Systematic Study of Pressure Fluctuation in the Riser of a Dual Inter-Connected Circulating Fluidized Bed: Using Single and Binary Particle Species
Abstract
1. Introduction
2. Materials and Methods
Pressure Fluctuation Analysis
3. Results and Discussion
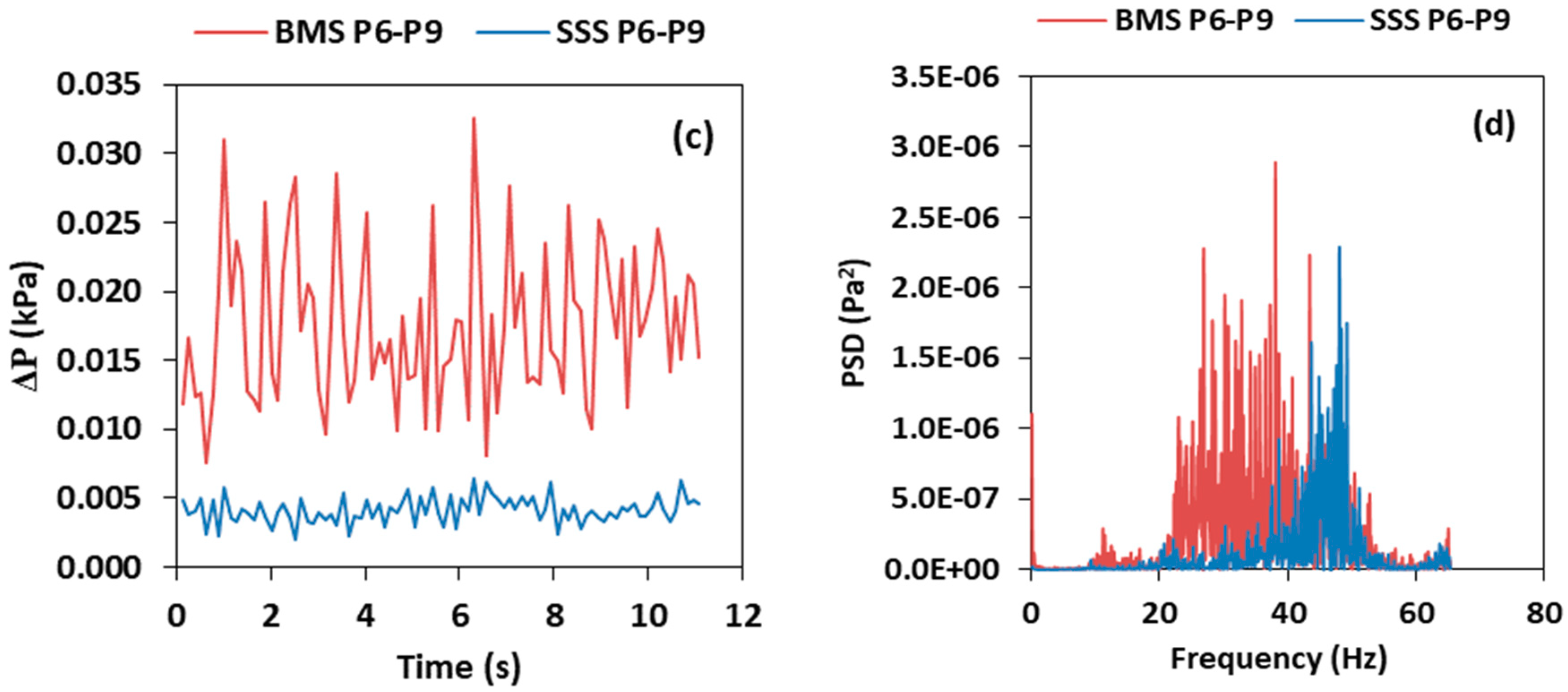
3.1. Single Species and Binary Mixtures Systems (BMS)
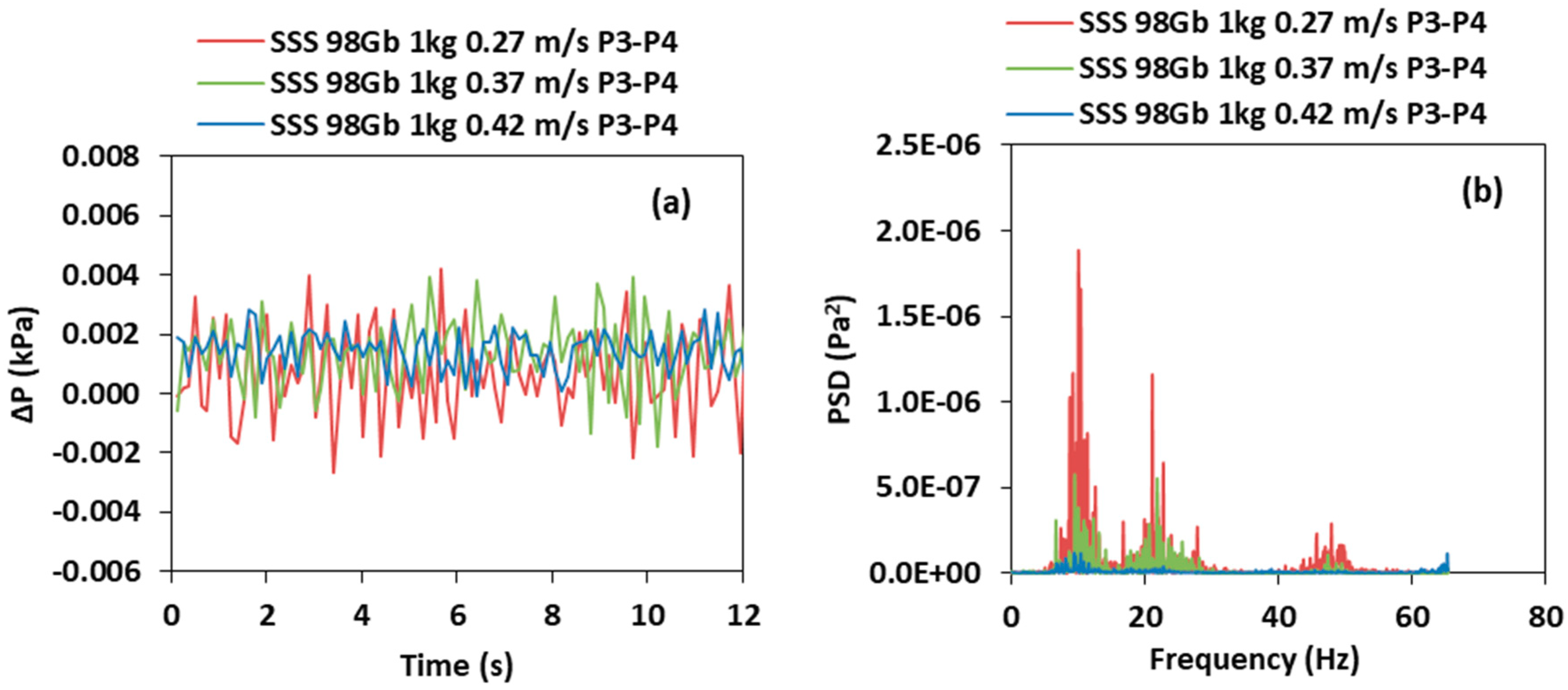
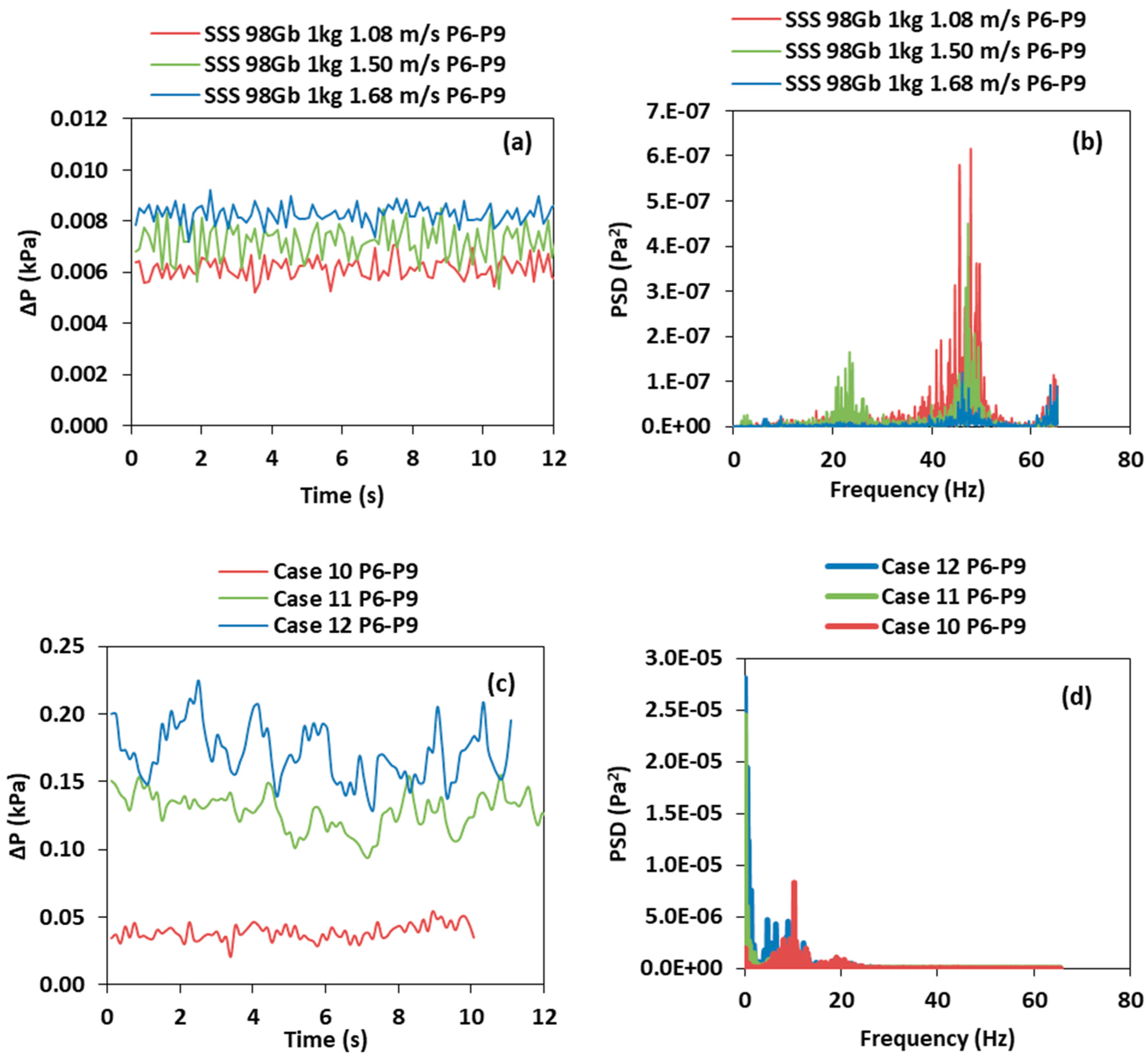
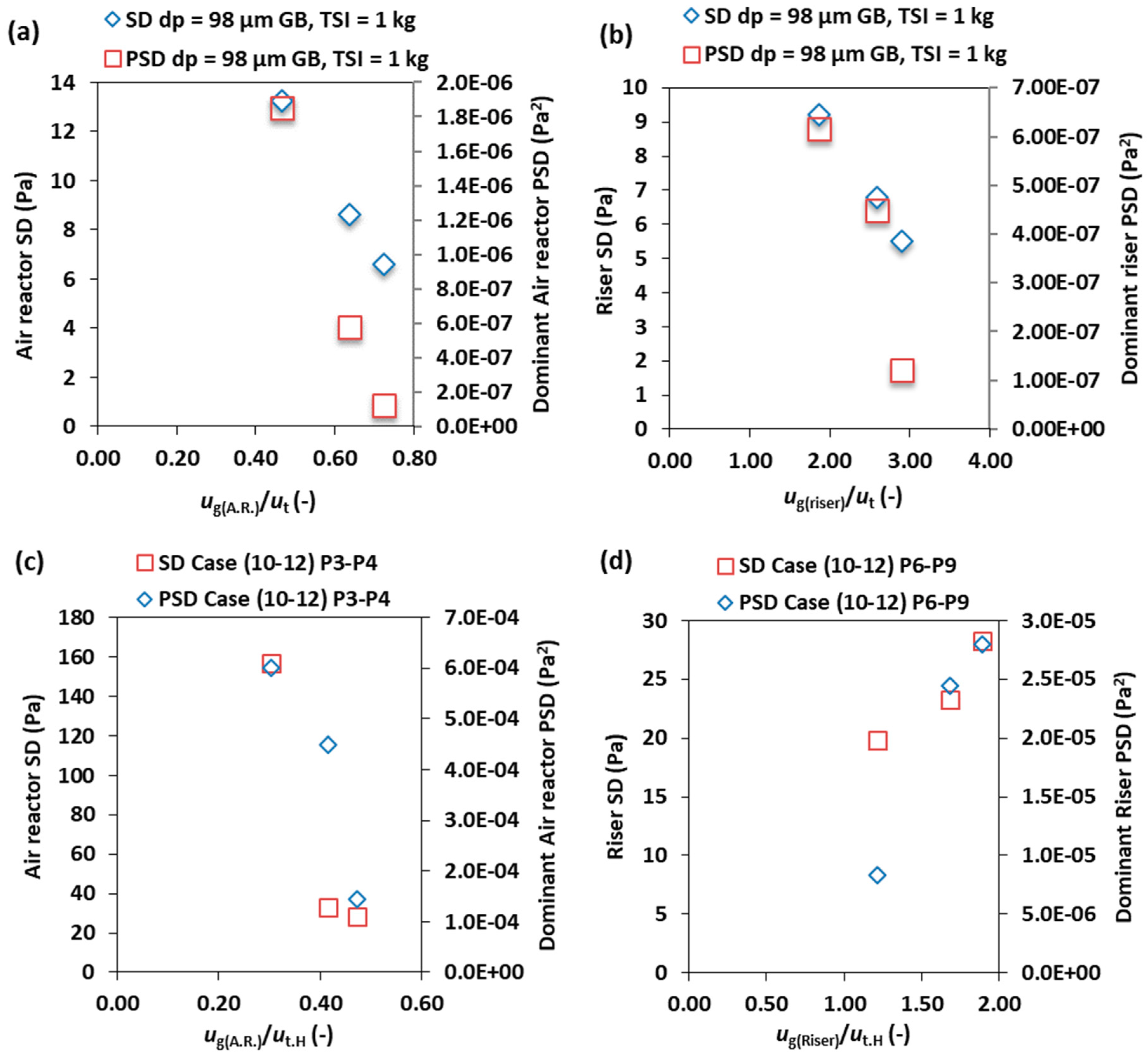
3.2. Effect of the Superficial Gas Velocity
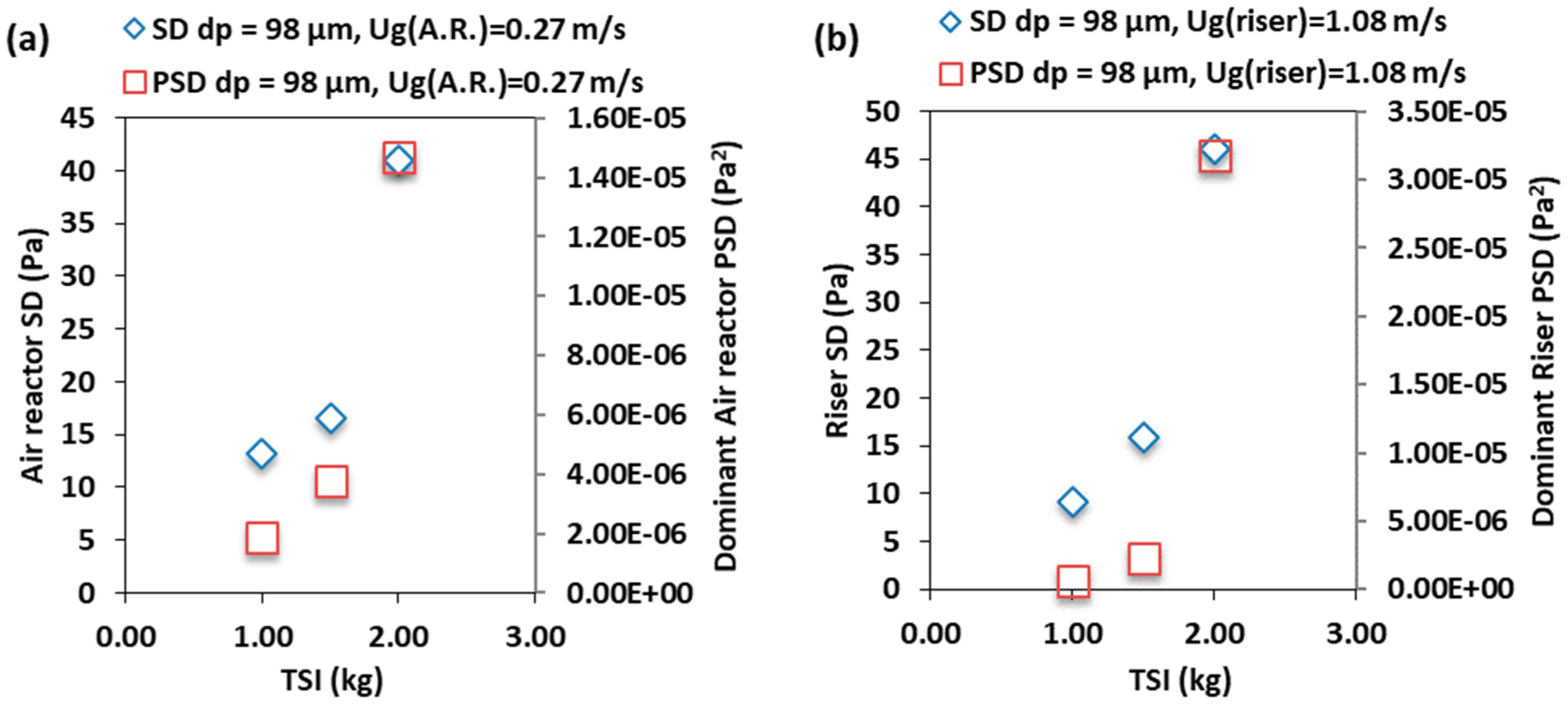
