Oil Recovery from Palm Kernel Meal Using Subcritical Water Extraction in a Stirred Tank Reactor
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Meal Preparation
2.3. Subcritical Water Extraction
2.4. Soxhlet Extraction
2.5. Gas Chromatography
2.6. Extraction Yield Determination
2.7. Experimental Execution
3. Results and Discussion
3.1. Experimental Region Exploration
3.2. Screening Experimentaion
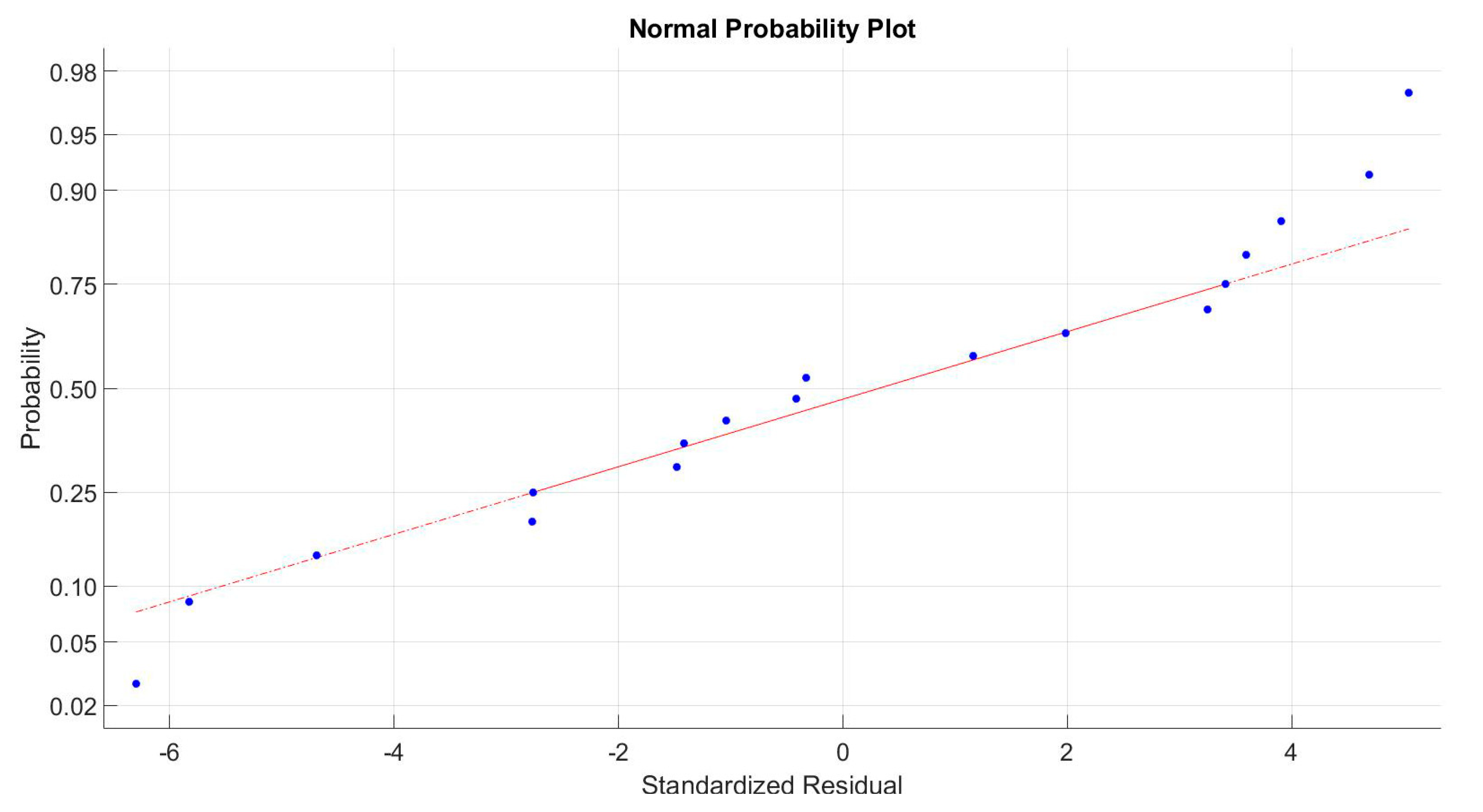
3.3. Statistical Analysis
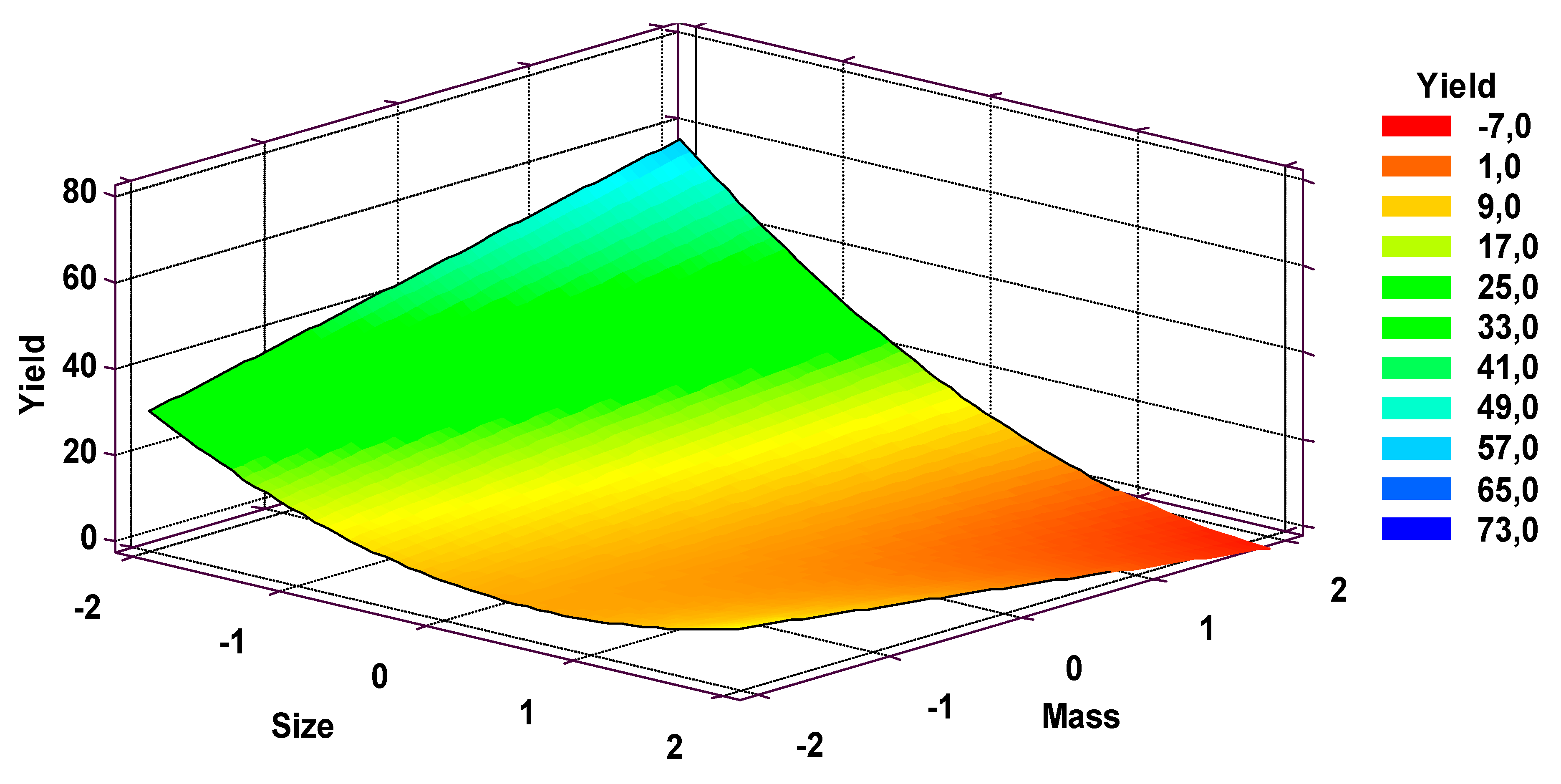
3.4. Response Surface Experimentation
3.5. Response Surface Statistical Analysis
3.6. Regression Model
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hutagalung, R.; Mahyuddin, M.; Vijchulata, J. Nutrient availability and utilization of feedstuffs for farm animals. In Feed Information and Animal Production; Commonwealth Agricultural Bureaux: Farnham Royal, UK, 1983; pp. 497–506. [Google Scholar]
- AGROPALMA. Available online: http://agropalmahn.com/wp-content/uploads/2013/12/Aceite-de-Palmiste-PKO.pdf (accessed on 1 July 2019).
