Optimization of Baicalin, Wogonoside, and Chlorogenic Acid Water Extraction Process from the Roots of Scutellariae Radix and Lonicerae japonicae Flos Using Response Surface Methodology (RSM)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Samples and Chemicals Reagents
2.3. Preparation of Standard Solution of Baicalin, Wogonoside, and Chlorogenic Acid
2.4. Sample Preparation and Extraction Protocol
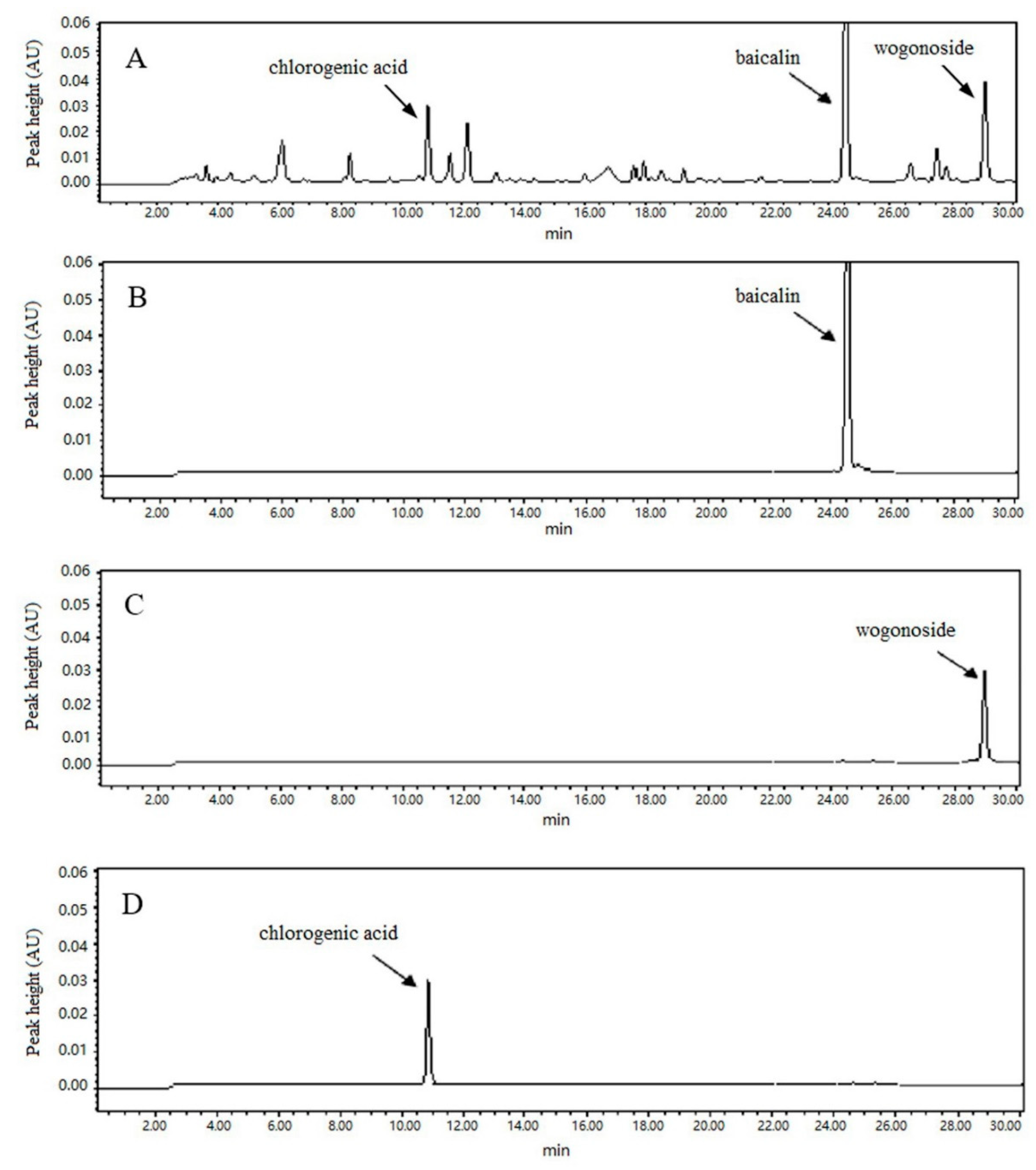
2.5. HPLC Quantification and Method Validation
2.6. Single-Factor Test Design
2.7. Box–Behnken Design (BBD) for Optimization of Extraction Conditions
2.8. Data Analysis
3. Results
3.1. High Performance Liquid Chromatography (HPLC) and Validation of Extraction Process
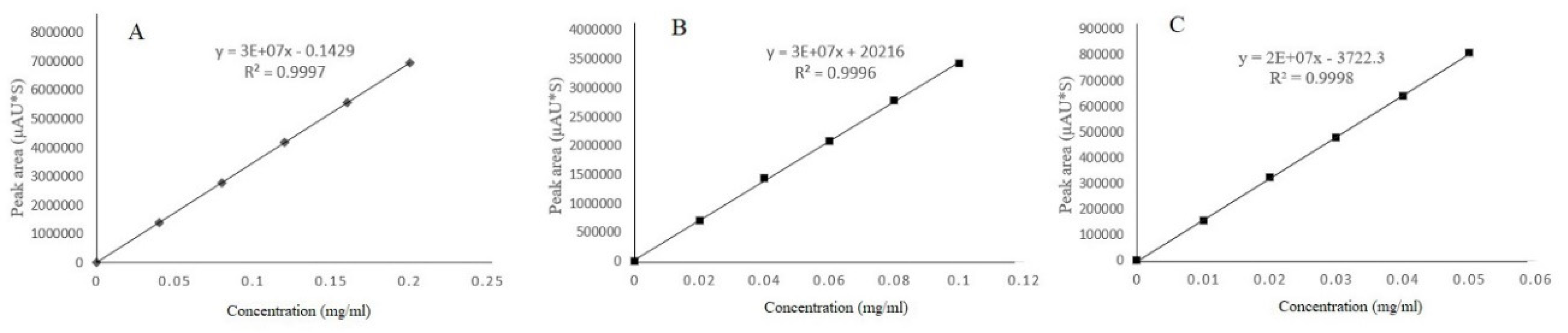
3.2. Standard Curve Determination and Range of Linearity
3.3. Precision Experiments
3.4. Stability, Reproducibility, and Recovery of Baicalin, Wogonoside, and Chlorogenic Acid
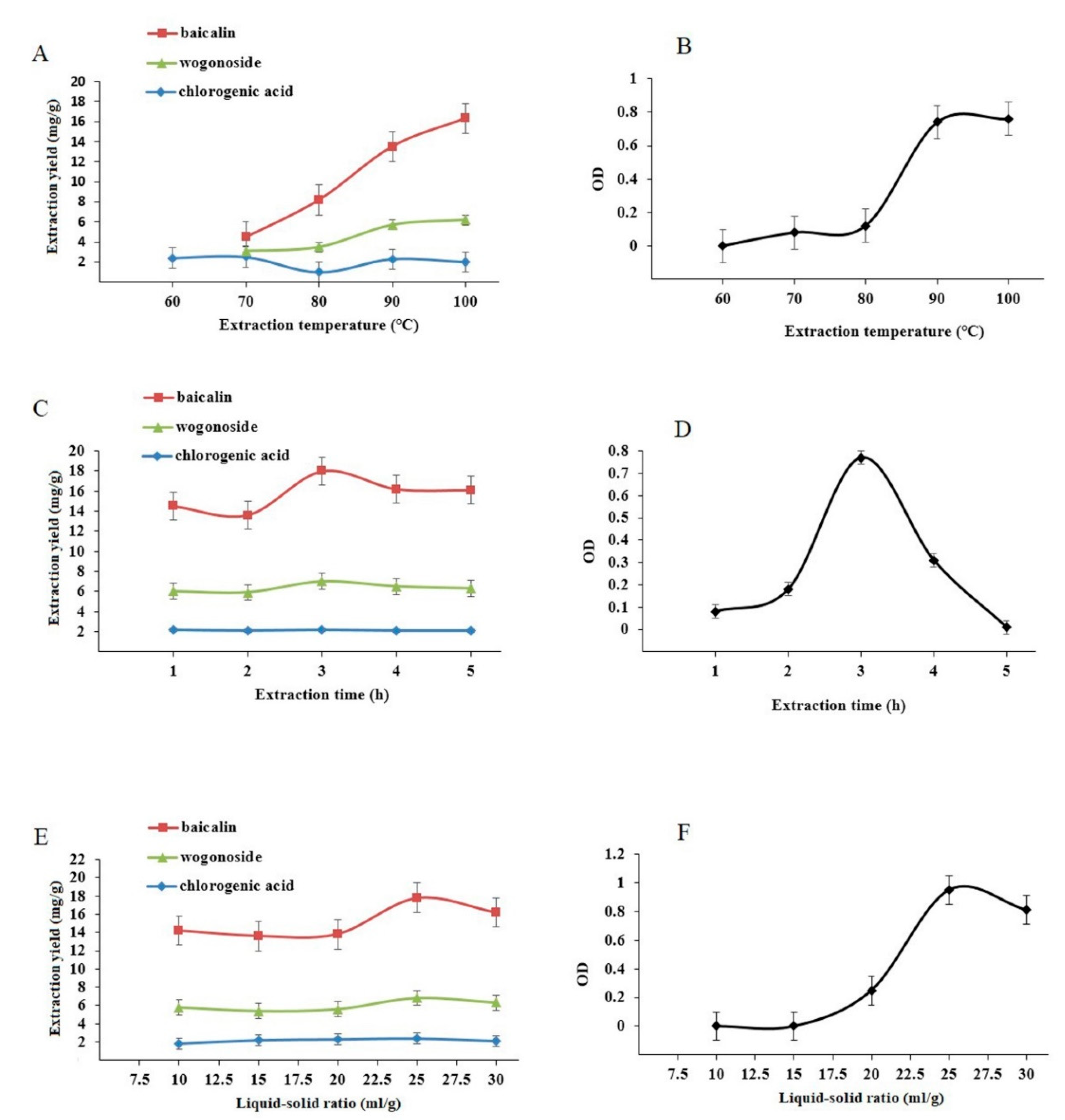
3.5. Single-Factor Experiments
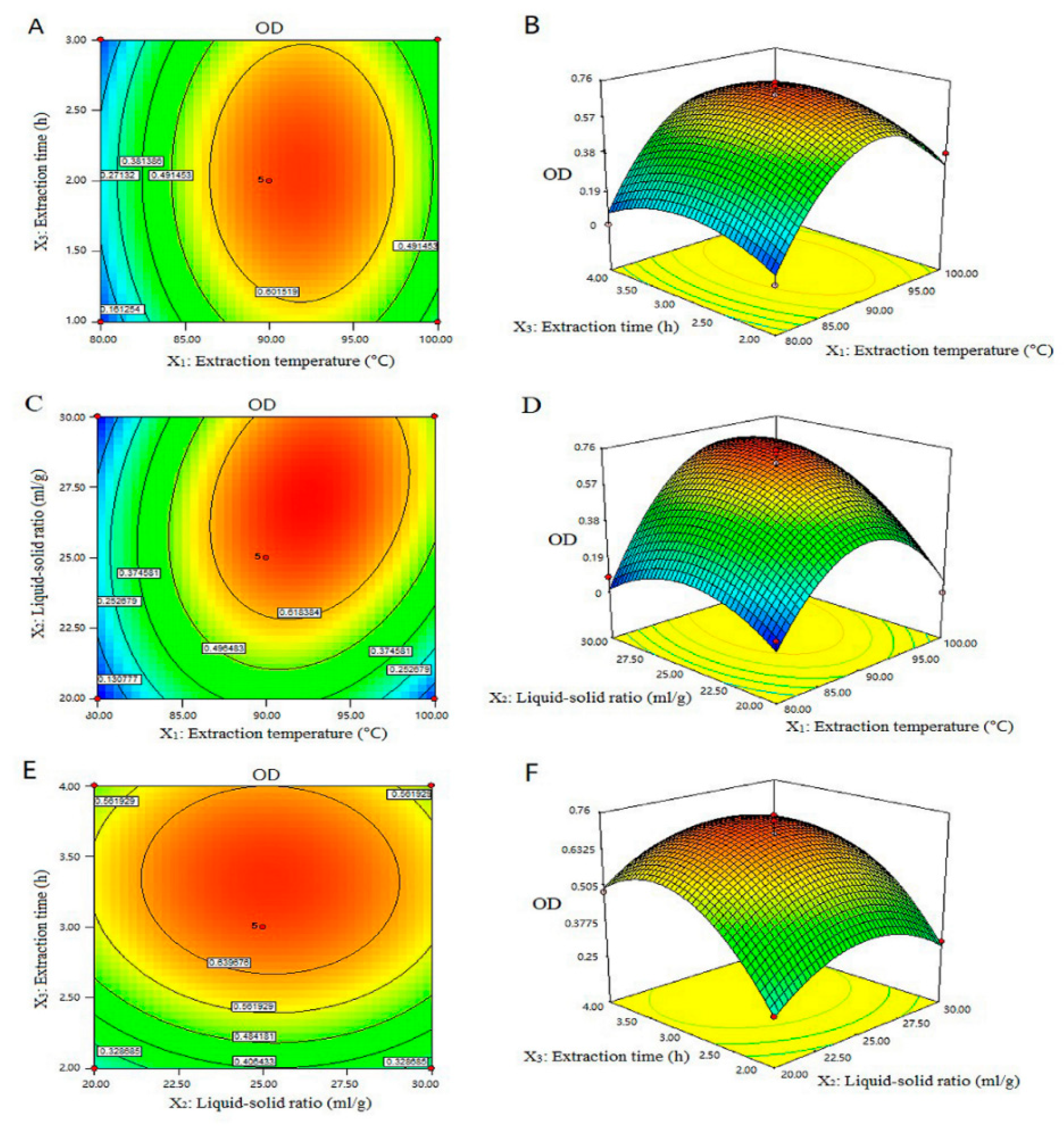
3.6. Optimization of the Extraction Parameters by Box–Behnken Design and Response Surface Methodology
0.011285X1X3 − 1.29500E − 003X2X3 − 3.65725E − 003X12 − 5.29400E − 003X22 − 0.17628X32
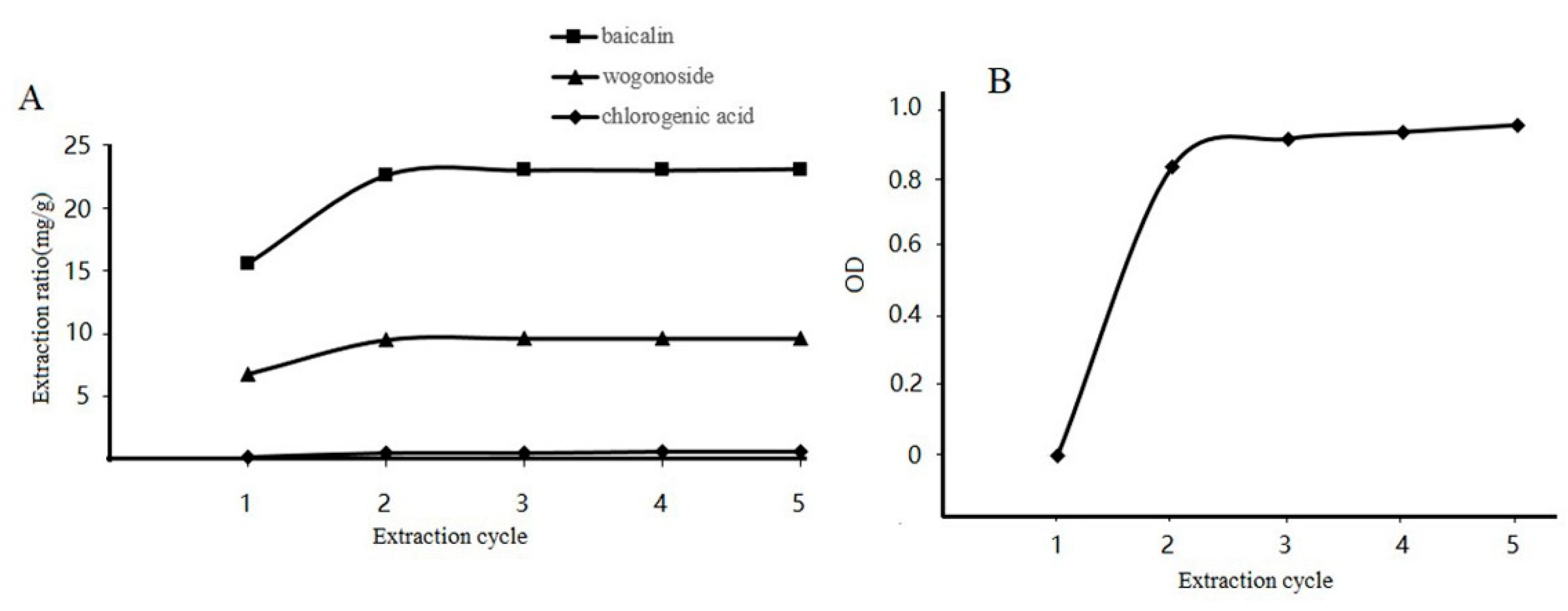
3.7. Effect of Extraction Cycle on Baicalin, Wogonoside, and Chlorogenic Acid Content
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Shang, X.; He, X.; He, X.; Li, M.; Zhang, R.; Fan, P.; Zhang, Q.; Jia, Z. The genus Scutellaria an ethnopharmacological and phytochemical review. J. Ethnopharmacol. 2010, 128, 279–313. [Google Scholar] [CrossRef] [PubMed]