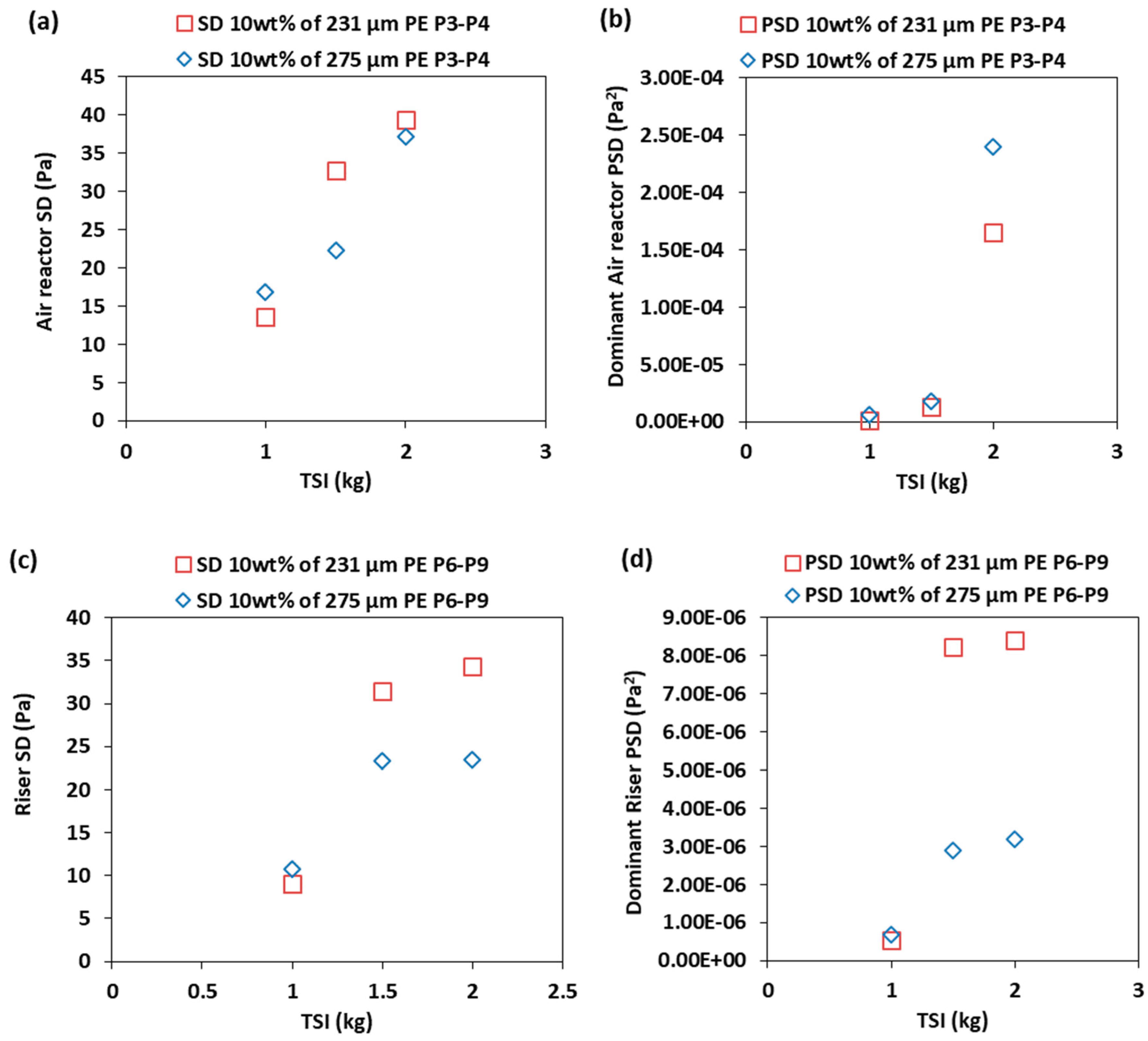
3.3. Effect of the Total Solids Inventory
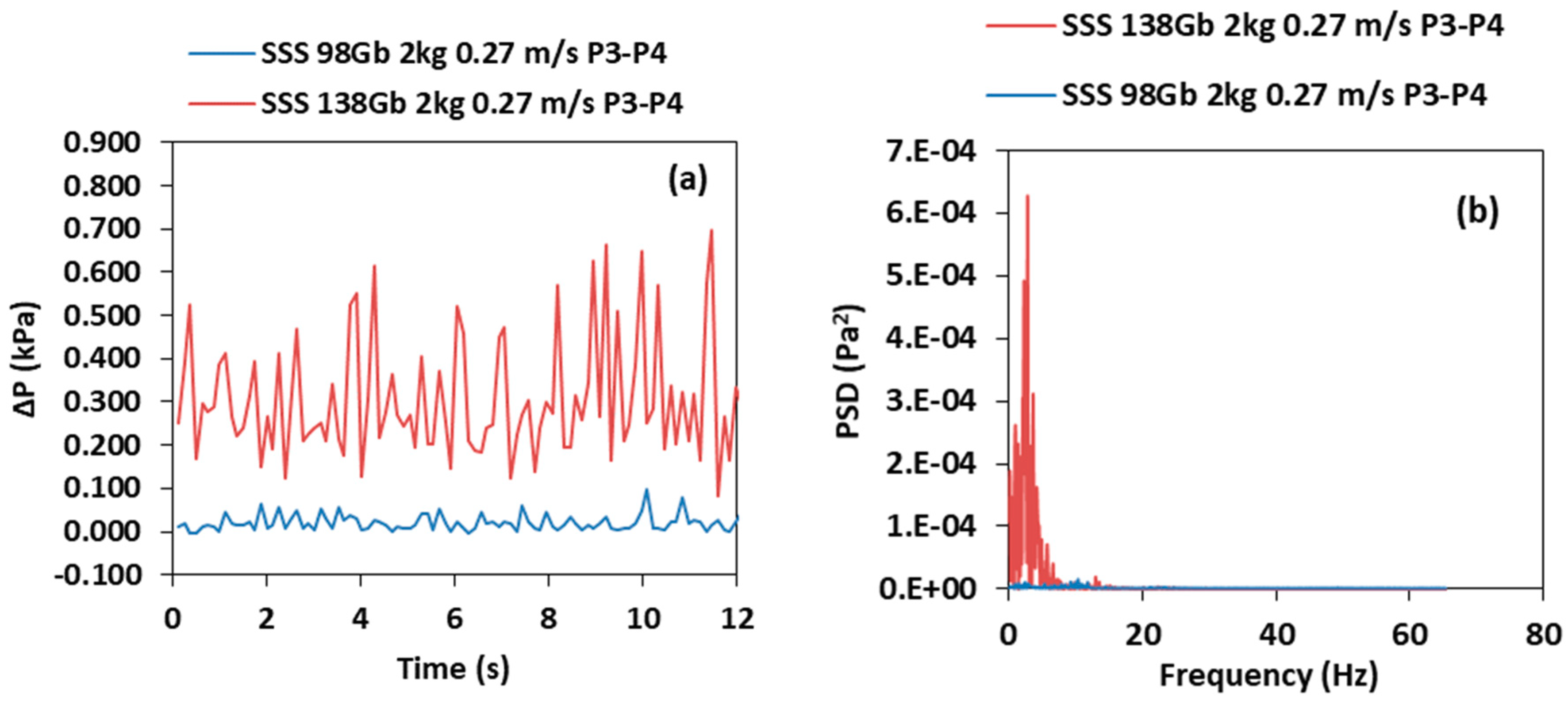
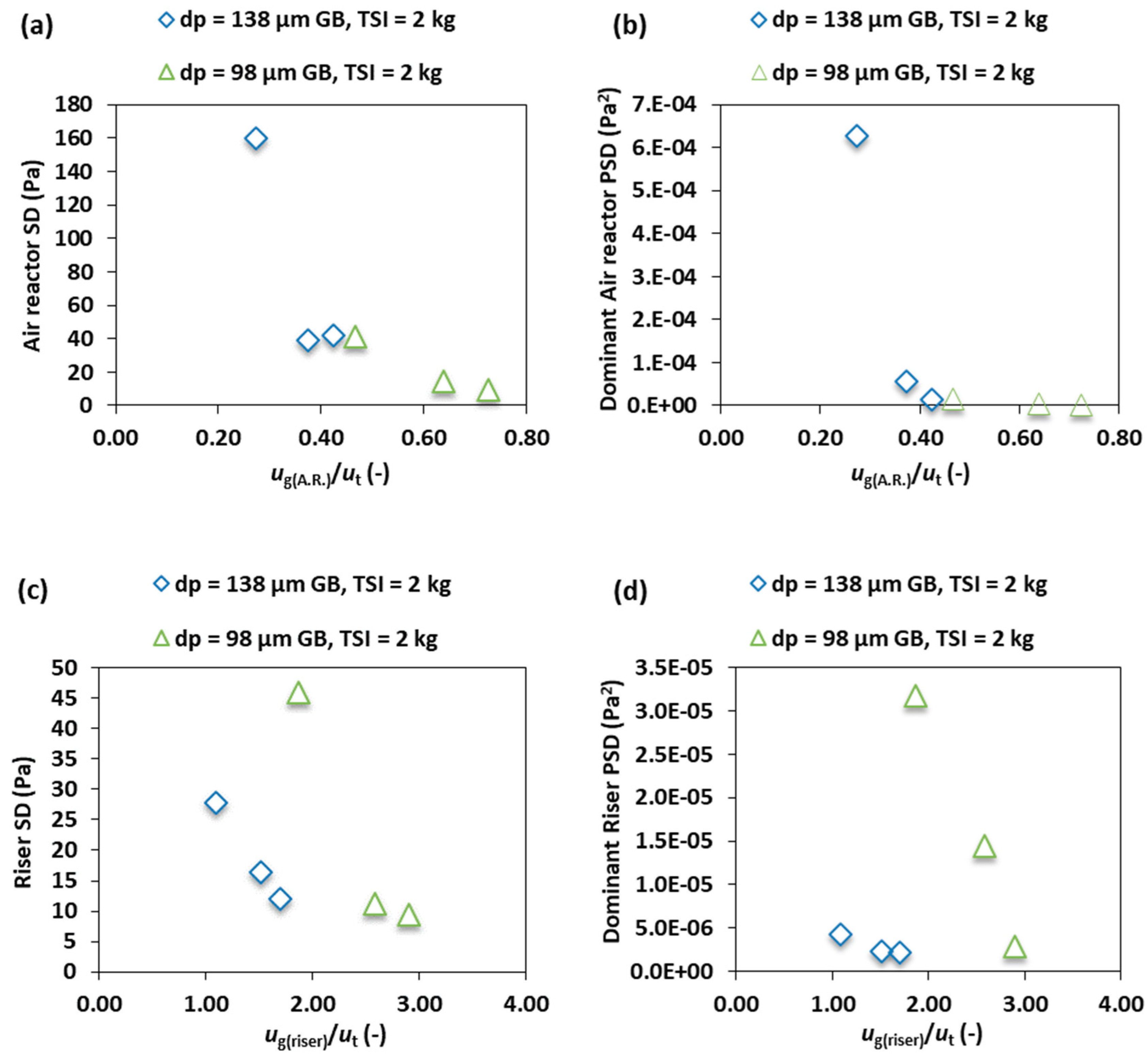
3.4. Effect of the Particle Size
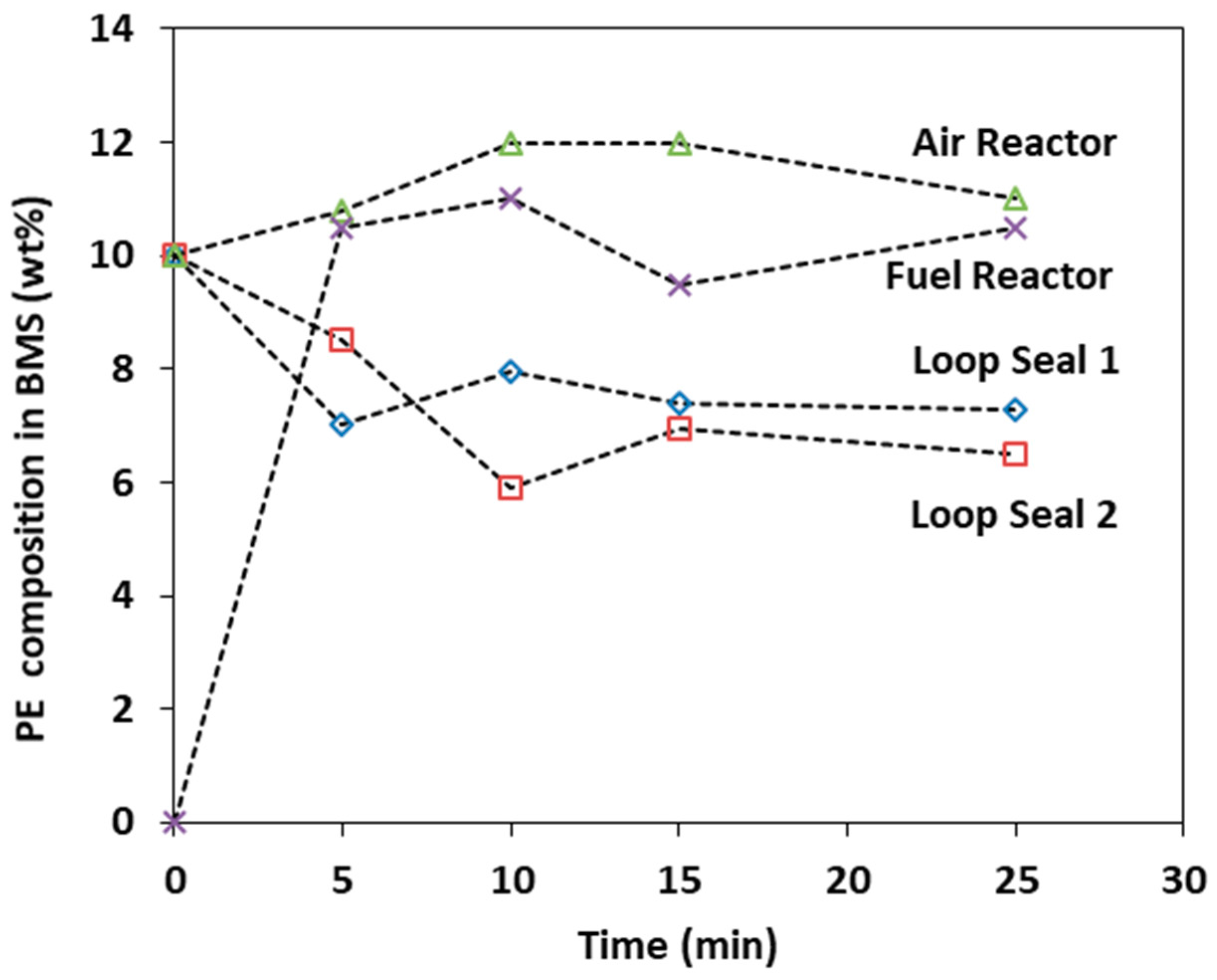
3.5. Effect of BMS Composition
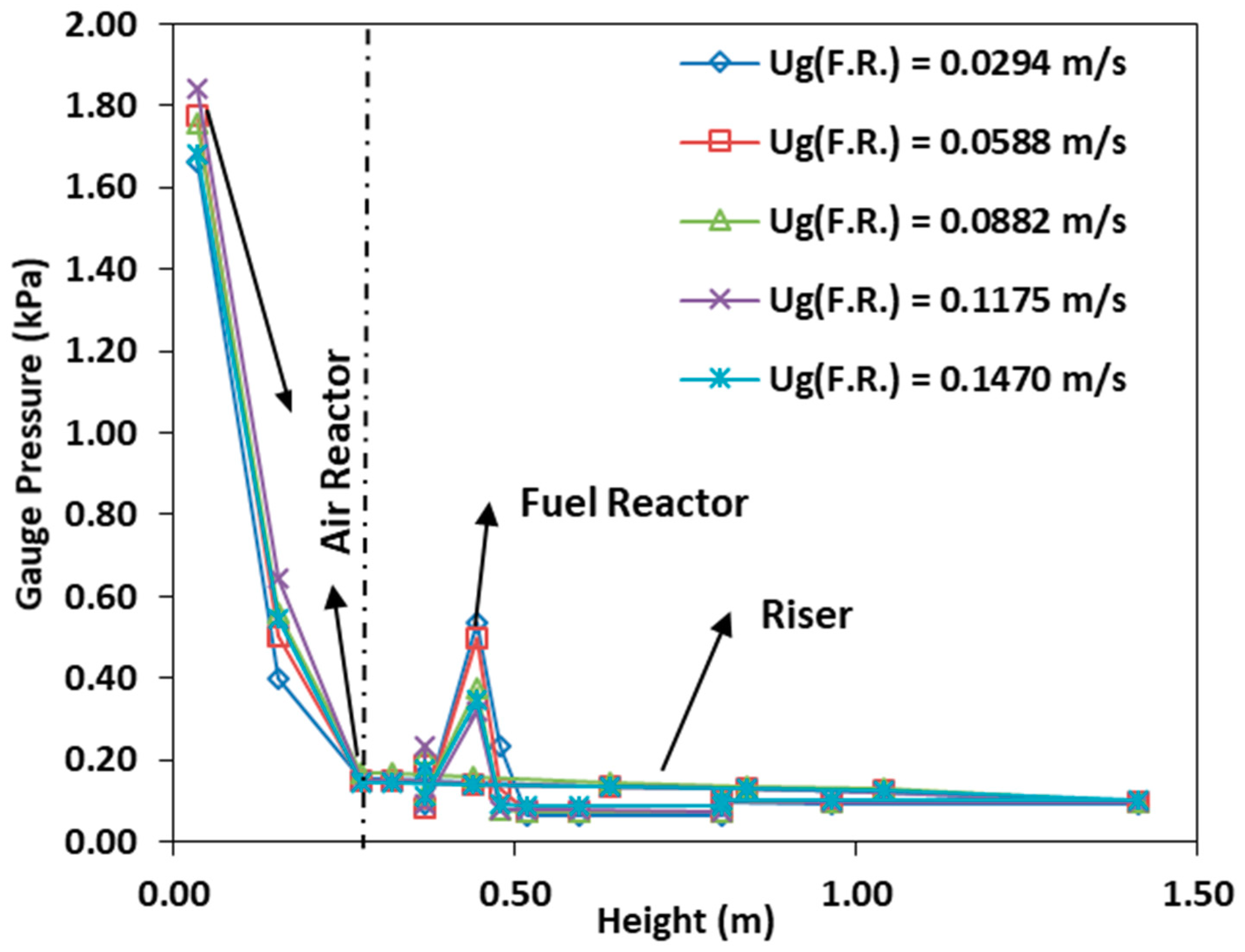
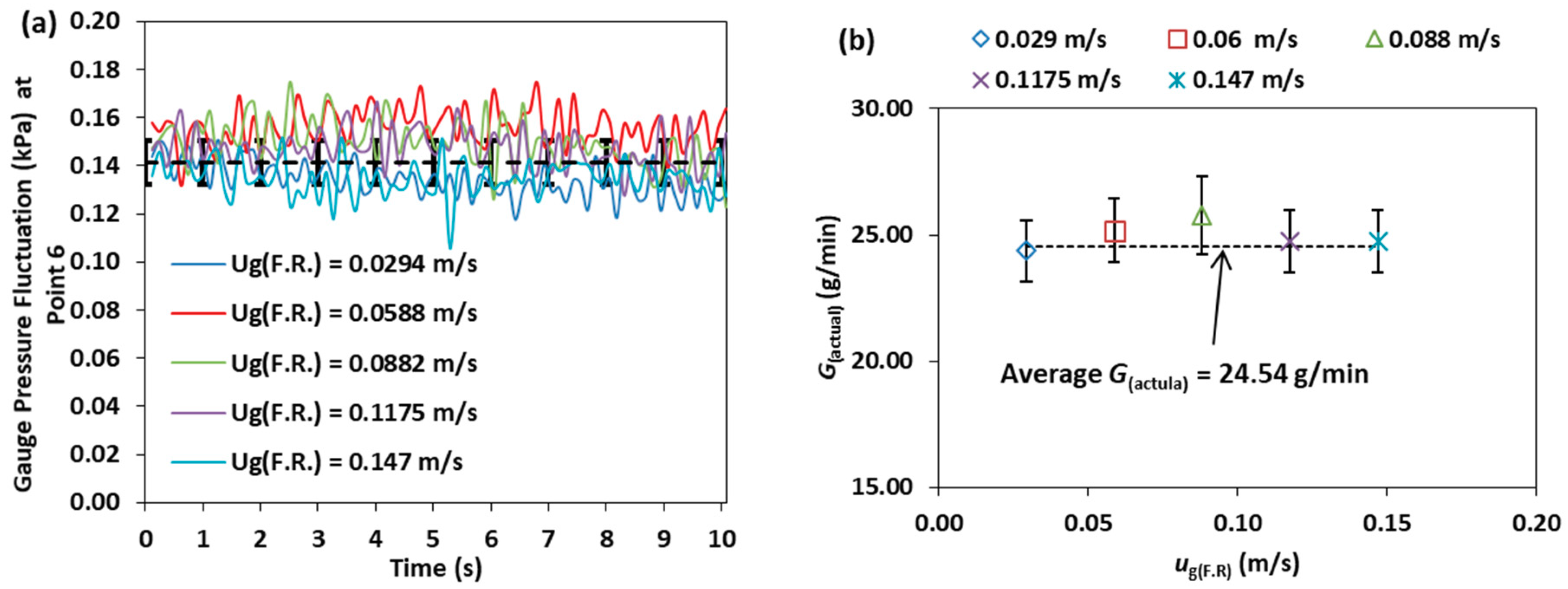
3.6. Effect of the Fuel Reactor Fluidization Velocity
4. Conclusions
- The introduction of a second species into the system influenced the intensity of the pressure fluctuation in the air reactor and the riser. In general, the