Simultaneous Removal of Calconcarboxylic Acid, NH4+ and PO43− from Pharmaceutical Effluent Using Iron Oxide-Biochar Nanocomposite Loaded with Pseudomonas putida
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Water Sample Collection
2.2. Preparation of the Iron Oxide/Biochar Nanocomposite
2.3. Bacteria Cultivation and Immobilization on the Iron Oxide/Biochar Nanocomposite
2.4. Industrial Wastewater Content and Batch Study
3. Results and Discussion
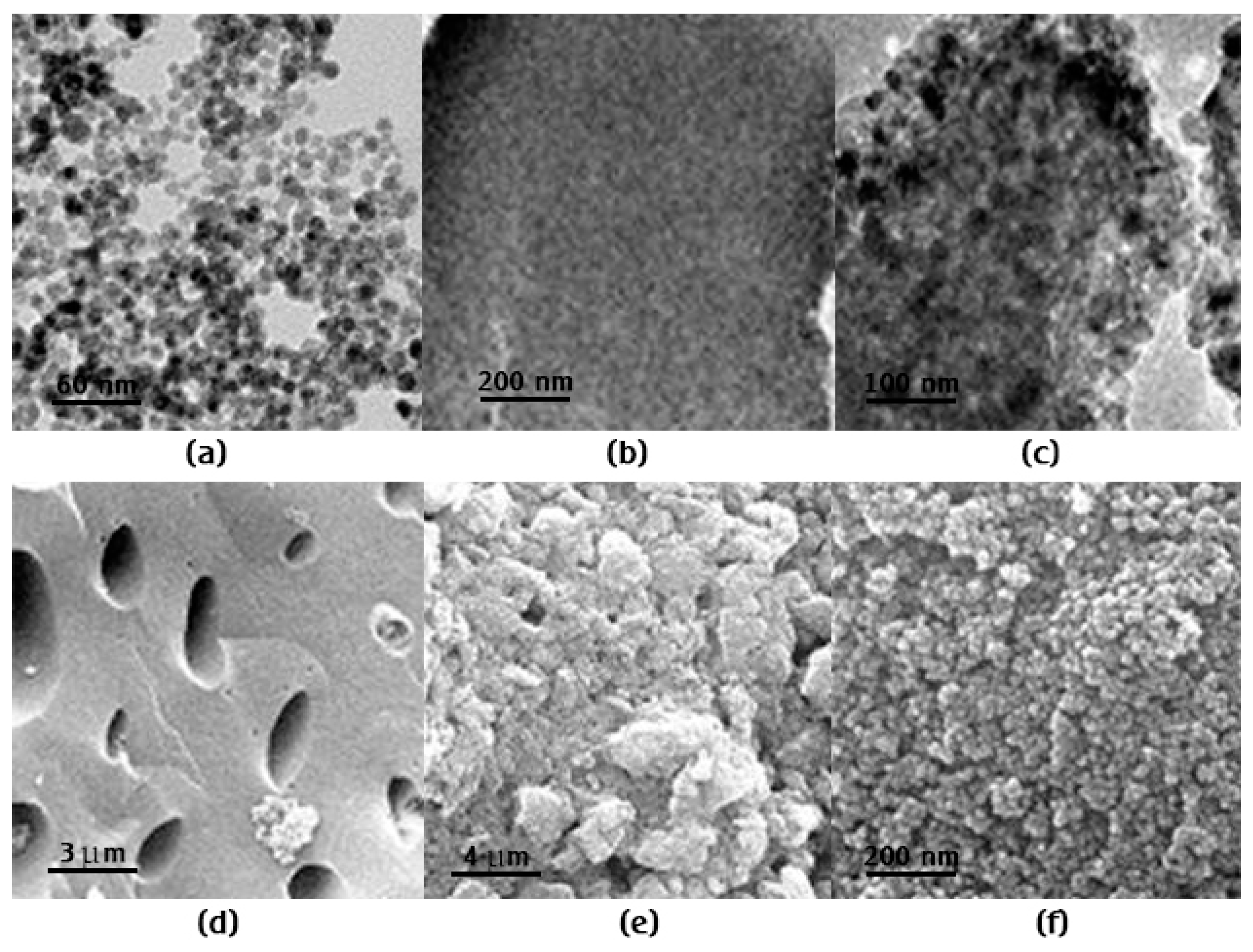
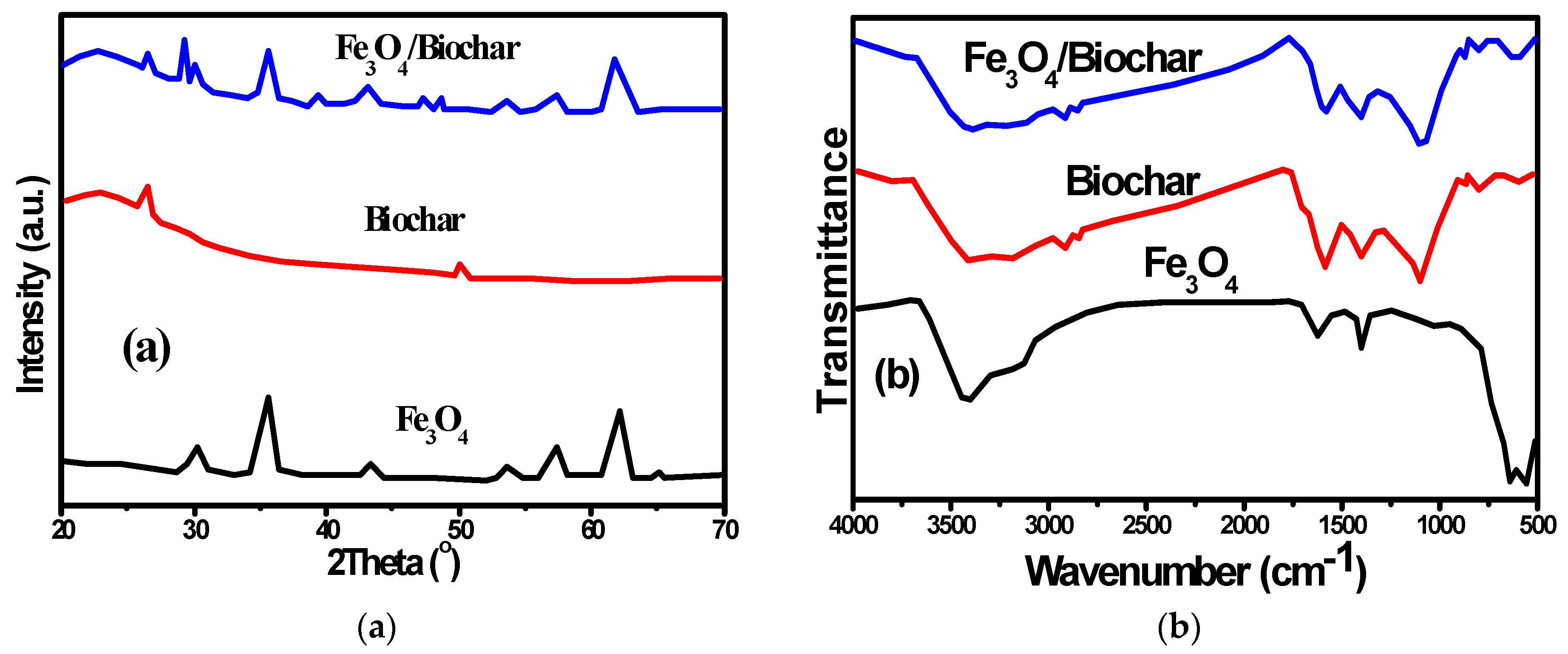
3.1. Characterization of the P. putida/Fe3O4/Biochar Composite
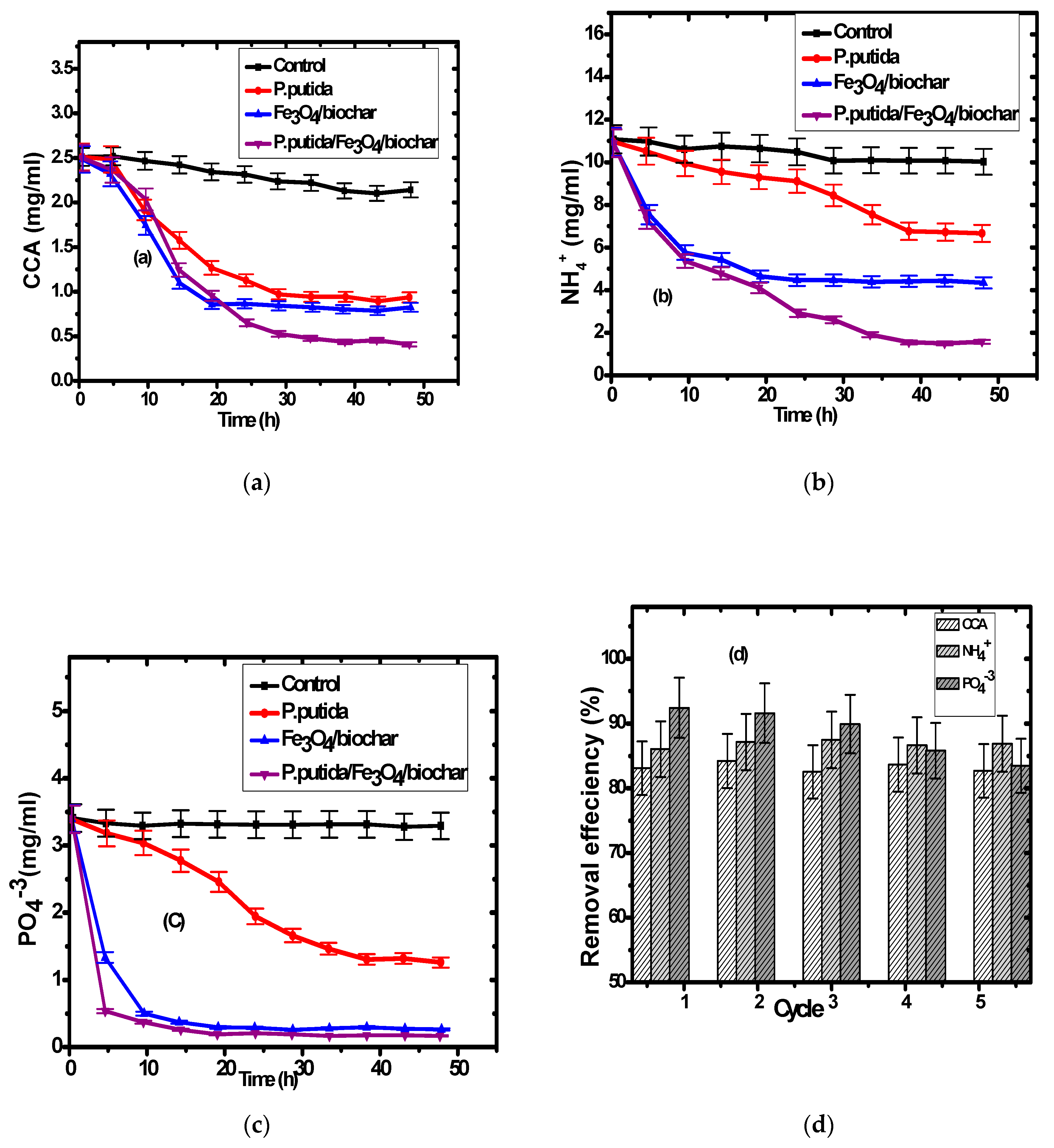
3.2. Pollutants Removal
3.3. Composite Reusability for Wastewater Treatment
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Patton, J.; Reeder, W. New indicator for titration of calcium with (ethylenedinitrilo) tetraacetate. Anal. Chem. 1956, 28, 1026–1028. [Google Scholar] [CrossRef]
- Sahoo, M.K.; Sinha, B.; Sharan, R.N. Metal ion-catalyzed mineralization and biodetoxification studies of Calconcarboxylic acid in aqueous solution: Effect of–COOH group. Desalin. Water Treat. 2015, 56, 1955–1963. [Google Scholar] [CrossRef]
- Shukla, V.Y.; Tipre, D.R.; Dave, S.R. Optimization of chromium (VI) detoxification by Pseudomonas aeruginosa and its application for treatment of industrial waste and contaminated soil. Bioremediat. J. 2014, 18, 128–135. [Google Scholar] [CrossRef]
- Vassilev, N.; Fenice, M.; Federici, F.; Azcon, R. Olive mill waster water treatment by immobilized cells of Aspergillus niger and its enrichment with soluble phosphate. Process Biochem. 1997, 32, 617–620. [Google Scholar] [CrossRef]