- Li-Weber, M. New therapeutic aspects of flavones: The anticancer properties of Scutellaria and its main active constituents Wogonin, Baicalein and Baicalin. Cancer Treat. Rev. 2009, 35, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Chen, X.Y.; Martin, C. Scutellaria baicalensis, the golden herb from the garden of Chinese medicinal plants. Sci. Bull. 2016, 61, 1391–1398. [Google Scholar] [CrossRef] [PubMed]
- Xue, T.H.; Roy, R. Studying traditional Chinese medicine. Science 2003, 300, 740–741. [Google Scholar] [CrossRef]
- Zhang, L.; Ren, Y.; Yang, C.; Guo, Y.; Zhang, X.; Hou, G.; Guo, X.; Sun, N.; Liu, Y. Wogonoside ameliorates lipopolysaccharide-induced acute lung injury in mice. Inflammation 2014, 37, 2006–2012. [Google Scholar] [CrossRef]
- Kovacs, G.; Kuzokina, I.N.; Szoke, E.; Kursinszki, L. HPLC determination of flavonoids in hairy-root cultures of Scutellaria baicalensis Georgi. Chromatographia 2004, 60, S81–S85. [Google Scholar] [CrossRef]
- Zhang, T.; Mi, M.T.; Tang, Y.; Zhao, J. The extraction of polyphenol contents of Chaenomeles sinensis and its effect on scavenging DPPH radical. Acta Nutr. Sin. 2007, 29, 485–489. [Google Scholar]
- Cai, H.; Cao, G.; Li, L.; Liu, X.; Ma, X.-Q.; Tu, S.-C.; Lou, Y.-J.; Qin, K.-M.; Li, S.-L.; Cai, B.-C. Profiling and characterization of volatile components from non-fumigated and sulfur-fumigated flos Lonicerae japonicae using comprehensive two-dimensional gas Chromatography time-of-flight mass spectrometry coupled with chemical group separation. Molecules 2013, 18, 1368–1382. [Google Scholar] [CrossRef]