pressure fluctuation in terms of SD and PSD are higher for BMS when compared to SSS. Increasing the wt.% (i.e., the composition of PE) increases the intensity of the pressure fluctuation and the percentage of the second species in the solid circulation rate.
- For SSS, the solids holdup and interaction of particles increases as the velocity decreases in the air reactor, which lead to a higher SD of the pressure fluctuation and PSD amplitude. Increasing the riser velocity increases the solids holdup on the riser; however, the SD and PSD amplitude decreases because more solids are transported upwards at a higher velocity and fewer solids fall downwards. Similarly, as the air reactor superficial gas velocity decreases in the BMS, the solids holdup increases of both species, therefore, higher SD of the pressure fluctuation and dominant PSD are observed. However, in the BMS, increasing the riser velocity increases the solids holdup of the larger particles in the riser. Thus, this will lead to increasing the SD and PSD of the dominant amplitude in the riser, which will follow the opposite trend in comparison with SSS.
- Increasing the total solids inventory for the SSS and BMS increases the pressure fluctuation in the air reactor and the riser of the CLC–CFM, because of increasing the solids holdup at constant superficial gas velocity.
- As the diameter of the particle increases the pressure fluctuation intensity, the frequency distribution and the PSD amplitude in the air reactor increases. Conversely, it decreases in the riser because the smaller particles are transported at a higher rate into the riser than the larger particles because of the terminal velocity differences.