- Liu, B.-F.; Jin, Y.-R.; Cui, Q.-F.; Xie, G.-J.; Wu, Y.-N.; Ren, N.-Q. Photo-fermentation hydrogen production by Rhodopseudomonas sp. nov. strain A7 isolated from the sludge in a bioreactor. Int. J. Hydrog. Energy 2015, 40, 8661–8668. [Google Scholar] [CrossRef]
- Nagadomi, H.; Kitamura, T.; Watanabe, M.; Sasaki, K. Simultaneous removal of chemical oxygen demand (COD), phosphate, nitrate and H2S in the synthetic sewage wastewater using porous ceramic immobilized photosynthetic bacteria. Biotechnol. Lett. 2000, 22, 1369–1374. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, G.; Dai, X.; He, C. Photosynthetic bacteria treatment of synthetic soybean wastewater: Direct degradation of macromolecules. Bioresour. Technol. 2010, 101, 7672–7674. [Google Scholar] [CrossRef]
- Idi, A.; Nor, M.H.M.; Wahab, M.F.A.; Ibrahim, Z. Photosynthetic bacteria: An eco-friendly and cheap tool for bioremediation. Rev. Environ. Sci. Bio/Technol. 2015, 14, 271–285. [Google Scholar] [CrossRef]
- Heisler, J.; Glibert, P.M.; Burkholder, J.M.; Anderson, D.; Cochlan, W.; Dennison, W.C.; Dortch, Q.; Gobler, C.J.; Heil, C.A.; Humphries, E. Eutrophication and harmful algal blooms: A scientific consensus. Harmful Algae 2008, 8, 3–13. [Google Scholar] [CrossRef]
- Barca, C.; Gérente, C.; Meyer, D.; Chazarenc, F.; Andrès, Y. Phosphate removal from synthetic and real wastewater using steel slags produced in Europe. Water Res. 2012, 46, 2376–2384. [Google Scholar] [CrossRef]
- Kim, J.Y.; Balathanigaimani, M.; Moon, H. Adsorptive removal of nitrate and phosphate using MCM-48, SBA-15, chitosan, and volcanic pumice. Water Air Soil Pollut. 2015, 226, 431. [Google Scholar] [CrossRef]
- Mazloomi, F.; Jalali, M. Ammonium removal from aqueous solutions by natural Iranian zeolite in the presence of organic acids, cations and anions. J. Environ. Chem. Eng. 2016, 4, 240–249. [Google Scholar] [CrossRef]