- JaaffarAhmad, M.; Pantzaris, T. Properties and Utilization of Palm Kernel Oil. Palmas 2002, 35, 11–23. [Google Scholar]
- Gámiz-Gracia, L.; Luque de Castro, M.D. Continuous subcritical water extraction of medicinal plant essential oil: Comparison with conventional techniques. Talanta 2000, 51, 1179–1185. [Google Scholar] [CrossRef]
- Holliday, R.L.; King, J.W.; List, G.R. Hydrolysis of Vegetable Oils in Sub- and Supercritical Water. Ind. Eng. Chem. Res. 1997, 36, 932–935. [Google Scholar] [CrossRef]
- Carr, A.G.; Mammucari, R.; Foster, N.R. A review of subcritical water as a solvent and its utilization for the processing of hydrophobic organic compounds. Chem. Eng. J. 2011, 172, 1–17. [Google Scholar] [CrossRef]
- Nezbeda, I.; Pavlíček, J. Application of primitive models of association: A simple theoretical equation of state of water. Fluid Phase Equilibria 1996, 116, 530–536. [Google Scholar] [CrossRef]
- Caffarena, E.R.; Grigera, J.R. On the hydrogen bond structure of water at different densities. Phys. A Stat. Mech. Appl. 2004, 342, 34–39. [Google Scholar] [CrossRef]
- Nakahara, M.; Matubayasi, N.; Wakai, C.; Tsujino, Y. Structure and dynamics of water: From ambient to supercritical. J. Mol. Liq. 2001, 90, 75–83. [Google Scholar] [CrossRef]
- Mazaheri, H.; Lee, K.T.; Bhatia, S.; Mohamed, A.R. Subcritical water liquefaction of oil palm fruit press fiber for the production of bio-oil: Effect of catalysts. Bioresour. Technol. 2010, 101, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Mazaheri, H.; Lee, K.T.; Bhatia, S.; Mohamed, A.R. Subcritical water liquefaction of oil palm fruit press fiber in the presence of sodium hydroxide: An optimization study using response surface methodology. Bioresour. Technol. 2010, 101, 9335–9341. [Google Scholar] [CrossRef] [PubMed]
- Mazaheri, H.; Lee, K.T.; Bhatia, S.; Mohamed, A.R. Sub/supercritical liquefaction of oil palm fruit press fiber for the production of bio-oil: Effect of solvents. Bioresour. Technol. 2010, 101, 7641–7647. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, J.; Kuang, S.K.; Amin, N.S. Liquefaction of empty palm fruit bunch (EPFB) in alkaline hot compressed water. Renew. Energy 2010, 35, 1220–1227. [Google Scholar] [CrossRef]
- Chan, Y.H.; Yusup, S.; Quitain, A.T.; Uemura, Y.; Sasaki, M. Bio-oil production from oil palm biomass via subcritical and supercritical hydrothermal liquefaction. J. Supercrit. Fluids 2014, 95, 407–412. [Google Scholar] [CrossRef]
- Kawamura, F.; Saary, N.S.; Hashim, R.; Sulaiman, O.; Hashida, K.; Otsuka, Y.; Nakamura, M.; Ohara, S. Subcritical Water Extraction of Low-molecular-weight Phenolic Compounds from Oil Palm Biomass. Jpn. Agric. Res. Q. 2014, 48, 355–362. [Google Scholar] [CrossRef]
