feasibility Study. Processes. 2019; 7(12):956. https://doi.org/10.3390/pr7120956
Chicago/Turabian StyleBeisl, Stefan, Julian Quehenberger, Donya Kamravamanesh, Oliver Spadiut, and Anton Friedl. 2019. "Exploitation of Wheat Straw Biorefinery Side Streams as Sustainable Substrates for Microorganisms: A Feasibility Study" Processes 7, no. 12: 956. https://doi.org/10.3390/pr7120956
APA StyleBeisl, S., Quehenberger, J., Kamravamanesh, D., Spadiut, O., & Friedl, A. (2019). Exploitation of Wheat Straw Biorefinery Side Streams as Sustainable Substrates for Microorganisms: A Feasibility Study. Processes, 7(12), 956. https://doi.org/10.3390/pr7120956