- Varying the fuel reactor fluidization velocity at constant air reactor fluidization velocity does not influence the air reactor and riser pressure profile, the intensity of the pressure fluctuation and the solid circulation rate.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| Symbols | |
| Ar | Archimedes number |
| dp | particle mean diameter (m) |
| din(riser) | riser inner diameter (mm) |
| din(A.R.) | air reactor inner diameter (mm) |
| g | gravitational acceleration (m/s2) |
| Gactual | actual solid circulation rate (g/min) |
| h | height (mm) |
| N | number of the sampling points |
| P | monitored period (s) |
| Redp(A.R.) | particles Reynolds number in the air reactor (–) |
| Redp(riser) | particles Reynolds number in the riser (–) |
| umf | the minimum fluidization velocity (m/s) |
| umf(mixture) | the minimum fluidization velocity of binary mixture (m/s) |
| ut | particle terminal velocity (m/s) |
| ut.H | terminal velocity of the particle with the higher ut in the binary mixture (m/s) |
| ut.L | terminal velocity of the particle with the lower ut in the binary mixture (m/s) |
| ug | interstitial riser gas velocity (m/s) |
| ug(riser) | riser gas velocity (m/s) |
| ug(A.R.) | air reactor gas velocity (m/s) |
| ug(F.R.) | fuel reactor gas velocity (m/s) |
| umf(mixture) | binary mixture minimum fluidization velocity (m/s) |
| t | time (s) |
| Greek letters | |
| X and Y | amplitude at the angular frequency |
| Δh | height between the two pressure ports (m) |
| ΔP | pressure drop between two points (kPa) |
| ΔPAverage | average pressure drop between over the entire sample (kPa) |
| ρf | fluid density (kg/m3) |
| ρp | solid or particle density (kg/m3) |
| ρ | density (kg/m3) |
| ϕ/ϕs | solids holdup /solids holdup (−) |
| μ | fluid viscosity (Pa. s) |
| ω | angular frequency (rad/sec) |
Abbreviations
| BMS | Binary-mixture system |
| CFB | Circulating fluidized bed |
| CFM | Cold-flow model |
| CLC | Chemical looping combustion |
| GB | Glass bead |
| PE | Polyethylene |
| PSD | Power spectrum density (Pa2) |
| SSS | Single-species system |
| SD | Standard deviation (Pa) |
| TSI | Total solids inventory (kg) |
Appendix A
Steady State Operation
References
- Castilho, G.J.; Cremasco, M.A.; de Martín, L.; Aragón, J.M. Experimental fluid dynamics study in a fluidized bed by deterministic chaos analysis. Part. Sci. Technol. 2011, 29, 179–196. [Google Scholar] [CrossRef]
- Sasic, S.; Leckner, B.; Johnsson, F. Characterization of fluid dynamics of fluidized beds by analysis of pressure fluctuations. Prog. Energy Combust. Sci. 2007, 33, 453–496. [Google Scholar] [CrossRef]
- Xiaodong, W.; Qing, L.; Duan, W. Effect of manganese doping on oxygen storage capacity of ceria- zirconia mixed oxides. J. Rare Earths 2006, 24, 549–553. [Google Scholar]
- Song, H.; Doroodchi, E.; Moghtaderi, B. Redox Characteristics of Fe–Ni/SiO2 Bimetallic Oxygen Carriers in CO under Conditions Pertinent to Chemical Looping Combustion. Energy Fuels 2011, 26, 75–84. [Google Scholar] [CrossRef]
- Feng, Y.Q.; Yu, A.B. An analysis of the chaotic motion of particles of different sizes in a gas fluidized bed. Particuology 2008, 6, 549–556. [Google Scholar] [CrossRef]
- Noda, K.; Uchida, S.; Makino, T.; Kamo, H. Minimum fluidization velocity of binary mixture of particles with large size ratio. Powder Technol. 1986, 46, 149–154. [Google Scholar] [CrossRef]
- Huilina, L.; Yuronga, H.; Gidaspow, D.; Lidana, Y.; Yukuna, Q. Size segregation of binary mixture of solids in bubbling fluidized beds. Powder Technol. 2003, 134, 86–97. [Google Scholar] [CrossRef]
- Feng, Y.Q.; Yu, A.B. Effect of bed thickness on the segregation behavior of particle mixtures in a gas fluidized bed. Ind. Eng. Chem. Res. 2010, 49, 3459–3468. [Google Scholar] [CrossRef]
- Huilin, L.; Yunhua, Z.; Ding, J.; Gidaspow, D.; Wei, L. Investigation of mixing/segregation of mixture of particles in gas-solid fluidized beds. Chem. Eng. Sci. 2007, 62, 301–317. [Google Scholar] [CrossRef]
- Rowe, P.N.; Nienow, A.W.; Agbim, A.J. The mechanisms by which particles segregate in gas fluidised beds-binary systems of near-spherical particles. Trans. Instn. Chem. Eng. 1972, 50, 310–323. [Google Scholar]
- Jang, H.T.; Park, T.S.; Cha, W.S. Mixing–segregation phenomena of binary system in a fluidized bed. J. Ind. Eng. Chem. 2010, 16, 390–394. [Google Scholar] [CrossRef]
- Gibilaro, L.G.; Rowe, P.N. A model for a segregation gas fliudised bed. Chem. Eng. Sci. 1974, 29, 1403–1412. [Google Scholar] [CrossRef]
- Kwant, G.; Prins, W.; Swaaij, W.P.M.v. Particle mixing and separation in a binary solids floating fluidized bed. Powder Technol. 1995, 82, 279–291. [Google Scholar] [CrossRef]
- Nienow, A.W.; Rowe, P.N.; Cheung, L.Y.L. A quantitative analysis of the mixing of two segregating powders of different density in a gas-fluidised bed. Powder Technol. 1978, 20, 89–97. [Google Scholar] [CrossRef]
- Olivieri, G.; Marzocchella, A.; Salatino, P. Segregation of fluidized binary mixtures of granular solids. Am. Inst. Chem. Eng. J. 2004, 50, 3095–3106. [Google Scholar] [CrossRef]
- Garcia, F.; Romero, A.; Villar, J.C.; Bell, a.A. A Study of segregation in a Gas-Solid Fluidized bed: Particles of differnt Density. Powder Technol. 1989, 58, 169–174. [Google Scholar] [CrossRef]
- Das, M.; Banerjee, M.; Saha, R.K. Segregation and mixing effects in the riser of a circulating fluidized bed. Powder Technol. 2007, 178, 179–186. [Google Scholar] [CrossRef]
- Palappan, K.G.; Sai, P.S.T. Studies on segregation of binary mixture of solids in a continuous fast fluidized bed Part I. Effect of particle density. Chem. Eng. Sci. 2008, 138, 358–366. [Google Scholar] [CrossRef]
- Palappan, K.G.; Sai, P.S.T. Studies on segregation of binary mixture of solids in continuous fast fluidized bed Part II. Effect of particle size. Chem. Eng. Sci. 2008, 139, 330–338. [Google Scholar] [CrossRef]
- Palappan, K.G.; Sai, P.S.T. Studies on segregation of binary mixture of solids in continuous fast fluidized bed Part III. Quantification of performance of the segregator. Chem. Eng. J. 2008, 145, 100–111. [Google Scholar] [CrossRef]
- Hirschberg, B.; Werther, J. Factors affecting solids segregation in circulating fluidized-bed riser. Am. Inst. Chem. Eng. J. 1998, 44, 25–34. [Google Scholar] [CrossRef]