- Cardenas-Toro, F.P.; Forster-Carneiro, T.; Rostagno, M.A.; Petenate, A.J.; Maugeri Filho, F.; Meireles, M.A.A. Integrated supercritical fluid extraction and subcritical water hydrolysis for the recovery of bioactive compounds from pressed palm fiber. J. Supercrit. Fluids 2014, 93, 42–48. [Google Scholar] [CrossRef]
- Norsyabilah, R.; Hanim, S.S.; Norsuhaila, M.H.; Noraishah, A.K.; Kartina, S. Subcritical Water Extraction of Monosaccharides from Oil Palm Fronds Hemicelluloses. Malays. J. Anal. Sci. 2013, 17, 272–275. [Google Scholar]
- Hanim, S.S.; Norsyabilah, R.; Suhaila, M.H.N.; Noraishah, A.; Kartina, A.K.S. Effects of temperature, time and pressure on the hemicelluloses yield extracted using subcritical water extraction. Procedia Eng. 2012, 42, 562–565. [Google Scholar] [CrossRef]
- Cocks, L.V.; van Rede, C. Laboratory Handbook for Oil and Fat Analysis; Acdemic Press Inc. Ltd.: New York, NY, USA, 1966. [Google Scholar]



| Run | Temperature (K) | Time (s) | Meal Mass (×10−3 kg) | Particle Size | Yield (%wt) |
|---|---|---|---|---|---|
| 1 | 428.15 | 720 | 12 | A | 0 |
| 2 | 418.15 | 720 | 20 | A | 0 |
| 3 | 428.15 | 480 | 12 | B | 8.22 |
| 4 | 428.15 | 480 | 20 | A | 1.85 |
| 5 | 418.15 | 480 | 20 | B | 16.19 |
| 6 | 418.15 | 720 | 12 | B | 10.87 |
| 7 | 428.15 | 720 | 20 | B | 24.55 |
| 8 | 418.15 | 480 | 12 | A | 5.44 |
| 9 | 428.15 | 480 | 20 | B | 7.4 |
| 10 | 418.15 | 720 | 12 | A | 3.82 |
| 11 | 418.15 | 720 | 20 | B | 24.56 |
| 12 | 418.15 | 480 | 20 | A | 2.47 |
| 13 | 428.15 | 480 | 12 | A | 2.80 |
| 14 | 428.15 | 720 | 20 | A | 8.51 |
| 15 | 418.15 | 480 | 12 | B | 13.03 |
| 16 | 428.15 | 720 | 12 | B | 13.08 |
| Source | Sum of Squares | Degrees of Freedom | Mean Square | F-Ratio | p-Value |
|---|---|---|---|---|---|
| Temperature | 6.21 | 1 | 6.21 | 0.26 | 0.6205 |
| Time | 48.96 | 1 | 48.96 | 2.05 | 0.1804 |
| Meal Mass | 49.95 | 1 | 49.95 | 2.09 | 0.1764 |
| Size | 540.68 | 1 | 540.68 | 22.60 | 0.0006 |
| Total Error | 263.22 | 11 | 23.93 | 0.26 | 0.6205 |
| Total | 909.02 | 15 |
| Source | Sum of Squares | Degrees of Freedom | Mean Square | F-Ratio | p-Value |
|---|---|---|---|---|---|
| B = Reaction Time | 48.96 | 1 | 48.96 | 3.12 | 0.0978 |
| C = Meal Mass | 49.95 | 1 | 49.95 | 3.18 | 0.0948 |
| D = Particle Size | 540.68 | 1 | 540.68 | 34.42 | 0.0000 |
| BC | 61.74 | 1 | 61.74 | 3.93 | 0.0661 |
| CD | 50.59 | 1 | 50.59 | 3.22 | 0.0929 |
| Total Error | 157.1 | 10 | 15.71 | ||
| Total | 909.02 | 15 |
| Run | Particle Size (mm) | Time (min) | Meal Mass (g) | Obtained Yield (%wt) |
|---|---|---|---|---|
| 1 | −1 | −1 | 1 | 21.99 |
| 2 | −1 | −1 | −1 | 18.60 |
| 3 | 1 | −1 | −1 | 8.08 |