- Wu, M.Z.; Xu, Y.L.; Li, F.; Wei, P.; Wang, Z.; Luo, Z.P.; Wang, R.; Zhang, J.F.; Lin, F.C.; Yang, J. Cloning and expression analysis of chlorogenic acid biosynthetic gene NtHQT1 from Nicotiana tabacum. Tob. Sci. Technol. 2015, 48, 1–6. [Google Scholar] [CrossRef]
- Nascu-Briciu, R.D.; Cobzac, S.C.; Baciu, S. Optimum ultrasound assisted extraction conditions of some flavonoids from green tea leaves. Control quality of green tea product by TLC fingerprinting. Anal. Lett. 2011, 44, 2865–2875. [Google Scholar] [CrossRef]
- Repolles, C.; Herrero-Martinez, J.M.; Rafols, C. Analysis of prominent flavonoid aglycones by high-performance liquid chromatography using a monolithic type column. J. Chromatogr. A. 2006, 1131, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Al-Temimi, A.; Choudahry, R. Determination of antioxidant activity in different kinds of plants in vivo and in vitro by using diverse technical methods. J. Nutr. Food Sci. 2013, 3, 1–6. [Google Scholar] [CrossRef]
- Herrera, M.C.; de Castro, M.D.L. Ultrasound-assisted extraction of phenolic compounds from strawberries prior to liquid chromatographic separation and photodiode array ultraviolet detection. J. Chromatogr. A. 2005, 1100, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bajer, T.; Adam, M.; Galla, L.; Ventura, K. Comparison of various extraction techniques for isolation and determination of isoflavonoids in plants. J. Sep. Sci. 2007, 30, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Elerath, J.; Pecht, M. A highly accurate method for assessing reliability of redundant arrays of inexpensive disks (RAID). IEEE Trans. Comput. 2009, 58, 289–299. [Google Scholar] [CrossRef]