- Alghamdi, Y.A.; Peng, Z.B.; Doroodchi, E.; Moghtaderi, B. CFD-DEM Simulation of Mixing and Segregation of Fluidized Binary Mixtures of Particles with Different Sizes and Densities. In Proceedings of the 21st International Conference on Fluidized Bed Combustion (FBC), Naples, Italy, 3–6 June 2012; pp. 1058–1065. [Google Scholar]
- Alghamdi, Y.A.; Doroodchi, E.; Moghtaderi, B. Mixing and segregation of binary oxygen carrier mixtures in a cold flow model of a chemical looping combustor. Chem. Eng. J. 2013, 223, 772–784. [Google Scholar] [CrossRef]
- Peng, Z.; Doroodchi, E.; Alghamdi, Y.; Moghtaderi, B. Mixing and segregation of solid mixtures in bubbling fluidized beds under conditions pertinent to the fuel reactor of a chemical looping system. Powder Technol. 2013, 235, 823–837. [Google Scholar] [CrossRef]
- Bai, D.; Shibuya, E.; Nakagawa, N.; Kato, K. Characterization of gas fluidization regimes using pressure fluctuations. Powder Technol. 1996, 87, 105–111. [Google Scholar] [CrossRef]
- Bi, H.T. A critical review of the complex pressure fluctuation phenomenon in gas–solids fluidized beds. Chem. Eng. Sci. 2007, 62, 3473–3493. [Google Scholar] [CrossRef]
- Jang, H.; Kim, S.; Cha, W.; Hong, S.; Doh, D. Pressure fluctuation properties in combustion of mixture of anthracite and bituminous coal in a fluidized bed. Korean J. Chem. Eng. 2003, 20, 138–144. [Google Scholar] [CrossRef]
- Johnsson, F.; Zijerveld, R.C.; Schouten, J.C.; van den Bleek, C.M.; Leckner, B. Characterization of fluidization regimes by time-series analysis of pressure fluctuations. Int. J. Multiph. Flow. 2000, 26, 663–715. [Google Scholar] [CrossRef]
- Van Ommen, J.R.; Sasic, S.; van der Schaaf, J.; Gheorghiu, S.; Johnsson, F.; Coppens, M.-O. Time-series analysis of pressure fluctuations in gas–solid fluidized beds—A review. Int. J. Multiph. Flow 2011, 37, 403–428. [Google Scholar] [CrossRef]
- Zhong, W.; Zhang, M. Pressure fluctuation frequency characteristics in a spout-fluid bed by modern ARM power spectrum analysis. Powder Technol. 2005, 152, 52–61. [Google Scholar] [CrossRef]
- Leu, L.P.; Wu, C.N. Prediction of pressure fluctuations and minimum fluidization velocity of binary mixtures of geldart group B particles in bubbling fluidized beds. Can. J. Chem. Eng. 2000, 78, 578–585. [Google Scholar] [CrossRef]
- Villa Briongos, J.; Aragón, J.M.; Palancar, M.C. Phase space structure and multi-resolution analysis of gas–solid fluidized bed hydrodynamics: Part I The EMD approach. Chem. Eng. Sci. 2006, 61, 6963–6980. [Google Scholar] [CrossRef]
- Briongos, J.V.; Aragón, J.M.; Palancar, M.C. Phase space structure and multi-resolution analysis of gas–solid fluidized bed hydrodynamics: Part II: Dynamic analysis. Chem. Eng. Sci. 2007, 62, 2865–2879. [Google Scholar] [CrossRef]
- Stringer, J. Is a fluidized bed a chaotic dynamic system? In Proceedings of the 10th International Conference on Fluidized Bed Combustion, San Francisco, CA, USA, 30 April–3 May 1989; pp. 265–272. [Google Scholar]
- Alberto, C.; Felipe, S.; Rocha, S.C.S. Time series analysis of pressure Fluctuation in gas-solid fluidized beds. Braz. J. Chem. Eng. Chem. Sci. 2004, 21, 497–507. [Google Scholar]
- Saxena, S.C.; Rao, N.S.; Tanjore, V.N. Diagnostic procedures for establishing the quality of fluidization of gas-solid systems. Exp. Therm. Fluid Sci. 1993, 6, 56–73. [Google Scholar] [CrossRef]
- Wang, S.; Wang, G.; Jiang, F.; Luo, M.; Li, H. Chemical looping combustion of coke oven gas by using Fe2O3/CuO with MgAl2O4 as oxygen carrier. Energy Environ. Sci. 2010, 3, 1353–1360. [Google Scholar] [CrossRef]
- Adánez, J.; Gayán, P.; Celaya, J.; Diego, L.d.; García-Labiano, F.; Abad, A. Chemical looping combustion in a 10 kW prototype using a CuO/Al2O3 oxygen carrier: Effect of operating conditions on methane combustion. Ind. Eng. Chem. Res. 2006, 45, 6075–6080. [Google Scholar] [CrossRef]
- Peng, Z.; Doroodchi, E.; Alghamdi, Y.A.; Shah, K.; Luo, C.; Moghtaderi, B. CFD–DEM simulation of solid circulation rate in the cold flow model of chemical looping systems. Chem. Eng. Res. Des. 2015, 95, 262–280. [Google Scholar] [CrossRef]
- Moghtaderi, B. Review of the recent chemical looping process developments for novel energy and fuel applications. Energy Fuels 2011, 26, 15–40. [Google Scholar] [CrossRef]
- Dahl, I.M.; Bakken, E.; Larring, Y.; Spjelkavik, A.I.; Håkonsen, S.F.; Blom, R. On the development of novel reactor concepts for chemical looping combustion. Energy Procedia 2009, 1513–1519. [Google Scholar] [CrossRef]
- Ishida, M.; Zheng, D.; Akehat, T. Evaluation of a chemical looping combustion power generation system by graphic exergy analysis. Energy Int. J. 1987, 12, 147–154. [Google Scholar] [CrossRef]
- Pröll, T.; Rupanovits, K.; Kolbitsch, P.; Bolhàr-Nordenkampf, J.; Hofbauer, H. Cold Flow Model Study on a Dual Circulating Fluidized Bed (DCFB) System for Chemical Looping Processes. Chem. Eng. Technol. 2009, 32, 418–424. [Google Scholar] [CrossRef]
- Kolbitsch, P.; Bolhàr-Nordenkampf, J.; Proll, T.; Hofbauer, H. Operating experience with chemical looping combustion in a 120 kW dual circulating fluidized bed (DCFB) unit. Int. J. Greenh. Gas Control 2010, 4, 180–185. [Google Scholar] [CrossRef]
- Kronberger, B.; Lyngfelt, A.; Löffler, G.; Hofbauer, H. Design and Fluid Dynamic Analysis of a Bench-Scale Combustion System with CO2 Separation−Chemical-Looping Combustion. Ind. Eng. Chem. Res. 2005, 44, 546–556. [Google Scholar] [CrossRef]
- Charitos, A.; Hawthorne, C.; Bidwe, A.R.; Korovesis, L.; Schuster, A.; Scheffknecht, G. Hydrodynamic analysis of a 10kWth Calcium looping daul fluidized bed for post-combustion CO2 capture. Powder Technol. 2010, 200, 117–127. [Google Scholar] [CrossRef]
- Lyngfelt, A.; Kronberger, B.; Adanez, J.; Morin, J.X.; Hurst, P. Development of oxygen carrier particles for chemical-looping combustion esign and operation of a 10 kW chemical-combustor. In Proceedings of the 7th International Conference on Greenhouse Gas Control Technologies, Vancouver, BC, Canada, 5–9 September 2004. [Google Scholar]
- Alghamdi, Y.; Peng, Z.; Zanganeh, J.; Moghtaderi, B.; Doroodchi, E. Hydrodynamics similarities in cold flow model of chemical looping combustors: An experimental study. Powder Technol. 2019, 343, 542–550. [Google Scholar] [CrossRef]
- Glicksman, L.R.; Hyre, M.; Woloshun, K. Simplified scaling relationships for fluidized beds. Powder Technol. 1993, 77, 177–199. [Google Scholar] [CrossRef]