- Zhang, T.; Li, Q.; Ding, L.; Ren, H.; Xu, K.; Wu, Y.; Sheng, D. Modeling assessment for ammonium nitrogen recovery from wastewater by chemical precipitation. J. Environ. Sci. 2011, 23, 881–890. [Google Scholar] [CrossRef]
- Lucas, D.; Barceló, D.; Rodriguez-Mozaz, S. Removal of pharmaceuticals from wastewater by fungal treatment and reduction of hazard quotients. Sci. Total Environ. 2016, 571, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Leong, K.Y.; Ng, H.Y. Anaerobic treatment of pharmaceutical wastewater: A critical review. Bioresour. Technol. 2017, 245, 1238–1244. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Bhattacharjee, C.; Sarkar, S. Studies on the performance of annular photo reactor (APR) for pharmaceutical wastewater treatment. J. Water Process Eng. 2017, 19, 26–34. [Google Scholar] [CrossRef]
- Zhan, J.; Li, Z.; Yu, G.; Pan, X.; Wang, J.; Zhu, W.; Han, X.; Wang, Y. Enhanced treatment of pharmaceutical wastewater by combining three-dimensional electrochemical process with ozonation to in situ regenerate granular activated carbon particle electrodes. Sep. Purif. Technol. 2019, 208, 12–18. [Google Scholar] [CrossRef]
- Kataria, N.; Garg, V. Green synthesis of Fe3O4 nanoparticles loaded sawdust carbon for cadmium (II) removal from water: Regeneration and mechanism. Chemosphere 2018, 208, 818–828. [Google Scholar] [CrossRef]
- Wu, J.; Huang, D.; Liu, X.; Meng, J.; Tang, C.; Xu, J. Remediation of As (III) and Cd (II) co-contamination and its mechanism in aqueous systems by a novel calcium-based magnetic biochar. J. Hazard. Mater. 2018, 348, 10–19. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. Biochar for Environmental Management: Science, Technology and Implementation; Routledge: Abingdon-on-Thames, UK, 2015. [Google Scholar]