- Trinh, T.K.; Kang, L.S. Application of response surface method as an experimental design to optimize coagulation test. Environ. Eng. Res. 2010, 15, 63–70. [Google Scholar] [CrossRef]
- Cui, X.; Muhammad, I.; Li, R.; Jin, H.; Guo, Z.; Yang, Y.; Hamid, S.; Li, J.; Cheng, P.; Zhang, X. Development of a UPLC-FLD Method for Detection of Aflatoxin B1 and M1 in Animal Tissue to Study the Effect of Curcumin on Mycotoxin Clearance Rates. Front. Pharmacol. 2017, 8, 650. [Google Scholar] [CrossRef]
- Ekberg, C.; Fermvik, A.; Retegan, T.; Skarnemark, G.; Foreman, M.R.S.; Hudson, M.J.; Englund, S.; Nilsson, M. An overview and historical look back at the solvent extraction using nitrogen donor ligands to extract and separate An (III) from Ln(III). Radiochim. Acta 2008, 96, 225–233. [Google Scholar] [CrossRef]
- Sheng, Z.; Zhao, J.; Muhammad, I.; Zhang, Y. Optimization of total phenolic content from Terminalia chebula Retz. fruits using response surface methodology and evaluation of their antioxidant activities. PLoS ONE 2018, 13, e0202368. [Google Scholar] [CrossRef]
- Feng, J.; Xu, W.; Tao, X.; Wei, H.; Cai, F.; Jiang, B.; Chen, W. Simultaneous determination of baicalin, baicalein, wogonin, berberine, palmatine and jatrorrhizine in rat plasma by liquid chromatography–tandem mass spectrometry and application in pharmacokinetic studies after oral administration of traditional Chinese medicinal preparations containing scutellaria–coptis herb couple. J. Pharm. Biomed. Anal. 2010, 53, 591–598. [Google Scholar]