- Glicksman, L.R.; Hyre, M.R.; Farrell, P.A. Dynamic similarity in fluidization. Int. J. Multiph. Flow 1994, 20 (Suppl. 1), 331–386. [Google Scholar] [CrossRef]
- Cho, P.; Mattisson, T.; Lyngfelt, A. Comparison of iron-, nickel-, copper- and manganese-based oxygen carriers for chemical-looping combustion. Fuel 2004, 83, 1215–1225. [Google Scholar] [CrossRef]
- Alghamdi, Y.A. Transport characteristics of binary mixture of particles in chemical looping combustion applications. Ph.D. Thesis, University of Newcastle, Callaghan, Australia, April 2016. [Google Scholar]
- Alghamdi, Y.; Peng, Z.; Shah, K.; Moghtaderi, B.; Doroodchi, E. Predicting the solid circulation rate in chemical looping combustion systems using pressure drop measurements. Powder Technol. 2015, 286, 572–581. [Google Scholar] [CrossRef]
- Alghamdi, Y.A.; Peng, Z.; shah, K.; Moghtaderi, B.; Doroodchi, E. A correlation for predicting solids holdup in the dilute pneumatic conveying flow regime of circulating and interconnected fluidised beds. Powder Technol. 2016, 297, 357–366. [Google Scholar] [CrossRef]
- Wen, C.Y.; Yu, Y.H. A Generalized Method for Predicting the Minimum Fluidization Velocity. Am. Inst. Chem. Eng. J. 1966, 12, 610–612. [Google Scholar] [CrossRef]
- Haider, A.; Levenspiel, O. Drag coefficient and terminal velocity of spherical and nonspherical particles. Powder Technol. 1989, 58, 63–70. [Google Scholar] [CrossRef]
- Cheung, L.; Nienow, A.W.; Rowe, P.N. Minimum fluidisation velocity of a binary mixture of different sized particles. Chem. Eng. Sci. 1974, 29, 1301–1303. [Google Scholar]










| dp, min (μm) | dp, max (μm) | dp, mean (μm) | ρp (kg/m3) | umfa (m/s) | utb (m/s) | Particle Type |
|---|---|---|---|---|---|---|
| 90 | 106 | 98 | 2462 | 0.0085 | 0.58 | GB |
| 106 | 125 | 116 | 2462 | 0.012 | 0.75 | GB |
| 125 | 150 | 138 | 2462 | 0.016 | 0.99 | GB |
| 212 | 250 | 231 | 939 | 0.018 | 0.89 | PE |
| 250 | 300 | 275 | 939 | 0.025 | 1.1 | PE |
| 300 | 355 | 328 | 939 | 0.036 | 1.35 | PE |
| System Type | TSI (kg) | GB dp (µm) | ug(A.R.) (m/s) | ug(A.R.)/ut | ug(riser) (m/s) | ug(riser)/ut | ug(F.R.) (m/s) |
|---|---|---|---|---|---|---|---|
| SSS | 1 | 98 | 0.27 | 0.46 | 1.08 | 1.88 | 0.088 |
| SSS | 1 | 98 | 0.37 | 0.64 | 1.5 | 2.56 | 0.088 |
| SSS | 1 | 98 | 0.42 | 0.72 | 1.68 | 2.90 | 0.088 |
| SSS | 1.5 | 98 | 0.27 | 0.46 | 1.08 | 1.88 | 0.088 |
| SSS | 1.5 | 98 | 0.37 | 0.64 | 1.5 | 2.56 | 0.088 |
| SSS | 1.5 | 98 | 0.42 | 0.72 | 1.68 | 2.90 | 0.088 |
| SSS | 2 | 98 | 0.27 | 0.46 | 1.08 | 1.88 | 0.088 |
| SSS | 2 | 98 | 0.37 | 0.64 | 1.5 | 2.56 | 0.088 |
| SSS | 2 | 98 | 0.42 | 0.72 | 1.68 | 2.90 | 0.088 |
| SSS | 1.75 | 138 | 0.27 | 0.27 | 1.08 | 1.08 | 0.088 |
| SSS | 1.75 | 138 | 0.37 | 0.37 | 1.5 | 1.52 | 0.088 |
| SSS | 1.75 | 138 | 0.42 | 0.42 | 1.68 | 1.70 | 0.088 |
| SSS | 2 | 138 | 0.27 | 0.27 | 1.08 | 1.08 | 0.088 |
| SSS | 2 | 138 | 0.37 | 0.37 | 1.5 | 1.52 | 0.088 |
| SSS | 2 | 138 | 0.42 | 0.42 | 1.68 | 1.70 | 0.088 |
| SSS | 2.25 | 138 | 0.27 | 0.27 | 1.08 | 1.08 | 0.088 |
| SSS | 2.25 | 138 | 0.37 | 0.37 | 1.5 | 1.52 | 0.088 |
| SSS | 2.25 | 138 | 0.42 | 0.42 | 1.68 | 1.70 | 0.088 |
| System Type | Case No. | TSI (kg) | PE dp (µm) | GB dp (µm) | dp(PE)/dp(GB) | PE Composition (wt%) | GB Composition (wt%) | ut.H/ut.L | ug(A.R.) (m/s) | ug(A.R.)/ut.H | ug(riser) (m/s) | u(riser.)/ut.H | umf(mixture) (m/s) a | ug(F.R.) (m/s) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BMS | 1 | 1 | 231 | 98 | 2.4 | 10 | 90 | 1.6 | 0.27 | 0.30 | 1.08 | 1.22 | 0.00769 | 0.088 |
| BMS | 2 | 1.5 | 231 | 98 | 2.4 | 10 | 90 | 1.6 | 0.27 | 0.30 | 1.08 | 1.22 | 0.00769 | 0.088 |
| BMS | 3 | 2 | 231 | 98 | 2.4 | 10 | 90 | 1.6 | 0.27 | 0.30 | 1.08 | 1.22 | 0.00769 | 0.088 |
| BMS | 4 | 1 | 275 | 98 | 2.8 | 10 | 90 | 2 | 0.27 | 0.25 | 1.08 | 0.98 | 0.00772 | 0.088 |
| BMS | 5 | 1.5 | 275 | 98 | 2.8 | 10 | 90 | 2 | 0.27 | 0.25 | 1.08 | 0.98 | 0.00772 | 0.088 |
| BMS | 6 | 2 | 275 | 98 | 2.8 | 10 | 90 | 2 | 0.27 | 0.25 | 1.08 | 0.98 | 0.00772 | 0.088 |
| BMS | 7 | 2 | 231 | 138 | 1.7 | 5 | 95 | 1.1 | 0.42 | 0.42 | 1.68 | 1.70 | 0.0150 | 0.088 |
| BMS | 8 | 2 | 231 | 138 | 1.7 | 10 | 90 | 1.1 | 0.42 | 0.42 | 1.68 | 1.70 | 0.0150 | 0.088 |
| BMS | 9 | 2 | 231 | 138 | 1.7 | 20 | 80 | 1.1 | 0.42 | 0.42 | 1.68 | 1.70 | 0.0150 | 0.088 |
| BMS | 10 | 2 | 231 | 116 | 2 | 10 | 90 | 1.2 | 0.27 | 0.30 | 1.08 | 1.20 | 0.0110 | 0.088 |
| BMS | 11 | 2 | 231 | 116 | 2 | 10 | 90 | 1.2 | 0.37 | 0.42 | 1.5 | 1.70 | 0.0110 | 0.088 |
| BMS | 12 | 2 | 231 | 116 | 2 | 10 | 90 | 1.2 | 0.42 | 0.47 | 1.68 | 1.89 | 0.0110 | 0.088 |
| BMS | 13 | 2 | 328 | 138 | 2.4 | 10 | 90 | 1.4 | 0.27 | 0.20 | 1.08 | 0.80 | 0.01512 | 0.088 |
| Effect of Increasing | SSS | BMS | ||||||
|---|---|---|---|---|---|---|---|---|
| Air Reactor | Riser | Air Reactor | Riser | |||||
| SD | PSD | SD | PSD | SD | PSD | SD | PSD | |
| ug(A.R.) and ug(riser) | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↑ | ↑ |
| TSI | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ |
| dp | ↑ | ↑ | ↓ | ↓ | ↓ | ↑ | ↓ | ↓ |
| BMS Wt.% (5, 10, 20%) | - | - | - | - | ↑ | ↑ | ↑ | ↑ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alghamdi, Y.A.; Peng, Z.; Luo, C.; Almutairi, Z.; Moghtaderi, B.; Doroodchi, E. Systematic Study of Pressure Fluctuation in the Riser of a Dual Inter-Connected Circulating Fluidized Bed: Using Single and Binary Particle Species. Processes 2019, 7, 890. https://doi.org/10.3390/pr7120890
Alghamdi YA, Peng Z, Luo C, Almutairi Z, Moghtaderi B, Doroodchi E. Systematic Study of Pressure Fluctuation in the Riser of a Dual Inter-Connected Circulating Fluidized Bed: Using Single and Binary Particle Species. Processes. 2019; 7(12):890. https://doi.org/10.3390/pr7120890
Chicago/Turabian StyleAlghamdi, Yusif A., Zhengbiao Peng, Caimao Luo, Zeyad Almutairi, Behdad Moghtaderi, and Elham Doroodchi. 2019. "Systematic Study of Pressure Fluctuation in the Riser of a Dual Inter-Connected Circulating Fluidized Bed: Using Single and Binary Particle Species" Processes 7, no. 12: 890. https://doi.org/10.3390/pr7120890
APA StyleAlghamdi, Y. A., Peng, Z., Luo, C., Almutairi, Z., Moghtaderi, B., & Doroodchi, E. (2019). Systematic Study of Pressure Fluctuation in the Riser of a Dual Inter-Connected Circulating Fluidized Bed: Using Single and Binary Particle Species. Processes, 7(12), 890. https://doi.org/10.3390/pr7120890