- Dominic Woolf, D.; Amonette, J.E.; Street-Perrott, F.A.; Lehmann, J.; Joseph, S. Sustainable biochar to mitigate global climate change. Nat. Commun. 2010, 1, 56. [Google Scholar] [CrossRef]
- Van Zwieten, L.; Kimber, S.; Morris, S.; Chan, K.Y.; Downie, A.; Rust, J.; Joseph, S.; Cowie, A. Effects of biochar from slow pyrolysis of papermill waste on agronomic performance and soil fertility. Plant Soil 2010, 327, 235–246. [Google Scholar] [CrossRef]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 203. [Google Scholar] [CrossRef] [PubMed]
- Sujana, P. The application of biochar to screen printing liquid waste polluted land, its effect in soil, mustard greens to heavy metals (Fe, Cr). RJTA 2018, 22, 224–234. [Google Scholar] [CrossRef]
- Sarpong, K.A.; Salazar, A.; Ortega, A.; Telles, K.; Djaman, K.; O’Neill, M.K.; Valles-Rosales, D.J.; Brewer, C.E. Pyrolysis of Wood Excelsior Residues for Biochar and Renewable Energy Production. In Proceedings of the 2018 ASABE Annual International Meeting: American Society of Agricultural and Biological Engineers, Detroit, MI, USA, 29 July–1 August 2018. [Google Scholar]
- Chen, Z.-H.; Du, X.L.; He, J.B.; Li, F.; Wang, H.; Li, Y.L.; Li, B.; Xin, S. Porous coconut shell carbon offering high retention and deep lithiation of sulfur for lithium–sulfur batteries. ACS Appl. Mater. Interfaces 2017, 9, 33855–33862. [Google Scholar] [CrossRef]
- El-Naggar, A.; Lee, S.S.; Awad, Y.M.; Yang, X.; Ryu, C.; Rizwan, M.; Ringklebe, J.; Tsang, D.C.W.; Ok, Y.S. Influence of soil properties and feedstocks on biochar potential for carbon mineralization and improvement of infertile soils. Geoderma 2018, 332, 100–108. [Google Scholar] [CrossRef]