- Ni, H.; Wu, Z.; Muhammad, I.; Lu, Z.; Li, J. Optimization of baicalin water extraction process from Scutellaria baicalensis (a traditional Chinese medicine) by using orthogonal test and HPLC. Rev. Bras. Farmacogn. 2017. [Google Scholar] [CrossRef]
- Sun, Y.; Li, T.; Yan, J.; Liu, J. Technology optimization for polysaccharides (POP) extraction from the fruiting bodies of Pleurotus ostreatus by Box–Behnken statistical design. Carbohydr. Polym. 2010, 80, 242–247. [Google Scholar] [CrossRef]
- Zhang, T.F.; Yang, J.F.; Lin, D.K.J. Small Box–Behnken design. Stat. Probab. Lett. 2011, 81, 1027–1033. [Google Scholar] [CrossRef]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response Surface Methodology (RSM) as a Tool for Optimization in Analytical Chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef]
- Pinho, C.; Melo, A.; Mansilha, C.; Ferreira, I.M.P.L.V.O. Optimization of Conditions for Anthocyanin Hydrolysis from Red Wine Using Response Surface Methodology (RSM). J. Agric. Food Chem. 2011, 59, 50–55. [Google Scholar] [CrossRef]
- Altemimi, A.; Watson, D.G.; Kinsel, M.; Lightfoot, D.A. Simultaneous extraction, optimization, and analysis of flavonoids and polyphenols from peach and pumpkin extracts using a TLC-densitometric method. Chem. Central J. 2015, 9, 39. [Google Scholar] [CrossRef] [Green Version]
| Independent Variables | Symbols | Coded Levels | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| Extraction temperature (°C) | X1 | 80 | 90 | 100 |