| 4 | 1 | 1 | 1 | 1.41 |
| 5 | 0 | 1.68179 | 0 | 7.16 |
| 6 | −1 | 1 | −1 | 18.20 |
| 7 | 0 | 0 | 0 | 13.52 |
| 8 | 0 | 0 | −1.68179 | 7.95 |
| 9 | 0 | 0 | 0 | 13.18 |
| 10 | 1 | −1 | 1 | 2.83 |
| 11 | 1 | 1 | −1 | 2.62 |
| 12 | 0 | 0 | 0 | 3.63 |
| 13 | 0 | 0 | 0 | 8.51 |
| 14 | −1.68179 | 0 | 0 | 37.97 |
| 15 | −1 | 1 | 1 | 30.59 |
| 16 | 0 | −1.68179 | 0 | 7.17 |
| 17 | 1.68179 | 0 | 0 | 0.43 |
| 18 | 0 | 0 | 1.68179 | 12.74 |
| Source | Sum of Squares | Degrees of Freedom | Mean Square | F-Ratio | p-Value |
|---|---|---|---|---|---|
| A = Particle Size | 1386.68 | 1 | 1386.68 | 127.11 | 0.0000 |
| B = Time | 0.12 | 1 | 0.12 | 0.01 | 0.9172 |
| C = Meal Mass | 22.00 | 1 | 22.00 | 2.17 | 0.1789 |
| AA | 154.69 | 1 | 154.69 | 15.25 | 0.0045 |
| AB | 28.27 | 1 | 28.27 | 2.79 | 0.1335 |
| AC | 61.61 | 1 | 61.61 | 6.07 | 0.0390 |
| BB | 7.27 | 1 | 7.27 | 0.72 | 0.4219 |
| BC | 21.39 | 1 | 21.39 | 2.11 | 0.1845 |
| CC | 1.70 | 1 | 1.70 | 0.17 | 0.6932 |
| Total Error | 81.13 | 8 | 10.14 | ||
| Total | 1764.86 | 17 |
| Source | Sum of Squares | Degrees of Freedom | Mean Square | F-Ratio | p-Value |
|---|---|---|---|---|---|
| A = Particle Size | 1386.68 | 1 | 1386.68 | 98.59 | 0.0000 |
| C = Meal Mass | 22.00 | 1 | 22.00 | 1.56 | 0.2425 |
| AA | 173.32 | 1 | 173.32 | 12.32 | 0.0066 |
| AC | 61.61 | 1 | 61.61 | 4.38 | 0.0659 |
| Lack-of-fit | 15.23 | 4 | 3.81 | 0.27 | 0.8896 |
| Total Error | 126.59 | 9 | 14.06 | ||
| Total | 1785.43 | 17 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bustillo Maury, J.; Aldana Rico, A.; García Pinto, C.; Hernández Medina, I.; Urueta Urueta, J.; King, J.W.; Bula Silvera, A. Oil Recovery from Palm Kernel Meal Using Subcritical Water Extraction in a Stirred Tank Reactor. Processes 2019, 7, 797. https://doi.org/10.3390/pr7110797
Bustillo Maury J, Aldana Rico A, García Pinto C, Hernández Medina I, Urueta Urueta J, King JW, Bula Silvera A. Oil Recovery from Palm Kernel Meal Using Subcritical Water Extraction in a Stirred Tank Reactor. Processes. 2019; 7(11):797. https://doi.org/10.3390/pr7110797
Chicago/Turabian StyleBustillo Maury, Johnnys, Andrés Aldana Rico, Cindy García Pinto, Ingrid Hernández Medina, Juan Urueta Urueta, Jerry W. King, and Antonio Bula Silvera. 2019. "Oil Recovery from Palm Kernel Meal Using Subcritical Water Extraction in a Stirred Tank Reactor" Processes 7, no. 11: 797. https://doi.org/10.3390/pr7110797
APA StyleBustillo Maury, J., Aldana Rico, A., García Pinto, C., Hernández Medina, I., Urueta Urueta, J., King, J. W., & Bula Silvera, A. (2019). Oil Recovery from Palm Kernel Meal Using Subcritical Water Extraction in a Stirred Tank Reactor. Processes, 7(11), 797. https://doi.org/10.3390/pr7110797