- Lin, Y.; Munroe, P.; Joseph, S.; Henderson, R.; Ziolkowski, A. Water extractable organic carbon in untreated and chemical treated biochars. Chemosphere 2012, 87, 151–157. [Google Scholar] [CrossRef]
- Qin, H.Z.; Liu, Y.Y.; Li, L.Q.; Pan, G.X.; Zhang, X.H.; Zheng, J.W. Adsorption of cadmium solution by biochar from household biowaste. J. Ecol. Rural. Environ. 2012, 28, 181–186. [Google Scholar]
- Xu, T.; Lou, L.; Luo, L.; Cao, R.; Duan, D.; Chen, Y. Effect of bamboo biochar on pentachlorophenol leachability and bioavailability in agricultural soil. Sci. Total Environ. 2012, 414, 727–731. [Google Scholar] [CrossRef]
- Akhavan, O.; Azimirad, R. Photocatalytic property of Fe2O3 nanograin chains coated by TiO2 nanolayer in visible light irradiation. Appl. Catal. A Gen. 2009, 369, 77–82. [Google Scholar] [CrossRef]
- Khusnutdinova, A.N.; Ovchenkova, E.P.; Khristova, A.P.; Laurinavichene, T.V.; Shastik, E.S.; Liu, J.; Tsygankov, A.A. New tolerant strains of purple nonsulfur bacteria for hydrogen production in a two-stage integrated system. Int. J. Hydrog. Energy 2012, 37, 8820–8827. [Google Scholar] [CrossRef]
- Kato, S.; Hashimoto, K.; Watanabe, K. Methanogenesis facilitated by electric syntrophy via (semi) conductive iron-oxide minerals. Environ. Microbiol. 2012, 14, 1646–1654. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cheng, X.; Sun, D.; Ren, Y.; Xu, G. Simultaneous nutrient and carbon removal from azo dye wastewater using a photorotating biological contactor reactor. J. Chem. Technol. Biotechnol. 2014, 89, 1545–1552. [Google Scholar] [CrossRef]
- Chen, J.; Yan, L.G.; Yu, H.Q.; Li, S.; Qin, L.L.; Liu, G.Q.; Li, Y.F.; Du, B. Efficient removal of phosphate by facile prepared magnetic diatomite and illite clay from aqueous solution. J. Environ. Chem. Eng. 2016, 287, 162–172. [Google Scholar] [CrossRef]