| Liquid–solid ratio (mL/g) | X2 | 20:1 | 25:1 | 30:1 |
| Extraction time (h) | X3 | 2 | 3 | 4 |
| Concentrations (mg/mL) | RSD% | ||
|---|---|---|---|
| 0.04 | 1,400,829.8 ± 11,486.7 | 0.82 | |
| Baicalin | 0.12 | 4,176,222.6 ± 11,373.6 | 0.27 |
| 0.20 | 6,904,145.2 ± 17,260.3 | 0.25 | |
| 0.02 | 399,412.4 ± 3708.5 | 0.93 | |
| Wogonoside | 0.06 | 1,196,842.2 ± 8566.3 | 0.72 |
| 0.10 | 2,065,237 ± 11,709.9 | 0.57 | |
| 0.01 | 56,175.3 ± 876.3 | 1.56 | |
| Chlorogenic acid | 0.03 | 165,526.9 ± 2284.9 | 1.38 |
| 0.05 | 478,578.2 ± 4508 | 0.94 |
| Stability | Reproducibility | |||
|---|---|---|---|---|
| RSD | RSD | |||
| Baicalin | 3,922,371 ± 44,077.5 | 1.12% | 3,969,422 ± 83,402.7 | 2.10% |
| Wogonoside | 1,582,977 ± 25,327.6 | 1.60% | 1,552,831 ± 34,628.1 | 2.23% |
| Chlorogenic acid | 235,760 ± 2678.3 | 1.14% | 236,604 ± 6881.4 | 2.91% |
| Weight of Compounds in the Extract Samples (mg) | Weight of Standard Substances Added (mg) | Measured Quantity (mg) | RSD% | ||
|---|---|---|---|---|---|
| Baicalin | 1.093 ± 0.0262 | 1 | 1.1937 ± 0.019 | 100.69 ± 1.23 | 1.22 |
| Wogonoside | 0.448 ± 0.0103 | 0.45 | 0.4938 ± 0.003 | 101.68 ± 2.05 | 2.02 |
| Chlorogenic acid | 0.153 ± 0.0032 | 0.15 | 0.3024 ± 0.006 | 99.6 ± 1.56 | 1.57 |
| No. | X1 (°C) | X2 (ml/g) | X3 (h) | Extraction Ratio (mg/g) | OD | ||
|---|---|---|---|---|---|---|---|
| Baicalin | Wogonoside | Chlorogenic Acid | |||||
| 1 | 80 | 20 | 3 | 8.46 | 4.58 | 2.54 | 0 |
| 2 | 100 | 20 | 3 | 17.02 | 5.24 | 1.90 | 0.39 |
| 3 | 80 | 30 | 3 | 8.46 | 7.58 | 2.52 | 0.00 |
| 4 | 100 | 30 | 3 | 17.30 | 5.34 | 1.92 | 0.41 |
| 5 | 80 | 25 | 2 | 8.52 | 4.00 | 2.36 | 0.06 |
| 6 | 100 | 25 | 2 | 18.58 | 3.78 | 1.74 | 0 |
| 7 | 80 | 25 | 4 | 8.54 | 4.10 | 2.62 | 0.09 |
| 8 | 100 | 25 | 4 | 17.10 | 5.58 | 1.98 | 0.48 |
| 9 | 90 | 20 | 2 | 12.88 | 4.72 | 1.88 | 0.25 |
| 10 | 90 | 30 | 2 | 14.18 | 4.94 | 1.90 | 0.31 |
| 11 | 90 | 20 | 4 | 13.38 | 5.62 | 2.18 | 0.48 |
| 12 | 90 | 30 | 4 | 13.44 | 5.72 | 2.22 | 0.51 |
| 13 | 90 | 25 | 3 | 14.64 | 6.30 | 2.38 | 0.66 |
| 14 | 90 | 25 | 3 | 15.04 | 7.04 | 2.42 | 0.75 |
| 15 | 90 | 25 | 3 | 14.92 | 6.38 | 2.40 | 0.69 |
| 16 | 90 | 25 | 3 | 15.44 | 6.56 | 2.44 | 0.73 |
| 17 | 90 | 25 | 3 | 14.38 | 6.26 | 2.40 | 0.65 |
| Source | Sum of Squares | Degree of Freedom | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 1.160 | 9 | 0.129 | 25.800 | 0.0001 * |
| X1 | 0.160 | 1 | 0.160 | 32.051 | 0.0008 * |
| X2 | 0.001 | 1 | 0.001 | 0.292 | 0.6059 |
| X3 | 0.111 | 1 | 0.111 | 22.271 | 0.0022 * |
| X1 X2 | 0.000 | 1 | 0.000 | 0.0367 | 0.8535 |
| X1 X3 | 0.051 | 1 | 0.051 | 10.191 | 0.0152 * |
| X2 X3 | 0.000 | 1 | 0.000 | 0.034 | 0.8599 |
| X12 | 0.563 | 1 | 0.563 | 112.67 | <0.0001 * |
| X22 | 0.074 | 1 | 0.074 | 14.755 | 0.0064 * |
| X32 | 0.131 | 1 | 0.131 | 26.175 | 0.0014 * |
| Residual | 0.035 | 7 | 0.005 | ||
| Lack of fit | 0.028 | 3 | 0.009 | 5.104 | 0.0747 |
| Pure error | 0.007 | 4 | 0.002 | ||
| Cor total | 1.196 | 16 |
| Optimum Condition | Extraction Ratio (mg/g) | OD | |||||
|---|---|---|---|---|---|---|---|