- Wang, W.; Ding, Y.; Wang, Y.; Song, X.; Ambrose, R.F.; Ullman, J.L.; Winfrey, B.K.; Wang, J.; Gong, J. Treatment of rich ammonia nitrogen wastewater with polyvinyl alcohol immobilized nitrifier biofortified constructed wetlands. Ecol. Eng. 2016, 94, 7–11. [Google Scholar] [CrossRef]
- Eldyasti, A.; Andalib, M.; Hafez, H.; Nakhla, G.; Zhu, J. Comparative modeling of biological nutrient removal from landfill leachate using a circulating fluidized bed bioreactor (CFBBR). J. Hazard. Mater. 2011, 187, 140–149. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
M. Siddeeg, S.; A. Tahoon, M.; Ben Rebah, F. Simultaneous Removal of Calconcarboxylic Acid, NH4+ and PO43− from Pharmaceutical Effluent Using Iron Oxide-Biochar Nanocomposite Loaded with Pseudomonas putida. Processes 2019, 7, 800. https://doi.org/10.3390/pr7110800
M. Siddeeg S, A. Tahoon M, Ben Rebah F. Simultaneous Removal of Calconcarboxylic Acid, NH4+ and PO43− from Pharmaceutical Effluent Using Iron Oxide-Biochar Nanocomposite Loaded with Pseudomonas putida. Processes. 2019; 7(11):800. https://doi.org/10.3390/pr7110800
Chicago/Turabian StyleM. Siddeeg, Saifeldin, Mohamed A. Tahoon, and Faouzi Ben Rebah. 2019. "Simultaneous Removal of Calconcarboxylic Acid, NH4+ and PO43− from Pharmaceutical Effluent Using Iron Oxide-Biochar Nanocomposite Loaded with Pseudomonas putida" Processes 7, no. 11: 800. https://doi.org/10.3390/pr7110800
APA StyleM. Siddeeg, S., A. Tahoon, M., & Ben Rebah, F. (2019). Simultaneous Removal of Calconcarboxylic Acid, NH4+ and PO43− from Pharmaceutical Effluent Using Iron Oxide-Biochar Nanocomposite Loaded with Pseudomonas putida. Processes, 7(11), 800. https://doi.org/10.3390/pr7110800