| Extraction Temperature (°C) | Liquid–Solid Ratio (mL/g) | Extraction Time (h) | Baicalin | Wogonoside | Chlorogenic Acid | Experimental | Predicted |
| 93 (92.58) | 25 (25.23) | 2.4 (2.42) | 15.6 | 6.2 | 2.6 | 0.759 ± 0.09 | 0.741 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Wang, R.; Sheng, Z.; Wu, Z.; Chen, C.; Ishfaq, M. Optimization of Baicalin, Wogonoside, and Chlorogenic Acid Water Extraction Process from the Roots of Scutellariae Radix and Lonicerae japonicae Flos Using Response Surface Methodology (RSM). Processes 2019, 7, 854. https://doi.org/10.3390/pr7110854
Li J, Wang R, Sheng Z, Wu Z, Chen C, Ishfaq M. Optimization of Baicalin, Wogonoside, and Chlorogenic Acid Water Extraction Process from the Roots of Scutellariae Radix and Lonicerae japonicae Flos Using Response Surface Methodology (RSM). Processes. 2019; 7(11):854. https://doi.org/10.3390/pr7110854
Chicago/Turabian StyleLi, Jichang, Rui Wang, Zunlai Sheng, Zhiyong Wu, Chunli Chen, and Muhammad Ishfaq. 2019. "Optimization of Baicalin, Wogonoside, and Chlorogenic Acid Water Extraction Process from the Roots of Scutellariae Radix and Lonicerae japonicae Flos Using Response Surface Methodology (RSM)" Processes 7, no. 11: 854. https://doi.org/10.3390/pr7110854
APA StyleLi, J., Wang, R., Sheng, Z., Wu, Z., Chen, C., & Ishfaq, M. (2019). Optimization of Baicalin, Wogonoside, and Chlorogenic Acid Water Extraction Process from the Roots of Scutellariae Radix and Lonicerae japonicae Flos Using Response Surface Methodology (RSM). Processes, 7(11), 854. https://doi.org/10.3390/pr7110854






